Abstract
Objectives
To explore neural connectivity changes associated with repetitive transcranial magnetic stimulation (rTMS) to the temporoparietal junction for patients with bothersome tinnitus.
Study Design
Randomized, double-blind, controlled clinical trial
Methods
30 patients with subjective, nonpulsatile tinnitus for 6 months duration or longer and a score of 36 or greater on the Tinnitus Handicap Inventory (THI) completed the study. Participants were randomized to receive either sham or active treatment with rTMS to the temporoparietal junction for either 2 or 4 weeks of therapy. Participants underwent resting state functional connectivity MRI (rs-fcMRI) before therapy and immediately following treatment. Functional connectivity changes between active and sham treatment groups were compared using regions of interest in auditory, default mode, ventral attention, and executive attention networks.
Results
Sixteen patients received active rTMS treatment; 14 patients received sham treatment. There were no differences between the active and sham groups in baseline functional connectivity. Neither treatment with rTMS nor sham therapy resulted in statistically significant functional connectivity changes in the examined brain networks.
Conclusions
The analysis did not identify any changes in neural connectivity following treatment in patients with bothersome tinnitus. These results are consistent with our findings of lack of symptom changes previously reported in the same group of patients. Measures of neural connectivity may inform future work using rTMS to better understand the possible benefits of neural stimulation for tinnitus.
Keywords: tinnitus, functional MRI, repetitive transcranial magnetic stimulation
Introduction
Tinnitus is the perception of sound without an acoustic stimulus. Tinnitus patients often suffer from depression, insomnia, and anxiety, and have difficulty with attention and concentration,1 leading to disability. Unfortunately, the pathophysiology of tinnitus is not fully understood and there is no cure or effective treatment for those who suffer from the disease.
While most early research in tinnitus focused on the role of peripheral dysfunction in the tinnitus percept, recently focus has shifted to the idea that differential neural connectivity may also explain the amount of bother experienced by tinnitus patients.2 In this context, it has been suggested that tinnitus is caused by excessive spontaneous activity in auditory cortex3 and that chronic tinnitus may be similar to neuropsychiatric syndromes related to plastic alterations of the brain.4 Auditory hyperactivity might be evident not only in focal changes in neural activation, but in functional connectivity across brain networks.5 If dysfunctional neural connectivity in auditory processing networks is a source of tinnitus distress, altering this activity may be an effective treatment.
Repetitive transcranial magnetic stimulation (rTMS) is an intervention that might be used to correct dysfunctional cortical networks. rTMS uses a magnetic field to create an electric current within the brain which depolarizes axons and activates neural networks;6 the repeated application of these pulses has the potential to induce changes in neural connectivity. Outside of tinnitus, functional MRI (fMRI) has identified changes in neural activity following rTMS therapy7,8 and rTMS is effective in treating medication resistant depression.9
Despite its promise, the results of studies examining the efficacy of rTMS in tinnitus are mixed.6 A critical decision rests on the site of rTMS stimulation (that is, which brain network to stimulate). Common targets are auditory cortical regions including left temporoparietal junction (TPJ) and auditory cortex in an effort to inhibit auditory hyperactivity. While some studies suggest symptom improvement following repeated sessions of rTMS to the auditory cortex or TPJ,10–18 a lack of improvement has been observed in several randomized controlled trials,19,20 including our own previously published randomized, controlled, cross-over trials.21,22
Our two previously published studies report symptom outcomes following either active or sham treatment with rTMS to the TPJ. Resting state functional connectivity MRI was obtained before and after treatment. Tinnitus patients show differences in functional connectivity as compared to healthy controls,2 and we hypothesize that neural connectivity changes following rTMS will correlate to symptom improvement. The proposed mechanism for intervention is that rTMS results in altered patterns of neural activity in cortical networks, and this altered network activity causes a reduction in symptoms. Under this view, there are at least two explanations for why rTMS to TPJ did not result in symptom improvement. The first is that rTMS did not result in changes to neural connectivity. If symptom improvement indeed follows from changes in brain connectivity, a lack of changes in neural activity would be consistent with a lack of improvement. A second explanation is that rTMS to TPJ effectively alters neural connectivity, but not in a network that is contributing to tinnitus bother. Critically, functional neuroimaging can distinguish between these two possibilities. A mechanistic understanding of the effects of rTMS on the TPJ will help explain the variability of prior results and help guide effective treatments.
