Abstract
AIM
Recent evidence suggests that ATP-mediated purinergic signaling at the level of the rostral ventrolateral medulla (RVLM) contributes to both central and peripheral chemoreceptor control of breathing and blood pressure: neurons in the retrotrapezoid nucleus (RTN) function as central chemoreceptors in part by responding to CO2-evoked ATP release by activation of yet unknown P2 receptors, and nearby catecholaminergic C1 neurons regulate blood pressure responses to peripheral chemoreceptor activation by a P2Y1 receptor dependent mechanism. However, potential contributions of purinergic signaling in the RTN to cardiorespiratory function in conscious animals have not been tested.
METHODS
Cardiorespiratory activity of unrestrained awake rats were measured in response to RTN injections of ATP, and during exposure to hypercapnia (7% CO2) or hypoxia (8% O2) under control conditions and after bilateral RTN injections of P2 receptor blockers (PPADS or MRS2179).
RESULTS
Unilateral injection of ATP into the RTN increased cardiorespiratory output by a P2-recepor dependent mechanism. We also show that bilateral RTN injections of a non-specific P2 receptor blocker (pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS) reduced the ventilatory response to hypercapnia (7% CO2) and hypoxia (8% O2) in unanesthetized rats. Conversely, bilateral injections of a specific P2Y1-receptor blocker (MRS2179) into the RTN had no measurable effect on ventilatory responses elicited by hypercapnia or hypoxia.
CONCLUSION
These data exclude P2Y1 receptor involvement in the chemosensory control of breathing at the level of the RTN, and show that ATP-mediated purinergic signaling contributes to central and peripheral chemoreflex control of breathing and blood pressure in awake rats.
Keywords: ATP, central autonomic pathways, breathing, medulla oblongata, RTN
Introduction
The retrotrapezoid nucleus (RTN) is located in the rostral ventrolateral medulla (RVLM) and is known to function as an important site of central chemoreception (Nattie and Li, 2012, Guyenet, 2014). Neurons in this region that express the transcription factor Phox2b are intrinsically CO2/H+-sensitive in vivo (Mulkey et al., 2004, Takakura et al., 2006) and in vitro (Onimaru and Dutschmann, 2012, Wang et al., 2013), are glutamatergic and project to all segments of the respiratory network to regulate both inspiratory and expiratory activity (Guyenet, 2014). Evidence also suggests that Phox2b expressing RTN neurons may also comprise the parafacial respiratory group (pFRG) and contribute to breathing automaticity (Onimaru et al., 2008, Thoby-Brisson et al., 2009, Pagliardini et al., 2011, Huckstepp et al., 2015, Marina et al., 2010), however, since our focus is on chemoreception, we will refer to this region as the RTN. Astrocytes in the RTN also appear to function as chemoreceptors by providing a CO2/H+-dependent purinergic drive, via direct gating of connexin 26 hemichannels (Meigh et al., 2013), to enhance activity of chemosensitive RTN neurons (Gourine et al., 2010, Huckstepp et al., 2010, Wenker et al., 2012). Although the majority of evidence suggesting that purinergic signaling contributes to RTN chemoreception were obtained from anesthetized animals or in vitro or in situ preparations (Thomas et al., 1999, Thomas and Spyer, 2000, Gourine and Spyer, 2003, Wenker et al., 2012, Huckstepp et al., 2010, Sobrinho et al., 2014), recent evidence suggests that CO2-evoked ATP release may also contribute to breathing in unanesthetized humans during quiet sleep (Meigh et al., 2014).
Purinergic signaling at the level of the RVLM also contributes to peripheral chemoreceptor modulation of breathing and blood pressure. For example, chemosensitive RTN neurons (Wenker et al., 2012, Moreira et al., 2015) and presympathetic C1 neurons (Moreira et al., 2015, Wenker et al., 2013) are activated by purinergic agonists. Application of purinergic agonists into the RVLM increased breathing and blood pressure in anesthetized rats (Wenker et al., 2013, Wenker et al., 2012, Thomas and Spyer, 2000), and the blockade of P2 receptors in the RVLM blunted cardiorespiratory responses to peripheral chemoreceptor activation in anesthetized rats (Wenker et al., 2013, Wenker et al., 2012). Evidence also suggests that inhibition of P2 receptors in the nearby Bötzinger and pre-Bözinger complex blunted the respiratory response evoked by peripheral chemoreceptor activation in awake rats (Moraes et al., 2011). Furthermore, the application of PPADS or TNP-ATP (P2 antagonists) had an equivalent effect attenuating the CO2-evoked increase on breathing in the ventral medullary surface chemosensitive areas in vitro (Gourine et al., 2005a). In addition, P2X2/3 knockout mice had ventilation attenuated during hypoxia (Gourine, 2005, Rong et al., 2003). These results provide compelling evidence that purinergic signaling at the level of the RVLM contributes to peripheral chemoreceptor control of breathing and blood pressure. However, the extent to which purinergic signaling contributes to central or peripheral chemoreceptor modulation of RTN neurons in the awake state has yet to be determined.
The main objective of the present study is to determine whether purinergic signaling in the RTN contributes to central or peripheral chemoreceptor activation of breathing and blood pressure in the unrestrained awake adult rats. To address this aim, we measured cardiorespiratory activity of unrestrained awake rats in response to RTN injections of ATP, and during exposure to hypercapnia (7% CO2) or hypoxia (8% O2) under control conditions and after bilateral RTN injections of P2 receptor blockers (PPADS or MRS2179). Considering that P2Y1 receptors regulate activity of nearby C1 cells and contribute to the blood pressure response elicited by peripheral chemoreceptor activation (Wenker et al., 2013), we chose to test the effects of P2 receptor blockade on RTN chemoreceptors in relative isolation by performing these experiments in C1-lesioned animals. We found that the stimulatory effects of RTN injections of ATP on cardiorespiratory activity could be blocked by prior ipsilateral RTN injection of a non-specific P2 receptor blocker (pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS) and a selective P2Y1 receptor blocker (MRS2197). We also found that bilateral RTN injections of PPADS, but not MRS2179, blunted the ventilatory response to both hypercapnia and hypoxia in unanesthetized rats. These results suggest that purinergic signaling at the level of the RTN contributes to both central and peripheral chemoreceptor control of breathing in unanesthetized awake rats. These results also exclude P2Y1 receptors as potential candidates responsible for this response.
Methods
Animals
Experiments were performed in 52 adult male Wistar rats weighing 300-350 g. Animals were used in accordance with the guidelines approved by the Animal Experimentation Ethics Committee of the Institute of Biomedical Science at the University of São Paulo (ICB/USP) (n0 70/2012). All efforts were made to minimize animal discomfort and the number of animals used.
Surgery and anesthesia
Rats were anesthetized with intraperitoneal injection of ketamine (Rhobifarma Indústria Farmacêutica Ltda, Hortolândia, SP, Brazil; 80 mg/kg of body weight) combined with xylazine (Sespo Indústria e Comércio Ltda, Paulínia, SP, Brazil; 7 mg/kg of body weight) and placed in a stereotaxic frame (model 900; David Kopf Instruments, Tujunga, CA, USA). Bilateral stainless-steel cannulas were implanted into the RTN using the following co-ordinates: 2.5 mm caudal to lambda, 1.8 mm lateral to the midline and 7.5 mm below the dura mater. The cannulas were fixed to the cranium using dental acrylic resin and jeweller’s screws. Rats received a prophylactic dose of penicillin (Fort Dodge Saúde Animal Ltda, Campinas, SP, Brazil) (30 000 IU) given by intramuscular injection and a subcutaneous injection of the analgesic Ketoflex (ketoprofen, Biofarm Química e Farmacêutica Ltda, Jaboticabal, SP, Brazil; 1%, 0.03 ml per rat) post surgically. After the surgery, the rats were maintained in individual boxes with free access to tap water and food pellets (Guabi rat chow; Paulínia, SP, Brazil) for at least 7 days before the experiments.
