Abstract
We studied 92 patients with transplant glomerulopathy to develop a prognostic index based on the risk factors for allograft failure within five years of diagnosis (Development cohort). During 60 months (median) follow up, 64 patients developed allograft failure. A chronic-inflammation score generated by combining Banff ci, ct and ti scores, serum creatinine and proteinuria at biopsy, were independent risk factors for allograft failure. Based on the Cox model, we developed a prognostic index and classified patients into risk groups. Compared to the low risk group (median allograft survival over 60 months from diagnosis), patients in the medium risk group had a hazard ratio of 2.83 (median survival 25 months), while those in the high risk group had a hazard ratio of 5.96 (median survival 3.7 months). We next evaluated the performance of the prognostic index in an independent external cohort of 47 patients with transplant glomerulopathy (Validation cohort). The hazard ratios were 2.18 (median survival 19 months) and 16.27 (median survival 1.6 months), respectively, for patients in the medium and high risk groups, compared to the low risk group (median survival 47 months). Our prognostic index model did well in measures of discrimination and calibration. Thus, risk stratification of transplant glomerulopathy based on our prognostic index may provide informative insight for both the patient and physician regarding prognosis and treatment.
Keywords: chronic allograft nephropathy, kidney biopsy
INTRODUCTION
Transplant Glomerulopathy (TG), defined, as a morphological lesion of kidney allograft characterized on light microscopy by the duplication of glomerular basement membrane in the absence of immune complex deposition, is an important cause of late allograft failure (1–4). The strong association with circulating antibodies directed against donor human leukocyte antigen (HLA) and prior acute antibody-mediated rejection, as well as new research pointing towards a possible crosstalk between endothelial cells and HLA antibody, implies chronic alloantibody mediated injury as a plausible cause of TG (1, 5–7).
Kidney recipients with TG have poor allograft survival compared with those who do not have TG (8). However factors associated with allograft failure have not been clearly defined (9). Recognizing such factors and risk stratification of patients is important and may help improve outcomes. The objective of our research was to develop a prognostic index (PI) based on the risk factors for allograft failure within five years of diagnosis and validate the PI in an independent external cohort of kidney transplant recipients with TG.
RESULTS
Characteristics of the study cohort
The study cohort (Development cohort) contained 92 clinically indicated kidney allograft biopsies from 92 kidney transplant recipients with TG from Cornell. We reviewed the results of 1606 consecutive clinically indicated kidney allograft biopsies at our center between January 2000 and June 2011 from 842 kidney transplant recipients and identified these 92 (6%) biopsies (Table 1). For patients with multiple allograft biopsies only the first biopsy with TG was included. Results on staining for complement split product 4d (C4d) were available on all and electron microscopy were available in 85 (92%) specimens. A single pathologist (SVS) evaluated the biopsies and categorized them using the Banff ’07 update of the Banff ’97 classification.
Table 1.
Characteristics of kidney allograft recipients
| Variables | N=92 patients |
|---|---|
| At the time of transplant | |
| Age (years), mean (SD) | 44 (15) |
| Women, N (%) | 37 (40) |
| Racial categories (Black), N (%) | 25 (27) |
| Cause of end stage kidney disease, N (%) | |
| Diabetes | 19 (21) |
| Hypertension | 18 (20) |
| Polycystic kidney disease | 6 (7) |
| IgA nephropathy | 5 (5) |
| Lupus nephritis | 5 (5) |
| Focal and segmental glomerulosclerosis | 4 (4) |
| Other glomerular diseases | 9 (10) |
| Others or Unknown | 26 (28) |
| Deceased donor organ, N (%) | 48 (52) |
| Cold ischemia time, hours, deceased donor, mean (SD) | 26 (10) |
| Human leukocyte antigen mismatches, mean (SD) | 5 (2) |
| Donor information available, N (%) | 73 (79) |
| Age (years), mean (SD) | 44 (15) |
| Women, N (%) | 38 (52) |
| Racial categories (black), N (%) | 13 (18) |
| Previous transplants, N (%) | 16 (17) |
| Panel Reactive antibodies (PRA)a, Data available, N (%) | 69 (73) |
| Peak PRA %, median (IQR) | 11 (0–100) |
| Pre-transplant PRA %, median (IQR) | 0 (0–80) |
| CDC cross match, Data available, N (%) | 92 (100) |
| T-cell positive, N (%) | 0 (0) |
| B-cell positive, N (%) | 10 (11) |
| Flow Cytometry cross match, Data available, N (%) | 30 (33) |
| T-cell positive | 11 (37) |
| B-cell positive | 14 (47) |
| Luminex platform DSA, Data available, N (%) | 17 (18) |
| DSA negative (MFI of the highest rank donor-specific bead <1000) | 11 (65) |
| DSA positive (MFI of the highest rank donor-specific bead >1000 | 6 (35) |
| Received desensitization therapy, N (%) | 15 (16) |
| Induction Immunosuppression, N (%) | 62 (67) |
| Antithymocyte globulin | 54 (87) |
| Interleukin receptor-2 antibodies | 8 (13) |