The objective of the current study was to systematically evaluate neural network changes in patients with bothersome chronic tinnitus who underwent rTMS treatment targeting the left TPJ, as compared to those who received sham therapy. We targeted the TPJ because it is thought to contribute to secondary and integrative auditory processes. This is the first study to evaluate functional connectivity before and after treatment in a randomized controlled trial of temporoparietal rTMS in tinnitus patients.
Materials and Methods
Design
This study was a double-blinded, randomized controlled trial. Participants were considered eligible for this study if they were between 18 and 60 years of age with subjective, nonpulsatile tinnitus for 6 months or longer. All participants were required to score at least a 30 on the Tinnitus Handicap Inventory (THI) assessment23 and a score of less than 14 on the Beck Depression Inventory.24 All participants gave informed consent to participate in the study under a protocol approved by the Washington University in Saint Louis Institutional Review Board.
Details of the methods of the study have been previously published.21,22 Using a block randomization code generated by a statistician, all patients who remained eligible for the study were assigned to receive either sham or active treatment, with both participants and physicians blinded to treatment group. The initial protocol for this study was approved for 2 consecutive weeks of treatment. Midway through the trial, the protocol was revised to allow for 4 consecutive weeks of treatment in order to evaluate increased length of treatment time. Here we collapse these two groups into a single analysis. Prior to initial treatment and immediately following completion of either 2 or 4 weeks of treatment, participants underwent a resting-state functional connectivity MRI.
This study used a cross-over design with a wash-out period between treatments (sham and active therapy). Following the first arm of treatment, participants underwent a wash-out period, followed by the second treatment. The focus of this manuscript is the evaluation and comparison of neuroimaging before and after the first arm of treatment.
Measurements
Participants completed multiple assessments including past medical and health information, tinnitus description, hearing history and exposures, audiometric exam, and neurocognitive assessments. Additionally, participants underwent resting-state functional connectivity MRI prior to, and immediately after completion of treatment.
rTMS procedure
Participants received 5 treatment sessions per week for 2 or 4 consecutive weeks. The coil was placed over the left TPJ for all subjects. The sham coil was identical in appearance and sound to the active treatment coil. The delivery stimulus was calculated by placing the coil over the motor cortex and calculating the threshold at which the thumb abductor was stimulated to contract. This was considered the motor threshold. Active treatment was delivered at 1 Hz at 110% of motor threshold. The coil was placed over the TPJ region and not the motor cortex for treatment, therefore a muscle response was not elicited during treatment and patients would not have been able to differentiate between active and sham treatment. Sessions were conducted with interval stimulation for a total of 42.5 minutes.
Neuroimaging
Resting –state functional connectivity MRI was collected prior and immediately after completion of either active or sham treatment on a Siemens 3T Trio scanner. Participants wore headphones to reduce background noise and remained awake, with their eyes closed, in the scanner. Images were collected using an echo-planar sequence (EPI) (Repetition time [TR] = 2200 ms, echo time [TE] = 27 ms, flip angle = 90°, 4 mm isotropic voxels). Each participant completed three runs of 164 volumes. T1-weighted structural images were also acquired using a MPRAGE sequence (TR = 2100 ms, TE = 3.93ms, flip angle = 7°, 1 × 1 × 1.25 mm voxels).
Analysis
Analysis of behavioral data has been previously described for the 2-week treatment group21 and the 4-week treatment group;22 here we report combined statistics for completeness. Pairs of pre- and post- treatment intervention MRI scans were available and usable for 30 subjects; participants who did not complete a post intervention scan were not included in any analysis. MRI data was processed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK) and the Automatic Analysis processing environment (version 4.1; http://www.automaticanalysis.org).25 The analysis was completed in an identical fashion to our prior study.26 The variance between successive EPI volumes was computed, and scans were aligned using rigid body transformations. Variance and motion parameters were used to identify scans exceeding any of the following: 0.5mm translation, 0.3° rotation, or a variance of 5 standard deviations from the mean scaled variance. These scans were later modeled out to limit the impact of participant motion. EPI images for each participant were coregistered to that participant’s structural image, spatially normalized to Montreal Neurological Institute (MNI) space,27 and smoothed with a 9 mm full-width half maximum isotropic Gaussian kernel. The statistical model included discrete cosine basis functions to bandpass filter the data from 0.01–0.1 Hz, the 6 movement parameters obtained from realignment, and their squares and volterra expansions. Using a singular value decomposition, the number of confounds was decreased to regressors that explained at least 99% of the variance. Estimates for the time-series of interest were calculated from the residuals from the model.