Pulsatile arterial pressure, mean arterial pressure (MAP) and heart rate (HR) were recorded in unanesthetized, freely moving rats as previously described (Favero et al., 2011, Takakura et al., 2013, Takakura et al., 2014). Briefly, 1 day before the experiments, under general anesthesia induced by intraperitoneal injection of ketamine (80 mg/kg of body weight) combined with xylazine (7 mg/kg of body weight), a polyethylene tube (PE-10 connected to PE-50; Clay Adams, Parsippany, NJ, USA) was inserted into the abdominal aorta through the femoral artery. The cannula was tunnelled subcutaneously to the back of the rats to allow access in unrestrained, freely moving animals. After they recovery from surgery, the freely moving rats were placed in boxes with free access to tap water and food pellets until the experiment day.
In vivo recordings of physiological variables
Twenty-four hours after the artery cannulation, the animals with bilateral cannula implanted in the RTN were adapted to the environment of the recording room, the arterial catheter was connected to a pressure transducer (MLT844, ADInstruments, Sydney, NSW, Australia) coupled to a preamplifier (Bridge Amp, ML221, ADInstruments, Sydney, NSW, Australia) that was connected to a Powerlab computer data acquisition system (PowerLab 16/30, ML880, ADInstruments).
Respiratory rate (fR, breaths/min) and tidal volume (VT, ml/kg) were measured by whole-body plethysmography as described in detail previously (Malan, 1973, Favero et al., 2011, Takakura et al., 2013, Takakura et al., 2014). All experiments were performed at room temperature (24-26°C). The rats were placed in a plexiglass recording chamber (5 L) that was flushed continuously with a mixture of 79% nitrogen and 21% oxygen (unless otherwise required by the protocol) at a rate of 1 L/min. Concentrations of O2 and CO2 in the chamber were monitored on-line using a fast-response O2/CO2 monitor (ADInstruments, NSW, Australia). The pressure signal was amplified, filtered, recorded, and analyzed off-line using Powerlab software (Powerlab 16/30, ML880/P, ADInstruments, NSW, Australia). Rectal temperature was measured before and at the end of the experiments, and the values were averaged. Measurements of fR and VT were made during 2 minute periods under control conditions and after 10 minute exposures to hypercapnia or hypoxia, when breathing stabilized. Changes in the fR, VT and minute ventilation (VE) (fR × VT; ml/min/kg) were averaged and expressed as means ± SEM.
Chemoreflex analysis
Unanesthetized rats were allowed at least 30-40 min to acclimatize to the chamber environment at normoxia/normocapnia (21% O2, 79% N2 and <0.5% CO2) before measurements of baseline arterial pressure, heart rate and VE were taken. Hypoxia was induced by lowering the O2 concentration in the inspired air down to a level of 8% for 10 min. Hypercapnia was induced by titrating CO2 into the respiratory mixture up to a level of 7% (21% O2, 69% N2) for 10 min.
Intraparenchymal injections
A Hamilton syringe (5 μL) connected by polyethylene tubing (PE-10) to an injection needle (1.5 mm longer than the guide cannulas) was used to deliver the following drugs into the RTN of awake freely moving rats: the non-specific P2 receptor antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS - Sigma Chemicals Co., St Louis, MO, USA; 5 mM in sterile saline, pH 7.4); the P2Y1-receptor antagonist (MRS2179 - Tocris USA; 100 μM in sterile saline, pH 7.4), ATP (Sigma Chemicals Co., St Louis, MO, USA; 10 mM in sterile saline, pH 7.4) and the P2Y1-receptor agonist (MRS2365 - Tocris USA; 100 μM in sterile saline, pH 7.4). All drug concentrations were selected based on previous studies (Wenker et al., 2013, Wenker et al., 2012, Sobrinho et al., 2014, Moraes et al., 2011).
To lesion the C1, we made bilateral RVLM injections of the saporin conjugate [Sar9, Met (O2)11]-dopamine β-hydroxylase (DβH-SAP; 2.4 ng/100 nL) (Advanced Targeting Systems, San Diego, CA, USA) as previously described (Schreihofer & Guyenet, 2000; Madden & Sved, 2003; Wenker et al., 2013). Animals were used for experiments 7 to 10 days after DβH-SAP treatment. Bilateral injections of toxin produced no observable behavioral effects and these rats gained weight normally.
Histology
At the end of each experiment, rats were deeply anesthetized with extra dose of thiopental sodium and perfused through the heart with saline (pH 7.4) followed by formaldehyde (4% in 0.1M phosphate buffer, pH 7.4). The brains were removed and stored in fixative for 24 h at 4°C. The medulla was cut in 40 μm thick coronal sections with a microtome (Leica SM2010R, Germany). Sections were stored at −20°C in a cryoprotectant solution. The injection sites were confirmed with an Axioskop 2 microscope (Zeiss, Oberkochen, Germany). Sections were aligned with respect to a reference section, which was the most caudal section containing an identifiable cluster of facial motor neurons. To this reference section was assigned a value of 11.6 mm caudal to bregma (bregma −11.6 mm, (Paxinos and Watson, 1998)). Levels rostral or caudal to this reference section were determined by adding or subtracting the number of intervening sections.
Tyrosine hydroxylase (TH) was detected with a mouse antibody (1:2000, Chemicon, Temecula, USA) and Phox2b with a rabbit antibody (1:800, gift from J.F. Brunet, Ecole Normale Superieure, Paris, France). These primary antibodies were detected by incubation with appropriate secondary antibodies to reveal TH (biotinylated goat anti-mouse, Invitrogen, Carlsbad, CA, USA) and Phox2b (biotinylated donkey anti-rabbit, Jackson, West Grove, PA, USA). The specificity of the antibodies has been validated previously (Takakura et al., 2008, Takakura et al., 2014, Taxini et al., 2011, Barna et al., 2012, Barna et al., 2014).
Statistics
Data are reported as mean ± standard error of the mean. Statistical analysis was performed using Sigma Stat version 3.0 software (Jandel Corporation, Point Richmond, CA, USA). T-test was performed to compare the antagonists (PPADS or MRS2179) to saline injections during chemoreflexes activation, and Kruskal-Wallis One Way Analysis of Variance on Ranks or one-way ANOVA followed by the Newman-Keuls multiple comparisons test was used to have the comparisons between resting, saline + ATP and antagonists (PPDS or MRS2179) + ATP injections (p < 0.05).
Results
Selective ablation of the C1 cells within the RVLM
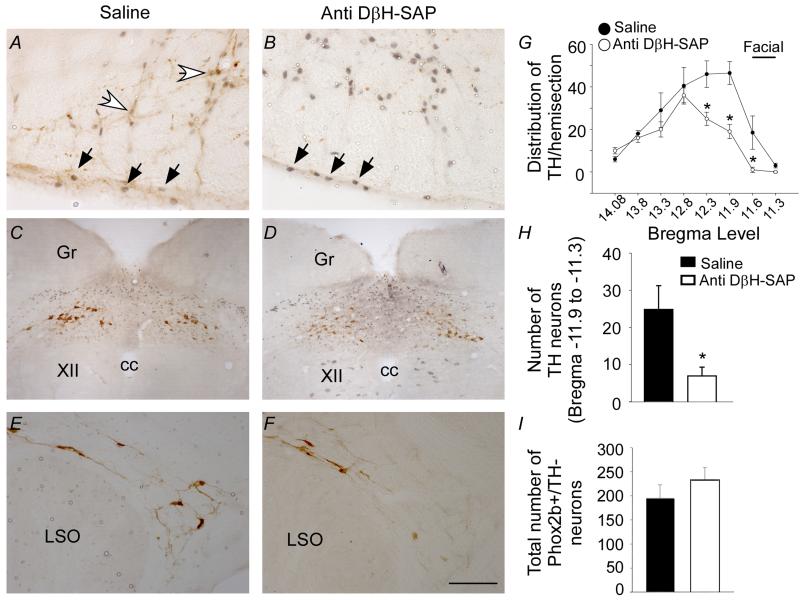
To study contributions to central and peripheral chemoreflexes of purinergic signaling only in RTN chemoreceptors, all the experiments were performed in C1-lesioned animals. We selectively destroyed C1 cells by making bilateral injections of the anti-DβH-saporin toxin into the RVLM at the level of the RTN. We chose this approach because the C1 cells are located in close proximity to RTN chemoreceptors, and C1 cells regulate blood pressure responses to peripheral chemoreceptor activation partly by a P2Y1 receptor dependent mechanism (Wenker et al., 2013). To assess the effect of anti-DβH-SAP treatment on the number of C1 cells and RTN chemoreceptors, we quantified tyrosine-hydroxylase (TH) and Phox2b-immunoreactivity. As expected, the number of C1 cells (TH+) at RTN level was significantly reduced in RVLM of anti-DβH-SAP treated animals (7 ± 2 vs. control: 25 ± 6; t(25) = 217.000, p = 0.017) (Figs. 1A-B, G-H); however, there was no quantifiable difference in the number of RTN chemoreceptors (i.e., Phox2b+/TH−) in control or anti-DβH-SAP treated animals (233 ± 25, vs. control 195 ± 28; t(70) = −0.995, p = 0.353) (Figs. 1A-B and I). Note that anti-DβH-SAP treated animals showed only a small reduction in catecholaminergic neurons at more caudal levels of the ventrolateral medulla (Fig. 1G) or in other brainstem regions (e.g., A2 or A5) (Figs. 1C-F), thus indicating that our C1 lesion model was specific to the RVLM at the level of the RTN. Furthermore, these results indicate that we can effectively reduce the number of C1 cells while sparing chemosensitive RTN neurons, therefore we consider this a valid model to test effects of purinergic signaling on RTN chemoreceptors in a situation of reduced number of C1 cells.
