Abstract
Purpose
In pre-clinical radiation research, it is challenging to localize soft tissue targets based on cone beam computed tomography (CBCT)-guidance. As a more effective method to localize soft tissue targets, we developed an online bioluminescence tomography (BLT) system for the small animal radiation research platform (SARRP). We demonstrated BLT-guided radiotherapy and validated targeting accuracy, based on a newly developed reconstruction algorithm.
Methods and Materials
The BLT system was designed to dock onto the SARRP for image acquisition and to be detached before radiation delivery. A 3-mirror system was devised to reflect the bioluminescence emitted from the subject to a stationary CCD camera. Multispectral BLT and the incomplete variables truncated conjugate gradient method with a permissible region shrinking strategy were employed as the optimization scheme to reconstruct bioluminescent source distributions. To validate BLT targeting accuracy, a small cylindrical light source with high CBCT contrast was placed in a phantom and also in the abdomen of a mouse carcass. The center of mass (CoM) of the source was recovered from BLT and used to guide radiation delivery. The accuracy of the BLT-guided targeting was validated with films and compared with the CBCT-guided delivery. In vivo experiments were conducted to demonstrate the BLT localization capability for various source geometries.
Results
Online BLT was able to recover the CoM of the imbedded light source with an average accuracy of 1 mm compared to CBCT localization. The difference between the BLT- and CBCT-guided irradiation shown on the films was consistent with the source localization revealed in the BLT and CBCT images. The in vivo results demonstrated that our BLT system could potentially be applied for multiple targets and tumors.
Conclusions
The online BLT/CBCT/SARRP system provides an effective solution for soft tissue targeting, particularly for small, non-palpable, or orthotopic tumor models.
INTRODUCTION
In radiation therapy, studies in small animal models are a key means to understand and develop treatments for a wide spectrum of diseases (1, 2). Several groups, including ours, have initiated efforts to develop small animal irradiators that mimic human treatment (3–12). In these systems, on-board computed tomography (CT) and cone-beam CT(CBCT) are used to guide radiotherapy. Our small animal radiation research platform (SARRP)(4, 13) employs CBCT to guide focal irradiation that is accurate to within 0.2 mm.
Although our experience and that of other investigators show that CT or CBCT is invaluable for guiding focal irradiation (6, 7, 14–16), localization of small and low contrast soft tissue targets remains challenging. At present, micro (μ)-magnetic resonance imaging, μ-positron emission tomography and μ-single photon emission computed tomography are the more common modalities that can provide improved localization accuracy for soft tissue targets (17). Cost, shielding and space limitations of the existing irradiators are all obstacles preventing implementation of these modalities. A compelling solution is molecular optical imaging, which has a compact footprint and additional advantages of lower cost and not using ionizing radiation.
Among possible optical modalities, bioluminescence imaging (BLI) has found extensive applications (18–21) and it is almost free of background. Due to the nonlinear relationship between signal strength, surface emittance and tissue optical properties, two-dimensional (2D) BLI measured on the object surface is limited in its ability to accurately localize a target in three-dimensions (3D). It has been shown, however, that with sophisticated reconstruction algorithms and mathematical models of light transport inside tissue, bioluminescence tomography (BLT) based on 2D BLI can reveal the 3D distribution of internal bioluminescent sources (22–27). With the wide availability of genetically engineered mouse models in oncologic and radiobiology research, we propose BLT as an attractive solution for soft tissue targeting which can be readily applied to existing animal models.
We had built an integrated CBCT and BLT system in a standalone configuration (28) for calibration method and reconstruction algorithm development. In this study, we introduced an online BLT/CBCT system for the SARRP and a significantly improved BLT algorithm. Because of the online design, we were able to demonstrate BLT-guided radiation therapy on the SARRP for the first time. Multispectral BLT (29), inverse calculation using the incomplete variables truncated conjugate gradient (IVTCG) method (30) and a permissible region shrinking strategy (31) were combined to enable fast and accurate BLT reconstruction. Studies were performed in a phantom and mouse carcasses with an implanted light source to show the capability of BLT-guided radiation therapy. In vivo experiments were conducted to demonstrate the potentiality of applying the BLT system for multiple targets and tumors.