For our primary analyses, we chose canonical regions of interest (ROIs)28 including: primary auditory cortex (left: [−38 −33 17], right: [58 −16 7]), secondary auditory (left TPJ: [−55 −40 14], right TPJ: [52 −33 8]), frontoparietal (left inferior frontal gyrus: [−42 38 21], right inferior frontal gyrus: [48 25 27]), cingulo-opercular (left anterior frontal operculum: [−51 8 −2], right anterior frontal operculum: [36 10 1]), and default (posterior cingulate: [8 −48 31], anterior cingulate: [12 36 20]) networks. The mean timeseries data was extracted from a 5mm radius sphere around each coordinate. These data were then entered in a timeseries model as above to identify regions of the brain in which activity was correlated to that of the seed region. Using a two sample t-test, the resulting parameter estimate maps for all participants were then entered into second-level group analyses to look at the activity for the active and sham treatment groups. Additionally, using paired samples t-tests, we analyzed the pre-treatment scans, post-treatment scans, and their comparison. A cluster-forming voxelwise threshold of p < 0.001, corrected for whole-brain significance at the cluster level (p < 0.05) using random field theory was utilized.29 Using Connectome Workbench, the results were displayed on an inflated cortical surface (v0.85; http://www.humanconnectome.org/software/connectome-workbench.html).
Additional exploratory analyses were conducted on a full set of 264 ROIs covering all regions of the brain.28 These analyses were Bonferroni corrected for multiple comparisons to account for the number of tests conducted.
Results
Patient Characteristics
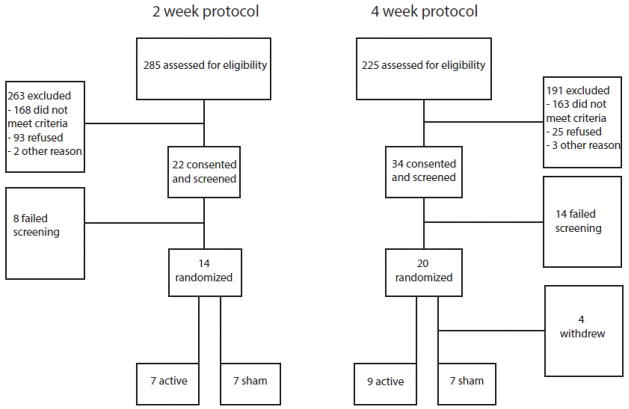
The flow diagram for patients we recruited is shown in Figure 1. In addition to participants reported in our previous publications,21,22 two additional patients randomized to receive 4 weeks of sham intervention are included in the current analysis. These patients did not complete the second arm of the study and were not included in previous analyses.
Figure 1.
Flow diagram of participation
Patient characteristics are shown in Table 1. There were no differences in baseline age or hearing between the active and sham groups, as measured by student’s t-test. Hearing was measured by grading audiogram results based on a 6 level scale: 1) Normal, 2) Slight, 3) Mild, 4) Moderate, 5) Moderate/Severe or 6) Severe hearing loss. Neither active treatment for 2 or 4 weeks was found to be more effective than sham treatment for reduction of symptoms of chronic bothersome tinnitus according to THI assessments.21,22 After including the 2 additional patients, Mann-Whitney U tests revealed no significant differences between baseline, post-intervention or post-intervention - baseline THI measurements between the sham and active rTMS groups. There were no serious adverse events reported.21,22 We do not believe that patients were aware of their randomization assignment because patients’ guesses as to which treatment they received were not better than chance.22
Table 1.
Demographics
| Sham | Active | |
|---|---|---|
| Age (median) | 53 | 50 |
| Gender (% male) | 71% | 75% |
| Baseline THI (median) | 52 | 51 |
| Post-treatment THI (median) | 43 | 42 |
| Hearing ranking* (mean) | 2.85 | 2.50 |
Hearing was graded as 1) Normal, 2) Slight hearing loss, 3) Mild hearing loss, 4) Moderate hearing loss, 5) Moderate/Severe hearing loss or 6) Severe hearing loss based on patients’ audiogram.