| After transplant and before the index allograft biopsy | |
| Delayed graft function, N (%) | 15 (16) |
| Calcineurin inhibitor based maintenance immunosuppression, N (%) | 89 (97) |
| Early corticosteroid withdrawal | 39 (42) |
| Thrombotic microangiopathy, N (%) | 4 (4) |
| Hepatitis C virus, N (%) | 15 (16) |
| Acute rejection, N (%) | 23 (25) |
| Acute rejection episodes, N | 28 |
| Acute T-cell mediated rejection episodes, N (%) | 13 (46) |
| Acute antibody-mediated rejection episodes, N (%) | 7 (25) |
| Mixed acute T-cell and antibody-mediated rejection episodes, N (%) | 8 (29) |
| At the time of index allograft biopsy | |
| Age, mean (SD) | 48 (14) |
| Time from transplantation to biopsy (months), median (IQR) | 43 (16–83) |
| Luminex platform DSA, Data available, N (%) | 37 (40) |
| DSA negative (MFI of the highest rank donor-specific bead <1000) | 11 (30) |
| DSA positive (MFI of the highest rank donor-specific bead >1000) | 26 (70) |
| Serum creatinine (mg/dl), median (IQR) | 2.75 (2.15–4.14) |
| Proteinuria >1 g/day, N (%) | 63 (68) |
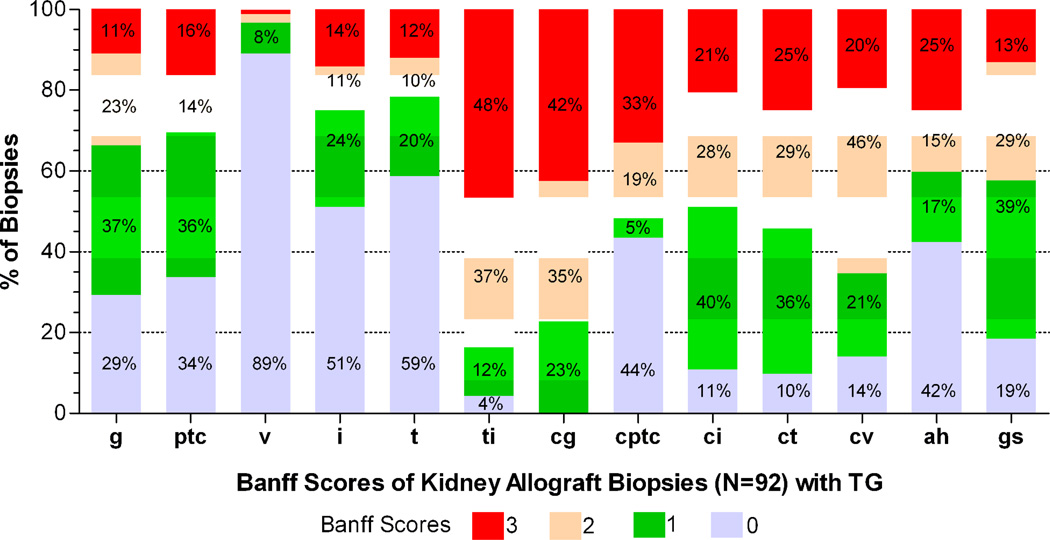
The median (inter-quartile range, IQR) time from transplantation to biopsy was 43 (16–83) months. The main reason for biopsy was an increase in serum creatinine in 64 (70%) and proteinuria in 28 (30%) patients. At the time of biopsy, serum creatinine was 2.75 (2.15–4.14) mg/dl, and the proteinuria was >1 g per day in 63 (69%) patients. The distribution of the histopathological features is shown in Figure 1. Sixty-nine of the 92 patients (75%) had evidence for chronic active antibody-mediated rejection; 38 with serological evidence of alloantibodies that included positive cross matches or Luminex platform-detected IgG antibodies directed against donor human leukocyte antigens (HLA); 34 with positive staining for peritubular capillary C4d and 56 with at least moderate microvascular inflammation (Banff score g+ptc≥2).
Figure 1. Histopathological characteristics of kidney transplant recipients with transplant glomerulopathy.
Stacked bar graph shows the distribution of histological scores of the 92 kidney allograft biopsies with transplant glomerulopathy from 92 kidney transplant recipients. A single pathologist (SVS) evaluated the biopsy specimens and categorized them using the Banff ’07 update of the Banff ’97 classification. The median (IQR) number of glomeruli per biopsy sample was 11 (8–17). Figure depicts the g score (glomerulitis), ptc score (peritubular capillary inflammation), v score (vascular inflammation), i score (interstitial inflammation), t score (tubulitis), ti score (total inflammation), cg score (chronic glomerulopathy), ci score (interstitial fibrosis), ct score (tubular atrophy), cv score (chronic vascular lesions) and ah score (arteriolar hyaline thickening). Also shown are cptc score (peritubular capillary basement membrane multilayering score [0: 1–2 basement membrane layers in peritubular capillaries assessed by electron microscopy, 1: 3–4 layers, 2: 5–6 layers and 3: >6 layers]), and the gs score (glomerulosclerosis score [0: ≤5% sclerosed glomeruli, 1: 6–25%, 2: 26–50% and 3: >50%]).
Treatment, follow up and clinical outcome
Patients diagnosed with TG were treated at the discretion of their transplant physician. Treatment consisted of anti-rejection therapy in 46 (50%) patients that included various combinations of high dose corticosteroids, intravenous immunoglobulin, plasmapheresis, antithymocyte globulin, rituximab and bortezomib with or without additional therapy with drugs that block the renin-angiotensin system. The other 46 (50%) patients did not receive anti-rejection therapy but were treated with addition of new or dose adjustment of their maintenance immunosuppressive medicines and drugs that block the renin angiotensin system
The primary outcome was allograft failure within 60 months following the diagnosis of TG. During a median follow up of 60 months from the diagnosis, 64 (70%) patients developed graft failure, 9 (3–26) months from the diagnosis. The 28 (30%) patients who did not have allograft failure were followed for 59 (37–60) months from the diagnosis. Time from transplantation to diagnosis was 48 (19–90) months in patients who eventually had allograft failure and was 37 (14–69) months in those who did not have allograft failure.
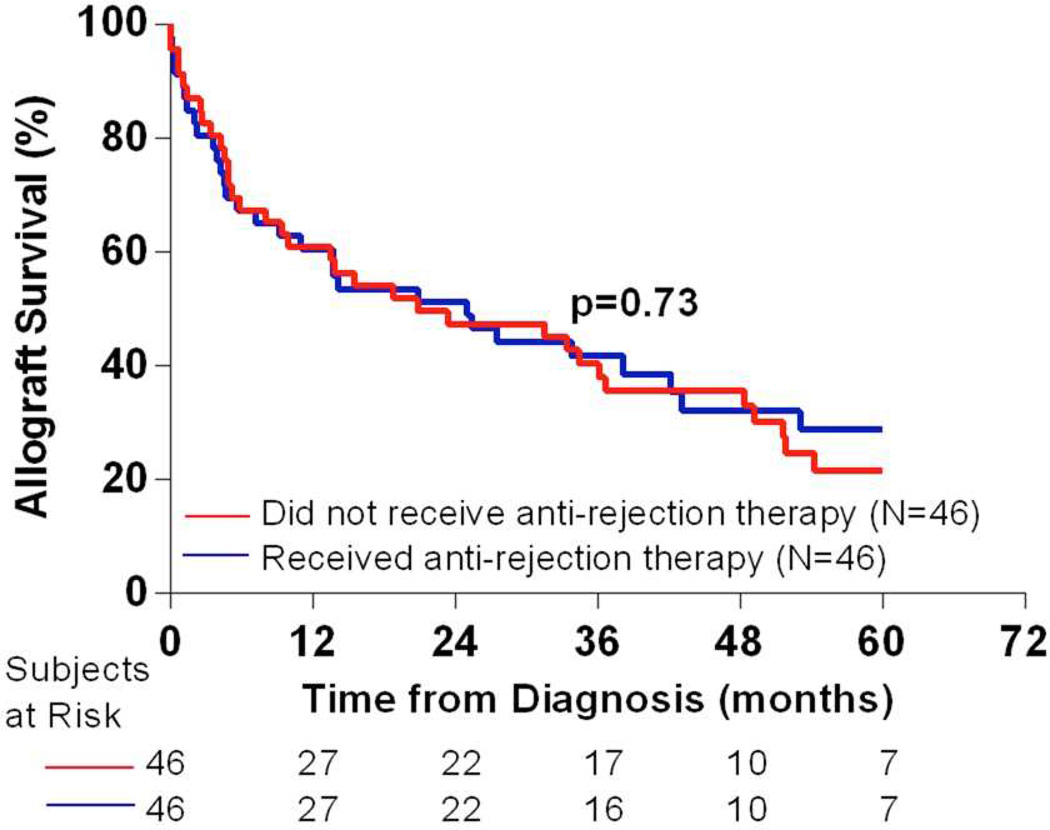
There was no difference in the outcome between patients who did and who did not receive anti-rejection therapy (Figure 2). Because patients were not randomly assigned to the two groups (anti-rejection therapy versus no anti-rejection therapy), we did propensity score (PS) analysis to mimic a quasi-randomized trial using the observational data. We used the baseline variables to generate PS, defined as the probability of receiving anti-rejection therapy. The resulting concordance index of the model was 0.89 suggesting that the model discriminated the two groups well. We then used the PS as a covariate in the Cox analysis. The difference in allograft outcome between the two groups, adjusted for the PS, was not statistically significant (hazard ratio [HR] 0.89, 95% confidence interval [0.45–1.76], p=0.74).