METHODS AND MATERIALS
SARRP-Optical system
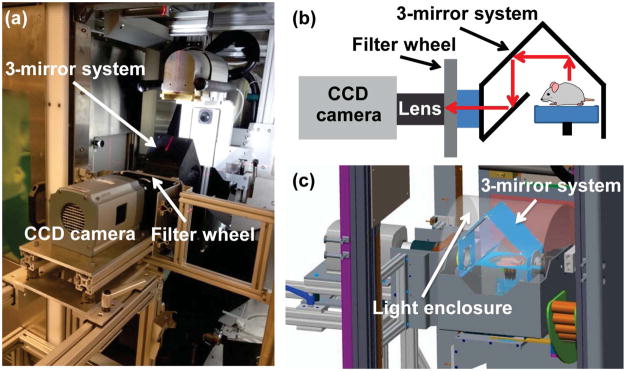
The second generation SARRP allows 360° isocentric gantry rotation and is equipped with CBCT and robotic controlled stages for animal positioning (4, 13). For CBCT imaging, a 20.5cm×20.5cm amorphous silicon flat panel detector with 200μm pixel pitch (Perkin Elmer, Waltham, MA, USA) is used to acquire 65kVp x-ray projection images from a 0.4mm focal spot. Figure 1a shows the BLT system consisting of a camera-filter-mirror assembly docked into the SARRP. A rotating 3-mirror system directs light emitted from the object to a stationary CCD camera and supports multi-view imaging (Figure 1b). The optical path from the SARRP isocenter, through the 3 mirrors, to the lens front surface is 42 cm, and the depth-of-field is 4 cm. Figure 1c illustrates the side view of the SARRP-BLT system. A light-tight dome covers the mirror and object. The CCD camera has a large 27.6mm×27.6mm back-illuminated sensor with 2048×2048 pixels, (or 13.5μm/pixel), (iKon-L 936, Andor Technology, Belfast, UK) and is fitted with a 35mm f/1.4 lens (Rokinon, New York, NY, USA) for optical image acquisition. The quantum efficiency of the CCD sensor is >90% over a wavelength range of 500–700nm. The camera operates at −80°C to minimize dark current and other thermal noise during image acquisition. Spectral discrimination is provided by four band-pass filters (590, 610, 630 and 650nm, 10 nm FWHM, Andover, Salem, NH, USA) mounted in a computer-controlled wheel (Edmund Optics, Barrington, NJ, USA). The procedures for calibrating the intensity nonuniformity arising from the lens vignetting effect and nonuniform pixel response of the CCD chip are described in the Supplementary section 1.
Figure 1.
(a) Online BLT/SARRP system; (b) Optical path from object to lens; (c) Light enclosure to cover the object and mirror-assembly.
BLT reconstruction
The diffusion approximation (DA) to the radiative transport equation is widely used to model light propagation in tissue when photon transport is dominated by scattering (32). In the continuous wave mode, the DA and the Robin-type boundary condition are expressed as
| (1) |
where Φ(r) is the photon fluence rate at location r in domain Ω, is the diffusion coefficient, μa and are absorption and reduced scattering coefficients, respectively, and S(r) is the bioluminescence source distribution. ξ represents points on the object boundary and coefficient A can be derived from Fresnel’s law (33) depending on the refractive index of tissue and air. n̂ is the unit vector pointing outward normal to the boundary ∂Ω.
Equation (1) can be further expressed in the form of Green’s functions which link the fluence rate at the object boundary and the bioluminescence source distribution (34, 35). For M measurements and N mesh nodes, the relationship can be expressed as
| (2) |
where [φ1, …, φM]T is a vector containing the fluence rate measured at the boundary, [s1, …, sN]T is the vector of unknown bioluminescence distribution and Gi,j is the Green’s function describing the relationship between the fluence rate φi at detector i on the surface and the source sj at node j.
Multispectral images were acquired to improve BLT reconstruction results (24, 29). Equation (2) can be rewritten as
| (3) |
where G(λk) is the Green’s function, extended from Eq. (2), at wavelength λk and η(λk) is the relative spectral weight which accounts for the source emission spectrum, the transmission of individual filters and CCD quantum efficiency at different wavelengths. A modified version of the open source software NIRFAST(33, 36) was used to generate the Green’s function. To avoid biasing the reconstruction algorithm by the larger signals at longer wavelength (due to lower attenuation) (27), the measurements φ and the weighted Green’s function G̃ at each wavelength were divided by the maximum value of the measurements φ, that is
| (4) |
Our approach to reconstruct the bioluminescence source distribution s is to minimize the deviation between the computed Ḡ s and measured fluence rate φ̄ at the object boundary. However, the BLT reconstruction is ill-posed and underdetermined with fewer measurements than unknowns. He et al.(30) introduced the IVTCG optimization algorithm and demonstrated that this algorithm can stably solve this minimization problem with an L1 regularization term. BLT minimization is given as,
| (5) |
where τ is a non-negative regularization parameter, denotes the square of the Euclidean norm, such as , and ||s||1 = Σi|si| is the L1 norm of s. We employed the finite element method where the imaging object was discretized into a 3D mesh from the SARRP CBCT as the framework to numerically build the Green’s function and system matrix.
To improve convergence and reduce the computation time, an iterative strategy was chosen to adaptively shrink the solution space (31). The initial permissible solution space is the whole mesh domain except the surface nodes. The permissible region reduction factor is defined as β = (N1/Nf)1/(Nit−1), where N1 is the initial number of nodes for the permissible region. In this study, Nit (number of iterations) was set to 20 and to include all the possible solutions, Nf (final number of nodes) was set to 1. The objective function
| (6) |
was calculated according to the reconstructed source distribution s(i) at the i-th iteration. The permissible region was shrunk at each iteration by first sorting the nodes in descending order of source strength, and selecting the nodes with high average source strength per 1mm3 volume until the number of nodes is equal to NR/β, where NR is the total number of nodes in the permissible region. The solution corresponding to the minimum of the objective function was selected. In this study, CCD counts per unit time per pixel area were chosen as the measurement quantity at the boundary and were not linked to absolute emittance. Further study to calibrate CCD counts to absolute emittance at the boundary is warranted (28). The optical and reconstruction parameters used in this study are listed in the Supplementary Table e1. A threshold 10% of the maximum source strength was applied for display of the BLT results shown in this work.