Figure 1. Injection of anti DβH-SAP into the RVLM selectively destroys C1 neurons.
A-B) photomicrographs at the level of the RVLM (−11.6 mm from Bregma) from control group (A) and anti-DβH-SAP group (B) of animals. Black arrows represent the Phox2b-positive neurons and white arrows represent the TH-positive neurons. C1 neurons were identified immunohistochemically as TH-positive neurons. RTN neurons were identified immunohistochemically as TH-negative and Phox2b-positive neurons. C-F) photomicrographs show normal TH and Phox2b immunolabeling in the nearby A2 (C-D) and A5 (E-F) regions. G-H) average number of TH+/Phox2b− neurons per section from 10 rats. Counts were made in 1 in 6 series of 40 μm coronal sections. I) average number of Phox2b+/TH− neurons. Scale bar in F represents 100 μm in figure A-B; 400 μm in figure C-D and 200 μm in figure E-F. Abbreviations: cc, central canal; Gr, gracile nucleus; XII, hypoglossal nucleus; LSO, lateral superior olive. * different from control group (p < 0.05), n = 5/group of rats.
Respiratory effects produced by ATP injections into the RTN region
To determine whether purinergic signaling in the RTN modulates cardiorespiratory activity in awake rats, we performed unilateral injections of ATP (10 mM, 100 nL) into awake rats while recording breathing and blood pressure. The center of each injection were located ~250 μm below the facial motor nucleus and 200-300 μm rostral to the caudal end of the RTN (Fig. 2A), a region that contains the highest density of CO2-sensitive RTN neurons (Takakura et al., 2014, Takakura and Moreira, 2011, Takakura et al., 2011, Takakura et al., 2006). Under resting conditions, application of ATP into the RTN of awake C1 lesioned rats (n = 11) elicited an immediate increase in respiratory output: tidal volume (VT) increased from 8.1 ± 0.7 to 16.4 ± 1.5 ml/kg (p = 0.001), respiratory frequency (fR) increased from 92 ± 4 to 145 ± 5 breaths/min (p = 0.001) and minute ventilation (VE) increased from 784 ± 87 to 2,386 ± 239 ml/kg/min (p = 0.001) (Figs. 2B-E). Under these same conditions, injection of ATP into the RTN of C1 lesioned animals also increased MAP (144 ± 6, vs. resting: 110 ± 3 mmHg, p = 0.001) without changing heart rate (HR) (411 ± 42, vs. resting: 368 ± 6 bpm, p = 0.351) (data not showed). Furthermore, injection of PPADS (5 mM, 100 nL) or a specific P2Y1 receptor blocker MRS2179 (100 μM, 100 nL) into this region did not change resting respiratory output, MAP or HR (data not shown). However, P2 antagonists treatment strongly inhibited cardiorespiratory responses of awake C1 lesioned rats to subsequent injections of ATP. For example, PPADS attenuated the ATP-mediated increase in VT (8.3 ± 0.7, vs. saline + ATP: 16.4 ± 1.5 ml/kg; p = 0.001), fR (100 ± 9, vs. saline + ATP: 145 ± 5 breaths/min; p = 0.001) and VE (839 ± 115, vs. saline + ATP: 2,386 ± 239 ml/kg/min; p = 0.001) (Figs. 2B-E). Bilateral injection of MRS2179 into the RTN also attenuated the ATP-mediated increase in VT (6.8 ± 1; p = 0.001), fR (112 ± 12; p = 0.001) and VE (788 ± 235; p = 0.001) (Figs. 2B-E). Considering that application of a selective P2Y1 receptor agonist into the RTN increased breathing in urethane-anesthetized animals (Wenker et al., 2013), we performed additional experiments to confirm that P2Y1 receptors were effectively blocked under our conditions, i.e., we tested effects of MRS2179 on the respiratory responses elicited by injection of a specific P2Y1 receptor agonist (MRS2365) into the RTN. We found that the increase in cardiorespiratory effects elicited by the unilateral RTN injection of MRS2365 (100 μM, 100 nL) was blunted by the prior injection of the P2Y1 antagonists MRS2179. For example, unilateral blockade of the P2Y1 receptors abolish the increase in VT (4.8 ± 0.2, vs. saline + MRS2365: 9.4 ± 1.4; p = 0.016), fR (91 ± 9, vs. saline + MRS2365: 148 ± 2; p = 0.001) and VE (432 ± 28, vs. saline + MRS2365: 1,392 ± 194 ml/kg/min; p = 0.014) (data not shown).
Figure 2). Purinergic blockade blunted the cardiorespiratory effects of ATP injections into RTN region in awake rats.
A) Photomicrograph of a coronal section showing the site of a unilateral injection in the RTN (arrow). B) Recordings showing the effect of PPADS (5 mM - 100 nL) and MRS2179 (100 μM - 100 nL) into the RTN region on changes in arterial pressure (AP) and inspiratory and expiratory flows induced by ATP (10 mM - 100 nL) injection. Changes in (C) tidal volume (VT, ml/kg), (D) respiratory frequency (fR, breaths/min) and (E) minute volume (VE, ml/kg/min) elicited by ATP injection in the RTN region during saline, PPADS or MRS2179 injections into the RTN. Scale in A = 200 μm. Abbreviations: py, pyramidal tract; 7, facial motor nucleus; * different from resting; + different from saline + ATP (p < 0.05); n = 6-12/group of rats.
Purinergic signaling in the RTN regulates cardiorespiratory responses to hypercapnia in awake rats
To determine if purinergic signaling in the RTN region contributes to CO2-induced cardiorespiratory responses in awake rats, we tested the effects of 7% CO2 on cardiorespiratory activity under control conditions and after RTN injections of PPADS. As previously described, under control conditions exposure to hypercapnia (7% CO2, 21% O2, balanced with N2) increased VT (13.1 ± 4.5, vs. resting 7.4 ± 1.8 ml/kg; t(22) = 94,000, p = 0.001), fR (128 ± 14, vs. resting 84 ± 11 breaths/min; t(22) = −8,435 p = 0.001) and VE (1,695 ± 654, vs. resting 627 ± 168 ml/kg/min; t(22) = 78,000, p = 0.001) (Figs. 3A-C) (Takakura et al., 2013, Takakura et al., 2014, Damasceno et al., 2014). Also consistent with previous data from urethane-anesthetized rats (Wenker et al., 2012, Wenker et al., 2013), bilateral RTN injections of PPADS (5 mM) reduced CO2-induced changes in VT (8.7 ± 0.6 vs. saline: 13.1 ± 1.3 ml/kg; t(16) = 2.295, p = 0.036) and VE (1,027 ± 89, vs. saline: 1,695 ± 189 ml/kg/min; t(16) = 36.000, p = 0.049) in awake rats (Figs. 3A-C). However; contrary to evidence from anesthetized rats (Wenker et al., 2012), PPADS had no effect on the hypercapnia tachypneic response of awake rats (118 ± 4, vs. saline: 128 ± 4 breaths/min; t(16) = 1.553, p = 0.140). Furthermore, unlike previous data from intact anesthetized rats (Wenker et al., 2012), we find that injections of PPADS into the RTN of C1 lesion animals did not significantly affect the CO2-induced MAP (102 ± 5, vs. saline 112 ± 4 mmHg; t(15) = 1.679, p = 0.114) and HR response (390 ± 9 vs. saline 409 ± 12 bpm; t(15) = 1.071, p = 0.301) (Figs. 3D-E). Bilateral RTN injections of MRS2179 (100 μM) had no effect on cardiorespiratory responses to CO2 (Figs. 3A-E). These results suggest that P2 receptors, but not the P2Y1 receptors, at the level of the RTN, contribute to the CO2 chemosensory control of breathing in awake and anesthetized rats (Wenker et al., 2012, Sobrinho et al., 2014).
