Abstract
The addition of platelet-rich plasma (PRP) to rotator cuff repair has not translated into improved outcomes after surgery. However, recent work stimulating ligament healing has demonstrated improved outcomes when PRP or whole blood is combined with an extracellular matrix carrier. The objective of this study was to evaluate the effect of three components of blood (plasma, platelets and macrophages) on the in vitro activity of ovine rotator cuff cells cultured in an extracellular matrix environment. Tenocytes were obtained from six ovine infraspinatus tendons and cultured over 14 days in an extracellular matrix scaffold with the following additives: 1) Plasma (PPP), 2) Plasma and platelets (PAP), 3) Plasma and macrophages (PPPM), 4) Plasma, platelets and macrophages (PAPM), 5) Phosphate buffered saline (PBS), and 6) PBS with macrophages (PBSM). Assays measuring cellular metabolism (AlamarBlue), proliferation (Quantitative DNA assay), synthesis of collagen and cytokines (SIRCOL, TNF-α and IL-10 ELISA, and MMP assay), and collagen gene expression (qPCR) were performed over the duration of the experiment, as well as histology at the conclusion. Plasma was found to stimulate cell attachment and spreading on the scaffold, as well as cellular proliferation. Platelets also stimulated cell proliferation, cellular metabolism, transition of cells to a myofibroblast phenotype and contraction of the scaffolds. The addition of macrophages did not have any significant effect on the sheep rotator cuff cells in vitro. In vivo studies are needed to determine if these changes in cellular function will translate into improved tendon healing.
Keywords: Platelet-rich plasma, rotator cuff, sheep, fibroblast, in vitro, macrophage
INTRODUCTION
Rotator cuff tendon tears are one of the most common musculoskeletal complaints with more than 75,000 patients undergoing surgery to repair the rotator cuff each year.1; 2 Despite advances in both open and arthroscopic surgical techniques, there is still a high rate of failure after rotator cuff repair, with up to 94% of repairs of large tears going on to re-tear.3; 4 The high rate of failure of surgical repair of rotator cuff tears has led to interest in biologic adjunctive therapies to augment repair. Some biologic options that have been explored include fibrin and collagen matrices, mesenchymal stem cells, growth factors in isolation or combination, and platelet-rich plasma (PRP).5; 6 PRP seems ideally suited as a therapy, as the alpha granules of platelets contain multiple growth factors important in wound healing, namely platelet derived growth factor (PDGF),7 transforming growth factor beta (TGF-b),8 insulin-like growth factor I (IGF-1),9 vascular endothelial growth factor (VEGF),10 hepatocyte growth factor (HGF),11 epidermal growth factor (EGF),12 and basic fibroblast growth factor (bFGF).13 Multiple studies have established the beneficial biological effects of platelets and plasma on fibroblasts in culture, including stimulation of gingival and skin fibroblasts.14; 15 PRP has also been found to increase DNA and glycosaminoglycan production by human rotator cuff cells 16 as well as type I and III collagen gene expression.17
Unfortunately, multiple randomized clinical trials investigating the use of PRP have been unable to extend these findings into improved patient outcomes after rotator cuff repair.18-21 Most recently, Rodeo et al. examined the use of a platelet-rich fibrin matrix as an adjuvant during arthroscopic repair, but found no difference from controls in rotator cuff healing, patient strength, or functional scores.20 Similarly, Weber et al. found no improvement in postoperative pain, motion, shoulder outcome scores, or cuff integrity based on magnetic resonance imaging (MRI).21 Similar findings were found in in vivo studies of anterior cruciate ligament (ACL) healing, where PRP alone had no benefit.22 However, when the blood cells were delivered in an extracellular matrix (ECM) scaffold, benefit was shown in large animal in vivo models.23; 24 The role of the ECM scaffold may be to physiologically activate the platelets 25; 26 as well as immobilize them in the wound site 27; 28 - two properties that may be important in the intraarticular wound site of a torn rotator cuff tendon as well.
The understanding of the complex role macrophages play in the inflammatory response and subsequent healing has continued to expand.29 Macrophages are a dynamic cell which can take on pro- and anti-inflammatory roles, and contribute to immune regulation, host defense, and wound healing.30 They are able to switch between multiple programs with differing receptor and cytokine expression based on their local environment.30; 31 These cells become the predominant cell type in the healing process with days of an injury and can persist in the wound for several weeks. In the later stages of the inflammatory phase, they adopt a wound healing role in response to the cues in the local environment.32 Macrophages exposed to PRP have been shown to decrease their expression of pro-inflammatory cytokines such as interleukin-1 (IL-1) and adopt a more anti-inflammatory role.33 Macrophages may therefore be an important intermediary in the effect of PRP and one of the ways that PRP exerts its wound healing effects.