Figure 2. Allograft survival in patients who did and who did not receive anti-rejection therapy after the diagnosis of transplant glomerulopathy.
Kaplan-Meier survival curves for patients with transplant glomerulopathy stratified by their treatment status. The allograft survival probabilities of the two groups compared by Mantel-Cox log-rank test were not statistically different.
Statistical analyses of risk factors
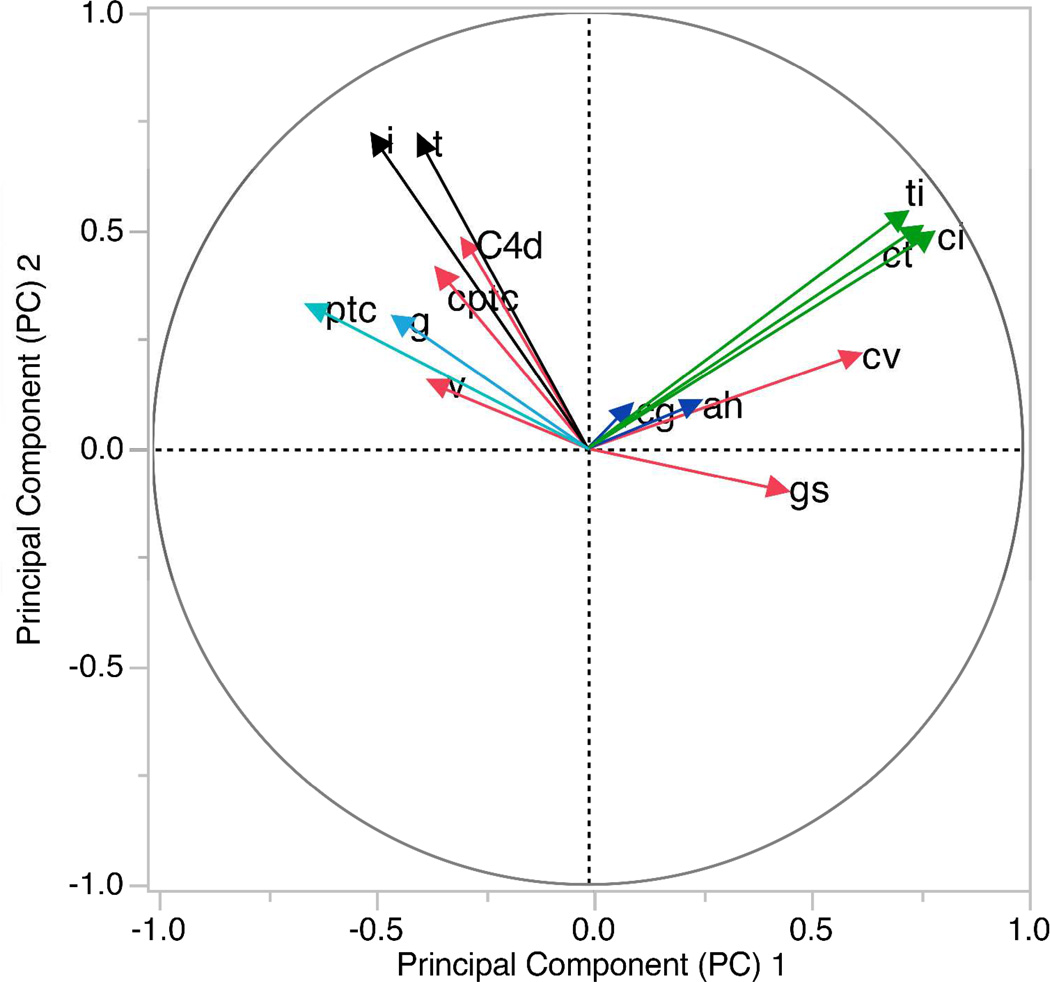
We included 19 variables at the time of the index biopsy in a univariate Cox regression analysis to determine the association of each variable with the allograft outcome. Six variables; serum creatinine, proteinuria, Banff scores t, ci and ct, as well gs score were statistically significant at p<0.1 (Table 2). Next, as a data reduction technique, we did principal component analyses (PCA) of 14 histopathology variables (Figure 3). Banff scores ci, ct and ti were loaded heavily on principal component (PC) 1. Hence we combined them and created a new variable (chronic-inflammation score, 0–9). As Banff scores i and t were loaded heavily on PC2, we combined both and created a new variable (acute-tubulointerstitial score, 0–6). Banff scores ah and cg that loaded on PC3 was combined as a new variable (chronic-arteriolar score, 1–6).
Table 2.