Neuroimaging results
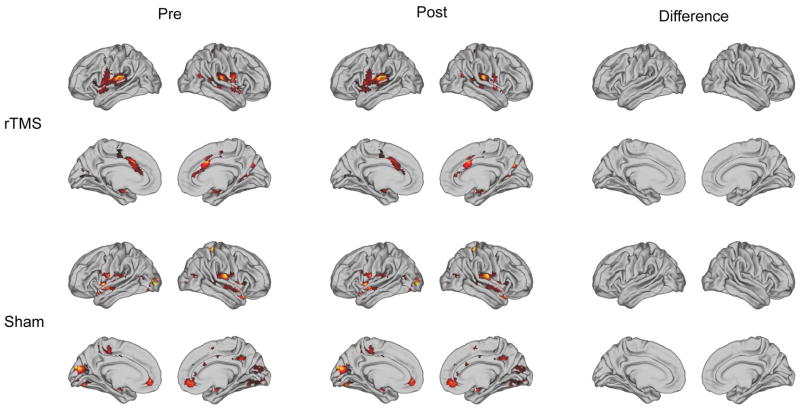
Two-sample t-tests were used to compare baseline functional connectivity of the active and sham groups, including a covariate to control for 2 or 4 week treatment. Even though the connectivity with each seed region identified an appropriate resting state network, there were no differences in connectivity between the active and sham groups using any of the seed regions shown in Figure 2. Additionally, analyses were done to compare post-treatment connectivity between the treatment and sham group. As an example, the results for the left auditory ROI are shown in Figure 3. There were no significant differences between active and sham, or pre- and post-, using any of these seed regions.
Figure 2.
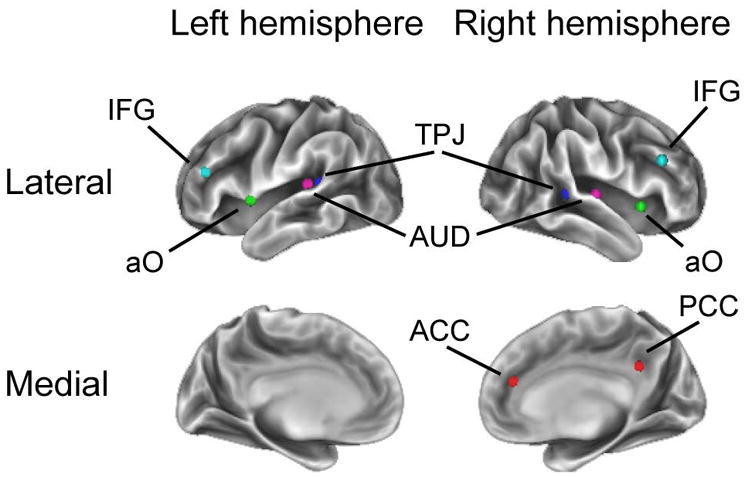
Regions of Interest (ROIs) used for functional connectivity analysis. TPJ = temporoparietal junction, IFG = inferior frontal gyrus, AUD = auditory cortex, ACC = anterior cingulate cortex, PCC = posterior cingulate cortex, aO = anterior operculum.
Figure 3.
Left auditory ROI: pre-treatment, post-treatment and difference between pre and post treatment resting state functional connectivity for active and sham therapy groups.
Finally, we conducted exploratory analyses on pairwise connectivity between seed regions covering the whole brain. We first performed paired samples t-tests comparing pre- and post- treatment for treatment and sham groups to look for connectivity changes following intervention in regions outside the attention networks that were the focus of our hypothesis-driven seed-based analysis above. No significant differences were found. In addition, regardless of group membership, we correlated pairwise functional neural connectivity with baseline THI, change in THI, baseline depression, and change in depression. Again, none of these correlations were statistically significant.
Discussion
Here we report the first study to examine changes in neural connectivity following either active rTMS directed towards the TPJ or sham treatment in a group of patients with bothersome tinnitus. Our previous reports suggest lack of symptom improvement following rTMS therapy to this region21,22 and our neuroimaging findings are consistent with these results. Using a seed-based analysis with identified brain regions thought to be affected in tinnitus patients, we did not identify any functional connectivity changes after treatment.
