Abstract
Objectives
To determine response rates for clinically significant weight loss (CWL) following different aerobic exercise training amounts and if enhanced cardiometabolic adaptations are observed with CWL compared to modest weight loss (MWL) or neither.
Methods
Participants (N=330) performed 6 months of aerobic training at 4kcals per kg per week (KKW), 8KKW or 12 KKW (50%, 100%, and 150% of recommended levels respectively). Weight loss was categorized as CWL (≥5%) or MWL (3.0% to 4.9%) or neither.
Results
The CWL response rate was greater in the 8KKW group (20.2%, CI: 13.0 to 27.5%) compared to 4KKW (10.3%, CI: 4.6 to 16.0%), but not compared to the 12KKW group (14.6%, CI: 7.6% to 21.6%). Reductions in HOMA-IR were observed in participants with CWL (−0.60, CI: −0.98 to −0.22) and with MWL (−0.48, CI:−0.87 to −0.10), but not those who achieved neither (−0.06, CI −0.22 to 0.10). No changes between groups were observed for cholesterol, fitness, or blood pressure.
Conclusions
Low response rates for CWL were observed following training, even at levels above recommended levels. Achieving MWL with exercise may represent a reasonable initial weight loss target since the improvement in insulin resistance with MWL are similar to what is achieved with CWL.
Keywords: Clinically significant weight loss, exercise training, obesity, insulin resistance
Introduction
Clinically significant weight loss (CWL) is defined as at least a 5% reduction in weight from the baseline level (1), and associated with improvements in cardiometabolic risk factors, such as beneficial changes in lipid profile and insulin sensitivity (1, 2, 3). Data from randomized controlled trials (where exercise sessions were supervised, the intervention was at least 12 weeks in duration, and no additional requirements for caloric restriction) suggest that participants who are overweight/obese achieve minimal to modest weight loss following aerobic exercise training (<2%) at levels consistent with physical activity (PA) recommendations (4, 5, 6, 7). Therefore, PA programs at or below current recommendations for PA in participants who are overweight/obese are unlikely to reach the threshold of CWL (1). Potential rationales explaining the low prevalence of CWL resulting from exercise training trials, despite the seemingly high energy expenditure associated with regular exercise training, include compensatory reductions in non-exercise PA and increases in caloric intake (8). Another important factor may be insufficient energy expenditure, as studies which have energy expenditure levels above PA guidelines have been shown to produce a greater overall weight loss (8, 9, 10).
While the overall mean percentage of weight loss from aerobic exercise training has been examined over an entire study population (1), individual variability exists in the overall response of weight loss with aerobic exercise training as several studies have shown that a sub-set of individuals are able to achieve greater weight loss following aerobic exercise training (11, 12). However, the response rates for CWL following different amounts of PA of aerobic exercise training relative to current recommended levels has not been previously quantified. This is clinically relevant to postmenopausal women who have elevated risk for cardiovascular disease (13, 14) and rates of obesity (15) compared to pre-menopausal women. The purpose of the present study was to: 1) Define the overall prevalence of CWL in postmenopausal women following aerobic exercise training consistent with public health recommendations, 50% below public health recommendations and 50% above public health recommendations; and 2) Compare the changes in cardiovascular risk factors in postmenopausal women able to achieve CWL compared to those that do not.
METHODS
The DREW study evaluated the effect of increasingly higher doses of energy expenditure on cardiorespiratory fitness in postmenopausal women (16). The protocol was reviewed and approved annually by the Cooper Institute institutional review board. Written informed consent was obtained prior to screening. Women recruited for this study were overweight or obese, sedentary (exercising < 20 minutes on ≤ 3 days per week and objective measurements of <8,000 steps/day), and had elevated systolic blood pressure. Notable exclusion criteria included the presence of significant CVD, conditions contraindicated for exercise training, elevated low density lipoprotein, and significant weight loss in the previous year (16).
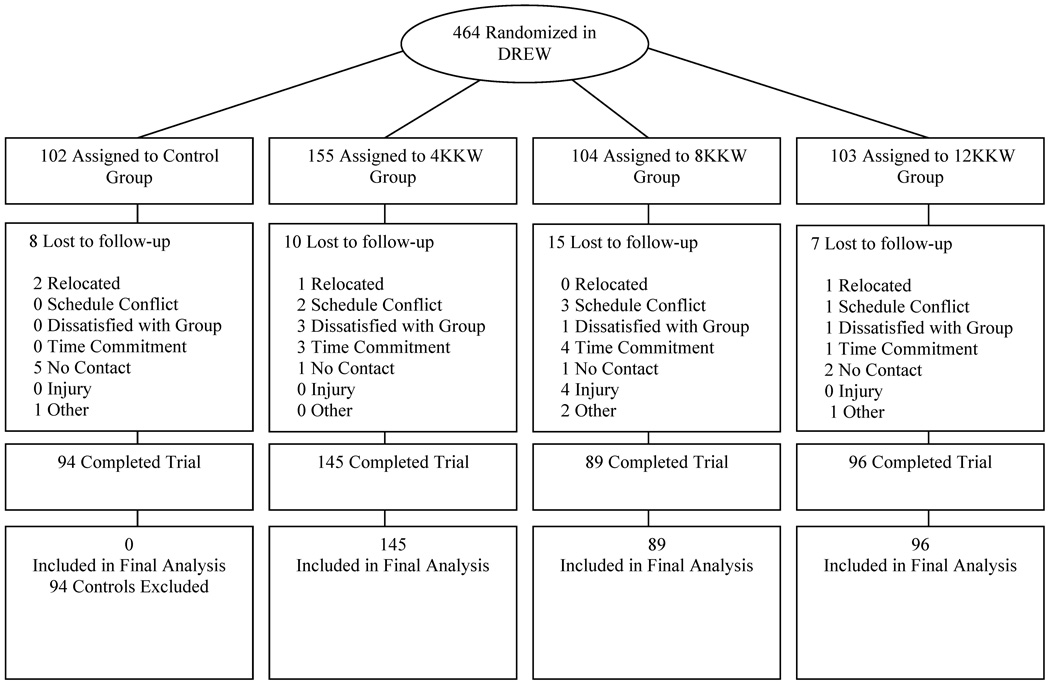
The present manuscript evaluates the effect of a specific dose of aerobic exercise training on the prevalence of CWL and the subsequent effects of exercise training with CWL on cardiometabolic variables. Therefore, we included exercisers from the DREW study who completed baseline and follow-up visits assessments. The consort diagram for the present study is shown in Figure 1. From the full sample of 464 participants, we included 330 participants in the final analysis (40 excluded for not completing baseline and follow-up assessment visits). Since the present paper focuses on the effects of exercise and weight loss, we excluded an additional 94 participants from the control group.