Association of individual variables with the allograft outcome determined by univariate Cox regression analyses
| Variable | Reference Group | Unit Change | Hazard Ratioa |
95% Confidence Interval |
p-value |
|---|---|---|---|---|---|
| Recipient age, years | - | Increase by 1 year | 1.00 | 0.98–1.02 | 0.75 |
| Time from transplant to biopsy, months | - | Increase by 1 month | 1.05 | 0.92–1.19 | 0.46 |
| Calendar year of diagnosis | Year 2011 | Decrease by 1 calendar year | 0.99 | 0.91–1.09 | 0.92 |
|
Serum creatinine mg/dl |
- | Increase by 1 mg/dl | 1.33 | 1.22–1.46 | <0.001 |
|
Proteinuria grams/day |
<1 gram/day | >1 gram/day | 1.87 | 1.05–3.32 | 0.03 |
| Glomerular inflammation (g score) |
Score=0 | Increase in score by 1 | 0.90 | 0.69–1.17 | 0.45 |
| Interstitial inflammation (i score) |
Score=0 | Increase in score by 1 | 1.15 | 0.91–1.44 | 0.24 |
|
Tubulitis (t score) |
Score=0 | Increase in score by 1 | 1.23 | 0.99–1.54 | 0.06 |
| Vascular inflammation (v score) |
Score=0 | Increase in score by 1 | 1.04 | 0.66–1.65 | 0.87 |
| Peritubular capillary inflammation (ptc score) |
Score=0 | Increase in score by 1 | 1.00 | 0.79–1.23 | 0.98 |
| Chronic glomerulopathy (cg score) |
Score=1 | Increase in score by 1 | 1.01 | 0.74–1.39 | 0.93 |
|
Interstitial fibrosis (ci score) |
Score=0 | Increase in score by 1 | 1.38 | 1.04–1.83 | 0.02 |
|
Tubular atrophy (ct score) |
Score=0 | Increase in score by 1 | 1.35 | 1.03–1.79 | 0.03 |
| Peritubular capillary basement membrane multilayering (cptc score) |
Score=0 | Increase in score by 1 | 0.86 | 0.70–1.04 | 0.12 |
| Total inflammation (ti score) |
Score=0 | Increase in score by 1 | 1.19 | 0.84–1.69 | 0.33 |
| Arteriolar hyaline thickening (ah score) |
Score=0 | Increase in score by 1 | 1.02 | 0.84–1.26 | 0.78 |
| Chronic vascular lesions (cv score) |
Score=0 | Increase in score by 1 | 1.02 | 0.78–1.33 | 0.89 |
|
Glomerulosclerosis (gs score) |
Score=0 | Increase in score by 1 | 1.28 | 0.97–1.69 | 0.08 |
| C4d staining | Negative | Positive | 0.93 | 0.55–1.55 | 0.77 |
| Analyses involving subgroups of TGb | |||||
| Definite Chronic Active ABMR (N=34) vs. Suspicious Chronic Active ABMR (N=35) |
[DSA+] and [C4d+ or g+ptc≥2] | (i) [DSA+] and [C4d- and g+ptc<2] or (ii) [DSA-/na] and [C4d+ or g+ptc≥2] |
0.62 | 0.34–1.12 | 0.11 |
| Definite Chronic Active ABMR (N=34) vs. Others (N=23) |
[DSA+] and [C4d+ or g+ptc≥2] | [DSA-/na] and [C4d- and g+ptc<2] | 0.96 | 0.54–1.78 | 0.79 |
| Suspicious Chronic Active ABMR (N=35) vs. Others (N=23) |
(i) [DSA+] and [C4d- and g+ptc<2] or (ii) [DSA-/na] and [C4d+ or g+ptc≥2] |
[DSA-/na] and [C4d- and g+ptc<2] | 1.63 | 0.86–3.07 | 0.13 |
| Definite/Suspicious Chronic Active ABMR (N=69) vs. Others (N=23) |
(i) [DSA+] and [C4d+ or g+ptc≥2] or (ii) [DSA+] and [C4d- and g+ptc<2] or (iii) [DSA-/na] and [C4d+ or g+ptc≥2] |
[DSA-/na] and [C4d- and g+ptc<2] | 1.26 | 0.74–2.15 | 0.84 |
The hazard ratio is the relative hazard for a unit change in the variable from the reference value. Variables that were statistically significant at a p-value of <0.1 are shown in bold.
Analysis of subgroups of TG based on their presumed etiology. Banff classification of chronic, active antibody-mediated rejection (ABMR) require all of the following three features to be present for the diagnosis; (i) Morphological evidence of chronic tissue injury (cg>0), (ii) Evidence of current/recurrent antibody interaction with vascular endothelium including linear C4d staining in peritubular capillaries or at least moderate microvascular inflammation (g+ptc≥2), and (iii) serological evidence of DSAs against HLA or other antigens. However, for the purpose of data analysis, similar to the criteria for acute/active ABMR, we categorized patients as definite chronic active ABMR when both ii and iii above was present along with i (by our inclusion criteria, all 92 patients had cg>0), or as suspicious chronic active ABMR when either ii or iii above was present along with i. We considered patients as DSA+ when they had evidence for circulating alloantibodies; (i) positive CDC or flow cytometry cross match or (ii) Luminex platform-detected circulating donor-specific anti-HLA IgG antibodies.
Figure 3. Principal component analysis of histopathological variables.
We did Principal Component Analysis (PCA) of 14 histopathological variables. The goal here was to identify variables that were closely associated with one another, so as to combine them as a single variable. In PCA a set of few new variables called principal components (PC) are generated that still reflects a large proportion of the information contained in the original dataset. Each PC is perpendicular to one another in a multidimensional space and thus is independent and uncorrelated. We extracted the first three PC that altogether explained 54% of the total variance. A two dimensional loading plot of PC1 and PC2 is depicted. PC1 explained 26% of the total variance and PC2 explained 17% of the total variance. Variables with the highest loading on PC1 (green) were Banff ci score, ct score and ti score. The correlation coefficient between the PC1 and ci score was 0.78, ct score was 0.75 and ti score was 0.72. Variables with the highest loading on PC2 (black) were Banff i score and t score. The correlation coefficient between the PC2 and i score was 0.71, and t score was 0.70. The variables with highest loading on PC3 (blue) were Banff ah score (0.62) and cg score (0.61). Based on these results we combined ci, ct and ti scores and created a new variable (chronic-inflammation score, 0–9). We combined t and i scores and created a new variable (acute-tubulointerstitial score, 0–6). We also combined ah and cg scores and created a new variable (chronic-arteriolar score, 1–6). These three new variables were included in the multivariate Cox proportional hazard analyses. We did PCA using JMP 11.0 (SAS Institute Inc., Cary, NC) software.
Next, based on criteria provided in Table 2, we categorized patients into three subgroups; definite chronic active antibody-mediated rejection (N=34), suspicious chronic active antibody-mediated rejection (N=35), and others (N=23), and analyzed if the etiology of TG was associated with outcome. Median time from transplantation to the diagnosis of TG was 31 months in patients with definite/suspicious chronic active antibody-mediated rejection while it was 76 months in the others (p<0.01). There was no statistically significant difference in outcome among the three subgroups. We then analyzed the 37 (40%) patients who had data available on circulating donor HLA-specific IgG antibodies (DSA), detected at the time of diagnosis by Luminex platform. For each patient, DSA results were entered in the statistical model in three different forms; presence (mean fluorescence intensity [MFI] >1000) or absence of DSA (dichotomous variable), MFI value of the highest rank donor-specific bead (continuous variable) or the sum of all MFI values (continuous variable). Our analyses revealed that DSA was not independently associated with allograft outcome (presence of DSA: N=26, HR 1.74 [0.64–4.68], p=0.28; highest MFI: median 7220, HR 1.01 per 1000 MFI [0.94–1.09], p=0.73; sum of all MFI: median 11471, HR 1.01 per 1000 MFI [0.98–1.03], p=0.60). Prior biopsy-confirmed acute rejection (N=23, 25%) or hepatitis C virus (HCV, N=15, 16%) did not influence allograft outcome. Ten of the 15 (67%) patients with HCV had features of definite or suspicious chronic active antibody-mediated rejection and none had immune complex deposits or thrombotic microangiopathy in the biopsy.
