Abstract
Vitamin D status has been associated with obesity, metabolic syndrome and several cancers including colon and breast. Since adipocytes express VDR and obesity is a known risk factor for cancer, vitamin D actions in adipose tissue may contribute to its cancer protective effects. In the mammary gland, signaling from adipocytes to epithelial cells is necessary for breast cancer initiation, but the impact of vitamin D on this cross-talk is unclear. To examine the role of VDR in adipose tissue, particularly in the context of the mammary gland, we crossed Vdr-flox mice with Fabp4-cre mice to generate mice with adipose-specific Vdr deletion (termed CVF mice). CVF mice and Fabp4-cre control mice (termed CN1 mice) were reared on high calcium “rescue” diets (for comparison to global VDRKO mice) or on high fat diets (to stimulate adiposity). Vdr expression was significantly reduced in adipose tissue of CVF mice compared to CN1 mice. In contrast to global VDRKO mice (which exhibit adipose atrophy), female CVF mice exhibited higher growth rates and increased visceral fat pad weight compared to control mice. Expression of Ucp1 and Pparg were elevated in white adipose tissue of CVF mice supporting these genes as Vdr targets in mature adipocytes. Adipose-specific Vdr deletion did not impair glucose tolerance or alter the weight of brown adipose tissue, liver, pancreas or bone in response to high fat feeding. In contrast to the effect of adipose-specific Vdr deletion on visceral fat pads, the weight of the subcutaneous (mammary) fat pad was not increased in high fat fed CVF female mice compared to control mice. Quantitative analysis of mammary ductal development on whole mounts and H&E stained sections indicated that adipose-deletion of Vdr significantly enhanced mammary epithelial density and branching. Collectively, these data support the hypothesis that Vdr in mature adipocytes alters the metabolic response to high fat diets and exerts anti-proliferative effects on the mammary epithelium.
Keywords: Mammary gland, adipose, western-style diet, vitamin D, VDR
INTRODUCTION
Adipocytes function in storage of energy reserves, secretion of adipokines that regulate appetite and control of thermogenesis. The major form of adipose tissue is termed white adipose tissue (WAT) and is located in subcutaneous and visceral depots. Brown adipose tissue (BAT) is a unique type of adipose tissue present in mammals that functions in body temperature regulation. The vitamin D receptor (VDR) is expressed in both WAT and BAT, and VDR knockout (VDRKO) mice exhibit reduced adiposity and resistance to weight gain when fed either low or high fat diets (1-5), supporting a role of VDR in energy metabolism. A caveat to the observed lean phenotype in VDRKO mice is that most of these studies were conducted in mice fed high dietary calcium, which has independently been shown to promote weight loss (6, 7). Furthermore, VDRKO mice exhibit additional phenotypes including alopecia and accelerated aging that could contribute to weight loss (8). In contrast to this phenotype in VDRKO mice, the majority of human studies suggest that vitamin D deficiency is correlated with obesity. In vitro experiments to address possible direct effects of the VDR ligand 1,25-dihydroxyvitamin D on adipocytes have also yielded contradictory results, with both enhancement and inhibition of adipogenesis reported in various model systems (9-12).
In addition to WAT and BAT depots, adipocytes are present within the stroma of many organs including mammary gland, pancreas, liver, and bone marrow where they contribute to normal development and physiology. Mammary adipocytes have been particularly well studied, as obesity is a known risk factor for breast cancer (13, 14) and significant cross-talk occurs between the epithelial and adipose tissue compartments in the gland (15, 16). Studies have demonstrated that adipocytes drive morphogenesis ex vivo and are essential for development of the mammary epithelium in vivo (15, 16). Cancer relevant signals released from mammary adipocytes that influence the epithelium include adipokines (leptin and adiponectin), inflammatory cytokines (IL-6), and growth factors. Since mammary adipocytes express VDR (17), we hypothesized that VDR signaling might regulate the cross-talk between adipose and epithelial compartments in the gland to promote breast cancer prevention. VDRKO mice display enhanced proliferation and branching of the mammary gland ductal network during puberty and pregnancy (18, 19), and both adipocyte and epithelial VDR have been implicated in regulation of glandular growth during puberty (20).
To investigate the role of VDR during adulthood on adiposity in general and the mammary adipose compartment in particular, we characterized the effects of chronic high fat feeding on mice with adipocyte-specific VDR deletion. In this model, the murine fatty acid binding protein 4 cre recombinase (Fabp4-cre) construct deletes VDR in the mammary adipose tissue but not in the epithelial compartment (20). A previous report demonstrated that adipose-specific VDR deletion resulted in abnormal pubertal mammary gland development (20), but no further characterization of these mice or their response to dietary manipulation has been reported. In the studies described here, we reared mice on high calcium rescue diets (for comparison to global VDRKO mice) or on high fat diets (to stimulate adiposity) and characterized body and tissue weights, glucose tolerance, and mammary gland morphology. The data support the concept that VDR in mature adipocytes suppresses post-weaning weight gain and exerts anti-proliferative effects on the mammary epithelium in female mice.