Figure 1.
Consort Diagram
Maximal exercise testing
Participants cycled at 30 watts (W) for 2 minutes, then 50 W for 4 minutes, followed by increases of 20 W every 2 minutes until they could no longer maintain a pedal cadence of 50 revolutions per minute. Respiratory gases were measured using a Parvomedics Truemax 2400 Metabolic Measurement Cart (Sandy, UT). Fitness measures were quantified in relative (mL O2•kg−1•min−1) and absolute VO2 peak (L O2/min). Two fitness tests were performed on different days at baseline and two tests were performed at follow-up (within 48–72 hours of each other). The average value for the two tests at each time point was used in the analyses unless only one of the two tests were completed at baseline or follow-up, in which case, we used the single value.
Non-exercise physical activity
Baseline PA level was quantified in steps per day measured using an Accusplit Eagle AE1620 (Livermore, CA) pedometer. Baseline PA was measured over the course of one week prior to randomization. Non-exercise PA was measured throughout the entire 6-month intervention period. Participants were not blinded to the pedometer data, and had to record step counts daily. Staff removed pedometers prior to exercise and returned the devices to participants after the session. Non-exercise PA was quantified in average daily step counts across the 6-month intervention.
Resting blood pressure
Resting blood pressure was evaluated with an automated blood pressure unit (Colin Medical Instruments, Plainfield, NJ) with the participant in the supine position after a 30 minute resting period (16).
Weight, CWL, and anthropometric measures
Weight was measured using an electronic scale (Siemens Medical Solutions, Malvern) with the participant only wearing a hospital gown. CWL was defined as those achieving ≥ 5% weight loss from baseline (1). We defined modest weight loss (MWL) as ≥3% to <5% from baseline (1). Body mass index (BMI) was calculated by dividing weight (kg) by height (meters) squared. Waist circumference was obtained halfway between the superior border of the pelvis and the anterior border of the last rib.
Glucose metabolism variables
Fasting glucose and insulin were measured after a 12 hour fast. Fasting glucose was evaluating using a hexokinase-glucose-6-phosphate dehydrogenase method. Insulin was measured by electrochemiluminescence. The homeostatic model of insulin resistance (HOMA-IR) was calculated from fasting glucose and insulin values(17) and HOMA-2 was calculated using the HOMA-2 calculator version 2.2 (18).
Caloric intake
Food frequency questionnaires (FFQ) (19) were used to measure estimated caloric intake. Participants were instructed to not make dietary modifications or begin dieting programs during the study (16).
Participant randomization
Following baseline testing, participants were randomized to the 4, 8, or 12 kcal/kg per week (KKW), or the non-exercise control group (16). The present analysis included 145 from the 4KKW group, 89 from the 8KKW group and 96 from the 12KKW group.
Exercise training
All groups expended 4KKW during the first week. Participants assigned to the 4KKW treatment arm continued to expend 4KKW for 6 months. All the other groups increased their energy expenditure by 1 KKW per week until they reached the exercise dose required for their group (i.e. 8KKW, 12KKW). Aerobic training was performed on semi-recumbent cycle ergometers and treadmills, and all sessions were directly supervised by study staff. Women in the exercise groups participated in 3 or 4 sessions each week for 6 months at a heart rate associated with 50% of peak VO2. The control group was asked to maintain their habitual PA level during the study.
Statistical procedure
Analyses were performed using SAS version 9.3 (Cary, NC). Descriptive data were tabulated as means (± SD), frequencies (%) or 95% confidence intervals (95% CI) as appropriate. A one-way analysis of variance (ANOVA) was used to compare baseline characteristics between groups for continuous variables, and a chi-square test (χ2) was used to compare baseline characteristics between categorical variables in: 1) the entire study sample and 2) Those achieving CWL, MWL or neither. A general linear model was utilized to determine the difference in the prevalence of CWL following exercise training in the 4, 8, and 12 KKW groups.