We did multivariate Cox proportional hazard regression analyses on six variables selected based on the results of univariate Cox analysis (serum creatinine, proteinuria and glomerulosclerosis score) or PCA (acute-tubulointerstitial score, chronic-inflammation score and chronic-arteriolar score) (Table 3). Creatinine (HR 1.35 [1.22–1.49], p<0.001), proteinuria (HR 1.62 [0.90–2.92], p=0.10) and chronic-inflammation score (HR 1.13 [1.02–1.25], p=0.01) constituted the final model. We confirmed the proportionality assumption of the Cox model by global test and by visual inspection of Schoenfeld residual plots.
Table 3.
Independent risk factors for allograft failure within 5 years after the diagnosis of transplant glomerulopathy
| Variable | Reference Category |
Unit Change |
Full Modela | Final Modelb | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio |
95% Confidence Interval |
p- value |
Hazard Ratio |
95% Confidence Interval |
p value |
|||
| Serum creatinine at biopsy, mg/dl | - | Increase by 1 mg/dl |
1.33 | 1.19–1.47 | <0.001 | 1.35 | 1.22–1.49 | <0.001 |
| Proteinuria at biopsy, grams/day | <1 gram/day |
>1 gram/day |
1.73 | 0.94–3.20 | 0.07 | 1.62 | 0.90–2.92 | 0.10 |
| Acute-tubulointerstitial score, i+t scores | i+t score=0 |
Increase in score by 1 |
1.04 | 0.91–1.19 | 0.49 | |||
| Chronic-inflammation score, ci+ct+ti scores | ci+ct+ti score=0 |
Increase in score by 1 |
1.12 | 1.01–1.24 | 0.03 | 1.13 | 1.02–1.25 | 0.01 |
| Chronic-arteriolar score, ah+cg scores | ah+cg score=1 |
Increase in score by 1 |
0.94 | 0.79–1.12 | 0.52 | |||
| Glomerulosclerosis score, gs score | Gs score=0 |
Increase in score by 1 |
1.12 | 0.81–1.55 | 0.45 | |||
Based on the results of the univariate Cox analyses (Table 2) and principal component analysis (Figure 3), we included six variables in a multivariate Cox proportional hazard regression analyses.
In order to develop a prognostic index for allograft failure, we developed a final model from the full model. For this purpose, we sequentially removed each variable from the full model and assessed the change in model fit by the Likelihood-ratio test to test the significance of the individual variables controlling for all other variables. We chose a p-value of <0.1 by the Likelihood-ratio test, to assess the change in model fit. Removal of acute-tubulointerstitial score, chronic-arteriolar score or glomerulosclerosis score from the full model did not impact the model (p>0.1), whereas removal of serum creatinine, proteinuria or chronic-inflammation score impacted the model (p<0.1). Hence, we retained serum creatinine, proteinuria, and chronic-inflammation score in the final model. We confirmed the proportionality assumption of the final Cox model by the global test and by visual inspection of the Schoenfeld residual plots. The coefficients (ln[Hazard Ratio]) of serum creatinine (0.29), proteinuria (0.48) and chronic-inflammation (0.12) in the final model constituted the prognostic index represented by the equation: (0.29*serum creatinine)+(0.48*proteinuria)+ (0.12*chronic-inflammation score).
Development of a prognostic index and generation of risk groups
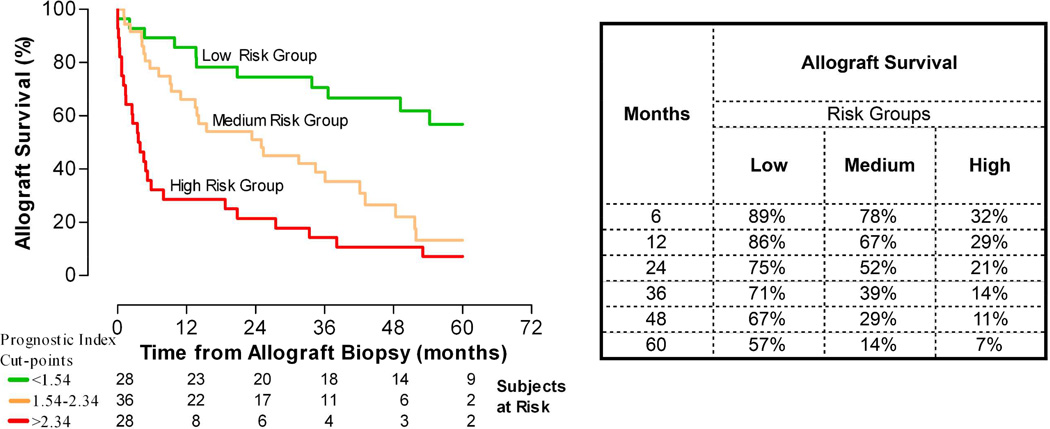
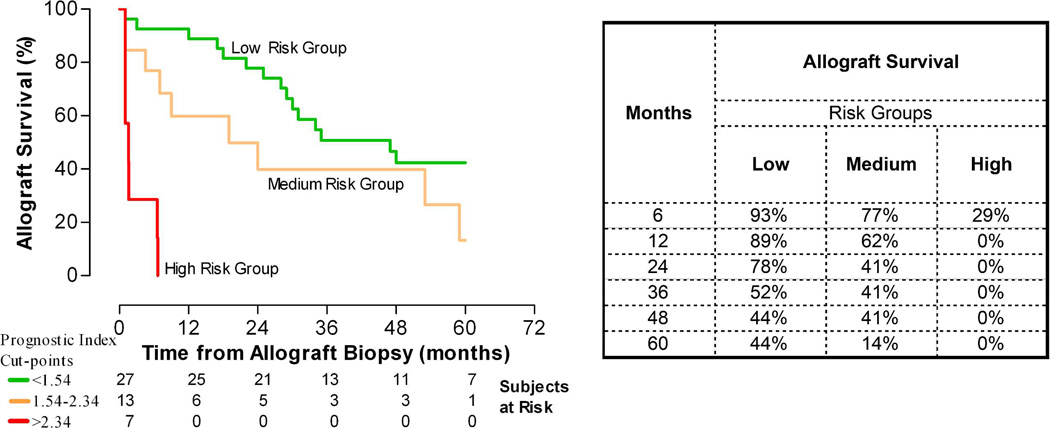
We developed a prognostic index (PI) for each patient from the linear predictor of the final Cox model. The PI was represented by the equation: (0.29*serum creatinine)+(0.48*proteinuria)+ (0.12*chronic-inflammation score). We did 10-fold cross validation of the model. Based on the cross validated estimates of the PI we arbitrarily divided the entire cohort into three risk groups of allograft failure; low risk (<30th percentile of the PI, cut off: 1.54), medium risk (30th-70th percentile) and high risk (>70th percentile of the PI, cut off: 2.34). The median allograft survival was >60 months from the diagnosis for the low risk group, 25 months and 3.7 months, respectively, for the medium and high risk groups (Figure 4). The HR for allograft failure in the medium and high risk groups were 2.83 (1.39–5.75) and 5.96 (2.91–12.19), respectively, compared to the low risk group.