The efficacy of rTMS as an intervention depends on how well it modulates the targeted brain areas, and the degree to which the targeted regions are responsible for symptoms. The use of fcMRI is critical to determine the degree to which neural activity has indeed been modulated. One pilot study used functional connectivity before and after rTMS therapy to the auditory cortex in tinnitus patients to evaluate neural changes; however, the results varied among the small number of tinnitus patients included in the study and are difficult to interpret.30 There have been mixed results regarding stimulation of the TPJ: Some studies support improvement of tinnitus symptoms,10,11,21 although multiple randomized controlled trials have not verified this finding.18–20,31
We propose that the lack of symptom improvement in the current study is due to lack of neural connectivity changes. The target of rTMS in the current study is the TPJ, which supports secondary and integrative auditory processing. The TPJ is also characterized as part of the ventral attention network, or bottom-up reorienting system. The ventral attention network is likely involved in prioritizing sensory input,32 which may be important for tinnitus patients who have difficulty ignoring their auditory percept. As compared to healthy controls, patients with bothersome tinnitus have been previously found to have altered activity in attention networks.3 There are multiple potential explanations for the lack of neural connectivity changes following rTMS in this study. One possibility is that rTMS therapy, while potentially therapeutic for some illnesses, may not modulate neural connectivity to a degree that can be captured using fcMRI. The literature regarding the investigation of imaging in patients with depression receiving rTMS is sparse and immediate post-treatment effects on fcMRI have not been verified. A second possible explanation is that the specific rTMS protocol used was not sufficient for modulation of the TPJ and associated networks. Finally, there are many areas of the brain that contribute to attentional processing which may serve as potential targets for rTMS therapy. The TPJ alone may not be the ideal target for rTMS therapy in tinnitus patients. It is possible that there are widespread cortical changes in tinnitus patients, which cannot be treated by a single localized therapy, or that an alternate attention network would serve as a more appropriate target.
Recent work has pointed to the dorsolateral prefrontal cortex (DLPFC), part of the executive attention system, as a potential target for tinnitus therapy. Randomized controlled trials comparing temporal cortex rTMS to combination therapy with temporal and prefrontal rTMS failed to identify statistically significant treatment effects immediately after treatment20,33 or at 3 month follow up.33 However, long-term follow-up of a few pilot studies has suggested that multi-site stimulation of the DLPFC, in addition to the temporal region or TPJ, results in improvement of tinnitus symptoms as compared to single-site stimulation of the temporal cortex.34,35 The use of rTMS for chronic tinnitus continues to be an area of active research as long-term neuroplastic changes and stable symptom improvements are of interest in this patient population.
Limitations of our study include generalizability, as our patient population was screened to exclude patients with active depression. Therefore, our participants may not be truly representative of the tinnitus population. Additionally, we chose to exclude tinnitus patients who are not bothered by their tinnitus, as we believe that the bothered tinnitus sub-group is most in need of active research. Again, patient selection may limit generalizability. Finally, although we did not see any significant changes using any of the seed regions included, it is possible that other differences in functional connectivity may exist between patient groups.
In the future, it would be informative to conduct a similar study using a randomized controlled design to evaluate functional connectivity at baseline and following rTMS therapy directed towards multiple attention networks, such as the dorsolateral prefrontal cortex and the TPJ. Effective rTMS therapy may require a multi-target approach in order to target the multiple dysfunctional networks likely responsible for tinnitus. We believe long-term symptom improvement is a result of changes in one or more neural networks and that neuroimaging can serve as a biomarker for these changes.
Conclusion
Consistent with a lack of symptom improvement following rTMS directed to the left TPJ, we did not find changes in neural connectivity following rTMS therapy. Rather than suggest that rTMS is ineffective for chronic bothersome tinnitus, our results suggest instead that the TPJ alone may not be an ideal target for tinnitus treatment. Further research is necessary to identify better targets for rTMS treatment in patients with bothersome tinnitus in order to create changes to cortical networks associated with symptom improvement.
Acknowledgments
This project was presented at the Triological Society Meeting held in Cornado Island, CA January 22–24, 2015.