Figure 3). PPADS, but not MRS2179, into the RTN reduced the effect of hypercapnia on breathing in awake rats.
Changes in (A) tidal volume (VT, ml/kg), (B) respiratory frequency (fR, breaths/min), (C) minute volume (VE, ml/kg/min), (D) mean arterial pressure (MAP, mmHg) and (E) heart rate (HR, bpm) elicited by hypercapnia (7% CO2) in animals that received saline, PPADS or MRS2179 injections into the RTN. F) Photomicrograph of a coronal section showing bilateral injections in the RTN and the computer-assisted plots of the center of the injection sites revealed by the presence of dye (coronal projection on plane Bregma −11.6 mm of the Paxinos atlas (Paxinos & Watson, 1998)). Abbreviations: py, pyramidal tract; Sp5, spinal trigeminal tract; 7, facial motor nucleus. Arrows indicate injection sites. Black dots represent the injections sites into the RTN and white dots represents the misplaced injections. Scale bar is 1 mm. *different from saline; (p < 0.05); n = 5-10/group of rats.
Purinergic signaling in the RTN differentially regulates cardiorespiratory responses to hypoxia in awake rats
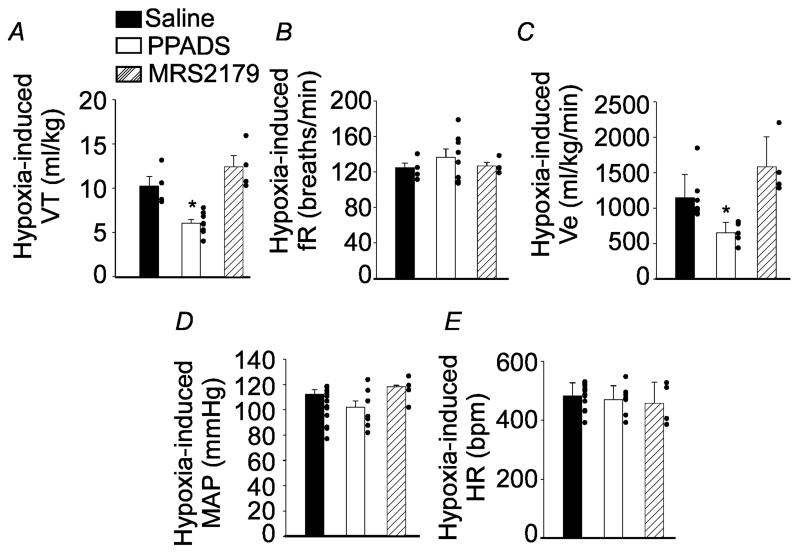
To determine whether purinergic signaling in the RTN contributes to cardiorespiratory responses to hypoxia in awake rats, we tested effects of hypoxia on cardiorespiratory activity under control conditions and after RTN injections of PPADS or MRS2179. Under control conditions, exposure to hypoxia (7% O2, balance N2) increased VT (7.7 ± 2, vs. resting 5.8 ± 2 ml/kg; t(20) = −2,250, p = 0.036), fR (125 ± 17, vs. resting 97 ± 25 breaths/min; t(20) = −3,375, p = 0.003) and VE (968 ± 344, vs. resting 555 ± 239 ml/kg/min; t(20) = −3,416, p = 0.002) (Figs. 4A-C). We found that bilateral RTN injections of PPADS (5 mM) decreased hypoxia-induced changes in VT (6 ± 0.5, vs. saline: 10.2 ± 1 ml/kg; t(13) = 4.670, p = 0.001) and VE (651 ± 151, vs. saline: 1,147 ± 330 ml/kg/min; t(13) = 15.000, p = 0.003) (Figs. 4A and C); however; contrary to the chemically (potassium cyanide) induced hypoxic response of anesthetized rats (Wenker et al., 2012, Wenker et al., 2013), PPADS had no effect on the hypoxia tachypneic response of awake rats (137 ± 9, vs. saline: 125 ± 5 breaths/min; t(13) =−0.938, p=0.37) (Fig. 4B). Injections of MRS2179 into the RTN had no effect on the ventilatory response to hypoxia (Figs. 4A-C). In addition, exposure to acute hypoxia for 10 minutes did not change MAP and HR (Figs. 4D-E) differently from anesthetized preparation (Gourine et al., 2005b, Wenker et al., 2012). Contrary to previous evidence from anesthetized rats with intact C1 regions (Wenker et al., 2012), MAP and HR responses to hypoxia were not affected by application of PPADS or MRS2179 into the RTN (Figs. 4D-E). These results suggest that purinergic signaling contributes to hypoxia activation of RTN chemoreceptors.
Figure 4). PPADS, but not MRS2179, into the RTN reduced the effect of hypoxia on breathing in awake rats.
Changes in (A) tidal volume (VT, ml/kg), (B) respiratory frequency (fR, breaths/min), (C) minute volume (VE, ml/kg/min), (D) mean arterial pressure (MAP, mmHg) and (E) heart rate (HR, bpm) elicited by hypoxia (8% O2) in animals that received saline, PPADS or MRS2179 injections into the RTN. *different from saline; (p < 0.05); n = 5-10/group of rats.
Cardiorespiratory responses to bilateral injections of P2 receptor antagonist outside the RTN region
Results from rats that received injections of P2 antagonist in sites outside the RTN were analyzed to confirm the specificity of the RTN region as the site where the purinergic antagonists injections produce the effects reported in the present study. Most of the injections, located outside the RTN region (5 out of 9), reached the rostroventrolateral medulla, including the presympathetic neurons and the expiratory Botzinger neurons (RVLM/BötC). Some injections (4 out of 9) were located in the parapyramidal region or in the lateral aspect of the ventrolateral medulla, close to the spinal trigeminal nucleus (Fig. 5F).
Figure 5). PPADS into the RVLM did not change the effect of hypercapnia on breathing in awake rats.
Changes in (A) tidal volume (VT, ml/kg), (B) respiratory frequency (fR, breaths/min), (C) minute volume (VE, ml/kg/min), (D) mean arterial pressure (MAP, mmHg) and (E) heart rate (HR, bpm) elicited by hypercapnia (7% CO2) in animals that received saline, or PPADS injections into the RVLM. F) Photomicrograph of a coronal section showing bilateral injections in the RVLM and the computer-assisted plots of the center of the injection sites revealed by the presence of dye (coronal projection on plane Bregma −12.3 mm of the Paxinos atlas (Paxinos and Watson, 1998). Abbreviations: Amb, nucleus ambigus; py, pyramidal tract; IO, inferior olive; Sp5, spinal trigeminal tract. Arrows indicate injection sites. Black dots represent the injections sites into the RVLM and white dots represent the misplaced injections. Scale bar is 1 mm. n = 5/group of rats.
PPADS (5 mM, 100 nL, n = 5) bilaterally injected into the RVLM/BötC or in the parapyramidal region or in the spinal trigeminal nucleus did not change MAP, HR and breathing at resting or during central chemoreflex activation (hypercapnia) (Figs. 5A-E).
Discussion
Our results show that blockade of P2 receptors, at the level of the RTN region, was able to reduce respiratory responses elicited by hypercapnia and hypoxia in unrestrained awake adult rats. Our evidence also suggests that P2Y1 receptors in the RTN are not required for respiratory responses to hypercapnia or hypoxia. These results demonstrate that in awake animals, purinergic signaling in the RTN region contributes to central and peripheral chemoreceptor control of breathing.