Figure 4. Kaplan-Meier analysis-estimated probability of allograft survival of the three risk groups of transplant glomerulopathy.
We used the linear predictor from the final Cox model to construct a prognostic index (PI) for each patient. Based on the PI we divided arbitrarily the entire cohort into three risk groups for allograft failure; low risk (<30th percentile of the PI, cut off: 1.54), medium risk (30th-70th percentile) and high risk (>70th percentile of the PI, cut off: 2.34). Figure depicts the Kaplan-Meier estimated probability of allograft survival for the three risk groups. The median allograft survival was >60 months from the diagnosis of TG for the low risk group, 25 months from the diagnosis for the medium risk group and 3.7 months from the diagnosis for the high risk group. The hazard ratios for allograft failure were 2.83 (1.39–5.75) and 5.96 (2.91–12.19) for the medium and high risk groups, respectively, compared to the low risk group. Table depicts the estimated allograft survival at various time points after the diagnosis.
Performance of the prognostic index in an independent external cohort
The Validation cohort included 47 clinically indicated kidney allograft biopsies from 47 kidney transplant recipients with TG from the Henri Mondor Hospital, Créteil, France (Supplementary Table 1). Criteria for diagnosis, follow up and outcome was similar to that of the Development cohort. Median follow up was 60 months from the diagnosis. Thirty-one (66%) patients developed graft failure at 17 (2–30) months from the diagnosis. The 16 (34%) patients who did not have allograft failure were followed for 60 (41–60) months from the diagnosis. We applied the same model (equation) generated in the Development cohort to the patients in the Validation cohort to calculate the PI and used the same cut off values to define the three risk groups in the Validation cohort (Supplementary Figure 1). The median allograft survival was 47, 19 and 1.6 months for the three risk groups (Figure 5). The HR was 2.18 (0.94–5.02) and 16.27 (4.62–57.28) for the medium and high risk groups, respectively, compared to the low risk group.
Figure 5. Kaplan-Meier analysis-estimated probability of allograft survival in an independent external cohort.
We used the same prognostic index cut off values to define the three risk groups in an independent external cohort of 47 kidney allograft recipients with transplant glomerulopathy. The median allograft survival was 47 months from the diagnosis for the low risk group, 19 months for the medium risk group and 1.6 months for the high risk group. The hazard ratios for allograft failure were 2.18 (0.94–5.02) and 16.27 (4.62–57.28) for the medium and high risk groups, respectively, compared to the low risk group. Table depicts the estimated allograft survival at various time points after the diagnosis.
Discrimination and calibration of the prognostic index model
Discrimination (separation) is the extent to which risk estimates from a model correctly distinguish two different patient prognoses. Harrell's c-index, the measure of discrimination, was 0.71 in the Development cohort and 0.78 in the Validation cohort, suggesting good discrimination of the model. Calibration is the prediction accuracy and reflects how survival probabilities from a model compare to that of actual outcome in the observed data. The p-value was 0.73 in the development cohort and 0.57 in the validation cohort, by the Likelihood ratio statistic of the added variable version of Grønnesby-Borgan test implemented in Stata software, suggesting good calibration of the model (Supplementary Figure 2).
DISCUSSION
In this largest cohort reported till date of kidney transplant recipients with TG, we generated a prognostic index and categorized patients into risk groups for allograft failure. Our model performed well in an independent external cohort of patients with TG with good discrimination and calibration. The risk factors that we have identified are traditional risk factors for any chronic kidney disease; but the novelty lies in its successful application to the most important cause of late graft failure.
In our study, the only histological feature associated with graft failure was a chronic-inflammation score that we generated, composed of Banff ci, ct and ti scores. Interstitial fibrosis and tubular atrophy (IFTA) is an end result of multiple different injuries in both native and transplant kidneys and has been consistently associated with poor kidney outcomes (10–14). In a recent analysis aimed at evaluating the histological risk factors for kidney allograft loss, the key finding was that regardless of the specific cause for chronic histological damage, the presence of IFTA had an additive and independent impact on graft outcomes including those patients with TG (2). In our analyses Banff ti score expectedly was strongly associated with ci and ct scores. The ti-score has been a better predictor of graft outcome when compared with the i-score, which assesses only the non-scarred areas of cortex (15,16). It is not surprising that none of the Banff acute scores contributed to the prognostic model, as TG is a result of chronic injury to the kidney rather than an acute process.
Transplant glomerulopathy develops due to repetitive microvasular endothelial injury that is primarily driven by alloantibodies. However, T-cell mediated injury, HCV and thrombotic microangiopathy may also contribute to its pathogenesis (6,7,17,18). Three-fourths of all our patients had evidence for definite or suspicious chronic antibody-mediated rejection and were diagnosed earlier than those who did not have such evidence. But after the diagnosis of TG, the time to graft failure was similar in both the groups, suggesting that fibrosis/inflammation, the final common pathway, determines the progression irrespective of the reasons for the development of TG. As Luminex platform became available at our center for routine use from 2008, not all patients had data available on circulating DSA detected by Luminex-based assay. However among patients who had DSA data available, allograft outcome was not statistically different between those who were positive or negative for DSA. While the presence of DSA compared with the absence of DSA in patients with TG is associated with reduced overall graft survival (19), it is controversial whether DSA continues to remain an independent risk factor for allograft outcome following the diagnosis of TG (20–23). The definition of ‘positive DSA’ in reported studies remains problematic, especially given the variability in the Luminex assays making the numerical value of any single measured mean MFI difficult to interpret (24,25). Contrary to an earlier study (4) we did not find HCV to impact the outcome of TG. None of our patients with HCV had immune complex deposits or thrombotic microangiopathy while two-thirds had features of antibody-mediated rejection, suggesting that TG in these patients was primarily as a result of injury due to alloantibodies.