The aim of the current study was to determine if the addition of platelets, plasma and/or macrophages to the 3-D environment of an ECM scaffold would stimulate ovine rotator cuff tendon cells to adhere to the matrix, proliferate and express a myofibroblast phenotype. We hypothesized that the addition of platelets would stimulate cell proliferation and collagen gene expression and that the addition of macrophages would also stimulate these same parameters.
METHODS
Rotator Cuff Tenocyte Isolation
Tissue from Dorsett Cross breed sheep from other institutional animal care and use committee (IACUC) approved studies at our institution was used in this experiment. Our institution does not require IACUC approval for obtaining tissues from animals euthanized after completion of other approved studies, and because no live animals were used in this study, no separate IACUC approval was needed. Infraspinatus tendon was sterilely harvested from six skeletally mature ovine shoulders.34 Tissue was obtained and processed immediately after euthanasia. 2 mm ×2 mm ×2 mm explants were placed in 6-well plates and cultured to establish six individual cell lines derived from the six animals. Explants and outgrowing cells were maintained in complete growth medium (CGM) of Dulbecco's Modification of Eagle's Media (Mediatech Inc., Herndon VA) with 10% fetal bovine serum (HyClone Inc., South Logan UT) and 1% antibiotic/antimycotic solution (Mediatech Inc., Manassas VA). Media was changed twice per week. Cells were grown to 80% confluence, passaged, and frozen. Third passage cells were used for all experiments.
Plasma and Platelet Preparations
Allogeneic ovine whole blood from a single donor was obtained from Lampire Biologicals (Pipersville, PA) in 10% acid-citrate-dextrose. Blood was centrifuged at 150x gravity (GH .8 rotor, Beckman GS-6 Centrifuge, Fullerton, CA) for 30 minutes. Platelet rich supernatant was collected and spun at 4000 rpm ×10 minutes to pellet the platelets. The remaining whole blood was spun at 3000×g to produce the platelet poor plasma (PPP) working solution. The platelet pellet was resuspended in PPP to create a suspension of platelets within the plasma (PAP). Cell counts (Table 1) were performed with the VetScan HM2 Hematology System (Abaxis, Union City, CA).
Table 1.
White cell and platelet counts for whole blood, PPP and PAP used in this experiment.
| WBC count (Cells/mL) | Platelet Count (Cells/mL) | |
|---|---|---|
| Whole Blood | 12.85×106 | 50×106 |
| Plasma | <1×105 | <1×105 |
| Platelets and Plasma | <4×105 | 136×106 |
Macrophage Isolation
Macrophages were isolated from ovine whole blood (Lampire Biologicals, Pipersville, PA). Peripheral blood mononuclear cells were isolated by density centrifugation using Ficoll-Paque (GE Healthcare Biosciences, Uppsala Sweden). Isolated cells were washed in PBS, suspended in RPMI, and plated on a T-75 culture flask at a density of 2.0 × 107 cells per flask. After two hours, cells were washed twice with PBS to remove non-adherent cells and maintained in culture with RPMI, 10% heat-inactivated FBS, and 1% antibiotic/ antimycotic solution. Macrophages were allowed to mature for seven days prior to use.
Extracellular Matrix Construct Preparation
Bovine knee capsule was harvested in a sterile fashion and solubilized in an acidic pepsin solution as previously described.24 Collagen content was adjusted to 8 mg/ml and neutralized to bring the final pH of the slurry to 7.4. Rotator cuff tenocytes were resuspended in PAP, PPP or PBS and macrophages added one half of the cell suspensions in each solution. The cell suspension was added to the neutralized collagen slurry at a concentration of 5×105 tenocytes/ml for experimental groups and 1×105 macrophages/ml for the macrophage groups. 1ml of cell/collagen mixture was pipetted into 3 cm long semi-cylindrical molds with a polyester mesh at each end to anchor the gels and placed in a humidified incubator at 37 °C and 5% CO2 for 1 h to achieve gelation. Constructs were cultured CGM and the medium was changed every three days. Cell composition of the scaffolds in each group is presented in Table 2.
Table 2.