BLT-guided irradiation
The workflow of online BLT-guided irradiation is described here: (1) dock BLT system onto the SARRP, (2) perform geometry calibration mapping the BLI coordinates to the CBCT coordinates, (see Supplementary section 1 for details of the calibration procedures), (3) acquire multispectral BLI, (4) detach the BLT system and acquire object CBCT, (5) use the NIRFAST package to generate the 3D mesh of the object from CBCT, and map the multispectral BLI to the mesh surface based on the geometry calibration. The 3D distribution of the bioluminescence source is then reconstructed and the CoM of the reconstructed source is calculated for radiation guidance. The total experiment time is approximately 40 minutes/animal. In this study, the CBCT volume was reconstructed with a voxel resolution of (0.2–0.25mm)3. Multispectral BLI were acquired at wavelengths of 590, 610, 630 and 650nm with 0.6mm/pixel. Although our system is capable of acquiring multiple projections of BLI by rotating the mirror assembly, single view BLI was used for the 3D reconstruction in this study. For BLT guided experiments, the mesh was set at approximately 10000 nodes and 60000 tetrahedral elements, and the element size varied between 0.1 – 0.5mm3.
To assess the BLT targeting accuracy, we performed experiments using a tissue simulating phantom and mouse carcasses. A cylindrical self-illuminated light source (Trigalight, tritium gas 12 year half-life, mb-microtec ag, Niederwangen, Switzerland) with high CBCT contrast, 0.9 mm diameter × 2 mm in length, was placed inside the phantom as well as the carcass abdomen. The mouse was euthanized just prior to Trigalight implantation and shaved for BLT measurement. The relative spectral weights of the source are 0.90, 1, 0.95, and 0.55 at the wavelengths 590, 610, 630 and 650 nm, respectively. The methods of phantom fabrication and measuring optical properties were published in a separate study (37). The mouse carcass was assumed to be optically homogeneous. To determine the optimal optical properties, several sets of optical properties from the literature (38–41) were initially tested by comparing the CoM of the reconstructed Trigalight in one carcass to that obtained by CBCT. Once the optimal values were determined, the same optical properties were applied for all carcasses. Gafchromic EBT3 films (Ashland, Covington, KY, USA) were used to verify the targeting accuracy for an anterior-posterior (AP) and lateral delivery by placing the films on the beam exit sides of the phantom and carcass. The details of the film measurements can be found in the Supplementary section 2. The source centroid position revealed from CBCT was regarded as the ground truth for target position. Both BLT- and CBCT-guided irradiation were performed and compared.
In vivo BLT study
To assess the capability of our BLT system for multiple sources and for tumors, two in vivo experiments were conducted. For the first experiment, a mouse surgically implanted with 2 larger cylindrical Trigalight sources (2 mm diameter × 6 mm length) in the abdomen was imaged with the BLT system. For the second experiment, a mouse bearing a subcutaneous tumor, firefly PC3-Luc prostate cancer cells, in the lower dorsal region was imaged. The details of the in vivo experiments can be found in the Supplementary section 3.
RESULTS
Phantom experiments
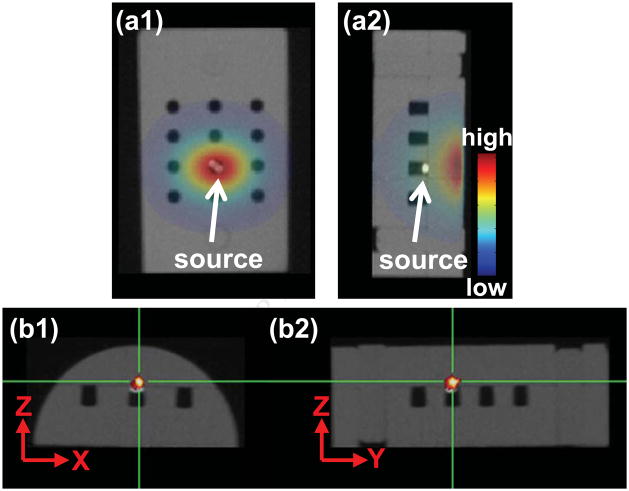
The Trigalight source was placed in one of the holes of the phantom (Figure 2a1). A small piece of Styrofoam was inserted underneath the source to minimize the air gap between source and detector points at the phantom surface. Note that the strongest intensity on the surface did not correspond to the true source position (Figure 2a2). Based on the single view (Figure 2a1), we can reconstruct the source in 3D (Figure 2b1 and b2). The largest CoM deviation between the “true” CBCT and BLT reconstructed positions of the Trigalight is in the depth direction, Z-axis, at 0.6mm. The average 3D offset between the true source center and BLT reconstructed CoM from 4 independent experiments is 0.6±0.1mm. The comparison of the measured and computed signals at the phantom surface can be found in Supplementary Figure e3.