There are no randomized trials for treatment of TG. Given the heterogeneity in treatment protocols, we used the propensity score approach, which mimics a quasi-randomized trial, as the best possible way to analyze the effect of anti-rejection therapy. Observational studies on anti-rejection treatment have suggested that varying combinations of IVIG, rituximab, plasmapheresis, bortezomib and steroids may improve graft outcomes (23,26,27). Our failure to show any tangible benefit of specific antirejection therapy supports our hypothesis that chronic allograft changes at the time of diagnosis dictate the overall prognosis. Moreover, despite improvement in our therapeutic armamentarium, we could not show an improvement in allograft survival over the years suggesting that we have probably not yet identified the correct target and timing for intervention.
Our analysis raises the question as to whether the key to intervention lies in earlier detection of TG before chronic injury. Occurrence of endothelial ultrastructural changes as an early marker of TG (28,29) and the association of endothelial cell transcripts with graft loss (21), taken in conjunction with our findings, suggest that the window for more effective treatment may be at the time of ultrastructural evidence of TG or alterations in endothelium-related molecular biomarkers but before the development of other traditional risk factors.
Our study has some limitations inherent to any retrospective study design. While we have identified variables that are clearly associated with allograft outcome among the plausible risk factors we chose to analyze, there may be many additional baseline and dynamic factors that are also strongly associated with allograft failure that have not been accounted for. Similarly, treatment was not standardized and the impact of a specific treatment regimen on the outcome could not be assessed. Finally, we did not have information on circulating DSA available on all patients. Our findings that DSA was not independently associated with allograft failure should not undermine the role of DSA or intervention directed against eliminating or reducing the circulating antibody burden, as it is possible that fibrosis could entirely be driven by alloantibody response. For a similar reason, our findings that Banff acute scores were not independently associated with allograft failure should not negate their role in driving the underlying chronic process.
To our knowledge this is the first study to develop a prognostic index in kidney transplant recipients with TG and to successfully validate it in an external cohort of patients. We believe that of our index will probably be useful for ascertaining the allograft prognosis for individual patients and in clinical trials designed to assess the benefit of various treatment regimens.
METHODS
Study cohort
This research, a retrospective cohort study at New York Presbyterian Hospital-Cornell and approved by our Institutional Review Board, is in accordance with the Principles of the Declaration of Istanbul. Our cohort of 92 kidney transplant recipients (Development cohort) was transplanted between 1969 and 2009. The independent external validation cohort consisted of 47 kidney allograft recipients cared for at the Henri-Mondor Hospital-Paris XII University, Créteil, France (Validation cohort). The Institutional Review Board of the Henri Mondor Hospital approved the study and its pathologist (DD), unaware of the results of the prognostic model or allograft outcome, evaluated the biopsies and categorized them using the Banff ’07 update of the Banff ’97 classification.
Clinical and laboratory data
The date of allograft biopsy was considered as the time of diagnosis of TG. Time to diagnosis was defined as the time from transplantation to the biopsy diagnosis of TG. At our center, CDC crossmatch was done on all patients whereas flow cytometry crossmatch was limited to sensitized patients. Data on DSA detected using microparticles with individual purified HLA antigens covalently bound as targets (One Lambda Inc, Canoga Park, CA) on the Luminex platform (available from 2008) were recorded.
Treatment and Outcome
Treatment with specific antirejection therapy included high dose corticosteroids with various combinations of intravenous immunoglobulin or plasmapheresis, antithymocyte globulin, rituximab, or bortezomib. Primary outcome was allograft failure following the diagnosis of TG, defined as persistent decline in estimated glomerular filtration rate of <15ml/min/1.73m2, repeat transplantation or initiation of maintenance dialysis treatment. Follow up time was defined as the time from the diagnosis to the primary outcome and was restricted to 60 months from the diagnosis. Individuals who were lost to follow up, who did not reach the primary outcome by June 2014 or who completed 60 months from the diagnosis were censored at their last follow up. In the validation cohort the follow up was similarly restricted to 60 months from the diagnosis.
Statistical analysis
To assess the impact of treatment on allograft outcome, we used PS method to mimic a quasi-randomized trial using the observational data. We defined PS as the probability of receiving anti-rejection therapy after the diagnosis of TG, conditional on observed baseline covariates. To develop the PI we first tested the statistical association of allograft outcome with 19 clinical, laboratory and histopathology variables by univariate Cox regression. We then used PCA to identify histological variables that were closely associated with one another, so as to combine them as a single variable. We extracted the first three PC. Based on the results of the PCA we combined two or more individual histological variables that loaded heavily on a PC to create a new variable. We included the variables that were statistically significant at p<0.1 in the univariate Cox regression and those that were generated from the PCA in a multivariate Cox proportional hazard regression analysis to develop a model predicting allograft outcome. We derived a PI for each patient by using the linear predictor from the Cox model, where the linear predictor is a weighted sum of the variables in the model, the weights being the regression coefficients. We did 10 fold cross validation of the model fit. Accordingly, the regression coefficients to calculate the PI for an individual patient was derived from a Cox model that did not include any data from that patient. Based on the cross validated estimates of the PI we arbitrarily divided the entire cohort into three risk groups for allograft failure and generated Kaplan-Meier survival curves. We evaluated the performance of our model in an independent external cohort of kidney transplant recipients with TG. We applied the same model (equation) generated in the Development cohort to the patients in the Validation cohort and derived a PI for each patient. We then applied the same cut off values of the PI used in the Development cohort and divided the patients in the Validation cohort into three risk groups. We assessed model discrimination by Harrell’s concordance statistic, the c-statistic modified for censored survival data and model calibration by the Likelihood ratio statistic of the added variable version of Grønnesby-Borgan test. We analyzed our data with Stata 11.2 (StataCorp., College Station, TX) and GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA) software.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Manikkam Suthanthiran for his guidance and support. We thank Dr. Joseph E. Schwartz for his advice about the statistical analysis.
Funding support:
Supported in part by an award from the National Institutes of Health (K08-DK087824) and the American Society of Transplantation (Faculty Development Grant Award) to T. Muthukumar and from the National Institutes of Health (Clinical and Translational Science Center Award UL1TR000457) to Weill Cornell Medical College
Footnotes
DISCLOSURE
None of the authors declare any relationship with companies that may have a financial interest in the information contained in this manuscript.
