Abstract
Wnt5a, a non-canonical Wnt ligand critical for outflow tract (OFT) morphogenesis, is expressed specifically in second heart field (SHF) progenitors in the caudal splanchnic mesoderm (SpM) near the inflow tract (IFT). Using a conditional Wnt5a gain of function (GOF) allele and Islet1-Cre, we broadly over-expressed Wnt5a throughout the SHF lineage, including the entire SpM between the IFT and OFT. Wnt5a over-expression in Wnt5a null mutants can rescue the cell polarity and actin polymerization defects as well as severe SpM shortening, but fails to rescue OFT shortening. Moreover, Wnt5a over-expression in wild-type background is able to cause OFT shortening. We find that Wnt5a over-expression does not perturb SHF cell proliferation, apoptosis or differentiation, but affects the deployment of SHF cells by causing them to accumulate into a large bulge at the rostral SpM and fail to enter the OFT. Our immunostaining suggests an inverse correlation between cell cohesion and Wnt5a level in the wild-type SpM. Ectopic Wnt5a expression in in the rostral SpM of Wn5a-GOF mutants diminishes the upregulation of adherens junction; whereas loss of Wnt5a in Wnt5a null mutants causes premature increase in adherens junction level in the caudal SpM. Over-expression of mouse Wnt5a in Xenopus animal cap cells also reduces C-cadherin distribution on the plasma membrane without affecting its overall protein level, suggesting that Wnt5a may play an evolutionarily conserved role in controlling the cell surface level of cadherin to modulate cell cohesion during tissue morphogenesis. Collectively, our data indicate that restricted expression of Wnt5a in the caudal SpM is essential for normal OFT morphogenesis, and uncover a novel function of spatially regulated cell cohesion by Wnt5a in driving the deployment of SHF cells from the SpM into OFT.
Keywords: Wnt5a, planar cell polarity, second heart field, outflow tract, morphogenesis, heart development, cell adhesion
Introduction
The heart arises from progenitor cells located in the crescent-shaped (in the mouse) or bilateral (in the chick) cardiogenic fields in the anterior lateral plate mesoderm (Dyer and Kirby, 2009; Evans et al., 2010). Embryo folding brings the lateral portions of the cardiogenic field to the ventral midline, allowing them to fuse and form the initial heart tube. Commonly referred to as the first heart field (FHF), the progenitor cells contributing to the initial heart tube differentiate early to give rise primarily to the left ventricle (LV) and atria. On the other hand, the progenitor cells residing in the more medial portion of the cardiogenic fields are shifted dorsally and remain undifferentiated during the initial heart tube formation, and are referred to as the second heart field (SHF). The SHF extends from the rostral pharyngeal mesoderm (PM) to the caudal splanchnic mesoderm (SpM), and is attached to heart tube only at its arterial (outflow) and venous (inflow) poles. SHF progenitors undergo extensive proliferation, and gradually differentiate and deploy into the heart tube to form the right ventricle (RV) and the outflow tract (OFT) at the arterial pole, and part of the atria and atrial septum at the venous pole (Dyer and Kirby, 2009; Evans et al., 2010; Vincent and Buckingham, 2010).
The myocardial cells within the early heart tube undergo prolonged proliferation arrest (van den Berg et al., 2009). The elongation of the heart tube, therefore, is driven primarily by addition of cells from the SHF. Sufficient elongation is necessary for cardiac morphogenesis such as rightward looping of the heart, and aligning the OFT over the inter-ventricular septum so that upon cardiac neural crest invasion, the OFT can be properly septated into the aorta and pulmonary artery and connected to the LV and RV, respectively. Aberrant OFT morphogenesis can cause a spectrum of conotruncal defects such as double outlet right ventricle (DORV), overriding aorta, transposition of the great arteries, pulmonary atresia and persistent truncus arteriosus (PTA) (Dyer and Kirby, 2009; Evans et al., 2010; Vincent and Buckingham, 2010).
Extensive studies in the field have delineated the signaling pathways and transcriptional networks that orchestrate cell proliferation and differentiation in the SHF to generate sufficient number of cardiomyocytes for the heart (Black, 2007; Vincent and Buckingham, 2010; Xin et al., 2013). However, relatively little is known how SHF cells are deployed into the heart. Our previous work has implicated a role of the non-canonical Wnt/ planar cell polarity (PCP) pathway in the deployment of SHF cells to the OFT. Initially identified in Drosophila, the PCP pathway coordinates cellular polarity in the plane of the epithelium, and regulates polarized cell behavior such as oriented cell intercalation and directional cell migration during convergent extension (CE) morphogenesis to modulate tissue shape and dimensions in vertebrates (Devenport, 2014; Goodrich and Strutt, 2011; Tada and Heisenberg, 2012; Zallen, 2007). The PCP pathway does not lead to β-catenin stabilization, but it utilizes some components of the canonical Wnt pathway, including the Frizzled (Fz) receptor and cytoplasmic protein Dishevelled (Dvl/Dsh), to function together with a set of distinct core PCP proteins like Van Gogh (Vang/Vangl). PCP signaling has been reported to regulate a diverse array of effectors, such as JNK, GTPase Rho/Rac/Cdc42/Rab5, and formin protein Daam1, and function in context- and tissue-specific manner to modulate cytoskeletal dynamics, cell cohesion, protrusive activity, etc. (Devenport, 2014; Gray et al., 2011; Habas et al., 2001; Wang et al., 2012). Studies in Xenopus and zebrafish have identified two non-canonical Wnts, Wnt5a and Wnt11, as the ligands that activate PCP signaling during tissue morphogenesis.
In the mouse, we and others have carried out tissue-specific gene ablation to demonstrate that PCP genes Dvl1/2 and Vangl2 are required specifically in the SHF lineage for OFT elongation and morphogenesis (Ramsbottom et al., 2014; Sinha et al., 2012). Furthermore, loss of either Wnt5a or Wnt11 also leads to severe conotruncal defects in mice (Schleiffarth et al., 2007; Zhou et al., 2007). Lineage tracing studies have revealed that Wnt11 starts to be expressed in the rostral SHF cells in the PM, and Wnt11-lineage populates the superior myocardial wall of the OFT and contributes specifically to the sub-aortic myocardium (Sinha et al., 2015b). Conversely, Wnt5a is expressed in the caudal SHF cells in the SpM, and loss of Wnt5a specifically diminishes the inferior myocardial wall of the OFT and its derivative, the sub-pulmonary myocardium (Sinha et al., 2015a). SHF progenitors in the caudal SpM of Dvl1/2 and Wnt5a null mutants display no cell proliferation or apoptosis defects, but lack the normal elongated and protrusive morphology, display defective actin organization, and are aggregated into compact of clusters instead of organizing into a cohesive sheet like the wild-type cells. These observations lead us to propose the first model on SHF deployment, in which Wnt5a, acting through PCP, induces oriented cell intercalation to incorporate SHF progenitors into a cohesive sheet at the caudal end of the SpM, thereby creating a pushing force to deploy SHF cells rostrally into the OFT (Sinha et al., 2015a; Sinha et al., 2012).
In the current study, we attempted to explore additional mechanisms underlying SHF deployment by taking a gain-of-function approach to over-express Wnt5a broadly in the SHF lineage. Our results no only provided additional support to our original model, but more importantly uncovered an additional role of restricted Wnt5a expression to spatially regulate cell cohesion to drive the deployment of SHF cells from the SpM into OFT.
Methods
Mouse strains
Rosa26Wnt5a mice were generated as described (Cha et al., 2014) and illustrated in Fig. 1A. A three-primer PCR strategy (Rosa10: CTCTGCTGCCTCCTGGCTTCT; Rosa11: CGAGGCGGATCACAAGCAATA and R26R2; GCGAAGAGTTTGTCCTCAACC) was used to genotype Rosa26Wnt5a mice. Genotyping of Wnt5 and Islet1-Cre were described previously (Cai et al., 2003; Yamaguchi et al., 1999).
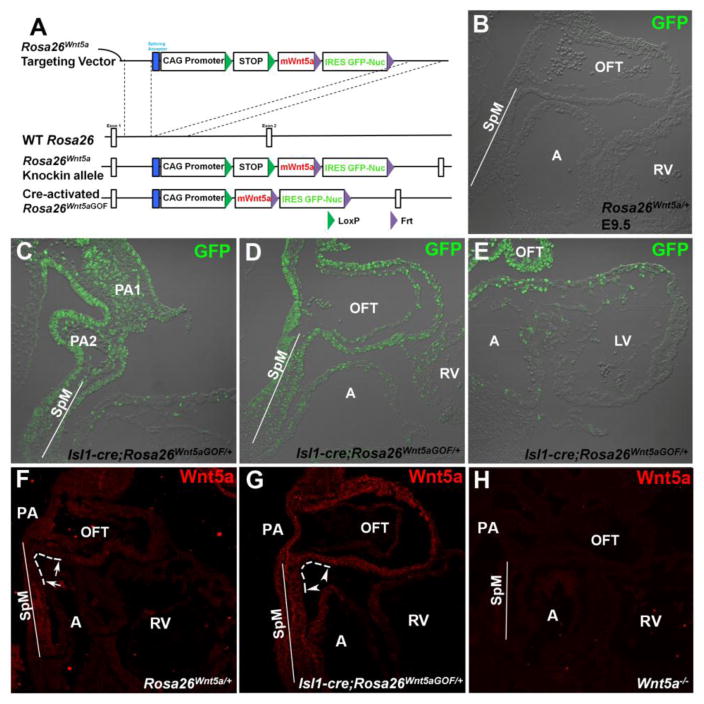
Figure 1.