Figure 2.
(a1,a2) The AP and lateral BLI overlaid with the CBCT of the phantom (half cylinder, 30mm diameter × 41mm height), which also shows the embedded light source. (b1,b2) The transverse and sagittal views of BLT slices overlaid with CBCT. The cross depicts the CoM of reconstructed bioluminescent source.
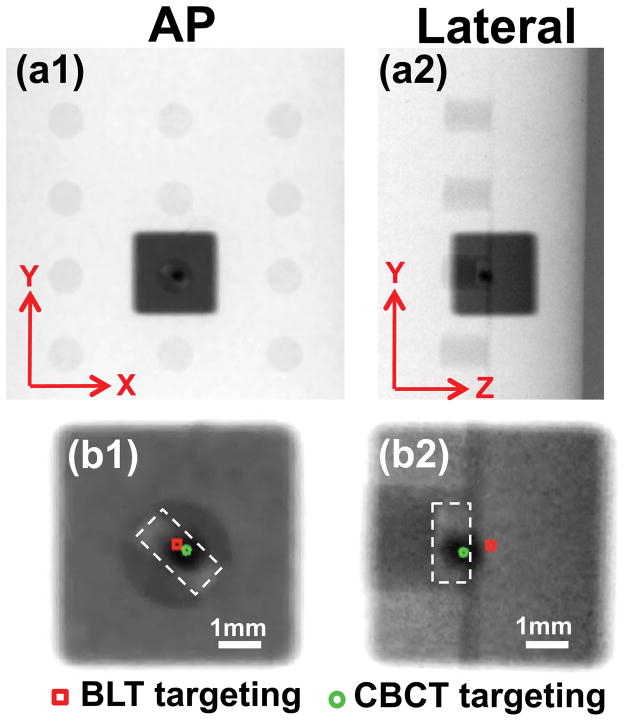
Figure 3 shows the film results for the irradiation based on the source position provided by BLT and CBCT reconstructions in Figure 2. A 0.5mm diameter cone and a 5mm × 5mm square collimator were used for CBCT- and BLT-guided delivery, respectively. In the X- and Y-axis, the offsets between the centers of the BLT- and CBCT-guided radiation fields are less than 0.2mm (Figures 3b1 and b2). Consistent with the BLT localization result in Figures 2b1 and b2, the largest targeting offset is observed in the lateral view, Z-axis 0.6mm (Figure 3b2).
Figure 3.
Film results of target validation in phantom: The BLT guided radiation was delivered with a 5mm × 5mm beam; CBCT guidance with a 0.5mm diameter beam. (a1,a2) show the results in the AP and lateral views. (b1,b2) are the corresponding enlarged views. The true source was contoured by the dashed line. The green circle marks the center of the CBCT guided radiation; red square marks the center of the BLT guided radiation.
Mouse carcass experiments
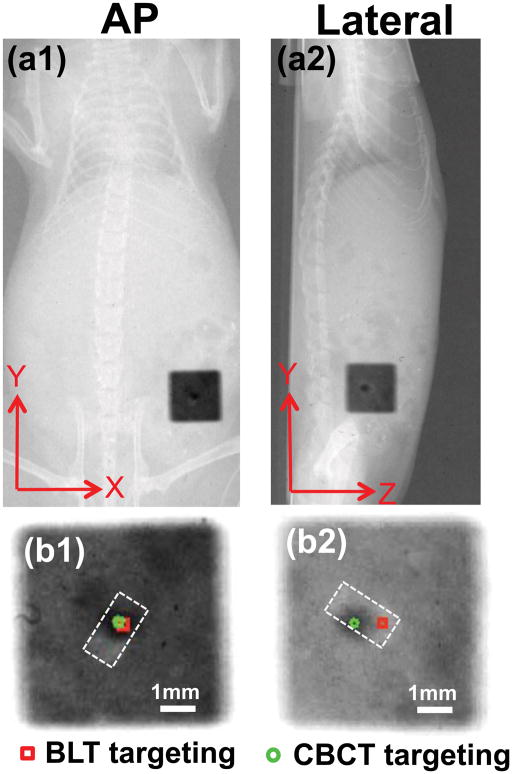
As observed in the phantom study, the location of the strongest surface bioluminescence intensity of the carcass did not reflect the true source position (Figures 4a1 and a2). Based on the single AP view (Figure 4a1), we can reconstruct the source in 3D, overlaid on the CBCT image (Figures 4b1–b3). The largest CoM deviation of 0.8mm is in the Z-axis (Figures 4b1 and b2). The average 3D offset between the true source center and BLT reconstructed CoM from 3 independent carcass experiments is 1.0±0.6mm. The fit between the measured and computed signals at the mouse surface are shown in Supplementary Figure e4. Figure 5 shows the film results of the BLT-guided irradiation compared to the CBCT-guided delivery. The targeting difference between the BLT and CBCT-guided irradiation is minimal in the X- and Y-axis (<0.2mm). The largest deviation of 0.8mm is clearly seen in the Z-axis (Figure 5b2), consistent with the reconstruction results in Figures 4b1 and b2.