Number of cells per scaffold in each group
| PBS | PBSM | PPP | PPPM | PAP | PAPM | |
|---|---|---|---|---|---|---|
| Tenocytes | 5×105 | 5×105 | 5×105 | 5×105 | 5×105 | 5×105 |
| Platelets | 0 | 0 | <1×105 | <1×105 | 54×106 | 54×106 |
| Macrophages | 0 | 1×105 | 0 | 1×105 | 0 | 1×105 |
AlamarBlue Metabolism Assay
AlamarBlue assay was performed on experimental days 0, 1, 3, 7, and 13. CGM was mixed with AlamarBlue® (AbD Serotec, Oxford UK) at a ratio of 9:1. Media was replaced with the AlamarBlue solution and incubated for 14 h, after which the absorbance of samples from each well were read at 570 nm and 600 nm (Spectramax250, Molecular Devices LLC, Sunnyvale CA) and percent reduction calculated per the manufacturer's protocol.
DNA Assay
DNA assay was performed on constructs harvested at 14 days. Constructs were digested in 1 ml of PBE/L-cystene/Papain digestion buffer and placed on a 60°C heat block until clear before freezing at −80 °C. DNA content was measured utilizing the Quant-iT™ PicoGreen® dsDNA Kit (Molecular Probes, Eugene OR). Florescence was read at an excitation of 480 nm and emission of 520 nm.
SIRCOL Collagen Assay
SIRCOL collagen assay (Biocolor Ltd, Carrickfergus, United Kingdom) was performed on each group at 1, 7 and 14 days according to manufacturer's instructions. Absorbance was measured at 560 nm.
Minimum Width
On day 14 the width of the construct on a digital image was measured at the narrowest point with NIH Image J 1.37V.
Quantitative RT-PCR (qPCR)
At 14 days, constructs were harvested and snap-frozen with liquid nitrogen. Samples were kept at −80°C until RNA extraction was performed. RNA was isolated after treatment with 1 ml of Trizol reagent and homogenization using the PureLink® RNA Mini Kit (Life Technologies, Carlsbad CA). Reverse transcription was performed with the SuperScript® III First Strand Synthesis System (Life Technologies, Carlsbad CA). qPCR was performed with the SYBRGreen PCR Master Mix Kit (Applied Biosystems, Warrington, United Kingdom). Targeted genes were types I and III procollagen (COL1A1 and COL3A1), and GAPDH was used as the reference gene (Table 3).
Table 3.
Ovine primer sequences for qPCR.
| Forward (5′-3′) | Reverse (5′-3′) | |
|---|---|---|
| COL1A1 | CCA GTC ACC TGC GTA CAG AAC GG | GCC AGT GTC TCC TTT GGG TCC |
| COL3A1 | GCT GGC TAC TTC TCG CTC TG | GTG GGC AAA CTG CAC AAC AT |
| GAPDH | CCA CTG GGG TCT TCA CTA CC | AAG CAG GGA TGA TGT TCT GG |
IL-10 and TNF-α Enzyme Linked Immunosorbent Assay (ELISA)
IL-10 and TNF-α cytokine concentrations were measured at day 3 utilizing ELISA kits (MyBiosource, San Diego CA). Absorbance was measured at 450 nm.
Matrix-metalloproteinase (MMP) Assay
Non-specific MMP activity assay (Abcam, Cambridge MA) was performed on media samples taken on day 3. 25 µl of samples and controls were added to 25 μl of 4-Aminophenylmercuric Acetate (APMA) solution to active MMPs present in the samples and incubated at 37 °C. 50 μl of MMP green substrate was then added to each sample and incubated for 60 minutes at room temperature. Florescence was read at an excitation of 490 nm and emission of 525 nm.
Histology
Constructs harvested at day 14 were fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin. 6 μm sections were cut using a microtome and representative sections stained with Gill's modified hematoxylin III and Eosin Y 1% alcoholic solution (EMD Chemicals Inc., Gibbstown, NJ). Immunohistochemical staining for α-smooth muscle actin (αSMA) was performed by Mass Histology Service, Inc. (Worchester, MA). In brief, after heat pretreatment, sections were treated with 3% hydrogen peroxide for 15 minutes then 2% horse serum for 20 minutes at room temperature. Sections were then incubated with rabbit anti- αSMA (ab5694, Abcam, Cambridge, MA) diluted 1:250 overnight at 4°C, then with vector rabbit impress secondary polymer for 45 minutes and 3,3′-diaminobenzidine for 3 minutes at room temperature. The sections were then counterstained with hematoxylin and imaged by a light microscope at 200X magnification.
Statistics
Data analysis was completed with STATA 12.0 (StataCorp, College Station TX). Multilevel mixed-effects linear regression was used to analyze all data, with platelet solution condition (PBS, PPP or PAP) and macrophage condition (with or without) as fixed effects, and cell line as a random effect. All results given as mean +/− standard error. A p-value of less than 0.05 was considered significant.