Inducing broad Wnt5a over-expression in the mouse second heart field (SHF) lineage. (A) Schematic diagram of the conditional Wnt5a gain-of-function (GOF) allele Rosa26Wnt5a. A targeting vector containing a CAG promoter and a LoxP flanked polyA sequence (STOP), followed by mouse Wnt5a (mWnt5a) and IRES- (internal ribosomal entry site) GFP-Nuc (nuclear GFP) sequence, was inserted into the first intron of the constitutive Rosa26 locus. The resulting Rosa26Wnt5a allele can be converted to the activated Rosa26Wnt5aGOF allele by Cre-mediated recombination that removes the floxed STOP sequence, thereby allowing Wnt5a and GFP to be expressed specifically in all Cre-expressing cells and their descendants. (B–E) Anti-GFP antibody staining to characterize Isl1-Cre induced activation of Rosa26Wnt5a. (B) In E9.5 control Rosa26Wnt5a/+ embryos carrying no Cre, no GFP protein is detected. (C) In Isl1-cre; Rosa26Wnt5aGOF/+ embryos, nuclear GFP expression is observed in SHF progenitor cells in the pharyngeal arches (PA) and the splanchnic mesoderm (SpM), as well as the neighboring endodermal cells. (D) GFP can also be detected in SHF descendants in the outflow tract (OFT), right ventricle (RV) and atria (A). (E) In the first heart field derived left ventricle (LV), however, GFP staining is largely absent with the exception of a few cardiomyocytes around the atrioventricular canal. (F–H) Anti-Wnt5a antibody staining to characterize the distribution of Wnt5a ligand. (F) In E9.5 wild-type or control Rosa26Wnt5a/+ embryos, endogenous Wnt5a is expressed at high level in the caudal SpM, but is absent in the rostral SpM and the adjacent inferior wall of the distal OFT (arrows in F, outlined by dotted white line). Low level of Wnt5a expression resumes in the proximal part of the OFT inferior wall. Low level of Wnt5a could also be detected in the PA and throughout the superior wall of the OFT. (G) In Isl1-Cre; Rosa26Wnt5aGOF/+ embryos, activation of Rosa26Wnt5a by Isl1-Cre leads to Wnt5a over-expression throughout the entire SpM and OFT, including the rostral SpM and distal inferior wall of the OFT where no endogenous Wnt5a is detected (compare arrowheads in G to arrows in F). (H) In Wnt5a−/− embryos, no Wnt5a staining is observed, demonstrating the specificity of the anti-Wnt5a antibody. OFT: outflow tract; RV: right ventricle; SpM: splanchnic mesoderm; A: atrium; PA: pharyngeal arch.
Animal care and use was in accordance with NIH guidelines and was approved by the Animal Care and Use Committee of the University of Alabama at Birmingham.
Embryo collection, staining and imaging
Embryos were dissected at appropriated embryonic stages and yolk sac was retained for genotyping. Embryos were fixed in 4% paraformaldehyde at 4°C overnight and stored in PBS until further use.
For OFT length measurement and quantification, E9.5 embryos with comparable somites were imaged and analyzed as previously described (Sinha et al., 2012).
For immunostaining, fixed embryos were cryo-embedded and cut to 10-um sections. Sections were blocked with 5% normal donkey serum in PBS followed by primary antibodies incubation overnight. The primary antibodies used in this study are: rabbit anti-GFP (A-11122, Invitrogen), rat anti-Wnt5a (MAB645, R&D Systems), mouse MF20 (DSHB), rat anti-N-cadherin (MNCD2, DSHB), mouse anti-α-catenin (610193, BD Transduction laboratories) and Rabbit anti-pHH3 (06-570, Millipore). Afterwards, sections were incubated with appropriate fluorescent secondary antibodies or FITC conjugated Phalloidin (Sigma) prior to be mounted with Vectashield DAPI medium (Vector Laboratory). Images were acquired with an Olympus FV1000 Laser Confocal Scanning microscope and analyzed with the FV10-ASW software. LWR analysis and angular measurement of SHF cells in the caudal SpM were performed as previously described (Sinha et al., 2015a).
In situ hybridization was performed following a previously described protocol (Sinha et al., 2015b).
Wholemount immunostaining and 3D reconstruction
SpM along with part of the distal OFT was dissected out from fixed embryos (Fig. 4A). Samples were blocked with 5% normal donkey serum in PBS and incubated with primary antibody (MF20) at 4°C overnight, washed and stained with fluorescent secondary antibody. Afterwards, samples were placed in an agarose plate with the ventral side facing up, and imaged under confocal microscope. Z-stack images were imported into ImageJ for 3D reconstruction.
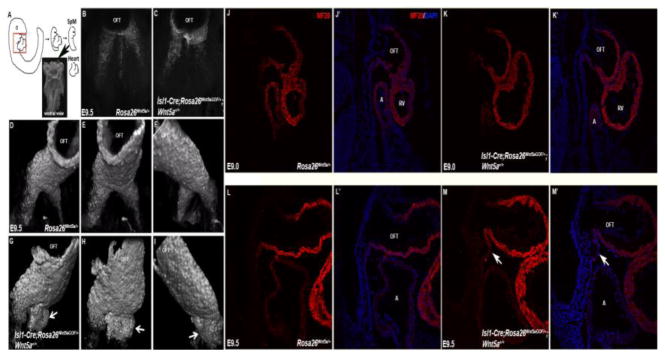
Figure 4.
SHF cells form aberrant bulge in the rostral SpM of Isl1-Cre; Rosa26Wnt5aGOF/+ mutants. (A) Schematic diagram showing how the SpM and distal OFT were isolated for staining and imaging to analyze myocardial differentiation. (B, C) The SpM and OFT from E9.5 control and Isl1-Cre; Rosa26Wnt5aGOF/+ mutant embryos were stained with MF20 and imaged ventrally under confocal microscope. (D–F) In control embryos, 3-D reconstruction of the z-stack images revealed that MF20-positive SHF progenitors were present as two narrow, lateral ridges in the rostral SpM, behind the OFT. (G–I) In Isl1-Cre; Rosa26Wnt5aGOF/+ mutants, however, the MF20 positive cells formed large, abnormal bulges under the OFT (arrows). (J–M′) Sagittal sections of E9.0 (J–K′) and E9.5 (L–M′) control (J & J′; L & L′) and Isl1-Cre; Rosa26Wnt5aGOF/+ mutant (K & K′; M & M′) embryos were stained with MF20 (red) and counterstained with DAPI (blue in J′, K′, L′, M′). The aberrant bulge formation could be observed in the rostral SpM of Isl1-Cre; Rosa26Wnt5aGOF/+ mutants at E9.5 (arrows in M & M′), but not at E9.0.
EdU pulse-chase labeling, detection and wholemount imaging
Pregnant female mouse was injected with 250ug of EdU (5-ethynyl-2′-deoxyuridine, Invitrogen) intraperitoneally at E9.25 to pulse label the proliferating cells. To detect the region where cells were proliferating, the female mouse was sacrificed two hours later to harvest embryo for wholemount EdU detection according to the user manual from the manufacturer (Invitrogen). To chase the EdU pulse-labeled proliferating cells, the female mouse was injected with 2.5mg thymidine (Sigma) after the two-hour EdU pulsing periord, and was sacrificed 18 hours later to harvest embryos at E10.0. After EdU detection, embryos were processed for MF20 wholemount staining before the SpM and OFT were dissected out for imaging with confocal microscopy. Z-stack images were imported into ImageJ for 3D reconstruction.
Semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR)
SpM micro-dissected from three E9.5 control or mutant embryos was used for RNA extraction with Trizol. cDNA was synthesized and used for semi-quantitative PCR as described previously (Wang et al., 2011).
Xenopus injection, animal cap immunostaining and western blotting
Two-cell stage Xenopus embryos were injected with 1ng of mWnt5a mRNA. At stage 8, animal caps were isolated, and either lysed for western blotting or processed for wholemount immunostaining using an anti-C-cad antibody (6B6, DSHB).
Results
Over-expression of Wnt5a in mouse SHF cells in the SpM
To overexpress Wnt5a specifically in the SHF lineage, we used a Wnt5a conditional gain-of-function (GOF) allele Rosa26Wnt5a (Fig. 1A). Detailed construction of Rosa26Wnt5a has been reported recently (Cha et al., 2014). Briefly, a cassette containing mouse Wnt5a cDNA (mWnt5a) and IRES-GFP-Nuc (nuclear GFP) was targeted into the constitutive Rosa26 locus, behind a CAG promoter (CMV enhancer + b-actin promoter) that promotes high-level, ubiquitous transcription. A polyadenylation stop sequence (STOP), flanked by LoxP sites, was inserted between the CAG promoter and Wnt5a to prevent Wnt5a and EGFP-Nuc transcription (Fig. 1A). Upon Cre-mediated recombination, however, the floxed STOP sequence is removed, converting Rosa26Wnt5a to its activated form Rosa26Wnt5aGOF and allowing Wnt5a and EGFP-Nuc to be expressed specifically in all Cre-expressing cells and their descendants (Fig. 1A).