REFERENCES
- 1.Hanf W, Bonder CS, Coates PT. Transplant glomerulopathy: the interaction of HLA antibodies and endothelium. J Immunol Res. 2014;2014:549315. doi: 10.1155/2014/549315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naesens M, Kuypers DR, De Vusser K, et al. The histology of kidney transplant failure: a long-term follow-up study. Transplantation. 2014;98:427–435. doi: 10.1097/TP.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 3.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 4.Baid-Agrawal S, Farris AB3rd, Pascual M, et al. Overlapping pathways to transplant glomerulopathy: chronic humoral rejection, hepatitis C infection, and thrombotic microangiopathy. Kidney Int. 2011;80:879–885. doi: 10.1038/ki.2011.194. [DOI] [PubMed] [Google Scholar]
- 5.Valenzuela NM, Reed EF. Antibodies in transplantation: the effects of HLA and non-HLA antibody binding and mechanisms of injury. Methods Mol Biol. 2013;1034:41–70. doi: 10.1007/978-1-62703-493-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sis B, Campbell PM, Mueller T, et al. Transplant glomerulopathy, late antibody-mediated rejection and the ABCD tetrad in kidney allograft biopsies for cause. Am J Transplant. 2007;7:1743–1752. doi: 10.1111/j.1600-6143.2007.01836.x. [DOI] [PubMed] [Google Scholar]
- 7.Remport A, Ivanyi B, Mathe Z, et al. Better understanding of transplant glomerulopathy secondary to chronic antibody-mediated rejection. Nephrol Dial Transplant. 2014 Dec 3; doi: 10.1093/ndt/gfu371. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8.Cosio FG, Gloor JM, Sethi S, et al. Transplant glomerulopathy. Am J Transplant. 2008;8:492–496. doi: 10.1111/j.1600-6143.2007.02104.x. [DOI] [PubMed] [Google Scholar]
- 9.Banfi G, Villa M, Cresseri D, et al. The clinical impact of chronic transplant glomerulopathy in cyclosporine era. Transplantation. 2005;80:1392–1397. doi: 10.1097/01.tp.0000181167.88133.d2. [DOI] [PubMed] [Google Scholar]
- 10.Haas M. Chronic allograft nephropathy or interstitial fibrosis and tubular atrophy: what is in a name? Curr Opin Nephrol Hypertens. 2014;23:245–250. doi: 10.1097/01.mnh.0000444811.26884.2d. [DOI] [PubMed] [Google Scholar]
- 11.Bogenschutz O, Bohle A, Batz C, et al. IgA nephritis: on the importance of morphological and clinical parameters in the long-term prognosis of 239 patients. Am J Nephrol. 1990;10:137–147. doi: 10.1159/000168068. [DOI] [PubMed] [Google Scholar]
- 12.Bohle A, Wehrmann M, Bogenschutz O, et al. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract. 1991;187:251–259. doi: 10.1016/s0344-0338(11)80780-6. [DOI] [PubMed] [Google Scholar]
- 13.Cattran DC, Coppo R, Cook HT, et al. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society: The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 14.Berden AE, Ferrario F, Hagen EC, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–1636. doi: 10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- 15.Mengel M, Reeve J, Bunnag S, et al. Scoring total inflammation is superior to the current Banff inflammation score in predicting outcome and the degree of molecular disturbance in renal allografts. Am J Transplant. 2009;9:1859–1867. doi: 10.1111/j.1600-6143.2009.02727.x. [DOI] [PubMed] [Google Scholar]
- 16.Mannon RB, Matas AJ, Grande J, et al. Inflammation in areas of tubular atrophy in kidney allograft biopsies: a potent predictor of allograft failure. Am J Transplant. 2010;10:2066–2073. doi: 10.1111/j.1600-6143.2010.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husain S, Sis B. Advances in the understanding of transplant glomerulopathy. Am J Kidney Dis. 2013;62:352–363. doi: 10.1053/j.ajkd.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Haas M. Transplant glomerulopathy: it's not always about chronic rejection. Kidney Int. 2011;80:801–803. doi: 10.1038/ki.2011.192. [DOI] [PubMed] [Google Scholar]
- 19.Eng HS, Bennett G, Chang SH, et al. Donor human leukocyte antigen specific antibodies predict development and define prognosis in transplant glomerulopathy. Hum Immunol. 2011;72:386–391. doi: 10.1016/j.humimm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Lopez Jimenez V, Fuentes L, Jimenez T, et al. Transplant glomerulopathy: clinical course and factors relating to graft survival. Transplant Proc. 2012;44:2599–2600. doi: 10.1016/j.transproceed.2012.09.068. [DOI] [PubMed] [Google Scholar]
- 21.Kamal L, Broin PÓ, Bao Y, et al. Clinical, histological, and molecular markers associated with allograft loss in transplant glomerulopathy patients. Transplantation. 2015 Feb 11; doi: 10.1097/TP.0000000000000598. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.Torres IB, Salcedo M, Moreso F, et al. Comparing transplant glomerulopathy in the absence of C4d deposition and donor-specific antibodies to chronic antibody-mediated rejection. Clin Transplant. 2014;28:1148–1154. doi: 10.1111/ctr.12433. [DOI] [PubMed] [Google Scholar]
- 23.Lesage J, Noël R, Lapointe I, et al. Donor-specific antibodies, c4d and their relationship with the prognosis of transplant glomerulopathy. Transplantation. 2015;99:69–76. doi: 10.1097/TP.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 24.Friedlander R, Putheti P, Diaz E, et al. On the detection of anti-HLA antibodies using single antigen bead Luminex assay: lot-to-lot variations in MFI. Transplantation. 2013;96:e24–e26. doi: 10.1097/TP.0b013e31829c2481. [DOI] [PubMed] [Google Scholar]
- 25.Tait BD, Süsal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 26.Kim MG, Kim YJ, Kwon HY, et al. Outcomes of combination therapy for chronic antibody-mediated rejection in renal transplantation. Nephrology (Carlton) 2013;18:820–826. doi: 10.1111/nep.12157. [DOI] [PubMed] [Google Scholar]
- 27.Kahwaji J, Najjar R, Kancherla D, et al. Histopathologic features of transplant glomerulopathy associated with response to therapy with intravenous immune globulin and rituximab. Clin Transplant. 2014;28:546–553. doi: 10.1111/ctr.12345. [DOI] [PubMed] [Google Scholar]
- 28.Haas M, Mirocha J. Early ultrastructural changes in renal allografts: correlation with antibody-mediated rejection and transplant glomerulopathy. Am J Transplant. 2011;11:2123–2131. doi: 10.1111/j.1600-6143.2011.03647.x. [DOI] [PubMed] [Google Scholar]
- 29.Wavamunno MD, O'Connell PJ, Vitalone M, et al. Transplant glomerulopathy: ultrastructural abnormalities occur early in longitudinal analysis of protocol biopsies. Am J Transplant. 2007;7:2757–2768. doi: 10.1111/j.1600-6143.2007.01995.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.