Anti-DβH-Saporin is selective to catecholaminergic C1 neurons
All experimental groups received the bilateral injection of the anti-DβH-Saporin toxin within the RTN region to eliminate the nearby blood-pressure regulating C1 neurons. It is well known that C1 neurons are located very close to the chemosensitive RTN neurons (Takakura et al., 2008, Abbott et al., 2009), and so any approach to study RTN function using microinjections would certainly also affect C1 neurons.
Further, since C1 neurons also express purinergic receptors (Yao et al., 2000, Yao and Lawrence, 2005, Korim et al., 2012, Moraes et al., 2011) including P2Y1 receptors (Wenker et al., 2013), it would be difficult to distinguish effects of purinergic signaling on RTN and C1 neurons in intact animals. Our C1 lesion model made this distinction possible. Injections of anti-DβH-Saporin effectively eliminated catecholaminergic C1 neurons without affecting the Phox2b-expressing RTN chemoreceptors in the region. However, non-catecholaminergic neurons present in the RTN region are retained in our C1 lesion model. These cells are known to provide excitatory input to sympathetic preganglionic neurons, however, the majority of bulbospinal VLM neurons with putative sympathoexcitatory function are the C1 neurons (Schreihofer and Guyenet, 1997).
Contribution of purinergic signaling in the RTN during hypercapnia
Bilateral injections of P2 receptor antagonists into the RTN of adult awake unrestrained rats decreased the ventilatory response to CO2. These results are consistent with previous evidence showing that direct application of PPADS in the ventral surface also decreased the hypercapnic ventilatory response of anesthetized rats (Sobrinho et al., 2014, Wenker et al., 2012, Gourine, 2005). The former studies showed dramatic reductions in both amplitude and frequency of the phrenic nerve activity after exposure to high levels of CO2 after delivery of P2 blockers into the RTN. Unlike the above studies, we observed only a reduction in tidal volume which corresponded with a change in minute volume after exposure to hypercapnia. These results are consistent with the possibility that CO2-dependent drive from the RTN primarily regulates tidal volume in unanesthetized animals (Nattie et al., 2001, Takakura et al., 2013). The suggestion that RTN neurons may control tidal volume was first proposed by Dr. Nattie´s laboratory in the mid 1990s (Li and Nattie, 1995, Li et al., 1999) based on observations that acidification of the RTN with acetazolamide produced changes in tidal volume without modification of the respiratory frequency. It is likely that CO2/H+-induced changes in respiratory frequency are controlled by neurons located more caudal in the ventral respiratory column region, and we believe that some of these neurons are depressed due to the anesthesia or in reduced (artificially perfused) preparations (Wenker et al., 2012, Sobrinho et al., 2014, Moraes et al., 2012).
It is becoming evident that CO2-evoked ATP release from RTN astrocytes contributes to neural mechanisms of respiratory activity (Gourine et al., 2010, Kasymov et al., 2013, Teschemacher et al., 2015). P2X and P2Y receptors subunits are expressed by respiratory neurons in the ventral respiratory column, including the RTN (Yao et al., 2001, Gourine et al., 2003, Funk, 2013, Wenker et al., 2013). In the present study, we show that ATP stimulates activity of RTN neurons in the awake unrestrained animals by a P2 receptor dependent mechanism. These results are in agreement with evidence from anesthetized rats that showed that microinjection of ATP into the RTN increased breathing in conjunction with a more mild increase in blood pressure. Both responses could be blocked by P2-receptor antagonists, thus further supporting the possibility that purinergic signaling in the ventrolateral medulla can modulate the activity of respiratory chemoreceptors (Thomas and Spyer, 2000, Gourine, 2005, Huckstepp et al., 2010, Wenker et al., 2012, Sobrinho et al., 2014) and presympathetic neurons in the VLM (Wenker et al., 2013). In addition, application of ATP potentiates respiratory frequency in rhythmically active in vitro preparations from neonatal rats (Lorier et al., 2004).
Bilateral injections of PPADS into the RTN of adult unrestrained awake rats decreased the ventilatory response to CO2 by 37%. These results are entirely consistent with previous evidence showing that application of PPADS onto the RTN also decreased the hypercapnic ventilatory response in anesthetized rats by ~30% (Gourine, 2005, Wenker et al., 2012). Injections of the P2Y1 antagonist MRS2179 into the RTN did not change the increase in breathing mediated by hypercapnia, suggesting that P2Y1 receptors are not necessary for CO2-dependent ventilatory responses in either awake unrestrained (present results) or anesthetized animals (Wenker et al., 2012).
We also found that hypercapnia had no noticeable effect on blood pressure in unrestrained awake rats. It is well established in anesthetized rats that hypercapnia produces biphasic changes in blood pressure, i.e., hypotension followed by hypertension. The initial systemic hypotension probably results from a direct effect of CO2 on vasculature smooth muscle cells and then a compensatory activation of sympathetic activity allows blood pressure to recover to near control levels during hypercapnia, and accounts for the increase in arterial pressure observed at the end of the hypercapnia episode (Moreira et al., 2006, Takakura and Moreira, 2011, Takakura et al., 2011). Injection of PPADS into the RTN region decreased the sympathetic-mediated pressure response to hypercapnia, suggesting a role of purinergic signaling in regulation of vascular tone during hypercapnia. Our evidence that hypercapnia has no noticeable effect on blood pressure in unrestrained awake rats is somewhat unexpected, but there are several issues that should be taken into consideration when interpreting these results. First, the experiments described here were performed in unanesthetized awake rats, whereas most previous studies used urethane-anesthetized rats. This is an issue because urethane may affect the neural processing of several physiological functions, including changes of the synaptic mechanisms in the brainstem at the level of the nucleus of the solitary tract, where the second order neurons are involved in the classical neural control of blood pressure (Accorsi-Mendonca et al., 2007). However, this possibility remains speculative and in need of further investigation. Second, studies performed in anesthetized animals typically also vagotomized their animals to prevent any influence of the mechanical ventilation in the breathing output, whereas for experiments in awake animals the vagus nerve is kept intact. RTN neurons receives inhibitory inputs from the central pattern generator (Guyenet et al., 2005)(Guyenet et al., 2005)(Guyenet et al., 2005)(Guyenet et al., 2005) and are inhibited by the activation of slowly adapting lung stretch receptors (SARs) through vagus nerve projections (Moreira et al., 2007, Takakura et al., 2007). Third, it is also possible that anesthetics directly affect ion channels contributing to vascular CO2-reactivity; however, this possibility remains speculative.
Contribution of purinergic signaling into the RTN during hypoxia
Several experimental models have shown that P2 receptors expressed by CO2/H+-sensitive RTN neurons contribute to central chemoreflex (Spyer and Thomas, 2000, Gourine, 2005, Gourine et al., 2010, Wenker et al., 2012, Wenker et al., 2013, Sobrinho et al., 2014). It is also known that purinergic signaling contributes to cardiorespiratory responses elicited by peripheral chemoreceptor activation (Gourine et al., 2005b, Moraes et al., 2011). Here, we add to this evidence by showing that purinergic signaling in the RTN contributes to peripheral chemoreceptor mediated respiratory responses in awake rats.
Respiratory mechanisms driven by peripheral chemoreflex activation are still controversial, in particular, the contribution of purinergic signaling between the nucleus of the solitary tract (NTS) and the RVLM, which includes the presympathetic neurons and RTN chemoreceptors neurons. Previous data showed that the pathway between the NTS and VLM region during peripheral chemoreflex activation involves the well-known excitatory glutamate neurotransmission, but also a purinergic transmission which is presumably mediated by P2Y1 receptors (Aicher et al., 1996, Stornetta et al., 2002, Takakura et al., 2006, Wenker et al., 2013). Here we show that bilateral application of P2 receptor blockers in the RTN decreased the hypoxic ventilatory response in awake unrestrained rats. These results are supported by studies in awake P2X2-deficient mice, which showed a respiratory depression during hypoxia (Rong et al., 2003). These results complement the data presented here and suggest that ATP acting presumably via P2X2 receptors within the RTN region during hypoxia challenge.