To activate Rosa26Wnt5a specifically in the SHF, we used Islet1-Cre (Isl1-Cre) known to be expressed broadly in SHF progenitors (Cai et al., 2003; Yang et al., 2006). Since activation of Rosa26Wnt5a would simultaneously induce both Wnt5a and nuclear GFP expression, we first performed immunostaining with an anti-GFP antibody to characterize the spatial pattern of Rosa26Wnt5a activation by Isl1-Cre. On sagittal sections of control E9.5 Rosa26Wnt5a/+ embryos that did not carry Cre, no GFP positive cells were detected (Fig. 1B). In contrast, in Isl1-Cre; Rosa26Wnt5aGOF/+ embryos, GFP-positive cells were present in SHF progenitors in the pharyngeal arches (PA) and splanchnic mesoderm (SpM), as well as in neighboring endodermal cells (Fig. 1C). Consistent with the previous lineages studies on Isl1-Cre, we also detected nuclear GFP in SHF descendants in the heart, including the outflow tract (OFT), right ventricle (RV) and atria (A) (Fig. 1D & E). In contrast, we did not see GFP positive cells in the majority of the first heart field derived left ventricle (LV), with the exception of a few cardiomyocytes around the atrioventricular canal (Fig. 1E).
To determine how Wnt5a protein distribution was altered by Isl1-Cre induced Rosa26Wnt5a activation, we performed immunostaining with an anti-Wnt5a antibody (Fig. 1F–H). This antibody is highly specific to Wnt5a because in Wnt5a null mutants (Wnt5a−/−, Fig. 1H), no staining could be detected. In control Rosa26Wnt5a/+ (Fig. 1F) or wild-type (not shown) embryos, endogenous Wnt5a protein is present at high level in the caudal SpM, but is diminished towards the rostral SpM. Low levels of Wnt5a can also be observed in the PA, the superior wall of the OFT, and the inferior wall of the proximal OFT (Fig. 1F, Suppl. Fig. 1A′). No endogenous Wnt5a, however, is found in the rostral SpM and the adjacent inferior wall of the distal OFT (demarcated by arrows and outlined with dotted line, Fig. 1F, Suppl. Fig. 1A′), the atria and RV (Fig. 1F). Overall, the pattern of Wnt5a protein distribution mimics that of Wnt5a mRNA detected by in situ hybridization (Schleiffarth et al., 2007; Sinha et al., 2012; Yamaguchi et al., 1999).
In Isl1-Cre; Rosa26Wnt5aGOf/+ embryos, however, we found high level of Wnt5a throughout the entire SpM and the OFT (Fig. 1G, Suppl. Fig. 1B′), including the rostral SpM and distal inferior wall of the OFT that were devoid of endogenous Wnt5a (compare arrowheads in Fig. 1G to arrows in Fig. 1F, Suppl. Fig. 1B′). Consistent with GFP staining, exogenous Wnt5a was also detected in SHF descendants in the atria and RV in Isl1-Cre; Rosa26Wnt5aGOF/+ embryos (Fig. 1G).
Wnt5a over-expression in the SHF rescued cell polarity and SpM shortening defect in Wnt5a−/− mutants
A key unanswered questions concerning Wnt5a is whether it functions in an instructive or permissive fashion to regulate tissue morphogenesis. In our previous studies, we proposed that restricted Wnt5a expression in the caudal SpM activated PCP signaling to promote oriented cell intercalation to convert multi-layered, mesenchymal-like SHF progenitors into an epithelial sheet, thereby generating the pushing force to extend the SpM and deploy SHF cells rostrally into the OFT (Fig. 8; (Sinha et al., 2015a; Sinha et al., 2012)). Consequently, in Wnt5a null mice, SHF cells in the caudal SpM display actin polymerization and polarity defects, and the SpM between the OFT and IFT is severely shortened by E9.5 (Fig. 2C′, (Sinha et al., 2015a; Sinha et al., 2012)).
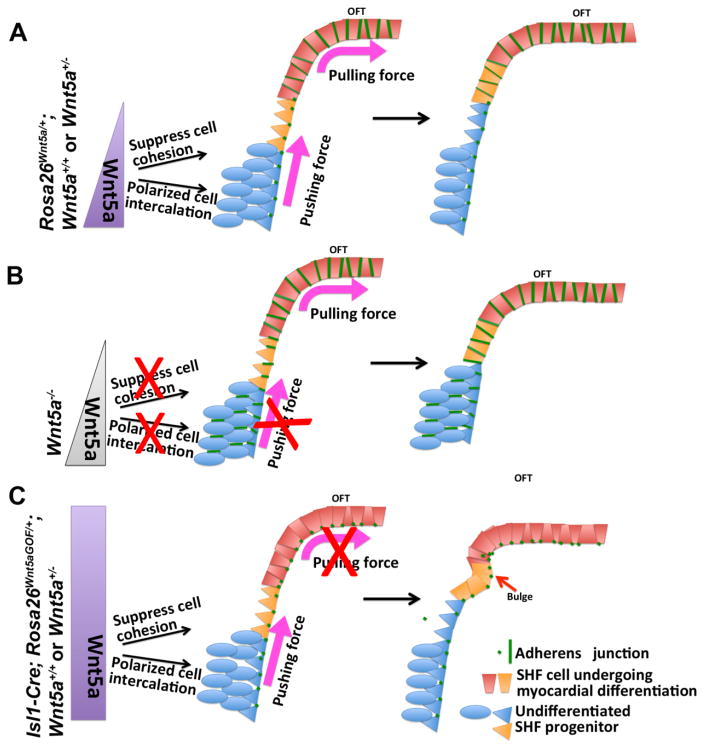
Figure 8.
An updated model for SHF deployment. (A) In wild-type embryos, Wnt5a expression in the caudal SpM activates PCP signaling to suppress adherens junction formation/ cell cohesion and promote polarized cell intercalation to convert SHF progenitors from multi-layered mesenchyme into an epithelial like sheet, thereby generating the pushing force to extend the SpM. In the rostral SpM and the adjacent distal OFT, lack of Wnt5a expression results in increased adherens junction formation/ cell cohesion, which creates the pulling force to deploy SHF cells into the OFT. (B) In Wnt5a null mutants, loss of Wnt5a in the caudal SpM fails to generate the pushing force, thereby leading to defects in SpM extension, SHF deployment, and OFT lengthening. (C) Conversely, in Wnt5a GOF mutants, ectopic Wnt5a expression in the rostral SpM disrupts the pulling force by inhibiting the normal up-regulation of cell cohesion, thereby leading to abnormal accumulation of SHF cells behind the OFT (bulge formation, red arrow), reduced SHF deployment, and insufficient lengthening of the OFT.
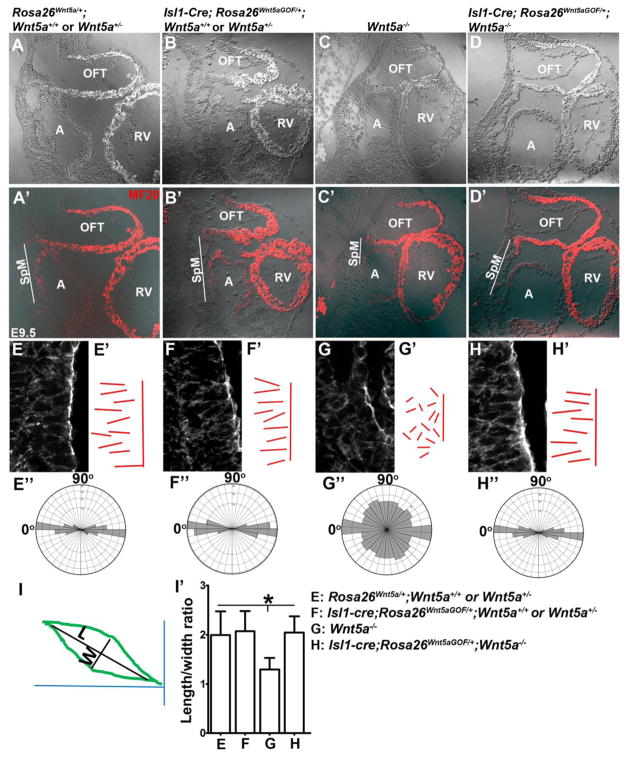
Figure 2.