RESULTS
AlamarBlue Assay
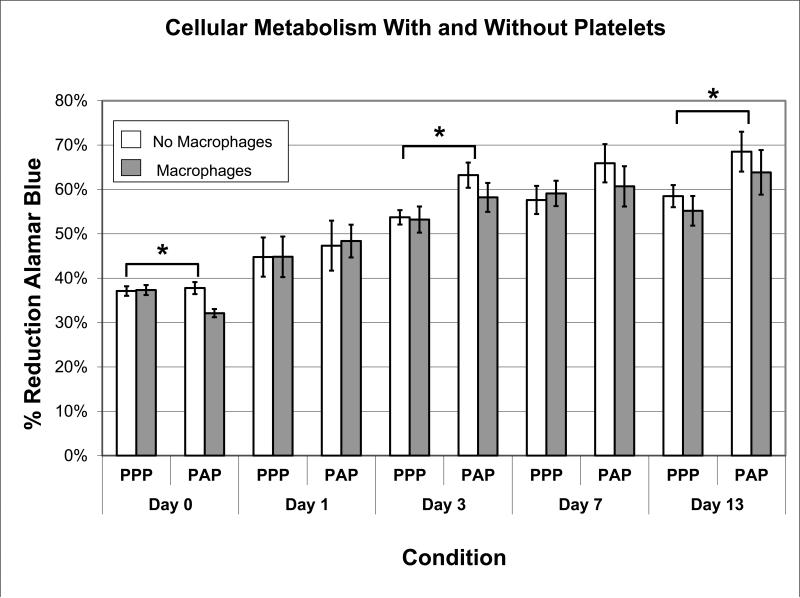
The addition of plasma alone resulted in a significant increase in cellular metabolism in the ECM scaffold compared to the PBS group at days 3, 7 and 13 (p<0.001 for all comparisons). The addition of platelets to the plasma resulted in greater cell metabolism than PPP at days 3 and 13 (Figure 1, p<0.001 for all comparisons), and a trend toward significance on day 7 (p=0.056). The addition of macrophages to PBS, to plasma or to platelets and plasma had no significant effect on metabolism at any time point. (p>0.3 for all comparisons).
Figure 1.
Proportion of AlamarBlue reduced (n=6 for all groups) over the course of the experiment. Error bars represent standard error. * = significant difference (P<0.05).
DNA Assay
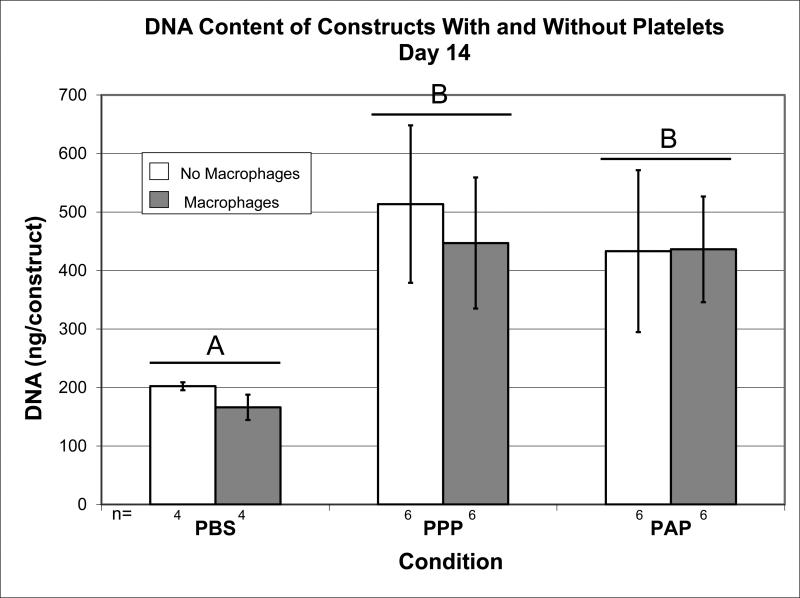
The addition of plasma resulted in a 2.5 fold increase in DNA content at day 14 (Figure 2; p<0.001) compared to the PBS group. The addition of platelets to the plasma resulted in no further increase in DNA content over plasma alone at 14 days of culture (p=0.424). The addition of macrophages to PBS, to plasma, or to platelets and plasma had no significant effect DNA content within the ECM scaffold at 14 days (p=0.329).
Figure 2.