During hypoxia, ATP will activate the primary peripheral chemosensory site (carotid bodies) to stimulate the afferent terminals which convey information about oxygen levels to the respiratory centers in the VLM (Funk, 2013). Therefore, we believe that ATP will be involved in the following chain of events: carotid body activation by low levels of O2 stimulates carotid sinus nerve terminals by sending information to the second-order neurons into the NTS; these second-order neurons will activate the respiratory neurons located in the ventral respiratory group (Alvares et al., 2014, Rong et al., 2003, Wenker et al., 2012, Wenker et al., 2013). We also showed that the P2Y1 blocker did not change the ventilatory response to hypoxia in awake unrestrained rats. However, we have recently published a study showing that P2Y1-receptors control activity of presympathetic C1 neurons (Wenker et al., 2013). Although these receptors do not influence cardiorespiratory responses to hypercapnia or hypoxia, injection of a P2Y1-receptor agonist into the C1 region mimicked effects of peripheral chemoreceptor activation by increasing breathing and blood pressure (Wenker et al., 2013, Wenker et al., 2012). Our evidence that blockade of P2Y1 in the RVLM did not affect the ventilatory response to hypoxia is not surprising since our experimental group had a lesion in the C1 neurons. In light of our evidence that P2Y1 receptors expressed on C1 cell mediate the peripheral chemoreceptor pressure response, we propose that differential expression of P2 receptors throughout the ventrolateral medulla could allow for parallel processing of respiratory and cardiovascular drive during hypoxia challenge.
Besides the role of glomus cells as an oxygen sensor to stimulate breathing, a recent study suggest that there is another specialized oxygen-sensitive cell type in the body, the astrocyte, that is tuned for rapid detection of physiological changes in brain oxygenation (Angelova et al., 2015). Our results suggest that systemic hypoxia could also activates astrocytes in the ventral medullary surface to release ATP contributing to the respiratory response to hypoxia.
Technical considerations
It is likely that RTN injections of ATP, which elicited strong cardiorespiratory responses in unrestrained awake adult rats, likely affected a larger distribution of cells as compared to endogenous purinergic signaling. In addition, RTN injections of P2-receptor blockers may have also affected purinergic signaling in nearby respiratory centers. For example, our injections sites were located approximately 500-800 μm from the ventral respiratory column, which contains a network of respiratory neurons (pre-Bötzinger complex and the ventral respiratory premotor neurons) that are responsible for generating and shaping of the respiratory rhythm, as well as neurons responsible for transmitting this rhythm to spinal motorneurons (MNs) (Duffin and van Alphen, 1995, Janczewski et al., 2013, Feldman et al., 2013). The respiratory and other neurons in the ventrolateral medullary formation in rat typically have dendrites that spread no further than 200 μm from their cell bodies in the rostrocaudal direction but exceptions (up to 500 μm spread) are not uncommon (Schreihofer and Guyenet, 1997). Furthermore, dendritic arborizations of some inspiratory MNs and pre-MNs extended beyond the border, resulting in an arrangement implying synaptic connections/integration outside the cell body nucleus. An important implication is that the functional extent of the pre-BötC defined by somatodendritic architecture encompasses a much larger reticular formation region than defined solely by cell body locations (Koizumi et al., 2008). Therefore it is conceivable that neurons located from 500 up to 800 μm caudal to the center of the injection sites might have been affected to various degrees by injections into the RTN.
Another important issue that we have taken into account is the high concentration of the PPADS used in the present study. At concentrations higher than 50 μM, PPADS can certainly antagonize glutamatergic transmission (Burnstock, 2007). However, the dose of PPADS used in the present study (5 mM) was chosen based on the literature and from previous studies from our group (de Paula et al., 2004, Moraes et al., 2011, Wenker et al., 2012, Wenker et al., 2013, Sobrinho et al., 2014). The dose of PPADS used was able to block the ED50 of ATP in the ventrolateral medulla. In addition, we also showed that the combination of PPADS with the broad spectrum ionotropic glutamatergic antagonist kynurenic acid produced a further blockade of the respiratory effects elicited by hypoxia (Wenker et al., 2013) which suggest that the dose of PPADS used was not enough to block all the glutamatergic receptors, especially in vivo experiments. However, we cannot rule out an interaction of glutamate and ATP at the level of the ventrolateral medulla on the modulation of breathing and future studies are necessary to better evaluate the crosstalk between glutamate and purines.
There are important caveats regarding the technology used in the present study that we tried to point out throughout the manuscript. Our results highlight the emerging view of the role of purines in the chemosensory control of breathing in conscious rats, but future studies will certainly dissect all the points raised to better evaluate our hypothesis.
Conclusions
In summary, the present results add to a growing body of evidence suggesting that purinergic signaling is important for respiratory function by showing that P2 receptors expressed by RTN neurons contribute to chemical drive to breathe in awake unrestrained adult rats (Fig. 6). It is well established that purinergic signaling contributes to autonomic and respiratory control by various mechanisms at several levels of the CNS (Wenker et al., 2012, Wenker et al., 2013, Moreira et al., 2015, Gourine et al., 2009). Disruption of the drive to breath is thought to contribute to mortality of certain pathologies, including sudden infant death syndrome (SIDS), stroke and epilepsy (Kinney et al., 2009, Massey et al., 2014, Davis et al., 2013). Additionally, in obstructive sleep apnea (OSA), the increase in the peripheral chemoreceptor sensitivity leads to a sympathoexcitation contributing to certain forms of hypertension and heart failure (Narkiewicz et al., 1999, Schultz et al., 2007).
Figure 6). Contribution of the purinergic signaling in the RTN region to chemosensory control of breathing.
Signals from central or peripheral chemoreceptors may affect the activity of several medullary areas, including the RTN, NTS and the ventral respiratory neurons (VRC), which affect motorneurons to respiratory muscles. The excitatory drive of ventrolateral medulla neurons operates via a direct glutamatergic and/or purinergic input from caudal NTS neurons and via a di-synaptic input that relays via the intrinsically chemosensitive neurons of RTN (Moreira et al., 2006, Takakura et al., 2006, Wenker et al., 2013). On the other hand, an essential step for hypercania-induced breathing is activation of RTN neurons which in turn send excitatory signals to activate the VRC neurons, possibly by purinergic signaling (Wenker et al., 2012, Wenker et al., 2013, Alvares et al., 2014). Chemosensitive RTN neurons are directly activated by CO2/H+ by mechanisms involving inhibition of TASK2 (Wang et al., 2013) and activation of a proton-activated receptor GPR2 (Kumar et al., 2015). In addition, RTN chemoreceptors receive CO2/H+-dependent excitatory purinergic drive from local astrocytes which sense CO2/H+ by inhibition of Kir4.1-containing channels (Wenker et al., 2010) and release ATP by mechanisms involving Ca2+-dependent exocytosis or Ca2+-independent release through connexin channels (Cx26 primarily) (Huckstepp et al., 2010). Abbreviations: ATP, adenosine triphosfate; CB, carotid body; Glut, glutamate; iGlut, ionotropic glutamatergic receptors; MN, motor neuron; NTS, nucleus of the solitary tract; P2X, ionotropic purinergic receptors; RTN, retrotrapezoid nucleus; VMS, ventral medullary surface; VRC, ventral respiratory column.
In recent years, purinergic signaling has been proposed to be an excellent system to target for therapies of numerous pathologies, mainly due to novel pharmacological agents being developed. (Jacobson and Boeynaems, 2010, Burnstock, 2014, Marina et al., 2013). Based on our recent data, we have proposed that P2-receptors could represent a therapeutic target for the treatment of respiratory diseases in which the chemosensory mechanisms are sensitized (Wenker et al., 2013). Most of the new purinergic pharmacological agents are ATP analogues and do not cross the blood brain barrier, making them less practical for use in the CNS, but better pharmacology, combined with further understanding of the specific purinergic receptor subtypes and signaling pathways involved in chemoreflex control by RTN may allow for novel therapeutic strategies for respiratory diseases.
Acknowledgements
This research was supported by public funding from São Paulo Research Foundation (FAPESP) (grants: 10/09776-3 to ACT; 2009/54888-7 and 13/10573-8 to TSM), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grants: 471263/2013-3 to ACT; 471283/2012-6 to TSM) and by funds from the National Institutes of Health Grants (HL104101, DKM) FAPESP felowship (2012/10337-0 to BFB) and CNPq fellowship (305533/2012-6 to TSM and 301651/2013-2 to ACT). We gratefully acknowledge J.F. Brunet (Departement de Biologie, Ecole Normale Superieure, Paris, France) for the Phox2b antibody.