Rescuing the cell polarity and SpM shortening defects in Wnt5a−/− mutants by Wnt5a over-expression (A′–D′) Immunofluorescent staining for myocardial marker MF20 was performed on sagittally sectioned E9.5 embryos to identify the atrium (A) and outflow tract (OFT). Fluorescent image was overlaid on DIC (differential interference contrast) image shown in (A)–(D). Compared to control embryos (A′), the length of the SpM between the OFT and atrium (outlined by white lines) was significantly shortened in Wnt5a−/− mutants (C′). This defect was rescued by over-expression of Wnt5a throughout the SpM in Isl1-Cre; Rosa26Wnt5aGOF/+; Wnt5a−/− embryos (D′). (E–H′) Phalloidin staining of the sagittal sections also revealed that SHF cells in the caudal SpM of control embryos were elongated and polarized, with their long axes oriented along the dorsal-ventral (D–V) axis of the embryo and perpendicular to the plane of the SpM (E, E′). These cells also display distinct actin filaments largely aligned along the D–V axis (E). In Wnt5a−/− embryos, the SHF cells in the caudal SpM display diminished and disorganized actin filaments, less elongated cell shape, and more randomized orientation of their long axis (G, G′). Isl1-Cre; Rosa26Wnt5aGOF/+ induced homogeneous Wnt5a expression in the SHF significantly rescued the elongation, orientation, and actin polymerization defects in the caudal SHF cells in Wnt5a−/− mutants (H, H′), but had no observable effect on the SHF cells in Wnt5a+/− or Wnt5a+/+ embryos (F, F′). To quantify cell polarity, lines were drawn along the long axis of each cell and represented in E′-H′. The length to width ratios (LWR) and angularity of each SHF cell in the SpM were calculated as depicted in (I). (E″–H″) Analyses with rose.net software indicated that in Rosa26Wnt5a/+ (E″), Isl1-Cre; Rosa26Wnt5aGOF/+; Wnt5a+/− or Wnt5a+/+ (F″) and Isl1-Cre; Rosa26Wnt5aGOF/+; Wnt5a−/− (H″) embryos, majority of the SHF cells (80%, 81% and 79% respectively) in the caudal SpM aligned their long axis within a ±20° arc perpendicular to the plane of the SpM (90°) and along the D–V axis of the embryo (0°). In Wnt5a−/− mutants, however, their orientations were randomized (G″). (I′) Measurement of the LWR of SHF cells in the caudal SpM. Rosa26Wnt5a/+: 1.99±0.48; Isl1-Cre; Rosa26Wnt5aGOF/+; Wnt5a+/− or Wnt5a+/+: 2.07±0.41; Wnt5a−/−: 1.29±0.23; Isl1cre; Rosa26Wnt5aGOF/+; Wnt5a−/−: 2.04±0.34. * indicated statistical significance with p<0.001.
Using the manifestation of these phenotypes as readout, we investigated whether homogeneous Wnt5a expression in the SpM of Isl1-Cre; Rosa26Wnt5aGOF/+ embryos could replace, or disrupt, endogenous Wnt5a function in the SHF. At E9.5, the length of the SpM between the OFT and IFT in embryos carrying Isl1-Cre; Rosa26Wnt5aGOF/+ in Wnt5a+/+ or Wnt5a+/− background is similar to that in control embryos (compare Fig. 2A′ to B′). In Wnt5a−/− background, however, Isl1-Cre; Rosa26Wnt5aGOF/+ induced homogeneous Wnt5a expression significantly rescued the severe SpM shortening defect in Wnt5a null mutants (compare Fig. 2D′ to C′).
We then examined the effect of homogeneous Wnt5a expression on actin organization and cell polarity of SHF progenitors. Consistent with our previous findings (Sinha et al., 2015a; Sinha et al., 2012), phalloidin staining on the sagittal sections of control Rosa26Wnt5a/+ embryos revealed that SHF cells in the caudal SpM normally display highly elongated and polarized morphology, with their long axes oriented perpendicular to the plane of the SpM and along the dorsal-ventral (D–V) axis of the embryo (Fig. 2E). These cells also display distinct actin filaments largely aligned along the D–V axis (Fig. 2E). In Wnt5a−/− embryos, however, the SHF cells in the caudal SpM display diminished and disorganized actin filaments, less elongated cell shape with significantly reduced length-to-width ratio (1.99+0.48 in control vs. 1.29+0.23 in Wnt5a−/−, p<0.001), and more randomized orientation of their long axis (Fig. 2G and I). Isl1-Cre; Rosa26Wnt5aGOF/+ induced homogeneous Wnt5a expression in Wnt5a−/− embryos significantly rescued the elongation, orientation, and actin polymerization defects in SHF cell in the caudal SpM (Fig. 2H and I), but had no detectable effect on those cells in Wnt5a+/− or Wnt5a+/+ embryos. Collectively, these data indicate that homogeneous over-expression of exogenous Wnt5a in SHF cells in the SpM does not perturb their normal morphogenesis, polarization and cytoskeletal organization, but can functionally replace endogenous Wnt5a to rescue the cell polarity, actin polymerization and SpM shortening defects in Wnt5a null embryos.
Ectopic Wnt5a expression in SHF progenitors perturbs their deployment and causes OFT shortening and heart-looping anomaly
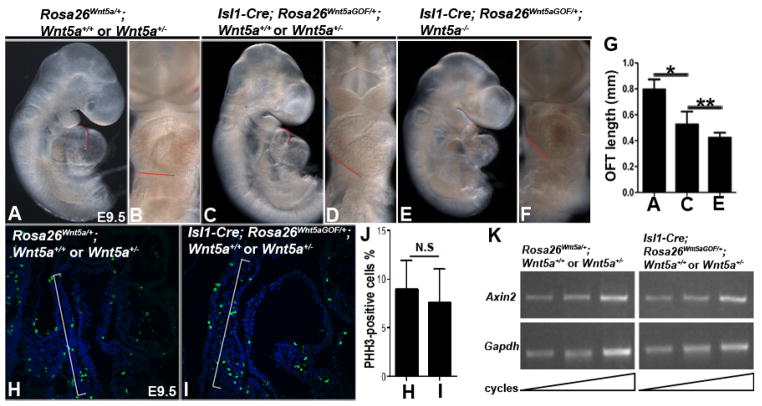
Our previous findings suggest that Wnt5a functions within SHF progenitors to promote their deployment, thereby promoting OFT lengthening and proper heart looping (Sinha et al., 2015a; Sinha et al., 2012). Given that exogenous Wnt5a expression induced by Isl1-Cre; Rosa26Wnt5aGOF/+ could rescue the SHF defects in Wnt5a−/− embryos, we expected that it might also rescue the OFT shortening and heart-looping defects. To our surprise, Isl1-Cre; Rosa26Wnt5aGOF/+ failed to rescue the OFT shortening and heart-looping defects in Wnt5a−/− mutants (Fig. 3E, G), and instead caused OFT shortening and aberrant heart looping in Wnt5a+/− or Wnt5a+/+ embryos by E9.5 (Fig. 3A–D, G). Therefore, exogenous Wnt5a expression induced by Isl1-Cre; Rosa26Wnt5aGOF/+ appears to exert a gain-of-function (GOF) or neomorphic effect to perturb OFT lengthening and heart looping, irrespective of the endogenous Wnt5a dosage. On the other hand, when we use Tnt-Cre, known to be expressed only in differentiated cardiomyocytes (Jiao et al., 2006), to activate Rosa26Wnt5a within the heart tube but not the SHF, we observed no OFT shortening defect (Suppl. Fig. 2). This result indicates that OFT shortening in Isl1-Cre; Rosa26Wnt5aGOF/+ embryos arises from defects in the SHF but not the OFT per se.
Figure 3.
Wnt5a over-expression in the SHF lineage caused OFT shortening and aberrant cardiac looping, but not defects in cell proliferation or canonical Wnt signaling in the SHF. (A, C, E) Lateral view of E9.5 Rosa26Wnt5a/+ control and Isl1-Cre; Rosa26Wnt5aGOF/+ mutant embryos. Quantification of OFT length along the inner curvature (traced with red lines in A, C, E) revealed significant shortening in Isl1-Cre; Rosa26Wnt5aGOF/+ embryos (G). (B, D, F) Frontal views showing aberrant cardiac looping and changes in the plane of ventricular alignment (red lines in B, D, F) in Isl1-Cre; Rosa26Wnt5aGOF/+ mutants. (H, I) E9.5 control and Isl1-Cre; Rosa26Wnt5aGOF/+ embryos were stained with mitotic marker anti-phospho-histone H3 (pHH3) (green) and counter-stained with nuclear dye DAPI (blue). (J) Quantification of the proliferation rate (the ratio of pHH3 positive nuclei to total nuclei) in SHF cells in the SpM (brackets in H, I) revealed no significant differences between the control and Isl1-Cre; Rosa26Wnt5aGOF/+ embryos. (K) To measure the canonical Wnt signaling activity, the SpM from E9.5 control and Isl1Cre; Rosa26Wnt5aGOF/+ embryos were micro-dissected and used for semi-quantitative RT-PCR analyses to measure the transcript level of Wnt target gene Axin2. Comparing to the control Gapdh, no significant difference in Axin2 level was found in Isl1-Cre; Rosa26Wnt5aGOF/+ embryos.
From E11.5, all Isl1-Cre; Rosa26Wnt5aGOF/+ embryos started to display pericardial edema indicative of compromised cardiac function, and died by E14.5 with severe edema (data not shown). This defect occurred irrespective of the endogenous Wnt5a genotype, suggesting that it also arose from a GOF effect owing to exogenous Wnt5a expression in SHF progenitors or their descendants in the heart. The cause of the cardiac insufficiency and embryonic lethality is currently unknown and still under investigation.