Amount of DNA (ng) measured by PicoGreen DNA assay at day 14. N as indicated. Error bars represent standard error. Bars capped with different letters significantly different at P<0.05.
SIRCOL Collagen Assay
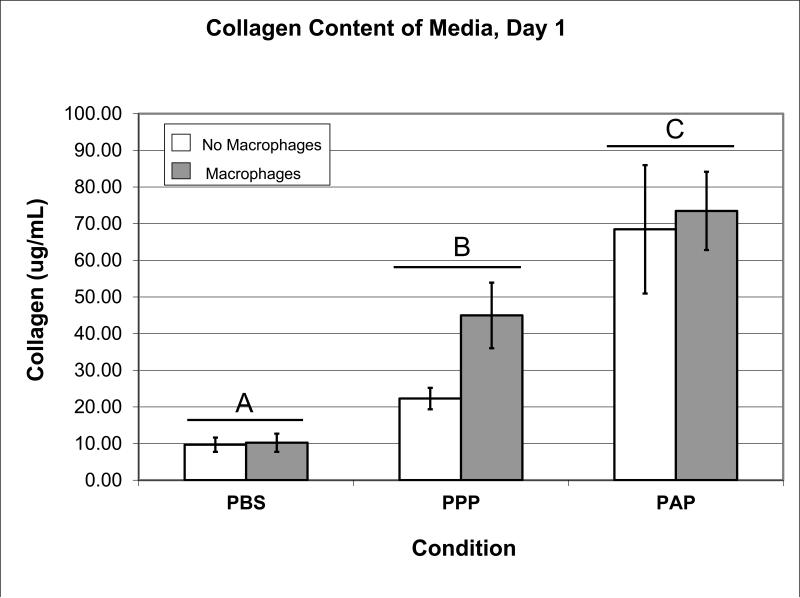
The addition of plasma resulted in a 3-fold increase in collagen expressed into the media (Figure 3; p=0.002) at day 1 of culture compared to PBS. The addition of platelets to the plasma resulted in a further 2-fold increase in collagen release compared to plasma alone (p<0.001) on day 1. The addition of macrophages to any of the groups had no significant effect on collagen content at any time point (p>0.13 for all comparisons). There were no significant main effects of plasma, or plasma and platelets on collagen content at any other time points.
Figure 3.
Amount of collagen (μg/ml) measured by SIRCOL at day 1 (n=6 for all groups). Error bars represent standard error. Bars capped with different letters significantly different at P<0.05.
Minimum Width
The addition of plasma resulted in no significant change over the PBS group in the contraction of the ECM scaffolds (Figure 4; p=0.093) at day 14 of culture. The addition of platelets to the plasma resulted in a significant decrease in the minimum width compared to PBS or plasma alone (p=0.004) on day 14. The addition of macrophages to PBS, to plasma, or to platelets and plasma had no significant construct width content at day 14 (p=0.556).
Figure 4.
Minimum width of constructs on day 14. Error bars represent standard error. N as indicated. Bars capped with different letters significantly different at P<0.05.
Quantitative RT-PCR
There were no significant effects of plasma, platelets and plasma, or macrophages on expression of COL1 or COL3 at day 14 (p>0.05 for all comparisons). However, the relative expression of COL1 in the PAP group was 53% greater than that in the PBS group at day 14 and approached significance (1739.69 vs. 1140.05, p=0.059).
IL-10 and TNF-α ELISA
There were no significant effects of plasma, platelets and plasma, or macrophages on IL-10 or TNF-α expression (p>0.05 for all comparisons).
MMP Assay
There were no significant effects of plasma, platelets and plasma, or macrophages on MMP expression (p>0.05 for all comparisons).
Histology
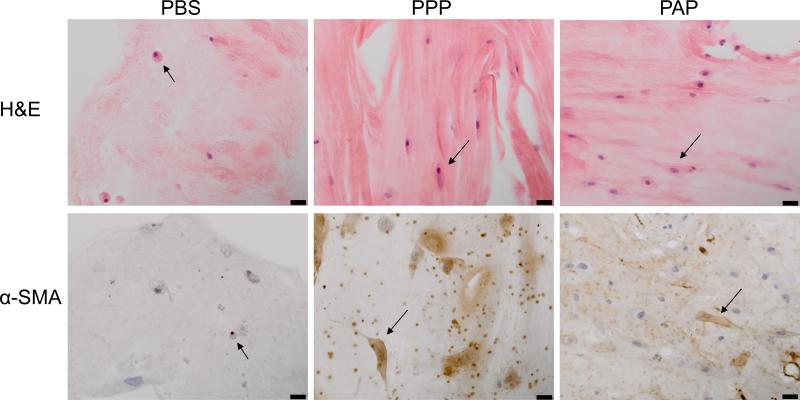
We observed that the cell density of H&E sections from constructs harvested at 14 days from the groups containing plasma or plasma and platelets was higher than in the PBS groups (Figure 5). In addition, more cells in the groups containing plasma exhibited cell spreading with cytoplasmic projections rather than a round cytoplasm. Staining for αSMA was also more pronounced in the PAP and PPP sections, with a minority of cells stained in the PBS group, and a majority of the cells stained in the PAP and PPP groups.