Footnotes
Conflict of Interest
We do not have conflict of interest.
Authors contributions
BFB, ACT, DKM and TSM designed research; BFB performed research and analyzed data and BFB, ACT, DKM and TSM wrote the paper.
References
- Abbott SB, Stornetta RL, Socolovsky CS, West GH, Guyenet PG. Photostimulation of channelrhodopsin-2 expressing ventrolateral medullary neurons increases sympathetic nerve activity and blood pressure in rats. J Physiol. 2009;587:5613–31. doi: 10.1113/jphysiol.2009.177535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accorsi-Mendonca D, Leao RM, Aguiar JF, Varanda WA, Machado BH. Am J Physiol Regul Integr Comp Physiol. United States: 2007. Urethane inhibits the GABAergic neurotransmission in the nucleus of the solitary tract of rat brain stem slices.) [DOI] [PubMed] [Google Scholar]
- Aicher SA, Saravay RH, Cravo S, Jeske I, Morrison SF, Reis DJ, Milner TA. J Comp Neurol. United States: 1996. Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: comparison with input from the caudal ventrolateral medulla.) [DOI] [PubMed] [Google Scholar]
- Alvares TS, Revill AL, Huxtable AG, Lorenz CD, Funk GD. J Physiol. The Authors; England: 2014. P2Y1 receptor-mediated potentiation of inspiratory motor output in neonatal rat in vitro.) 2014. The Journal of Physiology 2014 The Physiological Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova PR, Kasymov V, Christie I, Sheikhbahaei S, Turovsky E, Marina N, Korsak A, Zwicker J, Teschemacher AG, Ackland GL, Funk GD, Kasparov S, Abramov AY, Gourine AV. Functional Oxygen Sensitivity of Astrocytes. J Neurosci. 2015;35:10460–73. doi: 10.1523/JNEUROSCI.0045-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna BF, Takakura AC, Moreira TS. Pontomedullary and hypothalamic distribution of Fos-like immunoreactive neurons after acute exercise in rats. Neuroscience. 2012;212:120–30. doi: 10.1016/j.neuroscience.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Barna BF, Takakura AC, Moreira TS. Acute exercise-induced activation of Phox2b-expressing neurons of the retrotrapezoid nucleus in rats may involve the hypothalamus. Neuroscience. 2014;258:355–63. doi: 10.1016/j.neuroscience.2013.11.031. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling: from discovery to current developments. Exp Physiol. 2014;99:16–34. doi: 10.1113/expphysiol.2013.071951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasceno RS, Takakura AC, Moreira TS. Regulation of the chemosensory control of breathing by Kolliker-Fuse neurons. Am J Physiol Regul Integr Comp Physiol. 2014;307:R57–67. doi: 10.1152/ajpregu.00024.2014. [DOI] [PubMed] [Google Scholar]
- Davis AP, Billings ME, Longstreth WT, Jr., Khot SP. Early diagnosis and treatment of obstructive sleep apnea after stroke: Are we neglecting a modifiable stroke risk factor? Neurol Clin Pract. 2013;3:192–201. doi: 10.1212/CPJ.0b013e318296f274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula PM, Antunes VR, Bonagamba LG, Machado BH. Cardiovascular responses to microinjection of ATP into the nucleus tractus solitarii of awake rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1164–71. doi: 10.1152/ajpregu.00722.2003. [DOI] [PubMed] [Google Scholar]
- Duffin J, van Alphen J. Brain Res. Netherlands: 1995. Bilateral connections from ventral group inspiratory neurons to phrenic motoneurons in the rat determined by cross-correlation.) [DOI] [PubMed] [Google Scholar]
- Favero MT, Takakura AC, de Paula PM, Colombari E, Menani JV, Moreira TS. Chemosensory control by commissural nucleus of the solitary tract in rats. Respir Physiol Neurobiol. 2011;179:227–34. doi: 10.1016/j.resp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol. 2013;75:423–52. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD. Neuromodulation: purinergic signaling in respiratory control. Compr Physiol. 2013;3:331–63. doi: 10.1002/cphy.c120004. [DOI] [PubMed] [Google Scholar]
- Gourine AV. On the peripheral and central chemoreception and control of breathing: an emerging role of ATP. J Physiol. 2005;568:715–24. doi: 10.1113/jphysiol.2005.095968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Atkinson L, Deuchars J, Spyer KM. Purinergic signalling in the medullary mechanisms of respiratory control in the rat: respiratory neurones express the P2X2 receptor subunit. J Physiol. 2003;552:197–211. doi: 10.1113/jphysiol.2003.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–5. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005a;436:108–11. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. Release of ATP in the ventral medulla during hypoxia in rats: role in hypoxic ventilatory response. J Neurosci. 2005b;25:1211–8. doi: 10.1523/JNEUROSCI.3763-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Spyer KM. Chemosensitivity of medullary respiratory neurones. A role for ionotropic P2X and GABA(A) receptors. Adv Exp Med Biol. 2003;536:375–87. [PubMed] [Google Scholar]
- Gourine AV, Wood JD, Burnstock G. Purinergic signalling in autonomic control. Trends Neurosci. 2009;32:241–8. doi: 10.1016/j.tins.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol. 2014;4:1511–62. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. J Neurosci. United States: 2005. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, Cardoza KP, Henderson LE, Feldman JL. J Neurosci. United States: 2015. Role of parafacial nuclei in control of breathing in adult rats.) 2015 the authors 0270-6474/15/351052-16$15.00/0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol. 2010;588:3901–20. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Boeynaems JM. P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today. 2010;15:570–8. doi: 10.1016/j.drudis.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL. Role of inhibition in respiratory pattern generation. J Neurosci. 2013;33:5454–65. doi: 10.1523/JNEUROSCI.1595-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasymov V, Larina O, Castaldo C, Marina N, Patrushev M, Kasparov S, Gourine AV. J Neurosci. United States: 2013. Differential sensitivity of brainstem versus cortical astrocytes to changes in pH reveals functional regional specialization of astroglia.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–50. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Wilson CG, Wong S, Yamanishi T, Koshiya N, Smith JC. Functional Imaging, Spatial Reconstruction, and Biophysical Analysis of a Respiratory Motor Circuit Isolated In Vitro. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korim WS, Ferreira-Neto ML, Pedrino GR, Pilowsky PM, Cravo SL. Interaction of medullary P2 and glutamate receptors mediates the vasodilation in the hindlimb of rat. Purinergic Signal. 2012;8:715–28. doi: 10.1007/s11302-012-9318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, Ludwig MG, Perez-Reyes E, Mohebbi N, Bettoni C, Gassmann M, Suply T, et al. PHYSIOLOGY. Regulation of breathing by CO(2) requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science. 2015;348:1255–60. doi: 10.1126/science.aaa0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie EE. Prolonged stimulation of respiration by brain stem metabotropic glutamate receptors. J Appl Physiol (1985) 1995;79:1650–6. doi: 10.1152/jappl.1995.79.5.1650. [DOI] [PubMed] [Google Scholar]
- Li A, Randall M, Nattie EE. CO(2) microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol (1985) 1999;87:910–9. doi: 10.1152/jappl.1999.87.3.910. [DOI] [PubMed] [Google Scholar]
- Lorier AR, Peebles K, Brosenitsch T, Robinson DM, Housley GD, Funk GD. Respir Physiol Neurobiol. Netherlands: 2004. P2 receptors modulate respiratory rhythm but do not contribute to central CO2 sensitivity in vitro.) [DOI] [PubMed] [Google Scholar]
- Malan DH. The outcome problem in psychotherapy research. A historical review. Arch Gen Psychiatry. 1973;29:719–29. doi: 10.1001/archpsyc.1973.04200060005001. [DOI] [PubMed] [Google Scholar]
- Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci. 2010;30:12466–73. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Tang F, Figueiredo M, Mastitskaya S, Kasimov V, Mohamed-Ali V, Roloff E, Teschemacher AG, Gourine AV, Kasparov S. Purinergic signalling in the rostral ventro-lateral medulla controls sympathetic drive and contributes to the progression of heart failure following myocardial infarction in rats. Basic Res Cardiol. 2013;108:317. doi: 10.1007/s00395-012-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol. 2014;10:271–82. doi: 10.1038/nrneurol.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigh L, Greenhalgh SA, Rodgers TL, Cann MJ, Roper DI, Dale N. CO(2)directly modulates connexin 26 by formation of carbamate bridges between subunits. Elife. 2013;2:e01213. doi: 10.7554/eLife.01213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigh L, Hussain N, Mulkey DK, Dale N. Connexin26 hemichannels with a mutation that causes KID syndrome in humans lack sensitivity to CO2. Elife. 2014;3:e04249. doi: 10.7554/eLife.04249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes DJ, Bonagamba LG, Zoccal DB, Machado BH. Modulation of respiratory responses to chemoreflex activation by L-glutamate and ATP in the rostral ventrolateral medulla of awake rats. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1476–86. doi: 10.1152/ajpregu.00825.2010. [DOI] [PubMed] [Google Scholar]
- Moraes DJ, Zoccal DB, Machado BH. Sympathoexcitation during chemoreflex active expiration is mediated by L-glutamate in the RVLM/Botzinger complex of rats. J Neurophysiol. 2012;108:610–23. doi: 10.1152/jn.00057.2012. [DOI] [PubMed] [Google Scholar]
- Moreira TS, Takakura AC, Colombari E, Guyenet PG. J Physiol. England: 2006. Central chemoreceptors and sympathetic vasomotor outflow.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira TS, Takakura AC, Colombari E, Guyenet PG. J Neurophysiol. United States: 2007. Activation of 5-hydroxytryptamine type 3 receptor-expressing C-fiber vagal afferents inhibits retrotrapezoid nucleus chemoreceptors in rats.) [DOI] [PubMed] [Google Scholar]
- Moreira TS, Wenker IC, Sobrinho CR, Barna BF, Takakura AC, Mulkey DK. Independent purinergic mechanisms of central and peripheral chemoreception in the rostral ventrolateral medulla. J Physiol. 2015;593:1067–74. doi: 10.1113/jphysiol.2014.284430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–9. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–9. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreceptors: locations and functions. Compr Physiol. 2012;2:221–54. doi: 10.1002/cphy.c100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E, Shi J, Li A. Respir Physiol. Netherlands: 2001. Bicuculline dialysis in the retrotrapezoid nucleus (RTN) region stimulates breathing in the awake rat.) [DOI] [PubMed] [Google Scholar]
- Onimaru H, Dutschmann M. Calcium imaging of neuronal activity in the most rostral parafacial respiratory group of the newborn rat. J Physiol Sci. 2012;62:71–7. doi: 10.1007/s12576-011-0179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. J Neurosci. United States: 2008. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. J Neurosci. United States: 2011. Active expiration induced by excitation of ventral medulla in adult anesthetized rats.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brainin stereotaxic coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM, Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci. 2003;23:11315–21. doi: 10.1523/JNEUROSCI.23-36-11315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol. 1997;387:524–36. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Li YL, Ding Y. Arterial chemoreceptors and sympathetic nerve activity: implications for hypertension and heart failure. Hypertension. 2007;50:6–13. doi: 10.1161/HYPERTENSIONAHA.106.076083. [DOI] [PubMed] [Google Scholar]
- Sobrinho CR, Wenker IC, Poss EM, Takakura AC, Moreira TS, Mulkey DK. Purinergic signalling contributes to chemoreception in the retrotrapezoid nucleus but not the nucleus of the solitary tract or medullary raphe. J Physiol. 2014;592:1309–23. doi: 10.1113/jphysiol.2013.268490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyer KM, Thomas T. Sensing arterial CO(2) levels: a role for medullary P2X receptors. J Auton Nerv Syst. 2000;81:228–35. doi: 10.1016/s0165-1838(00)00118-1. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J Comp Neurol. 2002;444:207–20. doi: 10.1002/cne.10142. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Barna BF, Cruz JC, Colombari E, Moreira TS. Phox2b-expressing retrotrapezoid neurons and the integration of central and peripheral chemosensory control of breathing in conscious rats. Exp Physiol. 2014;99:571–85. doi: 10.1113/expphysiol.2013.076752. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Colombari E, Menani JV, Moreira TS. Ventrolateral medulla mechanisms involved in cardiorespiratory responses to central chemoreceptor activation in rats. Am J Physiol Regul Integr Comp Physiol. 2011;300:R501–10. doi: 10.1152/ajpregu.00220.2010. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS. Contribution of excitatory amino acid receptors of the retrotrapezoid nucleus to the sympathetic chemoreflex in rats. Exp Physiol. 2011;96:989–99. doi: 10.1113/expphysiol.2011.058842. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. J Physiol. England: 2006. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, De Paula PM, Menani JV, Colombari E. Control of breathing and blood pressure by parafacial neurons in conscious rats. Exp Physiol. 2013;98:304–15. doi: 10.1113/expphysiol.2012.065128. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol. 2008;586:2975–91. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, West GH, Gwilt JM, Colombari E, Stornetta RL, Guyenet PG. J Neurophysiol. United States: 2007. GABAergic pump cells of solitary tract nucleus innervate retrotrapezoid nucleus chemoreceptors.) [DOI] [PubMed] [Google Scholar]
- Taxini CL, Takakura AC, Gargaglioni LH, Moreira TS. Control of the central chemoreflex by A5 noradrenergic neurons in rats. Neuroscience. 2011;199:177–86. doi: 10.1016/j.neuroscience.2011.09.068. [DOI] [PubMed] [Google Scholar]
- Teschemacher AG, Gourine AV, Kasparov S. A Role for Astrocytes in Sensing the Brain Microenvironment and Neuro-Metabolic Integration. Neurochem Res. 2015 doi: 10.1007/s11064-015-1562-9. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Nat Neurosci. United States: 2009. Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex.) [DOI] [PubMed] [Google Scholar]
- Thomas T, Ralevic V, Gadd CA, Spyer KM. J Physiol. England: 1999. Central CO2 chemoreception: a mechanism involving P2 purinoceptors localized in the ventrolateral medulla of the anaesthetized rat.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Spyer KM. ATP as a mediator of mammalian central CO2 chemoreception. J Physiol. 2000;523(Pt 2):441–7. doi: 10.1111/j.1469-7793.2000.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Benamer N, Zanella S, Kumar NN, Shi Y, Bevengut M, Penton D, Guyenet PG, Lesage F, Gestreau C, Barhanin J, Bayliss DA. TASK-2 channels contribute to pH sensitivity of retrotrapezoid nucleus chemoreceptor neurons. J Neurosci. 2013;33:16033–44. doi: 10.1523/JNEUROSCI.2451-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol. 2010;104:3042–52. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Sobrinho CR, Takakura AC, Moreira TS, Mulkey DK. Regulation of ventral surface CO2/H+-sensitive neurons by purinergic signalling. J Physiol. 2012;590:2137–50. doi: 10.1113/jphysiol.2012.229666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Sobrinho CR, Takakura AC, Mulkey DK, Moreira TS. P2Y1 receptors expressed by C1 neurons determine peripheral chemoreceptor modulation of breathing, sympathetic activity, and blood pressure. Hypertension. 2013;62:263–73. doi: 10.1161/HYPERTENSIONAHA.113.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao ST, Barden JA, Finkelstein DI, Bennett MR, Lawrence AJ. J Comp Neurol. Wiley-Liss, Inc.; United States: 2000. Comparative study on the distribution patterns of P2X(1)-P2X(6) receptor immunoreactivity in the brainstem of the rat and the common marmoset (Callithrix jacchus): association with catecholamine cell groups.) 2000. [PubMed] [Google Scholar]
- Yao ST, Barden JA, Lawrence AJ. Neuroscience. United States: 2001. On the immunohistochemical distribution of ionotropic P2X receptors in the nucleus tractus solitarius of the rat.) [DOI] [PubMed] [Google Scholar]
- Yao ST, Lawrence AJ. Br J Pharmacol. England: 2005. Purinergic modulation of cardiovascular function in the rat locus coeruleus.) [DOI] [PMC free article] [PubMed] [Google Scholar]