In the current study, we focused on determining how Isl1-Cre; Rosa26Wnt5aGOF/+ caused OFT shortening defects by E9.5, prior to the manifestation of compromised cardiac function. To this end, we first examined cell proliferation in the SpM of Isl1-Cre; Rosa26Wnt5aGOF/+; Wnt5a+/− or Wnt5a+/+ embryos, since expansion of SHF progenitors was critical for generating sufficient number of cells for the OFT myocardium. Immunostaining with mitosis marker phospho-histone H3 (pHH3), however, revealed no significant change in the proliferation rate in SHF progenitors in the SpM (Fig. 3H–J). Secondly, we investigated canonical Wnt signaling since it plays a key role in SHF cell proliferation and differentiation (Ai et al., 2007; Cohen et al., 2007; Kwon et al., 2009; Lin et al., 2007), and its activity could potentially be inhibited by Wnt5a (Cohen et al., 2012; Mikels and Nusse, 2006; Topol et al., 2003). We micro-dissected the SpM from E9.5 embryos and performed semi-quantitative RT-PCR to measure the mRNA level of Axin2, a direct transcription target and reliable readout of the canonical Wnt pathway (Jho et al., 2002). We found no significant difference in the level of Axin2 between Isl1-Cre; Rosa26Wnt5aGOF/+ and control embryos, indicating that canonical Wnt signaling in SHF progenitors in the SpM was not perturbed (Fig. 3K).
To find alternative cause for the OFT shortening defect, we examined myocardial differentiation of SHF cells. We dissected E9.5 SpM along with the attached distal OFT, and performed whole-mount immunostaining with myocardial marker MF20 (Fig. 4A–C). Confocal microscopy and 3-dimentional (D) reconstruction revealed that in control embryos, MF20 positive cells were present as two ridges in the SpM caudal to the OFT (Fig. 4D–F), suggesting that SHF progenitors gradually differentiate into cardiomyocytes within these two ridges as they were deployed toward the OFT. In Isl1-Cre; Rosa26Wnt5aGOF/+ mutants, we observed abundant MF20 staining in the SpM and OFT, indicating that SHF cell differentiation was not perturbed. However, instead of organizing into two narrow ridges, the MF20 positive cells in Isl1-Cre; Rosa26Wnt5aGOF/+ mutants formed large, abnormal bulges between the rostral SpM and distal OFT (arrows in Fig. 4G–I).
Sagittal sectioning of the MF20 stained embryos confirmed that in E9.5 Isl1-Cre; Rosa26Wnt5aGOF/+ mutants, MF20-positive cells accumulated as a bulge at the junction between the SpM and the OFT (white arrow in Fig. 4M′). This aberrant bulge persists to E10.5 in Isl1-Cre; Rosa26Wnt5aGOF/+ mutants (Suppl. Fig. 3B), but is never observed in control littermates at either E9.5 or 10.5 (Fig. 4L, n=8; Suppl. Fig. 3A). Importantly, in younger Isl1-Cre; Rosa26Wnt5aGOF/+ mutants collected at E9.0 (~18 somites), we also did not observe such aberrant bulge (Fig. 4K), indicating that its formation followed the time course of SHF cell deployment from the SpM to the OFT.
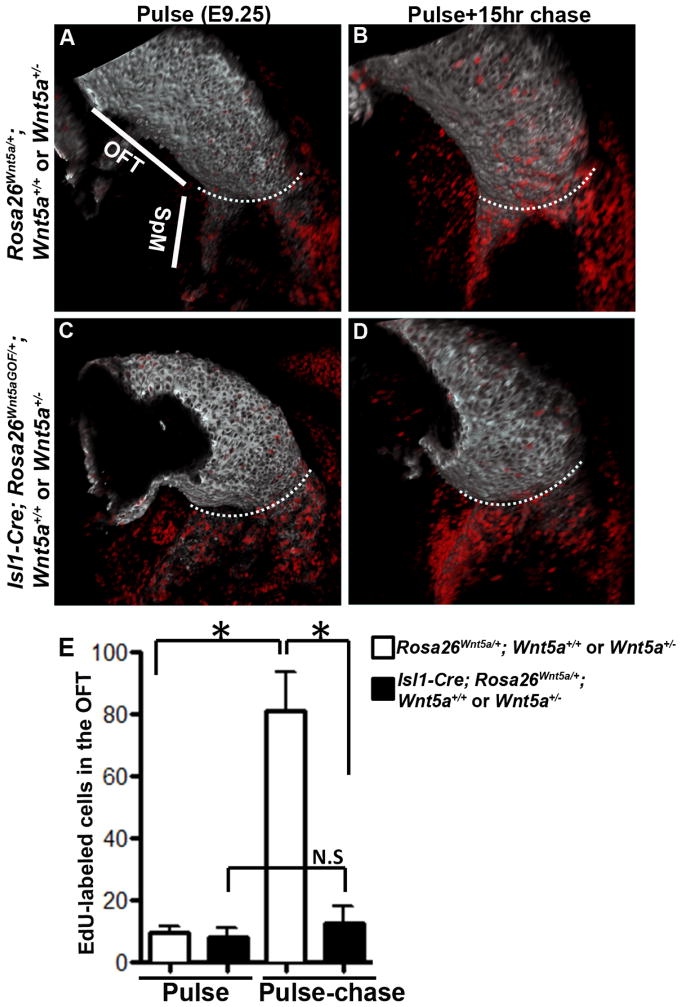
These results led us to further hypothesize that ectopic Wnt5a expression in Isl1-Cre; Rosa26Wnt5aGOF/+ embryos might block SHF cells from entering the OFT, resulting in their accumulation into aberrant bulge and failure to lengthen the OFT. To test this hypothesis, we investigated the deployment of SHF cells using EdU (5-ethynyl-2′-deoxyuridine) pulse-chase assay.
We first injected EdU to pulse label embryos for two hours at E9.25. After the pulse period, whole-mount EdU detection and MF20 staining revealed that in both control and Isl1-Cre; Rosa26Wnt5aGOF/+ mutant littermates, almost all the proliferating cells in the myocardial lineage (MF20/ EdU double-positive cells) were present in the SpM, but not in the OFT (Fig. 5A, C). This result is consistent with the previous chick study showing that myocardial progenitors are highly proliferative when in the SHF, but stop proliferating upon entering the OFT (van den Berg et al., 2009). This differential proliferation property makes it possible to use EdU pulse-chase assay to assess the deployment of rapidly proliferating SHF cells to the quiescent OFT myocardium. Therefore, after the 2-hour EdU pulse period at E9.25, we injected thymidine to terminate labeling and waited for a 15-hour chase period before collecting embryos for EdU detection. With the 15-hour chase, many EdU-labeled cells could be detected in the distal OFT myocardium in control littermates (Fig. 5B). In Isl1-Cre; Rosa26Wnt5aGOF/+ mutant littermates, however, significantly fewer EdU-labeled cells were present in the OFT (Fig. 5D, E), although large number of labeled cells were accumulated in the rostral SpM and behind the OFT. These results strongly support our hypothesis that in embryos carrying Isl1-Cre; Rosa26Wnt5aGOF/+, SHF cells fail to be deployed into the OFT and are instead accumulated at the junction between the SpM and the OFT.
Figure 5.
Deployment of SHF progenitors from the SpM to the OFT is inhibited in Wnt5a GOF mutants. (A, C) E9.25 embryos were pulsed labeled with EdU for 2 hours and processed for whole-mount EdU detection (red) and MF20 staining (grey). Confocal imaging and 3-D reconstruction showed that in both control (A) and Isl1-Cre; Rosa26Wnt5aGOF/+ mutant littermates (C), EdU labeled cells were present almost exclusively in the SpM, but not the OFT. (B, D) EdU pulse labeled E9.25 embryos were chased for 15 hours. After the chase, EdU-labeled cells could be detected in the distal OFT in control (B) but not in Isl1-Cre; Rosa26Wnt5aGOF/+ mutant littermates (D). For each image, the white dotted line marks the boundary between the OFT and the SpM. (E) For quantitative analysis of the total number of EdU-labeled cells in inferior OFT myocardium, 4–7 embryos from each group were imaged and EdU-positive cells were counted and analyzed pairwise with student t test.