Figure 4.
(a1,a2) AP and lateral BLI overlaid with the CBCT of the mouse carcass implanted with a light source. (b1,b2,b3) The transverse, sagittal and coronal views of BLT slices overlaid with CBCT. The cross depicts the CoM of reconstructed source.
Figure 5.
Film results for target validation in the mouse carcass. The same collimator settings as described in Figure 3 were used. (a1,a2) show the results in the AP and lateral views. (b1,b2) are the corresponding enlarged views. The true source was contoured by the dashed line. The green circle marks the center of the CBCT guided radiation; the red square marks the center of the BLT guided radiation.
In vivo experiments
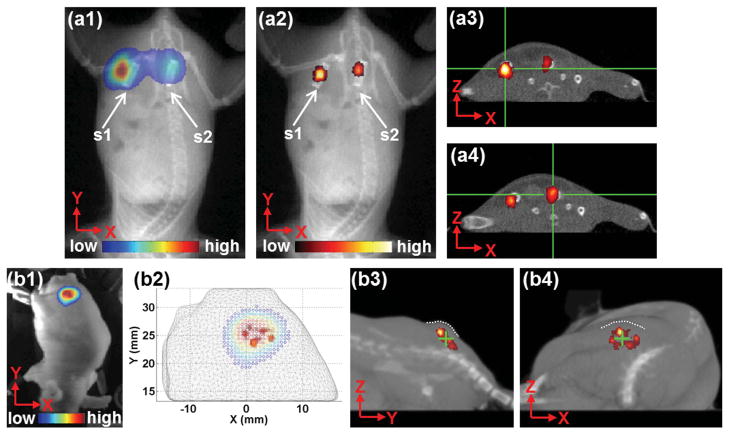
Figure 6 shows that the planar BLI can barely separate two sources, but BLT can clearly distinguish the sources (Figure 6a1vs. a2). The deviations of the BLT reconstructed CoM for source1 and 2 are 0.8 and 0.9 mm, respectively (Figure 6a3 and a4). Homogenous optical properties were assumed in this study, which possibly contributed to the higher strength of source 1 compared to source 2 (Figure 6a2).
Figure 6.
(a1–a4) Double source experiment; two identical cylindrical light sources, source1(s1) and source2(s2), were implanted in the mouse abdomen. (a1,a2) are the AP view of BLI, at 610nm, and BLT overlapped with CBCT projection, respectively. (a3,a4) are the transverse views of the BLT overlapped with CBCT slice for s1 and s2, respectively. The cross denotes the CoM of the reconstructed sources. (b1–b4) In vivo subcutaneous experiment; (b1) BLI overlaid with the photo image, (b2) the top view of BLI overlapped with the BLT reconstructed source onto the mouse mesh, (b3,b4) the sagittal and transverse views of BLT overlapped with the 3D rendering of the CBCT. The dash lines outline the tumor surface and cross represents the reconstructed CoM.
Figure 6b1 shows the surface bioluminescence signal emitted from the subcutaneous tumor. Because the cells were located subcutaneously and the tumor was palpable, in this case, the 2D BLI at the tumor surface is believed to represent the tumor location. As we expect, from the top view, the reconstructed 3D bioluminescent distributions (red dots) lie beneath the BLI intensity on the surface (Figure 6b2). The reconstructed bioluminescence CoM is also within the tumor as depicted by CBCT (Figure 6b3–b4).
DISCUSSION
Although BLI has been widely used in preclinical research, it has only been recently applied for radiation guidance (42–46). BLI alone is limited in guiding radiation delivery. Recent publications from our group (42, 43) revealed that a vertical beam line directed through the highest bioluminescence surface intensity deviated from the CoM of internal source by as much as 3.5mm. Due to the irregular phantom and animal surface, the optical paths from the bioluminescent source to different surface points can be highly variable such that the location of the strongest BLI signal does not reflect the true source position (Figures 2 and 4). Weersink et al.(46) recently integrated BLI onto the X-RAD 225Cx and asserted that 2D targeting based on BLI in the anterior/posterior direction can be applied to a single beam or parallel-opposed pair. Their approach alleviates the uncertainty in the depth position of the target but, as can be seen in Figures 2 and 4, the beam margin will need to be quite large to account for the non-intuitive relationship between 2D BLI intensity and the 3D location of the source.