We utilized an analysis of covariance (ANCOVA) to evaluate the change in cardiometabolic variables after exercise training between those who achieved CWL, achieved moderate weight loss (MWL), and those who did not achieve either. Covariates within the statistical model included baseline value and total energy expenditure from exercise training (to adjust the analyses for the dose of exercise training), and all tests were two sided. For lipid outcomes, the models are additionally adjusted for the use of the presence of lipid lowering medications at baseline. For blood pressure outcomes, the models were additionally adjusted for the presence of blood pressure medications at baseline. All ANCOVA analyses were verified for equal group variance, independence of covariate and treatment effects, and homogeneity of regression slopes. The results are presented in adjusted least squared means with 95% confidence intervals.
Lastly, we performed a logistic regression analyses to evaluate participant baseline factors or physical activity factors associated with increased likelihood of achieving CWL or at least MWL (weight loss greater than >3.0%). The goal of the analysis was select variables that were clinically relevant or could be discernable by a clinician, public health or exercise professional. Therefore, we included the following dichotomous variables into the model: obesity status, African American race, presence of low habitual physical activity levels at baseline (<5000 steps/day), senior (age ≥ 65 yrs.), impaired glucose tolerance (baseline glucose>100 mg/dL), exercising at or above physical activity guidelines (exercise groups: 8KKW, 12 KKW), or the presence of antihypertensive, cholesterol lowering or hormone replacement medications. Results are reported in odds ratios with 95% confidence intervals.
Results
Baseline characteristics across exercise groups are shown in Table 1. The sample had a mean (SD) age of 57.3 (6.5) years, a BMI of 31.9 (5.4) kg/m2 and a mean weight of 83.9 (11.6) kg and was 30.6% African American. In terms of baseline medications, 32.1% of the sample was on cholesterol lowering medication, 43.8% of the sample was on hormone replacement therapy, and 29.5% were on anti-hypertensive medications. No significant differences were observed for continuous or categorical variables at baseline across randomization groups or when data demographic data was analyzed based on whether participants obtained CWL, MWL or neither (Table 2).
Table 1.
Demographic characteristics
| Variable | 4 KKW (n=145) |
8 KKW (n=89) |
12 KKW (n=96) |
|---|---|---|---|
| Age (yrs.) | 58.0 (6.5) | 56.8 (8.5) | 56.7 (6.5) |
| Ethnicity (%), n | |||
| Caucasian | 60.7 (88) | 59.6 (53) | 72.9 (70) |
| African American | 33.1 (48) | 32.6 (29) | 25.0 (24) |
| Hispanic/Other | 0.06 (9) | 0.08 (7) | 0.02 (2) |
| Weight (kg) | 83.7 (11.4) | 85.0 (12.3) | 83.4 (11.3) |
| Systolic blood pressure (mmHg) | 138.8 (13.3) | 139.8 (13.6) | 138.1 (13.0) |
| Diastolic blood pressure (mmHg) | 80.7 (9.0) | 81.2 (8.4) | 83.4 (11.3) |
| BMI (kg/m2) | 32.2 (4.0) | 31.1 (3.6) | 31.1 (3.6) |
| Glucose (mg/dL) | 94.1 (8.6) | 94.4 (9.1) | 95.0 (8.5) |
| Insulin (pmol/L) | 74.3 (41.3) | 75.7 (42.1) | 70.3 (40.9) |
| HOMA-IR | 2.9 (1.8) | 3.0 (1.8) | 2.8 (1.7) |
| Total cholesterol (mg/dL) | 201.1 (31.8) | 201.7 (28.9) | 202.7 (28.6) |
| Low density lipoprotein (mg/dL) | 117.3 (27.5) | 118.4 (25.1) | 120.3 (28.6) |
| High density lipoprotein (mg/dL) | 57.9 (14.7) | 57.4 (15.4) | 57.7 (13.7) |
| Triglycerides (mg/dL) | 128.8 (59.3) | 129.6 (59.9) | 126.8 (71.5) |
| VO2 max (ml·kg·min) | 15.4 (3.0) | 15.0 (2.4) | 15.9 (3.0) |
| VO2 max (L/min) | 1.3 (0.3) | 1.3 (0.2) | 1.3 (0.2) |
| Nutrient intake (kcals/day) | 2204.6 (982.4) | 2297.1 (941.4) | 2306.3 (1057.1) |
| Medications (%), n | |||
| Anti-hypertensive | 26.9 (39) | 31.8 (28) | 31.3 (30) |
| Cholesterol lowering | 35.9 (52) | 28.1 (25) | 30.2 (29) |
| Hormone replacement therapy | 42.3 (58) | 43.4 (36) | 46.3 (44) |
Continuous variables are presented as mean (SD), Categorical variables are presented as (%), n
Table 2.
Demographic characteristics in participants obtaining clinically significant weight loss, moderate weight loss or neither.