Wnt5a expression affects adherens junctions in SHF cells in the SpM
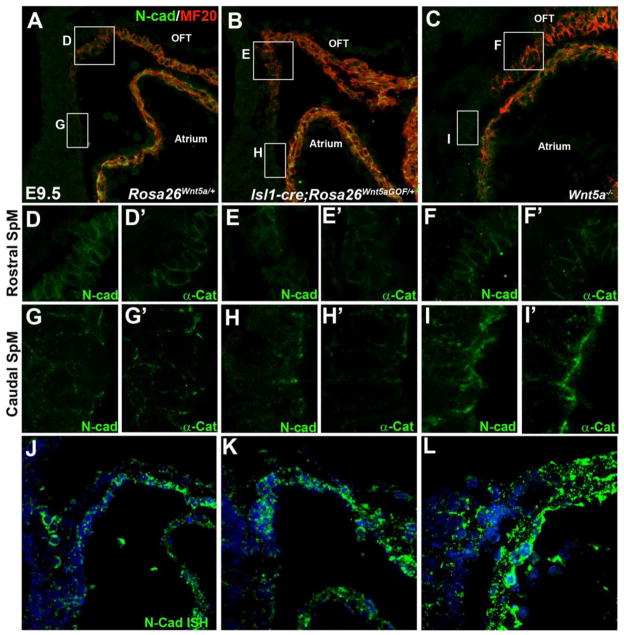
Our analyses of Isl1-Cre; Rosa26Wnt5aGOF/+ mutants revealed that both SHF deployment defect and aberrant bulge formation occurred at the junction between the rostral SpM and distal OFT, a region that lacked endogenous Wnt5a expression (Fig. 1F) but ectopically expressed Wnt5a in the mutants (Fig. 1G). The spatial correlation suggested that the deployment defect might be directly triggered by ectopic Wnt5a-induced changes at the cellular level. Interestingly, prior studies in zebrafish and Xenopus have identified a role of non-canonical Wnt pathway in regulating cadherin mediated cell cohesion (Choi and Han, 2002; Ulrich et al., 2005), and N-cadherin (N-cad) has been shown to be important for cardiac development (Linask et al., 1997; Radice et al., 1997; Soh et al., 2014). To test whether perturbation of N-cad mediated cell cohesion might underlie the defects in Isl1-Cre; Rosa26Wnt5aGOF/+ mutants, we performed double immunostaining with anti N-cad and MF20 antibodies. In E9.5 control embryos, SHF progenitors in the caudal SpM were negative for MF20, and have low level of N-cad that was localized primarily at the apical cell-cell junctions (Fig. 6A, 6G; Suppl. Fig. 4C, C′, D, D′). As SHF cells move to the rostral SpM, they became positive for myocardial marker MF20, and N-cad was expanded to localize along the entire boundaries between adjacent cells (Fig. 6A, 6D; Suppl. Fig. 4A, A′, B, B′). Immunostaining of adjacent sections showed a similar increase of α-Catenin (αCat) localization at the cell boundaries in the rostral SpM (Fig. 6A, D′, G′). Therefore, there appears to be a gradual increase in adherens junction formation and cell cohesion from the caudal to the rostral SpM as SHF cells undergo gradual myocardial differentiation and deployment towards the OFT.
Figure 6.
Wnt5a expression affects cell cohesion in the SHF. Sagittal sections of E9.5 control (A, Rosa26Wnt5a/+), Wnt5a GOF mutant (B, Isl1-Cre; Rosa26Wnt5aGOF/+; Wnt5a+/− or Wnt5a+/+), and Wnt5a−/− mutant (C) embryos were double stained with anti N-cadherin (N-cad) and MF20 antibodies. White square boxes in (A–C) denote the rostral SpM in each embryo and the enlarged views are shown in (D–F), respectively. (D′–F′) show adjacent sections stained with anti α-Catenin (α-Cat) antibody. White rectangle boxes in (A–C) denote the caudal SpM, and the enlarged views are represented in (G–I), respectively. (G′–I′) show adjacent sections stained with anti α-Cat antibody. (J), (K), (L): Fluorescent N-cad in situ hybridization on sections adjacent to (A), (B), and (C), respectively.
In Isl1-Cre; Rosa26Wnt5aGOF/+ mutants, we found that N-cad and α-Cat distribution was not perturbed in SHF cells in the caudal SpM (Fig. 6H, 6H′; Suppl. Fig. 5G–H′), but were significantly diminished at the cell boundaries in the MF-20 positive SHF cells in the rostral SpM and the adjacent distal OFT (Fig. 6E, 6E′; Suppl. Fig. 5E–F′). This result suggests that in the Isl1-Cre; Rosa26Wnt5aGOF/+ mutants, ectopic Wnt5a expression in the rostral SpM/distal OFT may have blocked a normal up-regulation of adheren junction formation and cell cohesion that are important for the deployment of SHF cells into the OFT, leading to aberrant bulge formation at the junction between the SpM and OFT (Fig. 4).
The above results promoted us to test whether endogenous Wnt5a might also play a role in modulating adherens junction/ cell cohesion in the SHF. To this end, we examined N-cad expression in Wnt5a−/− embryos. Interestingly, in the caudal SpM where endogenous Wnt5a is highly expressed (Fig. 1F), we found that SHF cells in Wnt5a−/− embryos displayed increased levels of N-cad and α-Cat (Fig. 6I, 6I′) at cell-cell contact. Staining on transverse sections from the caudal SpM showed that N-cad appeared to be localized more extensively along the lateral cell-cell junction in Wnt5a−/− embryos (Suppl. Fig. 5K–L′), in contrast to the apically restricted N-cad distribution in wild-type embryos (Suppl. Fig. 5C–D′). Conversely, in the rostral SpM where endogenous Wnt5a is not expressed (Fig. 1F), Wnt5a−/− embryos displayed no appreciable difference in N-cad/ α-Cat level or localization (Fig. 6F, 6F′; Suppl. Fig. 5I–J′).
Taken together, our studies with both Wnt5a gain- and loss-of-function mutants in mice support the idea that Wnt5a modulates adherens junction/ cell cohesion in the SHF by suppressing N-cad level or distribution at the cell-cell junction.
Wnt5a attenuates cadherin distribution at the cell surface
To further understand how Wnt5a might regulate N-cad in the SHF, we first performed fluorescence in situ hybridization to determine N-cad transcript level. We did not find significant difference in the level of N-cad mRNA in the SpM between control embryos and Isl1-Cre; Rosa26Wnt5aGOF/+ or Wnt5a−/− mutants (Fig. 6J–L), suggesting that Wnt5a regulated N-cad post-transcriptionally.
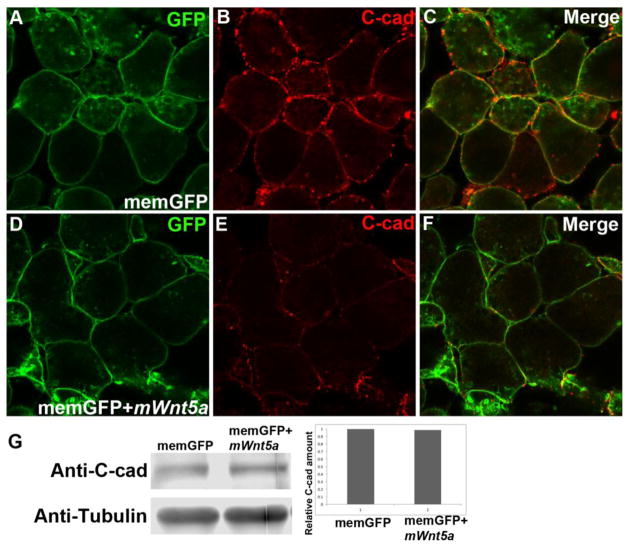
To test this idea further, we expressed mouse Wnt5a in Xenopus embryos, a classic system for studying non-canonical Wnt signaling. We injected mRNA of membrane GFP (memGFP), either alone or together with mouse Wnt5a mRNA (mWnt5a), into two-cell stage Xenopus embryos, and dissected animal cap at stage 8–9. In cells from memGFP single-injected embryos, C-cadherin (C-cad) was present as either distinct dots on the cell surface that had no contact with surrounding cells, or as contiguous lines along the junctions between cells that established contacts (Fig. 7B). In cells from mWnt5a co-injected embryos, however, the overall level of C-cad on the plasma membrane appeared to be significantly reduced (Fig. 7E), reminiscent of the defects associated with ectopic Wnt5a expression in Isl1-Cre; Rosa26Wnt5aGOF/+ mutant mice (Fig. 6E). Western blot analysis, however, revealed that the total amount C-cad protein was similar between memGFP single-injected and mWnt5a co-injected animal caps (Fig. 7G). Therefore, Wnt5a is most likely to regulate cadherin trafficking, localization or stability at the plasma membrane, but not its expression at the transcriptional or translational level.
Figure 7.
Mouse Wnt5a overexpression attenuates plasma membrane localization of C-cadherin in Xenopus animal cap cells. Two-cell stage Xenopus embryos were injected with mRNA for membrane GFP (memGFP), either alone (A) or together with mouse Wnt5a mRNA (mWnt5a) (D). Animal caps were dissected at stage 8 and stained with anti C-cad antibody (B & C; merged views in E & F). (G) Animal caps from injected embryos were lysed for immunoblotting analysis to quantify the amount of C-cad protein. α-tubulin was used as loading control.
Discussion
Wnt5a signaling in the caudal SpM generates a pushing force for SHF deployment
Numerous studies have established that SHF progenitors, residing in the PM and SpM, are added gradually to the heart tube to form the right ventricular and OFT myocardium (Cai et al., 2003; Kelly et al., 2001; Mjaatvedt et al., 2001; Waldo et al., 2001). Sufficient addition of SHF cells is critical for heart tube elongation, cardiac looping and conotruncal development, but we know very little about the mechanisms that underlie the deployment process per se. Our previous work, using loss-of-functional mouse mutants and targeted antibody injection in chick embryos, has implicated Wnt5a-initiated PCP signaling as one of the key mechanisms that promote the deployment of SHF cells from the SpM region to the OFT (Sinha et al., 2015a; Sinha et al., 2012). We proposed that in the caudal SpM, Wnt5a signals through the PCP pathway to induce polarized elongation and actin polymerization in SHF cells, thereby promoting CE-like, oriented intercalation to incorporate loosely packed, mesenchymal-like SHF cells into an epithelial like sheet. This morphogenetic event in turn generates the pushing force to extend the SpM rostrally and deploy the SHF cells into the inferior myocardial wall of the OFT (Fig. 8; (Sinha et al., 2015a; Sinha et al., 2012)).