We recently developed a standalone CBCT/BLT system (28) for calibration method and algorithm development. In this study, we introduce an online BLT-SARRP system to demonstrate BLT-guided radiation for the first time. Compared with the earlier work from Yang et al. (28), this paper integrates multispectral BLT (25, 29), the IVTCG method (30) and permissible source region (31) developed by our group and collaborators to enable fast and accurate CoM reconstruction. Furthermore, the new algorithm enables better conformality of the reconstructed source distributions without a predefined region of interest (see Figure 2 vs. Figure 8 from Yang el al. (28)). The computation time for our mouse carcass experiments was only 3 minutes using a 64-bit laptop with an Intel Core i7-3920XM 2.9GHz processor and 32GB memory; a time short enough to support online guidance of irradiation. Another positive efficiency factor with our approach is the use of single view BLI data for reconstruction. Dehghani et al.(25) demonstrated in numerical simulations with a mouse model that spectrally resolved single view BLT can be used to reconstruct a 2.5mm radius source within 1mm accuracy up to 12.5mm in depth. While we and other (25) have shown that single view data can provide excellent BLT reconstruction results, it may explain why our CoM offset was most noticeable in the “depth” direction. The depth uncertainty can likely be reduced with more camera views, which may also be necessary for metastatic nodules or deep-seated tumors.
Our adoption of the CoM approach is a first step to improve soft tissue targeting. The film validation results show that BLT can be used to guide irradiation of a target based on its CoM which agreed with the CoM derived from CBCT to about 1mm. Once the target CoM is located, our irradiation studies also confirmed that the SARRP can deliver radiation accurately to the target location to within 0.2mm (13). These capabilities are highly significant for pre-clinical radiation research involving targets with low CT contrast. Advanced conformal beam arrangements can now be used and would be much superior to the limited beam arrangements based on 2D BLI guidance (42–44, 46). The light source used in this study allows direct validation by CBCT, which would not be possible with an actual tumor model. However, in a real life specimen, the tumor shape can be more complex. The results of our in vivo studies demonstrate that our BLT system and algorithm have the potential to guide radiation for multiple targets or tumors. The in vivo results for delineating two cylindrical light sources implanted into a live mouse abdomen are promising (Figure 6 a2–a4), although the results are presented as cluster of points that emphasizes our algorithm optimized toward CoM reconstruction. A more complete BLT solution, such as higher order radiative transport equation, is expected to provide better target delineation (41, 47). Applying BLT for subcutaneous model is an initial step toward in vivo image-guidance (Figure 6 b2–b4). Our ultimate goal is to apply BLT guidance to the orthotopic model, which provides a more realistic environment for human cancer study.
In our study, the light source was placed in the abdomen which is a relatively optically homogenous region compared to highly heterogeneous areas such as lung and we selected somewhat pre-optimized optical properties for our carcass study. The use of diffuse optical tomography (27) in situ or organ-dependent optical properties will provide a more accurate description of the heterogeneous mouse. We recognize that the current online system requires manual docking. A parallel engineering effort is in progress to provide automated deployment of the BLT system onto the SARRP with better mechanical reproducibility to reduce calibration frequency (48).
In summary, our integrated BLT/SARRP system provides a new opportunity for soft tissue targeting in small animal models with an overall 1mm targeting accuracy that was achieved in both phantom and mouse carcass models. We also demonstrated the feasibility of applying the BLT-guided system for multiple targets and in vivo tumors.
Supplementary Material
SUMMARY.
To overcome the limitations of cone beam computed tomography (CBCT) in localizing soft tissue targets, we developed online 3D bioluminescence tomography (BLT) for the small animal radiation platform. The optical system is capable of locating a target’s center of mass with an average accuracy of 1 mm. The integrated online BLT/CBCT system provides an effective solution for soft tissue targeting, particularly for small, non-palpable, or orthotopic tumor models.
Acknowledgments
The authors would like to thank Esteban Velarde and Katriana Nugent for their help with SARRP and animal handling. This work is supported by NIH R01CA158100, China NNSFC 61401264 and Xstrahl Ltd.
Footnotes