| Variable | CWL (≥5.0% WL) (n=47) |
MWL (≥3.0% to ≤5.0% WL) (n=43) |
No CWL or MWL (<3.0% WL) (n= 240) |
|---|---|---|---|
| Age (yrs.) | 56.9 (5.6) | 56.5 (7.0) | 57.5 (6.6) |
| Ethnicity % (n) | |||
| Caucasian | 76.6 (36) | 60.5 (26) | 62.1 (149) |
| African American | 21.3 (10) | 30.2 (13) | 32.5 (78) |
| Hispanic/Other | 2.1 (1) | 9.3 (4) | 5.4 (13.0) |
| Weight (kg) | 85.8 (12.0) | 86.8 (12.0) | 83.1 (11.6) |
| Systolic blood pressure (mmHg) | 140.3 (14.7) | 138.9 (11.9) | 138.6 (13.3) |
| Diastolic blood pressure (mmHg) | 80.0 (8.2) | 82.7 (8.5) | 80.7 (8.9) |
| BMI (kg/m2) | 32.1 (3.5) | 32.7 (4.2) | 31.8 (5.8) |
| Glucose (mg/dL) | 92.9 (8.6) | 95.9 (8.0) | 94.5 (8.8) |
| Insulin (pmol/L) | 64.5 (32.7) | 75.6 (41.7) | 74.8 (42.6) |
| HOMA-IR | 2.5 (1.3) | 3.0 (1.8) | 3.0 (1.8) |
| Total cholesterol (mg/dL) | 200.5 (29.7) | 199.1 (33.7) | 202.4 (29.5) |
| Low density lipoprotein (mg/dL) | 118.2 (29.5) | 116.8 (29.3) | 118.8 (25.7) |
| High density lipoprotein (mg/dL) | 56.8 (15.6) | 55.5 (13.2) | 58.3 (14.6) |
| Triglycerides (mg/dL) | 127.7 (5.7) | 131.9 (62.9) | 127.9 (64.7) |
| VO2 max (ml·kg−1·min−1) | 15.1 (2.6) | 15.0 (2.7) | 15.6 (2.9) |
| VO2 max (L/min) | 1.3 (0.3) | 1.3 (0.2) | 1.3 (0.2) |
| Nutrient Intake (kcals/day) | 2281.1 (990.3) | 1979.4 (1107.6) | 2380.1 (872.6) |
| steps per day | 5058.4 (1993.2) | 4941.8 (1587.9) | 4771.1 (1919.0) |
| Medications % (n) | |||
| Anti-hypertensive | 21.3 (10) | 32.6 (14) | 30.4 (73) |
| Cholesterol lowering | 27.6 (13) | 25.6 (11) | 34.2 (82) |
| Hormone replacement therapy | 35.6 (16) | 46.3 (19) | 44.9 (103) |
Continuous variables are presented as mean (SD), Categorical variables are presented as (%), n
Prevalence of CWL
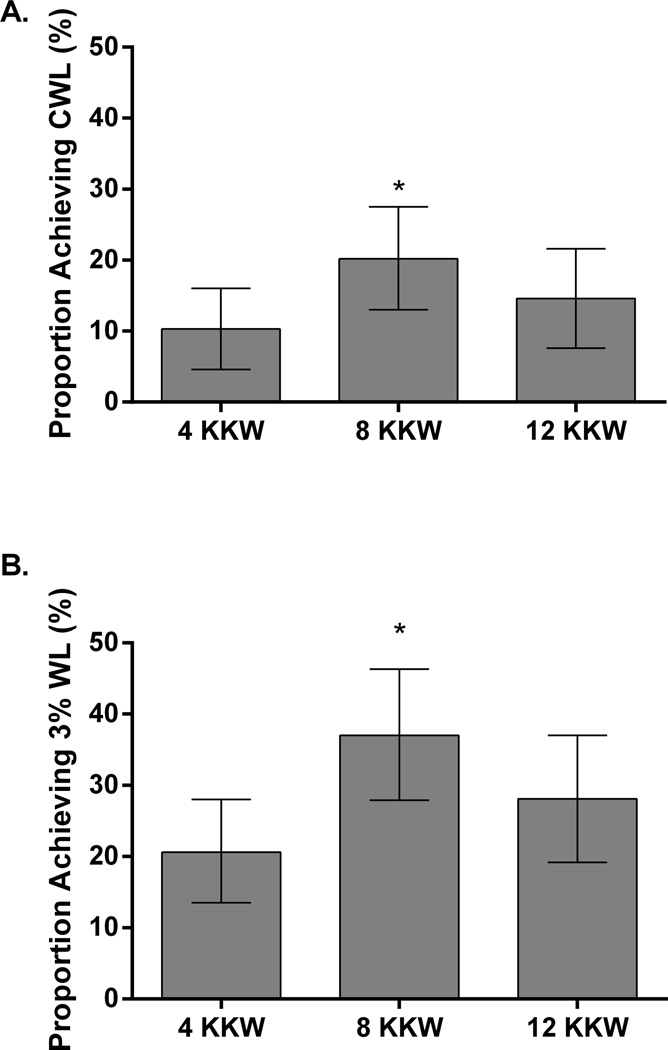
The prevalence of CWL and MWL in the study sample was 14.2% and 13.0%, respectively. The prevalence of CWL and MWL across exercise dose groups are shown in Figure 2. The proportion of women who achieved CWL was greater in the 8 KKW group (20.2%, CI: 13.0 to 27.5%) compared to the 4 KKW (10.3%, CI: 4.6 to 16.0%) (p=0.036), but not significantly different from the 12 KKW groups (14.6%, CI: 7.6% to 21.6%) (p= 0.356). Exercise training adherence was similar in both participants who achieved CWL (99.3%) and participants who did not achieve CWL (97.5 %) (p=0.215). The proportion of individuals who achieved at least MWL (MWL and CWL combined ([≥3% weight loss]) was greater in the 8 KKW group (37.0%, CI: 27.9 to 46.3) compared to the 4 KKW (20.6%, CI: 13.5 to 28.0) (p=0.006), but not significantly different compared the 12 KKW groups (28.1%, CI: 19.2 to 37.0, p=0.202)).
Figure 2.