Figure 5.
Representative histology of constructs at day 14 (200x magnification). Note the number and morphology of fibroblasts (arrows), and the increased positive IHC staining (brown) for α-SMA actin in the PPP and PAP groups. Scale bars = 20μm.
DISCUSSION
The purpose of this study was to determine the individual and combined contributions of plasma, platelets and macrophages on the cellular processes important in rotator cuff healing: proliferation, matrix production and scaffold remodeling. Plasma was found to stimulate cell attachment and spreading on the matrix as seen histologically on H&E staining, as well as cellular proliferation measured by the DNA assay. Platelets also stimulated cell proliferation, as well as cellular metabolism measured by the AlamarBlue assay, transition of cells to a myofibroblast phenotype seen by αSMA staining, and contraction of the scaffolds as measured by the minimum width of the constructs. However, the addition of macrophages in the absence of other white blood cells (WBCs) did not impact the in vitro activity of sheep rotator cuff tenocytes, even in the presence of platelets or plasma. Therefore, this study supports our hypothesis that platelets have a positive effect on rotator cuff biology in vitro on the ovine cells; however, contrary to our hypothesis, the addition of macrophages in this in vitro model did not increase the metabolic and proliferative properties of the rotator cuff tenocytes.
The finding of increased tenocyte proliferation with platelets and plasma is consistent with previous work examining rotator cuff tenocytes. Sadoghi et al. recently found that PRP significantly increases DNA and glycosaminoglycan production of human rotator cuff cells in culture, with 1 fold and 5 fold increases in platelet count showing significantly higher biological responses than a 10 fold increase.16 Additionally, there was a trend in our study to increased COL1 gene expression with platelets and plasma, also in agreement with prior studies 14; 17. Work by Jo et al. found tenocytes harvested from degenerative tears undergoing repair and cultured in the presence of PRP had increased proliferation and types I and III collagen gene expression, with concentrations of 4000 and 8000 × 103 platelets/μL showing the greatest increase in proliferation.17 These findings for a model in which the tenocytes and blood components are incorporated into an extracellular matrix carrier are thus consistent with prior 2-D in vitro studies.
This study establishes the positive in vitro biologic effects of 2-3X platelets and plasma on sheep rotator cuff tenocytes in an extracellular matrix carrier. There was an overall increase in cell metabolism in the platelet and plasma preparation groups on days 1, 3, 7 and 13, and both platelet and plasma groups had an increase in DNA quantity, suggesting increased cell proliferation. This corresponds to the increased number of cells seen on histology at day 14 in the PPP and PAP groups. COL1 gene expression was increased only in the presence of both platelets and plasma. These findings are consistent with prior work in which Cheng et al. found that plasma alone was sufficient for increased proliferation and metabolism of ACL cells in culture, however platelets and plasma stimulated increased COL1 gene production.35 Additionally we observed an increase in contraction of the collagen scaffold in the platelet and plasma groups at day 14, a process that is important during wound healing.36 Based on the increased presence of myofibroblasts in the same set of constructs, we suspect this is due to cell-mediated contraction rather than degradation. In addition, MMP activity in the media was equivalent in all groups, making differential degradation less likely.