Conflicts of interest: Drs. John Wong and Iulian Iordachita receive royalty payment from a licensing agreement between Xstrahl Ltd and Johns Hopkins University. John Wong also has a consultant agreement with Xstrahl Ltd.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alcorn S, Walker AJ, Gandhi N, et al. Molecularly targeted agents as radiosensitizers in cancer therapy--focus on prostate cancer. Int J Mol Sci. 2013;14:14800–14832. doi: 10.3390/ijms140714800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirsch DG. Using genetically engineered mice for radiation research. Radiat Res. 2011;176:275–279. doi: 10.1667/rrxx35.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou H, Rodriguez M, van den Haak F, et al. Development of a micro-computed tomography-based image-guided conformal radiotherapy system for small animals. Int J Radiat Oncol Biol Phys. 2010;78:297–305. doi: 10.1016/j.ijrobp.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong J, Armour E, Kazanzides P, et al. High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities. Int J Radiat Oncol Biol Phys. 2008;71:1591–1599. doi: 10.1016/j.ijrobp.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tryggestad E, Armour M, Iordachita I, et al. A comprehensive system for dosimetric commissioning and Monte Carlo validation for the small animal radiation research platform. Phys Med Biol. 2009;54:5341–5357. doi: 10.1088/0031-9155/54/17/017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarkson R, Lindsay PE, Ansell S, et al. Characterization of image quality and image-guidance performance of a preclinical microirradiator. Med Phys. 2011;38:845–856. doi: 10.1118/1.3533947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stojadinovic S, Low DA, Hope AJ, et al. MicroRT-small animal conformal irradiator. Med Phys. 2007;34:4706–4716. doi: 10.1118/1.2799887. [DOI] [PubMed] [Google Scholar]
- 8.Graves EE, Zhou H, Chatterjee R, et al. Design and evaluation of a variable aperture collimator for conformal radiotherapy of small animals using a microCT scanner. Med Phys. 2007;34:4359–4367. doi: 10.1118/1.2789498. [DOI] [PubMed] [Google Scholar]
- 9.Pidikiti R, Stojadinovic S, Speiser M, et al. Dosimetric characterization of an image-guided stereotactic small animal irradiator. Phys Med Biol. 2011;56:2585–2599. doi: 10.1088/0031-9155/56/8/016. [DOI] [PubMed] [Google Scholar]
- 10.Verhaegen F, Granton P, Tryggestad E. Small animal radiotherapy research platforms. Phys Med Biol. 2011;56:R55–83. doi: 10.1088/0031-9155/56/12/R01. [DOI] [PubMed] [Google Scholar]
- 11.Tillner F, Thute P, Butof R, et al. Pre-clinical research in small animals using radiotherapy technology--a bidirectional translational approach. Z Med Phys. 2014;24:335–351. doi: 10.1016/j.zemedi.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Verhaegen F, van Hoof S, Granton PV, et al. A review of treatment planning for precision image-guided photon beam pre-clinical animal radiation studies. Z Med Phys. 2014;24:323–334. doi: 10.1016/j.zemedi.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Matinfar M, Ford E, Iordachita I, et al. Image-guided small animal radiation research platform: calibration of treatment beam alignment. Phys Med Biol. 2009;54:891–905. doi: 10.1088/0031-9155/54/4/005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumann BC, Benci JL, Santoiemma PP, et al. An integrated method for reproducible and accurate image-guided stereotactic cranial irradiation of brain tumors using the small animal radiation research platform. Transl Oncol. 2012;5:230–237. doi: 10.1593/tlo.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford EC, Achanta P, Purger D, et al. Localized CT-guided irradiation inhibits neurogenesis in specific regions of the adult mouse brain. Radiat Res. 2011;175:774–783. doi: 10.1667/RR2214.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redmond KJ, Achanta P, Grossman SA, et al. A radiotherapy technique to limit dose to neural progenitor cell niches without compromising tumor coverage. J Neurooncol. 2011;104:579–587. doi: 10.1007/s11060-011-0530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunha L, Horvath I, Ferreira S, et al. Preclinical imaging: an essential ally in modern biosciences. Mol Diagn Ther. 2014;18:153–173. doi: 10.1007/s40291-013-0062-3. [DOI] [PubMed] [Google Scholar]
- 18.Thorne SH, Contag CH. Using in vivo bioluminescence imaging to shed light on cancer biology. Proc IEEE. 2005;93:750–762. [Google Scholar]
- 19.O’Neill K, Lyons SK, Gallagher WM, et al. Bioluminescent imaging: a critical tool in pre-clinical oncology research. J Pathol. 2010;220:317–327. doi: 10.1002/path.2656. [DOI] [PubMed] [Google Scholar]
- 20.Close DM, Xu T, Sayler GS, et al. In vivo bioluminescent imaging (BLI): noninvasive visualization and interrogation of biological processes in living animals. Sensors (Basel) 2011;11:180–206. doi: 10.3390/s110100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contag PR. Whole-animal cellular and molecular imaging to accelerate drug development. Drug Discov Today. 2002;7:555–562. doi: 10.1016/s1359-6446(02)02268-7. [DOI] [PubMed] [Google Scholar]
- 22.Ntziachristos V, Ripoll J, Wang LV, et al. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005;23:313–320. doi: 10.1038/nbt1074. [DOI] [PubMed] [Google Scholar]
- 23.Wang G, Cong W, Durairaj K, et al. In vivo mouse studies with bioluminescence tomography. Opt Express. 2006;14:7801–7809. doi: 10.1364/oe.14.007801. [DOI] [PubMed] [Google Scholar]
- 24.Kuo C, Coquoz O, Troy TL, et al. Three-dimensional reconstruction of in vivo bioluminescent sources based on multispectral imaging. J Biomed Opt. 2007;12:024007. doi: 10.1117/1.2717898. [DOI] [PubMed] [Google Scholar]
- 25.Dehghani H, Davis SC, Pogue BW. Spectrally resolved bioluminescence tomography using the reciprocity approach. Med Phys. 2008;35:4863–4871. doi: 10.1118/1.2982138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darne C, Lu Y, Sevick-Muraca EM. Small animal fluorescence and bioluminescence tomography: a review of approaches, algorithms and technology update. Phys Med Biol. 2014;59:R1–64. doi: 10.1088/0031-9155/59/1/R1. [DOI] [PubMed] [Google Scholar]