The prevalence of CWL (panel A) and MWL (panel B) in response to different doses of exercise training. * Indicates significant difference compared to the 4KKW group. CWL: Clinically significant weight loss (weight loss >5%), MWL: Modest weight loss (weight loss 3% to 4.9%)
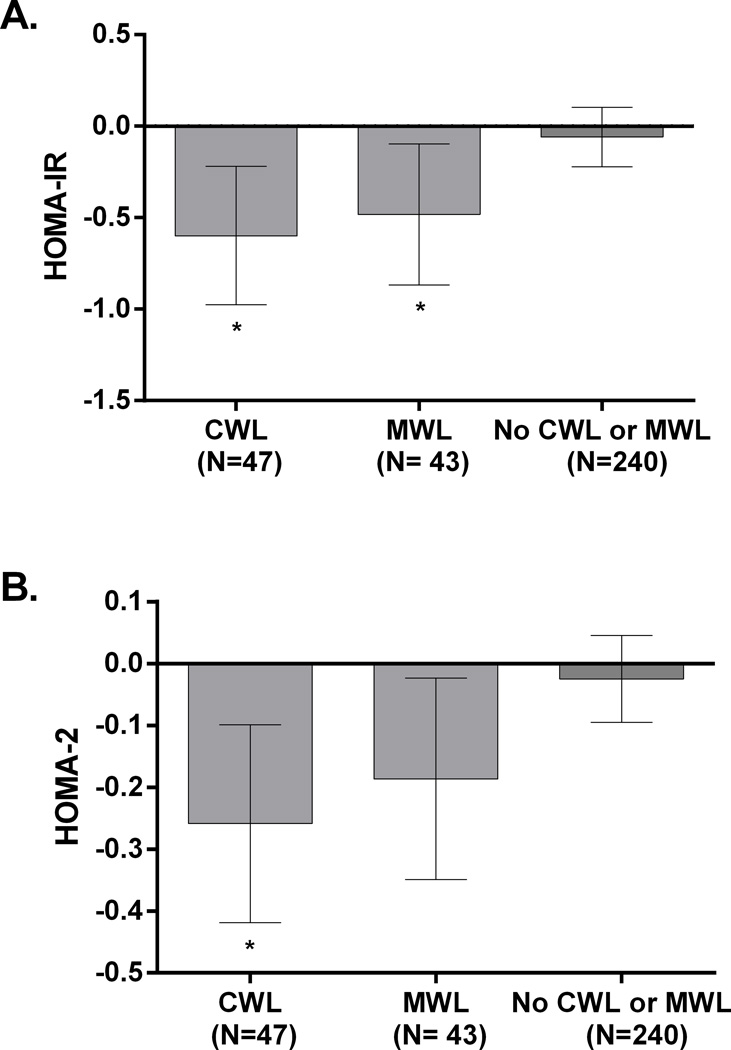
Effects of CWL and MWL on anthropometric and cardiometabolic risk factors: The changes in CVD risk factors in those who were classified with CWL (n= 47), MWL (n=43) and those who did not achieve either (n=240) are shown in Table 3. A greater reduction in waist circumference, weight and percent weight loss was observed in women who achieved CWL compared to MWL, and compared to those who achieved neither. Participants who achieved CWL had significant reductions in insulin levels (p=0.007), compared to those achieving neither MWL nor CWL. The change in insulin levels in those achieving MWL approached significance (p=0.07). No significant changes between groups were observed for the change in glucose, total cholesterol, low density lipoprotein, triglycerides, high density lipoprotein, systolic blood pressure, or absolute fitness or step values obtained across the 6 month intervention (all p> 0.05). The changes in HOMA-IR and HOMA-2 in women who achieved CWL, MWL or neither are shown in Figure 3. Women who achieved either CWL (−0.60, CI: −0.98 to −0.22, p=0.048) or MWL (−0.48, CI: −0.87 to −0.10, p= 0.017) had greater changes in HOMA-IR compared to participants who did not (−0.60, CI: −0.22 to 0.10) (Figure 3A). Women who achieved either CWL (−0.26, CI: −0.42 to −0.10, p=0.007) had greater changes in HOMA-2 compared to participants who did not (−0.02, CI: −0.09 to 0.05). Women categorized with MWL (−0.18, CI: −0.35 to −0.02) had a reduction in HOMA-2 that approached significance (p= 0.061) compared to women who did not achieve MWL or CWL (Figure 3B).
Table 3.