We further hypothesized the further addition of macrophages specifically might add an important mediator in the complicated inflammatory and healing process due to the important role macrophages play in the normal response to injury and wound healing. Although recent work has shown that macrophages cultured in the presence of platelets and plasma resulted in increased release of anti-inflammatory cytokines RANTES and LXA4 by platelets, and decreased production of monocyte chemotactic protein-1 37 and IL-1 33 by macrophages, our study suggests that the addition of macrophages in this model was not sufficient to translate these inflammation-modifying effects into increased in vitro cellular activity at the level of the tenocytes. This may be due to our manufactured in vitro environment, which lacks fresh sources of monocytes and macrophages over time and also lacks the presence of neutrophils, which may be an important co-factor for macrophage function in the wound site.38
This study has several additional limitations. First, only one concentration of macrophages was used in all cultures. The concentration was chosen based on the relatively low concentration of macrophages and monocytes in peripheral blood. It is possible a different concentration of macrophages would have produced different effects, and altering the macrophage concentration is a potential area of future study. Second, as the cells were cultured within an extracellular matrix scaffold, any products synthesized by the cells may have been trapped within the hydrogel, affecting the amount measured in the culture media. We suspect this contributed to the lack of significant findings in the IL-10 and TNF-α assays. Sample size was selected based on prior studies of platelets and plasma on ligament biology, although no formal power analysis was performed. Our study may not have been adequately powered to detect smaller differences. Finally, our in-vitro environment is limited by the use of passaged cells, the lack of mechanical load on the constructs, as well as the use of tendon cells from skeletally mature, healthy sheep, rather than human degenerated rotator cuff tendon. These limitations in the model should be kept in mind when trying to extrapolate our results to cells in degenerative tears of the human rotator cuff.
In conclusion, the addition of plasma and platelets each had anabolic effects on the ovine rotator cuff tenocytes; however, the addition of macrophages did not increase the observed anabolic effects of platelets or plasma in this model. These findings suggest that platelet therapy may indeed have clinical application in the rotator cuff. Further research into the proper application of platelets, such as via an extracellular matrix scaffold, may help lead to improved clinical outcomes and improved rates of healing. Our model may be useful for continued exploration of the use and application of platelets and plasma to augment rotator cuff healing using the translational model of bench to bedside research.
Acknowledgements
This study was supported by a grant from the National Institute of Health (NIAMS R01 AR054099-06), as well as support from the Translational Research Program at Boston Children's Hospital. This investigation was also supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award (F32 AR061186). The authors would also like to acknowledge the staff at Animal Research Children's Hospital (ARCH) for their assistance. This work was conducted with support from Harvard Catalyst (The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170-05 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health. MMM is an inventor on patents owned by Boston Children's Hospital in the area of collagen scaffolds for wound healing.
Footnotes
Contributions:
All authors contributed significantly to this work. B.A.K. and M.M.M. conceived the project, designed the experiments and interpreted the results. B.A.K, C.M.H, and B.L.P. performed all experiments and analysis. B.A.K. wrote the manuscript. C.M.H, B.L.P, and M.M.M provided critical edits of the manuscript.
REFERENCES
- 1.Gomoll AH, Katz JN, Warner JJ, et al. Rotator cuff disorders: recognition and management among patients with shoulder pain. Arthritis Rheum. 2004;50:3751–3761. doi: 10.1002/art.20668. [DOI] [PubMed] [Google Scholar]
- 2.Vitale MA, Vitale MG, Zivin JG, et al. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16:181–187. doi: 10.1016/j.jse.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Zumstein MA, Jost B, Hempel J, et al. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90:2423–2431. doi: 10.2106/JBJS.G.00677. [DOI] [PubMed] [Google Scholar]
- 5.Edwards SL, Lynch TS, Saltzman MD, et al. Biologic and pharmacologic augmentation of rotator cuff repairs. J Am Acad Orthop Surg. 2011;19:583–589. doi: 10.5435/00124635-201110000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res. 2008;466:622–633. doi: 10.1007/s11999-007-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan DR, Chao FC, Stiles CD, et al. Platelet alpha granules contain a growth factor for fibroblasts. Blood. 1979;53:1043–1052. [PubMed] [Google Scholar]
- 8.Assoian RK, Komoriya A, Meyers CA, et al. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258:7155–7160. [PubMed] [Google Scholar]