This research was supported by grant R01DC009095 from the National Institutes of Deafness and Other Communication Disorders. This research and publication was supported, in part, by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences, and the “Development Of Clinician/Researchers In Academic ENT” T32DC00022 from the National Institutes of Deafness and Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of interest: none
Level of evidence: 1b
Trial registration: clinicaltrials.gov Identifier: NCT00567892
Financial disclosures: This research was supported by grant R01DC009095 from the National Institutes of Deafness and Other Communication Disorders. This research and publication was supported, in part, by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences, and the “Development Of Clinician/Researchers In Academic ENT” T32DC00022 from the National Institutes of Deafness and Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Dobie R. Tinnitus: Theory and Management. Ontario: BC Decker; 2004. Overview: Suffering from tinnitus. [Google Scholar]
- 2.Burton H, Wineland A, Bhattacharya M, Nicklaus J, Garcia K, Piccirillo J. Altered networks in bothersome tinnitus: a functional connectivity study. BMC Neurosci. 2012;13:1–15. doi: 10.1186/1471-2202-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaltenbach J. Neurophysiologic mechanisms of tinnitus. Journal of the American Academy of Audiology. 2000;11:125–37. [PubMed] [Google Scholar]
- 4.Mühlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proceedings of the National Academy of Sciences. 1998;95:10340–3. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt S, Akrofi K, Carpenter-Thompson J, Husain F. Default Mode, Dorsal Attention and Auditory Resting State Networks Exhibit Differential Functional Connectivity in Tinnitus and Hearing Loss. PLoS One. 2013:8. doi: 10.1371/journal.pone.0076488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefaucheur J-P, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clinical Neurophysiology. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso EF, Fregni F, Martins Maia F, et al. rTMS treatment for depression in Parkinson’s disease increases BOLD responses in the left prefrontal cortex. The International Journal of Neuropsychopharmacology. 2008;11:173–83. doi: 10.1017/S1461145707007961. [DOI] [PubMed] [Google Scholar]
- 8.Martin L, Borckardt JJ, Reeves ST, et al. A Pilot Functional MRI Study of the Effects of Prefrontal rTMS on Pain Perception. Pain Medicine. 2013;14:999–1009. doi: 10.1111/pme.12129. [DOI] [PubMed] [Google Scholar]
- 9.Gaynes B, Lloyd S, Lux L. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J Clin Psychiatry. 2014:477–89. doi: 10.4088/JCP.13r08815. [DOI] [PubMed] [Google Scholar]
- 10.Anders M, Dvorakova J, Rathova L, et al. Efficacy of repetitive transcranial magnetic stimulation for the treatment of refractory chronic tinnitus: A randomized, placebo controlled study. Neuroendocrinology Letters. 2010;31:238–49. [PubMed] [Google Scholar]
- 11.Bilici S, Yigit O, Taskin U, Gor A, Yilmaz E. Medium-term results of combined treatment with transcranial magnetic stimulation and antidepressant drug for chronic tinnitus. Eur Arch Otorhinolaryngol. 2013:1–7. doi: 10.1007/s00405-013-2851-z. [DOI] [PubMed] [Google Scholar]
- 12.Chung HK, Tsai CH, Lin YC, et al. Effectiveness of theta-burst repetitive transcranial magnetic stimulation for treating chronic tinnitus. Audiology and Neurotology. 2012;17:112–20. doi: 10.1159/000330882. [DOI] [PubMed] [Google Scholar]
- 13.Khedr EM, Rothwell JC, Ahmed MA, El-Atar A. Effect of daily repetitive transcranial magnetic stimulation for treatment of tinnitus: Comparison of different stimulus frequencies. Journal of Neurology, Neurosurgery and Psychiatry. 2008;79:212–5. doi: 10.1136/jnnp.2007.127712. [DOI] [PubMed] [Google Scholar]
- 14.Kleinjung T, Eichhammer P, Langguth B, et al. Long-term effects of repetitive transcranial magnetic stimulation (rTMS) in patients with chronic tinnitus. Otolaryngology - Head and Neck Surgery. 2005;132:566–9. doi: 10.1016/j.otohns.2004.09.134. [DOI] [PubMed] [Google Scholar]
- 15.Lee HY, Yoo SD, Ryu EW, Byun JY, Yeo SG, Park MS. Short term effects of repetitive transcranial magnetic stimulation in patients with catastrophic intractable tinnitus: Preliminary report. Clinical and Experimental Otorhinolaryngology. 2013;6:63–7. doi: 10.3342/ceo.2013.6.2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcondes RA, Sanchez TG, Kii MA, et al. Repetitive transcranial magnetic stimulation improve tinnitus in normal hearing patients: A double-blind controlled, clinical and neuroimaging outcome study. European Journal of Neurology. 2010;17:38–44. doi: 10.1111/j.1468-1331.2009.02730.x. [DOI] [PubMed] [Google Scholar]