Change in cardiovascular risk factors in women achieving clinically significant weight loss, moderate weight loss or neither
| CWL (≥5.0% WL) (n=47) |
MWL (≥3.0% to ≤5.0% WL) (n=43) |
No CWL or MWL (<3.0% WL) (n= 240) |
|
|---|---|---|---|
| ΔWeight (kg) | −7.6 (CI: −8.1 to −7.3)† | −3.5 (CI: −4.1 to −2.9)† | 0.04 (−0.2 to 0.3)† |
| ΔPercent weight loss (%) | −8.9 (CI: −9.6 to −8.3)† | −4.1 (CI: −4.8 to −3.4)† | 0.1 (CI: −0.2 to 0.37)† |
| ΔWaist circumference (cm) | −6.0 (CI: −7.9 to −4.1)* | −3.6 (CI: −5.7 to −1.6) | −2.0 (CI: −2.8 to −1.1) |
| ΔHigh density lipoprotein (mg/dL) | 1.9 (CI: −2.8 to 6.7) | 1.0 (CI: −4.1 to 6.0) | −0.1 (CI: −3.2 to 2.9) |
| ΔLow density lipoprotein (mg/dL) | −3.6 (−15.0 to 7.9) | 0.2 (−12.3 to 12.7) | −0.3 (−7.6 to 7.1) |
| ΔTriglycerides (mg/dL) | −16.7 (CI: −44.8 to 11.5) | −10.1 (−40.4 to 20.2) | 3.6 (−14.4 to 21.6) |
| ΔTotal cholesterol (mg/dL) | −4.8 (CI: −18.5 to 8.9) | −1.6 (CI: −16.5 to 13.4) | 0.6 (CI: −8.1 to 9.4) |
| ΔSystolic blood pressure (mmHg) | −2.9 (CI: −6.4 to 0.6) | 1.0 (CI: −2.7 to −4.6) | −0.8 (CI: −2.4 to 0.9) |
| ΔDiastolic blood pressure (mmHg) | −1.5 (CI: −3.5 to 0.6) | 1.7 (CI: −0.4 to 3.9)ζ | 0.6 (CI: −0.4 to 1.5) |
| ΔGlucose (mg/dL) | −2.0 (−3.9 to 0.01) | −2.3 (CI: −4.4 to −0.2) | −1.0 (CI: −1.8 to 0.1) |
| ΔInsulin (pmol/L) | −13.5 (CI: −22.1 to −5.0)* | −9.4 (CI: −18.1 to −0.6) | −0.6 (−4.3 to 3.1) |
| ΔVO2 peak (L/min) | 0.06 (CI: 0.02 to 0.10) | 0.04 (CI: −0.01 to 0.08) | 0.07 (CI: 0.05 to 0.09) |
| ΔNutrient intake (kcals/day) | −637.75 (CI: −599.1 to −367.1) | −427.7 (CI: −717.8 to −137.7) | −637.8 (CI:(−902.4 to −373.1) |
| Δ Steps | 555.8 (CI: 356.0 to 755.6) | 672.5 (CI: 222.2 to 1122.8) | 657.2 (CI: 237.9 to 1076.6) |
| Steps during intervention (steps/day) | 5530.7 (CI: 5111.4 to 5950.1) | 5546.0 (CI: 5095.7 to 5996.3) | 5429.3 (CI: 5229.5 to 5629.1) |
Indicates significant difference compared to no CWL or MWL group;
indicates significant difference between MWL and CWL;
indicates all groups significantly different from all groups
Figure 3.
Change HOMA-IR (panel A) and HOMA-2 (panel B) in postmenopausal women achieving clinically significant weight loss, moderate weight loss or neither. *Indicates significant difference compared to No CWL or MWL group. CWL: Clinically significant weight loss (weight loss >5%), MWL: Modest weight loss (weight loss 3% to 4.9%)
Logistic regression analyses for predicting CWL or MWL
None of the variables included in the logistic regression model (baseline [<5000 steps/day], senior [age ≥ 65 yrs.], impaired glucose tolerance [baseline glucose>100 mg/dL], exercising at or above physical activity guidelines) predicted achieving clinically significant weight loss (all ps>0.05). However, exercising above physical activity guidelines during the intervention was associated with a greater odds of achieving at least MWL (1.8, CI: 1.08 to 3.03), however no other variables within the model were significant (all ps>0.05).
Discussion
While the mean percentage weight loss from controlled exercise training studies have been evaluated for CWL (4, 5, 6, 7), no study to our knowledge has evaluated the prevalence of CWL in response to 3 defined doses of aerobic exercise training. The primary findings of this study are: 1) The overall prevalence of CWL and MWL among postmenopausal women who were overweight and obese was low (14.2% and 13.0 %, respectively or 27.2% with at least MWL); 2) The exercise dose consistent with public health recommendations for PA was associated with the greatest prevalence of CWL and MWL; 3) Women achieving CWL or MWL with exercise training had greater overall improvements in HOMA-IR (likely mediated through reductions in plasma insulin) compared to those who did not, but no additional improvements were observed for other CVD risk factors.
Our results reaffirm evidence from previous well-controlled randomized controlled exercise training trials (4, 5, 6, 7) trials that aerobic training at recommended levels is associated with minimal weight loss, as we observed 1.7% mean weight loss in the entire study sample, which is well below the criteria for CWL. Similarly, only 27.2% of postmenopausal women achieved at least MWL and only 14.2% of women obtained CWL. The results of the present study are in concert with a recent American College of Sports Medicine position stand on weight loss and maintenance (1), which stated that CWL is unlikely to occur at levels of exercise at or below public health guidelines. Thomas et al. (8) performed mathematical modelling on the results from several exercise training interventions and found that the low overall response in weight loss may be due to inadequate energy expenditure and increased caloric intake. Several studies in which exercise training was supervised by study staff (without additional requirements for caloric restriction) have observed a greater magnitude of weight loss with aerobic exercise training levels above the minimum physical activity recommendations (9, 10, 20). Potential rationales for differences in results across studies may be due to the fact that the DREW participants were older, postmenopausal, and had a lower total exercise-related energy expenditure per week compared to studies with larger magnitudes of weight loss.
Based on our results, clinicians should advise postmenopausal women that CWL is unlikely to occur due to aerobic exercise alone, and assure that patients have realistic expectations for weight loss if they are exercising without also engaging in caloric restriction. However, we feel it is important to emphasize that many improvements in CV health and other health parameters are obtained with exercise training without caloric restriction, such as improvements in cardiorespiratory fitness (4), endothelial function (21, 22), visceral adiposity (23), and quality of life (24). This is important to communicate to individuals who are successful in exercising regularly, but are unable to reach weight loss goals.