In the current study, we show that endogenous Wnt5a protein is expressed in a highly restricted fashion in the caudal SpM (Fig. 1). In Wnt5a null mutants, expressing exogenous Wnt5a broadly in the SHF lineage using Rosa26Wnt5aGOF and Isl1-Cre can rescue not only the cellular defects in the caudal SpM, including polarization, elongation and actin polymerization defects in the SHF cells, but also the severe shortening of the SpM between the atrium and the OFT (Fig. 2). These results support our model that Wnt5a regulates polarized cell intercalation in the SHF to promote the extension of the SpM.
The restricted expression of Wnt5a in wild-type embryos suggests that endogenous Wnt5a may be present in a caudal to rostral gradient in the SpM. In contrast, Isl1-Cre; Rosa26Wnt5aGOF induced exogenous Wnt5a expression is homogenous throughout the SpM (Fig. 1). The fact that it can replace endogenous Wnt5a and restore cell polarization and SpM lengthening in Wnt5a null mutants imply that a presumptive Wnt5a gradient may not be required for establishing cell polarity and oriented cell behavior. This interpretation would be consistent with the idea that Wnt5a activated PCP signaling functions in a permissive fashion to enable the cells to respond to an instructive cue, such as a gradient of another signaling molecule. For instance, during Xenopus CE, a gradient of Activin along the A–P axis has been proposed to specify cell polarity for medial-laterally oriented cell intercalation, and PCP signaling enables the response to the Activin gradient (Ninomiya et al., 2004). Given that many signaling pathways, such as Fgf, Tgfβ/Bmp and Shh, are known to be active in the SHF, it will be important to determine in the future whether any of them may provide the instructive cue needed to polarize the SHF cells in the SpM.
Another interesting finding we made in the current study is a potential role of Wnt5a in modulating cell cohesion in the SHF. Loss of endogenous Wnt5a expression in the caudal SpM causes increased N-cad and α-Cat membrane localization in SHF cells in this region, whereas ectopic Wnt5a expression in the rostral SpM reduces N-cad and α-Cat membrane localization (Fig. 6). These results suggest that endogenous Wnt5a may have a direct role in down-regulating cell cohesion in the caudal SpM, and may explain why SHF cells in the caudal SpM of Wnt5a null mutants form abnormal, compact clusters (Sinha et al., 2012). Low cell cohesion maintained by Wnt5a may increase dynamic cell re-arrangement to facilitate oriented cell interaction in the caudal SpM, similar to the proposed role of XWnt5a during Xenopus CE (Choi and Han, 2002; Kuhl et al., 2000; Torres et al., 1996).
In Xenopus, XWnt5a is thought to act through Cdc42 to down-regulate cell cohesion in mesodermal cells and animal explants undergoing CE (Choi and Han, 2002). In gastrulating zebrafish, another non-canonical Wnt, Wnt11, promotes Rab5-mediated endocytosis of E-cadherin (E-cad) to modulate cell cohesion in mesendodermal cells (Ulrich et al., 2005). Our results show that mouse Wnt5a regulates cadherin at post-transcriptional/ post-translational level, and that it can play a conserved role in Xenopus explant to down-regulate C-cad localization at the plasma membrane without affecting its protein level (Fig. 6 and 7). Therefore, in light of the Xenopus and zebrafish studies, our data are most consistent with the idea that mouse Wnt5a also modulate cell cohesion through regulating cadherin trafficking or retention at the plasma membrane. More detailed mechanistic studies will need to be carried out to test this idea in the future. Given the critical requirement of Wnt5a in the development of many additional tissues/organs in mammals, it will also be important to determine whether Wnt5a modulates cell cohesion in other developmental context.
Up-regulation of cell cohesion in the rostral SpM/ distal OFT may serve as a pulling force for SHF deployment
Isl1-Cre; Rosa26Wnt5aGOF induced exogenous Wnt5a expression in the SHF of Wnt5a null mutants is able to rescue the SpM shortening defect, but fails to rescue the OFT shortening defect. Furthermore, in Wnt5a+/+ or Wnt5a+/− background, Isl1-Cre; Rosa26Wnt5aGOF is able to cause severe OFT shortening defects. We interpret this result to mean that while Isl1-Cre; Rosa26Wnt5aGOF can substitute for endogenous Wnt5a function in SHF progenitors in the SpM, it also exerts a gain of function effect that perturbs OFT lengthening.
We think that Isl1-Cre; Rosa26Wnt5aGOF perturbs OFT lengthening primarily through blocking SHF deployment at the rostral SpM for the following reasons. First, we observe no abnormalities in SHF proliferation, apoptosis or differentiation in Isl1-Cre; Rosa26Wnt5aGOF embryos (Fig. 3). Instead, we find that differentiated myocardial cells are accumulated into an aberrant bulge at the junction between the rostral SpM and the OFT (Fig. 4). Secondly, the aberrant bulge only forms after E9.0 following the onset of SHF deployment, suggesting that it may arise from a consequence of failed SHF deployment. Thirdly, our EdU pulse-chase experiments indeed demonstrate that in Isl1-Cre; Rosa26Wnt5aGOF embryos, SHF cells in the SpM fail to move into the OFT efficiently, and are instead accumulated at the junction between the rostral SpM and the OFT (Fig. 5).
Based on the known properties of non-canonical Wnt/ PCP signaling, Isl1-Cre; Rosa26Wnt5aGOF may exert gain of function effect to block SHF deployment by either increasing Wnt5a expression level to perturb the function of endogenous Wnt5a, or inducing ectopic Wnt5a expression to cause spatially aberrant pathway activation. We think that the former is unlikely because in Isl1-Cre; Rosa26Wnt5aGOF embryos we find no evidence for disrupted endogenous Wnt5a function. For instance, in the caudal SpM where endogenous Wnt5a is expressed (Fig. 1F), SHF cells in Isl1-Cre; Rosa26Wnt5aGOF; Wnt5a+/+ embryos display normal cell polarity and actin polymerization, in contrast to the defective polarity and actin polarization when endogenous Wnt5a function is disrupted in Wnt5a−/− embryos (Fig. 2). Our unpublished data also show that in the hind limb, where Isl1-Cre is highly expressed (Yang et al., 2006), Isl1-Cre; Rosa26Wnt5aGOF; Wnt5a+/+ embryos display normal skeletal formation. This is also in direct contrast to the severe limb skeletal defects resulted from loss of endogenous Wnt5a in Wnt5a−/− embryos (Yamaguchi et al., 1999). Collectively, these data lead us to reason that Rosa26Wnt5aGOF induced Wnt5a over-expression is not high enough to perturb the normal function of endogenous Wnt5a.
In contrast, our data are more supportive of the alternate idea that the SHF deployment defect in Isl1-Cre; Rosa26Wnt5aGOF mutants is triggered by ectopic Wnt5a expression. Our immunostaining clearly shows that in Isl1-Cre; Rosa26Wnt5aGOF embryos, Wnt5a expression is expanded to the rostral SpM and the adjacent distal OFT, where no endogenous Wnt5a is expressed (Fig. 1). Coincided with the rostral expansion of Wnt5a expression, both N-cad and α-Cat distribution at the cell-cell contact is abnormally reduced, suggesting that signaling events downstream of Wnt5a have been activated to aberrantly down-regulate adherens junction formation/ cell cohesion (Fig. 6). Interestingly, in wild-type embryos there appears to be a gradual increase of adherens junction formation in SHF cells from the caudal to the rostral SpM, as indicated by enhanced N-cad and α-Cat distribution at the cell-cell contract (Fig. 6, Suppl. Fig. 4 & 5). In a recent report, E-cadherin is also found to be expressed in SHF cells in the rostral, but not the caudal, SpM (Francou et al., 2014). Therefore, we reason that there may be an increasing gradient of cell cohesion from the caudal SpM to rostral SpM/ distal OFT, which may act as a pulling force to deploy SHF cells rostrally into the OFT (Fig. 8A). Ectopic Wnt5a expression in Isl1-Cre; Rosa26Wnt5aGOF mutants may perturb this pulling force by inhibiting the normal up-regulation of cell cohesion at the rostral SpM/ distal OFT (Fig. 8C), thereby leading to reduced SHF deployment, abnormal accumulation of SHF cells behind the OFT (Fig. 5), and insufficient lengthening of the OFT (Fig. 3).
Previous studies have demonstrated that N-cad mediated cell cohesion is crucial for cardiac development, structural integrity and function. Similar to our finding in the SHF in the mouse, Linask et al. have also observed an increase in cell cohesion during myocardial differentiation in the first heart field in avian embryos: N-cad and α/β-catenin are initially restricted to the apical cell junctions in chick precardiac mesoderm, but display markedly enhanced and even distribution throughout cell boundaries upon myocardial differentiation and formation of tubular heart (Linask, 1992; Linask et al., 1997). Germ-line deletion of N-cad in mice does not completely block myocardial differentiation since a primitive heart tube is able to form, although with no clear SHF derived structures. The myocardium integrity and cardiac function is also severely compromised, and N-cad null mutants die between E9.5 and 10.5 (Radice et al., 1997). These phenotypes imply that while N-cad is indispensible for maintaining the structural integrity in differentiated myocardium, it also has a role in the development of the SHF. Indeed, when N-cad is deleted specifically in the SHF lineage using Mef2c-Cre, the OFT is severely shortened and the RV is reduced in size, indicative of reduced SHF contribution to the heart (Soh et al., 2014). These defects are attributed to a role of N-cad in sustaining SHF progenitor proliferation and preventing their premature differentiation. Our work differs from the prior studies in that in our Wnt5a GOF mutants, ectopic Wnt5a expression in the SHF reduces, but does not completely eliminate, N-cad mediated cell cohesion. Therefore, we are able to bypass the proliferation/differentiation defect in N-cad loss-of-function mutants, and to identify a more subtle, yet critical, role of N-cad in the deployment of SHF cells into the OFT.