- 9.Karey KP, Sirbasku DA. Human platelet-derived mitogens. II. Subcellular localization of insulinlike growth factor I to the alpha-granule and release in response to thrombin. Blood. 1989;74:1093–1100. [PubMed] [Google Scholar]
- 10.Banks RE, Forbes MA, Kinsey SE, et al. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer. 1998;77:956–964. doi: 10.1038/bjc.1998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura T, Nawa K, Ichihara A, et al. Purification and subunit structure of hepatocyte growth factor from rat platelets. FEBS Lett. 1987;224:311–316. doi: 10.1016/0014-5793(87)80475-1. [DOI] [PubMed] [Google Scholar]
- 12.Assoian RK, Grotendorst GR, Miller DM, et al. Cellular transformation by coordinated action of three peptide growth factors from human platelets. Nature. 1984;309:804–806. doi: 10.1038/309804a0. [DOI] [PubMed] [Google Scholar]
- 13.Brunner G, Nguyen H, Gabrilove J, et al. Basic fibroblast growth factor expression in human bone marrow and peripheral blood cells. Blood. 1993;81:631–638. [PubMed] [Google Scholar]
- 14.Cho JW, Kim SA, Lee KS. Platelet-rich plasma induces increased expression of G1 cell cycle regulators, type I collagen, and matrix metalloproteinase-1 in human skin fibroblasts. Int J Mol Med. 2012;29:32–36. doi: 10.3892/ijmm.2011.803. [DOI] [PubMed] [Google Scholar]
- 15.Graziani F, Ivanovski S, Cei S, et al. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17:212–219. doi: 10.1111/j.1600-0501.2005.01203.x. [DOI] [PubMed] [Google Scholar]
- 16.Sadoghi P, Lohberger B, Aigner B, et al. Effect of platelet-rich plasma on the biologic activity of the human rotator-cuff fibroblasts: A controlled in vitro study. J Orthop Res. 2013;31:1249–1253. doi: 10.1002/jor.22360. [DOI] [PubMed] [Google Scholar]
- 17.Jo CH, Kim JE, Yoon KS, et al. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012;40:1035–1045. doi: 10.1177/0363546512437525. [DOI] [PubMed] [Google Scholar]
- 18.Bergeson AG, Tashjian RZ, Greis PE, et al. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40:286–293. doi: 10.1177/0363546511424402. [DOI] [PubMed] [Google Scholar]
- 19.Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39:258–265. doi: 10.1177/0363546510390780. [DOI] [PubMed] [Google Scholar]
- 20.Rodeo SA, Delos D, Williams RJ, et al. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40:1234–1241. doi: 10.1177/0363546512442924. [DOI] [PubMed] [Google Scholar]
- 21.Weber SC, Kauffman JI, Parise C, et al. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41:263–270. doi: 10.1177/0363546512467621. [DOI] [PubMed] [Google Scholar]
- 22.Murray MM, Palmer M, Abreu E, et al. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res. 2009;27:639–645. doi: 10.1002/jor.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41:1762–1770. doi: 10.1177/0363546513483446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vavken P, Fleming BC, Mastrangelo AN, et al. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy. 2012;28:672–680. doi: 10.1016/j.arthro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fufa D, Shealy B, Jacobson M, et al. Activation of platelet-rich plasma using soluble type I collagen. J Oral Maxillofac Surg. 2008;66:684–690. doi: 10.1016/j.joms.2007.06.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison S, Vavken P, Kevy S, et al. Platelet activation by collagen provides sustained release of anabolic cytokines. Am J Sports Med. 2011;39:729–734. doi: 10.1177/0363546511401576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastrangelo AN, Haus BM, Vavken P, et al. Immature animals have higher cellular density in the healing anterior cruciate ligament than adolescent or adult animals. J Orthop Res. 2010;28:1100–1106. doi: 10.1002/jor.21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray MM, Spindler KP, Ballard P, et al. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 29.Menzies FM, Henriquez FL, Alexander J, et al. Sequential expression of macrophage anti-microbial/inflammatory and wound healing markers following innate, alternative and classical activation. Clin Exp Immunol. 2010;160:369–379. doi: 10.1111/j.1365-2249.2009.04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 33.Woodall J, Jr., Tucci M, Mishra A, et al. Cellular effects of platelet rich plasmainterleukin1 release from prp treated macrophages. Biomed Sci Instrum. 2008;44:489–494. [PubMed] [Google Scholar]
- 34.Turner AS. Experiences with sheep as an animal model for shoulder surgery: strengths and shortcomings. J Shoulder Elbow Surg. 2007;16:S158–163. doi: 10.1016/j.jse.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Cheng M, Wang H, Yoshida R, et al. Platelets and plasma proteins are both required to stimulate collagen gene expression by anterior cruciate ligament cells in three-dimensional culture. Tissue Eng Part A. 2010;16:1479–1489. doi: 10.1089/ten.tea.2009.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ngo P, Ramalingam P, Phillips JA, et al. Collagen gel contraction assay. Methods Mol Biol. 2006;341:103–109. doi: 10.1385/1-59745-113-4:103. [DOI] [PubMed] [Google Scholar]
- 37.El-Sharkawy H, Kantarci A, Deady J, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78:661–669. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 38.Mikolajczyk TP, Skrzeczynska-Moncznik JE, Zarebski MA, et al. Interaction of human peripheral blood monocytes with apoptotic polymorphonuclear cells. Immunology. 2009;128:103–113. doi: 10.1111/j.1365-2567.2009.03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]