In the present study, the greatest prevalence of CWL was observed in the group that exercised at the dose of exercise consistent with PA recommendations(25) (20.2%). Although exercising at a greater dose of exercise (50% above public health guidelines) has been shown to result in greater improvements in cardiorespiratory fitness as well as other risk factors for CVD based on our previous reports (4, 26, 27), the prevalence of CWL was not increased by additional exercise-related energy expenditure. This result does not appear to be due to compensatory changes in non-exercise PA as similar pedometer measured step counts were observed during the 6-month intervention in participants who achieved CWL compared to those who did not. However, several other elements of non-exercise PA were not measured in the present study (e.g. total energy expenditure, moderate to vigorous PA, total sedentary time, pattern of PA). In addition, our observation that the proportion of individual achieving either CWL or MWL did not increase with higher amounts of exercise training may be due to increased compensation for weight loss in the 12 KKW group. Church et al. (11) reported an increased weight compensation for absolute weight loss in the DREW population in women exercising above public health recommendations. Similarly, King et al. (12) observed increased energy intake in adults who were overweight and obese in response to 12 weeks of aerobic exercise training. Thus, future studies should evaluate the potential etiologies and causes of weight compensation, and whether this phenomenon is specific to postmenopausal women.
A secondary aim of the present study was to evaluate if obtaining CWL with exercise training resulted in greater improvements in cardiometabolic risk factors compared to those who did not lose significant amounts of weight. Women obtaining CWL or MWL with aerobic exercise training had greater reductions in HOMA-IR and HOMA-2, surrogate markers of glucose metabolism, compared to individuals who did not lose at least modest amounts of weight (< 3%). Importantly, similar improvements in HOMA-IR were observed between those achieving CWL or MWL. Similar results were obtained with the use of the HOMA-2 model with exception that the reduction in insulin resistance approached significance compared to the group that did not achieve CWL or MWL. Our results suggest that weight change with exercise training is an important component of the improvement in insulin resistance following training. Additionally, CWL may carry a somewhat greater effect on insulin resistance than MWL given that the HOMA-2 model approached significance for MWL group compared to those not achieving MWL and CWL. However, our results are suggestive that the MWL with exercise training does carry some benefit in improving insulin resistance in postmenopausal women who are overweight or obese, and likely other major risk factors for CVD (endothelial function, cardiorespiratory fitness, etc.) (4, 21, 28). However, no other cardiometabolic risk factors (including lipids, blood pressure, or fitness) were improved further with CWL or MWL with exercise training. We additionally performed analyses looking at whether effects differed for those above clinical thresholds (e.g. dyslipidemia, hypertension, etc.), however no significant effects were observed (data not shown). Clinicians should consider initially targeting MWL for patients with obesity as it appears that this will result in an improvement in insulin resistance, especially for individuals who have difficulty achieving a higher magnitude of weight loss. However, more consistent effects may be observed with CWL when achievable.
Strengths of the present study include that DREW was a randomized controlled trial where all exercise training sessions were supervised by study staff to confirm adherence to the required energy expenditure of each group and exercise groups were based on public health recommendations for PA. Non-exercise PA was measured objectively at baseline and throughout the entire 6 month intervention by pedometers. Limitations of the present analysis are that it was retrospective, the only measure of non-exercise PA was steps counts collected with pedometers, and the results may not be generalizable to men, premenopausal women, or other age groups. Additionally, DREW did not collect a more sensitive measure of insulin action (e.g. oral glucose tolerance test, hyperinulinemic euglycemic clamp) or more sophisticated measurements of body composition (body fat was assessed by calipers in DREW, however there were concerns regarding the standardization of the technique during data collection and therefore these data were not presented in this manuscript). Lastly, the scope of our findings is limited to the cardiovascular variables evaluated in the DREW study. Thus, other indicators of health (e.g. physical function, musculoskeletal variables, and other cardiovascular risk factors) which may have relevance to individuals with obesity may have different relationships with MWL or CWL.
The results of the present study suggests that CWL is a somewhat rare phenomenon in postmenopausal women participating in aerobic exercise training, and was the most prevalent in women exercising at a dose consistent with PA recommendations. Although, more consistent improvements in insulin resistance measures were observed in postmenopausal women who obtained CWL (improvement in both HOMA-IR and HOMA-2), improvements in insulin resistance were evident in women achieving MWL (improvement in HOMA-IR only), which represents a more attainable weight loss target. Future studies should evaluate the clinical meaningfulness of CWL and MWL with more sensitive measures of insulin action/other CVD risk factors, other indicators of health (physical function, musculoskeletal measures), and potential etiologies for the lack of greater weight loss observed in exercise levels above public health recommendations in postmenopausal women.
Study importance questions.
What is already known about this topic
Aerobic exercise training can result in weight loss, but is unlikely to meet the threshold for clinically significant weight loss
Results from previous studies have discussed the mean reductions in weight across an entire study population, however the percentage of those that have achieved clinically significant weight loss has not been quantified following exercise training or at different exercise training amounts relative to public health guidelines
No studies have directly compared the effects of exercise training with no weight loss, modest weight loss and clinically significant weight loss on cardiometabolic variables
What does this study add
Provides the percentage of individuals achieving clinically significant or modest weight loss at three different amounts of aerobic exercise training (50, 100, and 150% of physical activity guidelines)
Provides the effect of clinically significant and modest weight loss on insulin resistance, lipids, blood pressure, and fitness compared to those that were not able to achieve weight loss
Provides information on whether certain demographic factors predict which individuals achieve clinically significant and modest weight loss
Acknowledgements
The authors would like to thank the participants of the DREW study and all research staff that assisted in the data collection.