- 17.Mennemeier M, Chelette KC, Allen S, et al. Variable changes in PET activity before and after rTMS treatment for tinnitus. The Laryngoscope. 2011;121:815–22. doi: 10.1002/lary.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi S, De Capua A, Ulivelli M, et al. Effects of repetitive transcranial magnetic stimulation on chronic tinnitus: A randomised, crossover, double blind, placebo controlled study. Journal of Neurology, Neurosurgery and Psychiatry. 2007;78:857–63. doi: 10.1136/jnnp.2006.105007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoekstra CEL, Versnel H, Neggers SFW, Niesten MEF, Van Zanten GA. Bilateral low-frequency repetitive transcranial magnetic stimulation of the auditory cortex in tinnitus patients is not effective: A randomised controlled trial. Audiology and Neurotology. 2013;18:362–73. doi: 10.1159/000354977. [DOI] [PubMed] [Google Scholar]
- 20.Langguth B, Landgrebe M, Frank E, et al. Efficacy of different protocols of transcranial magnetic stimulation for the treatment of tinnitus: Pooled analysis of two randomized controlled studies. World Journal of Biological Psychiatry. 2014;15:276–85. doi: 10.3109/15622975.2012.708438. [DOI] [PubMed] [Google Scholar]
- 21.Piccirillo JF, Garcia KS, Nicklaus J, et al. Low-frequency repetitive transcranial magnetic stimulation to the temporoparietal junction for tinnitus. Archives of Otolaryngology - Head and Neck Surgery. 2011;137:221–8. doi: 10.1001/archoto.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccirillo JF, Kallogjeri D, Nicklaus J, et al. Low-frequency repetitive transcranial magnetic stimulation to the temporoparietal junction for tinnitus: Four-week stimulation trial. JAMA Otolaryngology - Head and Neck Surgery. 2013;139:388–95. doi: 10.1001/jamaoto.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Archives of Otolaryngology -- Head & Neck Surgery. 1996;122:143–8. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- 24.Beck AT, Ward CH, Mendelson MM, Mock JJ, Erbaugh JJ. AN inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 25.Cusack R, Vicente-Grabovetsky A, Mitchell DJ, et al. Automatic analysis (aa): efficient neuroimaging workflows and parallel processing using Matlab and XML. Name: Frontiers in Neuroinformatics. 2014;8:90. doi: 10.3389/fninf.2014.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roland LT, Lenze EJ, Hardin FM, et al. Effects of Mindfulness Based Stress Reduction Therapy on Subjective Bother and Neural Connectivity in Chronic Tinnitus. Otolaryngology -- Head and Neck Surgery. 2015 doi: 10.1177/0194599815571556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72:665–78. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 30.Lefaucheur JP, Brugières P, Guimont F, et al. Navigated rTMS for the treatment of tinnitus: A pilot study with assessment by fMRI and AEPs. Neurophysiologie Clinique/Clinical Neurophysiology. 2012;42:95–109. doi: 10.1016/j.neucli.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Plewnia C, Vonthein R, Wasserka B, et al. Treatment of chronic tinnitus with theta burst stimulation: A randomized controlled trial. Neurology. 2012;78:1628–34. doi: 10.1212/WNL.0b013e3182574ef9. [DOI] [PubMed] [Google Scholar]
- 32.Petersen S, Posner M. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreuzer PM, Landgrebe M, Schecklmann M, Poeppl TB, Vielsmeier V, Hajak G. Can temporal repetitive transcranial magnetic stimulation be enhanced by targeting affective components of tinnitus with frontal rTMS? A randomized controlled pilot trial. Front Syst Neurosci. 2011:5. doi: 10.3389/fnsys.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleinjung T, Eichhammer P, Landgrebe M, et al. Combined temporal and prefrontal transcranial magnetic stimulation for tinnitus treatment: A pilot study. Otolaryngology - Head and Neck Surgery. 2008;138:497–501. doi: 10.1016/j.otohns.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Lehner A, Schecklmann M, Poeppl T, et al. Multisite rTMS for the Treatment of Chronic Tinnitus: Stimulation of the Cortical Tinnitus Network—A Pilot Study. Brain Topogr. 2013;26:501–10. doi: 10.1007/s10548-012-0268-4. [DOI] [PubMed] [Google Scholar]