- 27.Naser MA, Patterson MS. Improved bioluminescence and fluorescence reconstruction algorithms using diffuse optical tomography, normalized data, and optimized selection of the permissible source region. Biomed Opt Express. 2011;2:169–184. doi: 10.1364/BOE.2.000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Wang KK, Eslami S, et al. Systematic calibration of an integrated x-ray and optical tomography system for preclinical radiation research. Med Phys. 2015;42:1710. doi: 10.1118/1.4914860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dehghani H, Davis SC, Jiang S, et al. Spectrally resolved bioluminescence optical tomography. Opt Lett. 2006;31:365–367. doi: 10.1364/ol.31.000365. [DOI] [PubMed] [Google Scholar]
- 30.He X, Liang J, Wang X, et al. Sparse reconstruction for quantitative bioluminescence tomography based on the incomplete variables truncated conjugate gradient method. Opt Express. 2010;18:24825–24841. doi: 10.1364/OE.18.024825. [DOI] [PubMed] [Google Scholar]
- 31.Naser MA, Patterson MS. Bioluminescence tomography using eigenvectors expansion and iterative solution for the optimized permissible source region. Biomed Opt Express. 2011;2:3179–3193. doi: 10.1364/BOE.2.003179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arridge SR. Optical tomography in medical imaging. Inverse Probl. 1999;15:R41. [Google Scholar]
- 33.Dehghani H, Eames ME, Yalavarthy PK, et al. Near infrared optical tomography using NIRFAST: Algorithm for numerical model and image reconstruction. Commun Numer Methods Eng. 2008;25:711–732. doi: 10.1002/cnm.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cong W, Durairaj K, Wang LV, et al. A born-type approximation method for bioluminescence tomography. Med Phys. 2006;33:679–686. doi: 10.1118/1.2168293. [DOI] [PubMed] [Google Scholar]
- 35.Naser MA, Patterson MS. Algorithms for bioluminescence tomography incorporating anatomical information and reconstruction of tissue optical properties. Biomed Opt Express. 2010;1:512–526. doi: 10.1364/BOE.1.000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jermyn M, Ghadyani H, Mastanduno MA, et al. Fast segmentation and high-quality three-dimensional volume mesh creation from medical images for diffuse optical tomography. J Biomed Opt. 2013;18:86007. doi: 10.1117/1.JBO.18.8.086007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pekar J, Patterson MS. Fabrication and characterization of phantoms with tissue-like optical properties from 500 to 700nm. Med Laser Appl. 2010;25:147–153. [Google Scholar]
- 38.Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol. 2013;58:R37–61. doi: 10.1088/0031-9155/58/11/R37. [DOI] [PubMed] [Google Scholar]
- 39.Alexandrakis G, Rannou FR, Chatziioannou AF. Tomographic bioluminescence imaging by use of a combined optical-PET (OPET) system: a computer simulation feasibility study. Phys Med Biol. 2005;50:4225–4241. doi: 10.1088/0031-9155/50/17/021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Virostko J, Powers AC, Jansen ED. Validation of luminescent source reconstruction using single-view spectrally resolved bioluminescence images. Appl Opt. 2007;46:2540–2547. doi: 10.1364/ao.46.002540. [DOI] [PubMed] [Google Scholar]
- 41.Klose AD, Beattie BJ, Dehghani H, et al. In vivo bioluminescence tomography with a blocking-off finite-difference SP3 method and MRI/CT coregistration. Med Phys. 2010;37:329–338. doi: 10.1118/1.3273034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuli R, Surmak A, Reyes J, et al. Development of a novel preclinical pancreatic cancer research model: bioluminescence image-guided focal irradiation and tumor monitoring of orthotopic xenografts. Transl Oncol. 2012;5:77–84. doi: 10.1593/tlo.11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuli R, Armour M, Surmak A, et al. Accuracy of off-line bioluminescence imaging to localize targets in preclinical radiation research. Radiat Res. 2013;179:416–421. doi: 10.1667/RR2999.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee CJ, Spalding AC, Ben-Josef E, et al. In vivo bioluminescent imaging of irradiated orthotopic pancreatic cancer xenografts in nonobese diabetic-severe combined immunodeficient mice: a novel method for targeting and assaying efficacy of ionizing radiation. Transl Oncol. 2010;3:153–159. doi: 10.1593/tlo.09184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butterworth KT, Redmond KM, McMahon SJ, et al. Conventional in vivo irradiation procedures are insufficient to accurately determine tumor responses to non-uniform radiation fields. Int J Radiat Biol. 2015;91:257–261. doi: 10.3109/09553002.2014.980468. [DOI] [PubMed] [Google Scholar]
- 46.Weersink RA, Ansell S, Wang A, et al. Integration of optical imaging with a small animal irradiator. Med Phys. 2014;41:102701. doi: 10.1118/1.4894730. [DOI] [PubMed] [Google Scholar]
- 47.Klose AD. Light Scattering Review 7. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012. Multi-spectral luminescence tomography with the simplified spherical harmonics equations; pp. 37–67. [Google Scholar]
- 48.Li M, He X, Eslami S, et al. A Dual-use Imaging System for Pre-clinical Small Animal Radiation Research. Proc 37th Annu Int Conf of the IEEE Engineering in Medicine and Biology Society (EMBS); 2015; pp. 6904–6907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.