Funding: National Heart Lung, and Blood Institute grant (HL66262) and unrestricted research support from The Coca-Cola Company
Footnotes
Conflict of interest: The authors have no conflict of interests regarding the results published in this manuscript
References
- 1.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. Appropriate Physical Activity Intervention Strategies for Weight Loss and Prevention of Weight Regain for Adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 2.Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes Relat Metab Disord. 2005;29:1153–1167. doi: 10.1038/sj.ijo.0802982. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn G. Effect of Degree of Weight Loss on Health Benefits. Obes Res. 1995;3:211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 4.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of Different Doses of Physical Activity on Cardiorespiratory Fitness Among Sedentary, Overweight or Obese Postmenopausal Women With Elevated Blood Pressure. JAMA. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 5.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, et al. Effects of the Amount and Intensity of Exercise on Plasma Lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 6.Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, et al. Effects of Aerobic and Resistance Training on Hemoglobin A1c Levels in Patients With Type 2 Diabetes. JAMA: The Journal of the American Medical Association. 2010;304:2253–2262. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilmore JH, Després J-P, Stanforth PR, Mandel S, Rice T, Gagnon J, et al. Alterations in body weight and composition consequent to 20 wk of endurance training: the HERITAGE Family Study. The American Journal of Clinical Nutrition. 1999;70:346–352. doi: 10.1093/ajcn/70.3.346. [DOI] [PubMed] [Google Scholar]
- 8.Thomas DM, Bouchard C, Church T, Slentz C, Kraus WE, Redman LM, et al. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes Rev. 2012;13:835–847. doi: 10.1111/j.1467-789X.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly JE, Hill JO, Jacobsen DJ, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: The midwest exercise trial. Arch Intern Med. 2003;163:1343–1350. doi: 10.1001/archinte.163.11.1343. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly JE, Honas JJ, Smith BK, Mayo MS, Gibson CA, Sullivan DK, et al. Aerobic exercise alone results in clinically significant weight loss for men and women: Midwest exercise trial 2. Obesity. 2013;21:E219–E228. doi: 10.1002/oby.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in Weight, Waist Circumference and Compensatory Responses with Different Doses of Exercise among Sedentary, Overweight Postmenopausal Women. PLoS ONE. 2009;4:e4515. doi: 10.1371/journal.pone.0004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes. 2008;32:177–184. doi: 10.1038/sj.ijo.0803712. [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB. Metabolic risk factors for coronary heart disease in women: Perspective from the Framingham Study. Am Heart J. 1987;114:413–419. doi: 10.1016/0002-8703(87)90511-4. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and Risk of Cardiovascular Disease. Ann Intern Med. 1976;85:447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 15.Dubnov G, Brzezinski A, Berry EM. Weight control and the management of obesity after menopause: the role of physical activity. Maturitas. 2003;44:89–101. doi: 10.1016/s0378-5122(02)00328-6. [DOI] [PubMed] [Google Scholar]
- 16.Morss GM, Jordan AN, Skinner JS, Dunn AL, Church TS, Earnest CP, et al. Dose-Response to Exercise in Women Aged 45–75 yr (DREW): Design and Rationale. Med Sci Sports Exerc. 2004;36:336–344. doi: 10.1249/01.MSS.0000113738.06267.E5. [DOI] [PubMed] [Google Scholar]
- 17.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 19.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92:686–693. [PubMed] [Google Scholar]
- 20.Ross R, Dagnone D, Jones PJH, Smith H, Paddags A, Hudson R, et al. Reduction in Obesity and Related Comorbid Conditions after Diet-Induced Weight Loss or Exercise-Induced Weight Loss in Men. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 21.Swift DL, Earnest CP, Blair SN, Church TS. The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: results from the DREW study. Br J Sports Med. 2012;46:753–758. doi: 10.1136/bjsports-2011-090025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deljanin Ilic M, Ilic S, Kocic G, Pavlovic R, Stojanovic I. Exercise Training Improves Endothelial Function in Hypertensive Postmenopausal Women. J Hypertens. 2010;28:252. [Google Scholar]
- 23.Irving BA, Davis CK, Brock DW, Weltman JY, Swift D, Barrett EJ, et al. Effect of Exercise Training Intensity on Abdominal Visceral Fat and Body Composition. Med Sci Sports Exerc. 2008;40:1863–1872. doi: 10.1249/MSS.0b013e3181801d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin CK, Church TS, Thompson AM, Earnest CP, Blair SN. Exercise dose and quality of life: A randomized controlled trial. Arch Intern Med. 2009;169:269–278. doi: 10.1001/archinternmed.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. 2008:1–3. [Google Scholar]
- 26.Swift DL, Johannsen NM, Earnest CP, Blair SN, Church TS. Effect of Different Doses of Aerobic Exercise Training on Total Bilirubin Levels. Med Sci Sports Exerc. 2012;44:569–574. doi: 10.1249/MSS.0b013e3182357dd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johannsen NM, Swift DL, Johnson WD, Dixit VD, Earnest CP, Blair SN, et al. Effect of Different Doses of Aerobic Exercise on Total White Blood Cell (WBC) and WBC Subfraction Number in Postmenopausal Women: Results from DREW. PLoS ONE. 2012;7:e31319. doi: 10.1371/journal.pone.0031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swift DL, Lavie CJ, Johannsen NM, Arena R, Earnest CP, O'Keefe JH, et al. Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circ J. 2013;77:281–292. doi: 10.1253/circj.cj-13-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]