Conclusion
In summary, based on both loss- and gain-of-function studies of Wnt5a in mice, we propose an updated and integrated model on SHF deployment, as illustrated in Fig. 8A. In the caudal SpM close to the atria, Wnt5a activates PCP signaling to suppress cell cohesion and regulate polarized cell intercalation to convert SHF progenitors from multilayered mesenchyme into an epithelial like sheet, thereby generating the pushing force to extend the SpM. In the rostral SpM and the adjacent distal OFT, loss of Wnt5a expression results in increased cell cohesion, which creates the pulling force to move SHF cells into the OFT. The combined pushing and pulling force provides the necessary mechanical force to deploy SHF cells efficiently from the SpM into the heart tube to promote OFT elongation, cardiac looping and proper conotruncal formation.
Supplementary Material
Highlights.
Endogenous Wnt5a is expressed specifically in SHF progenitors in the caudal SpM;
Expressing Wnt5a throughout the SHF lineage rescues caudal SpM defects in Wnt5a−/− mutants but blocks SHF cells in the rostral SpM from entering the OFT;
Wnt5a expression is inversely correlated with adherens junction level in the SpM;
Expressing mouse Wnt5a in Xenopus down-regulates cadherin at cell surface;
Spatial regulation of cell cohesion by Wnt5a may promote SHF deployment to the OFT.
Acknowledgments
We thank Dr. Masatoshi Takeichi for the N-cadherin probe for in situ hybridization. This work was supported by grants from the National Institute of Health (R01 HL109130) and American Heart Association (14GRNT20380467) to J.W., and an AHA pre-doctoral fellowship (12PRE12060081) to T.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, Klein WH, Martin JF. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci U S A. 2007;104:9319–9324. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BL. Transcriptional pathways in second heart field development. Semin Cell Dev Biol. 2007;18:67–76. doi: 10.1016/j.semcdb.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Developmental cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Bartos A, Park C, Sun X, Li Y, Cha SW, Ajima R, Ho HY, Yamaguchi TP, Dey SK. Appropriate crypt formation in the uterus for embryo homing and implantation requires Wnt5a-ROR signaling. Cell Rep. 2014;8:382–392. doi: 10.1016/j.celrep.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SC, Han JK. Xenopus Cdc42 regulates convergent extension movements during gastrulation through Wnt/Ca2+ signaling pathway. Developmental biology. 2002;244:342–357. doi: 10.1006/dbio.2002.0602. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Miller MF, Wang Z, Moon RT, Morrisey EE. Wnt5a and Wnt11 are essential for second heart field progenitor development. Development. 2012;139:1931–1940. doi: 10.1242/dev.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. The Journal of clinical investigation. 2007;117:1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D. The cell biology of planar cell polarity. J Cell Biol. 2014;207:171–179. doi: 10.1083/jcb.201408039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer LA, Kirby ML. The role of secondary heart field in cardiac development. Developmental biology. 2009;336:137–144. doi: 10.1016/j.ydbio.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Yelon D, Conlon FL, Kirby ML. Myocardial lineage development. Circulation research. 2010;107:1428–1444. doi: 10.1161/CIRCRESAHA.110.227405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francou A, Saint-Michel E, Mesbah K, Kelly RG. TBX1 regulates epithelial polarity and dynamic basal filopodia in the second heart field. Development. 2014;141:4320–4331. doi: 10.1242/dev.115022. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Developmental cell. 2011;21:120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao K, Langworthy M, Batts L, Brown CB, Moses HL, Baldwin HS. Tgfbeta signaling is required for atrioventricular cushion mesenchyme remodeling during in vivo cardiac development. Development. 2006;133:4585–4593. doi: 10.1242/dev.02597. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Developmental cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nature cell biology. 2009;11:951–957. doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R, Rosenfeld MG, Chen J, Evans SM. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci U S A. 2007;104:9313–9318. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linask KK. N-cadherin localization in early heart development and polar expression of Na+,K(+)-ATPase, and integrin during pericardial coelom formation and epithelialization of the differentiating myocardium. Developmental biology. 1992;151:213–224. doi: 10.1016/0012-1606(92)90228-9. [DOI] [PubMed] [Google Scholar]
- Linask KK, Knudsen KA, Gui YH. N-cadherin-catenin interaction: necessary component of cardiac cell compartmentalization during early vertebrate heart development. Developmental biology. 1997;185:148–164. doi: 10.1006/dbio.1997.8570. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR. The outflow tract of the heart is recruited from a novel heart-forming field. Developmental biology. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- Ninomiya H, Elinson RP, Winklbauer R. Antero-posterior tissue polarity links mesoderm convergent extension to axial patterning. Nature. 2004;430:364–367. doi: 10.1038/nature02620. [DOI] [PubMed] [Google Scholar]
- Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Developmental biology. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Ramsbottom SA, Sharma V, Rhee HJ, Eley L, Phillips HM, Rigby HF, Dean C, Chaudhry B, Henderson DJ. Vangl2-regulated polarisation of second heart field-derived cells is required for outflow tract lengthening during cardiac development. PLoS Genet. 2014;10:e1004871. doi: 10.1371/journal.pgen.1004871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiffarth JR, Person AD, Martinsen BJ, Sukovich DJ, Neumann A, Baker CV, Lohr JL, Cornfield DN, Ekker SC, Petryk A. Wnt5a is required for cardiac outflow tract septation in mice. Pediatr Res. 2007;61:386–391. doi: 10.1203/pdr.0b013e3180323810. [DOI] [PubMed] [Google Scholar]
- Sinha T, Li D, Theveniau-Ruissy M, Hutson MR, Kelly RG, Wang J. Loss of Wnt5a disrupts second heart field cell deployment and may contribute to OFT malformations in DiGeorge syndrome. Human molecular genetics. 2015a;24:1704–1716. doi: 10.1093/hmg/ddu584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha T, Lin L, Li D, Davis J, Evans S, Wynshaw-Boris A, Wang J. Mapping the dynamic expression of Wnt11 and the lineage contribution of Wnt11-expressing cells during early mouse development. Developmental biology. 2015b;398:177–192. doi: 10.1016/j.ydbio.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha T, Wang B, Evans S, Wynshaw-Boris A, Wang J. Disheveled mediated planar cell polarity signaling is required in the second heart field lineage for outflow tract morphogenesis. Developmental biology. 2012;370:135–144. doi: 10.1016/j.ydbio.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh BS, Buac K, Xu H, Li E, Ng SY, Wu H, Chmielowiec J, Jiang X, Bu L, Li RA, Cowan C, Chien KR. N-cadherin prevents the premature differentiation of anterior heart field progenitors in the pharyngeal mesodermal microenvironment. Cell Res. 2014;24:1420–1432. doi: 10.1038/cr.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Heisenberg CP. Convergent extension: using collective cell migration and cell intercalation to shape embryos. Development. 2012;139:3897–3904. doi: 10.1242/dev.073007. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Yang-Snyder JA, Purcell SM, DeMarais AA, McGrew LL, Moon RT. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Developmental cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- van den Berg G, Abu-Issa R, de Boer BA, Hutson MR, de Boer PA, Soufan AT, Ruijter JM, Kirby ML, van den Hoff MJ, Moorman AF. A caudal proliferating growth center contributes to both poles of the forming heart tube. Circulation research. 2009;104:179–188. doi: 10.1161/CIRCRESAHA.108.185843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Current topics in developmental biology. 2010;90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Wang B, Sinha T, Jiao K, Serra R, Wang J. Disruption of PCP signaling causes limb morphogenesis and skeletal defects and may underlie Robinow syndrome and brachydactyly type B. Human molecular genetics. 2011;20:271–285. doi: 10.1093/hmg/ddq462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sinha T, Wynshaw-Boris A. Wnt signaling in mammalian development: lessons from mouse genetics. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Olson EN, Bassel-Duby R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat Rev Mol Cell Biol. 2013;14:529–541. doi: 10.1038/nrm3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yang L, Cai CL, Lin L, Qyang Y, Chung C, Monteiro RM, Mummery CL, Fishman GI, Cogen A, Evans S. Isl1Cre reveals a common Bmp pathway in heart and limb development. Development. 2006;133:1575–1585. doi: 10.1242/dev.02322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Zhou W, Lin L, Majumdar A, Li X, Zhang X, Liu W, Etheridge L, Shi Y, Martin J, Van de Ven W, Kaartinen V, Wynshaw-Boris A, McMahon AP, Rosenfeld MG, Evans SM. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nature genetics. 2007;39:1225–1234. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.