METHODS
Mice and Diets
Mice possessing loxP sites flanking exon 2 of the VDR, originally obtained from Dr. Shigeki Kato, were bred with B6.Cg-Tg(Fabp4-cre)1Rev/J mice (Jackson Laboratory, Bar Harbor, ME) which express Cre recombinase under the control of the mouse fatty acid binding protein 4 (Fabp4) promoter. This promoter is predominantly expressed during differentiation of adipocytes and is frequently used for adipose-specific tissue engineering (21, 22). All mice were bred on the C57Bl/6 background. The phenotype of the adipose-specific Cre-Vdr-Flox animals (termed CVF) was compared to that of the Fabp4-cre strain (termed CN1). It is important to note that CVF mice did not present with phenotypic rickets or bone defects when fed standard rodent diets, negating the necessity for the high calcium rescue diet and enabling the use of high fat western-style diets commonly used for studies of obesity. At weaning, males and females of each genotype were given free access to either a western style diet containing 40% fat by energy or 23% fat by weight (#D12079B, Research Diets, New Brunswick, NJ) or the high calcium rescue diet containing 16% fat by energy or 7% fat by weight (Research Diets #D10070302) which is commonly used for maintenance of global VDRKO mice. Body weights and food intake were assessed as described (2) and mice were sacrificed between the ages of 7.5-11 months. All procedures were conducted under protocols approved by the University at Albany IACUC.
Glucose Tolerance Testing
After a 6 h fast, adult mice between the ages of 7.5-11 months were weighed and baseline blood glucose was measured with the Alpha Trak 2 Blood Glucose Monitoring System. Mice were injected intraperitoneally (ip) with a 10% D-glucose solution (10 μl/g body weight) and blood glucose readings were taken at 15, 30, 60, and 120 min post-injection. Data was plotted as a percentage of baseline reading and the SUMPRODUCT function in Microsoft Excel was used to implement the trapezoidal rule of approximating the definite integral and calculate area under the curve as a measure of glucose tolerance.
Serum and Tissue Analyses
At necropsy, blood removed by cardiac puncture was collected in serum separator tubes for analysis of 25-hydroxyvitamin D (25D; 25-OH Vitamin D ELISA Assay Kit, Eagle Biosciences) and leptin (Mouse Leptin ELISA Kit, Sigma-Aldrich). Tissues were weighed and processed for histology or molecular analyses as follows: liver, left inguinal mammary gland, abdominal WAT, intrascapular BAT, femur, and pancreas were fixed in formalin, paraffin embedded, and stained with hematoxylin and eosin (H&E); right inguinal mammary glands were fixed in Carnoy's fixative for whole mounting as described (19) and analyzed for ductal outgrowth, epithelial density, and mammary branching. Images were processed using the ImageJ Cell Counter tool and terminal ductal branch points within the field of view were manually counted. Portions of WAT, BAT, and intact thoracic mammary glands were also snap frozen in liquid nitrogen for RNA extraction using Tri Reagent (Sigma-Aldrich) and RNA easy columns (Qiagen) according to manufacturer's directions. qPCR with a primer set directed against exon 2 of the mouse Vdr was used to determine the degree of Vdr knockdown in abdominal WAT. Other genes evaluated by qPCR included Fabp4, Pparg, Ucp1, Esr, ProgR, Acaca, Leptin, Dok1 and Steap4.
Statistical Analysis
Data are expressed as mean ± standard deviation. Differences between means were assessed with GraphPad Instat or Prism 5 by unpaired Student's t-test or one-way ANOVA with the Bonferroni multiple comparisons post-test. For data with unequal variances, the unpaired t test with Welch's correction was utilized. Diet-genotype interactions were assessed by two-way ANOVA. Slopes of growth curves were compared by linear regression analysis with Graph Pad Prism. In all cases, p<0.05 was considered statistically significant.
RESULTS
Confirmation of VDR deletion in adipose tissue
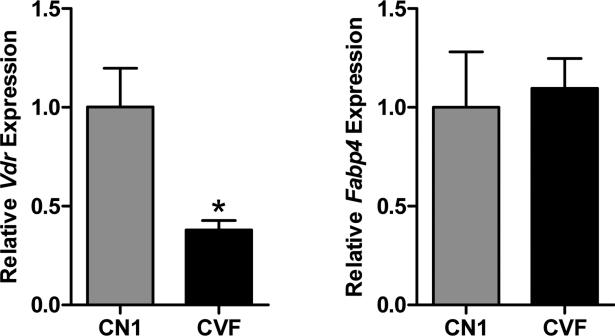
The efficiency of VDR ablation within the adipose tissue was assessed by qPCR of RNA isolated from the abdominal (visceral) WAT collected from CVF and CN1 mice fed the high fat diet. As shown in Figure 1, Vdr expression was detectable but significantly lower in tissue from CVF mice compared to that of CN1 mice. Equivalent expression of Fabp4 (the promoter of which is used to drive Cre expression in this model) was observed. The residual Vdr mRNA detected in adipose tissue from CVF mice likely reflects that of undifferentiated mesenchymal stem cells and preadipocytes that do not express Fabp4 as well as non-adipose cell types known to be present in WAT such as endothelial and immune cells (23). It is worth noting that high fat diets such as those used here promote accumulation of macrophages, dendritic cells and T cells (all of which express VDR) in adipose tissue (24). Previous studies have utilized western blotting to demonstrate that the VDR protein is undetectable in the subcutaneous adipose tissue of CVF mice fed standard rodent chow (20), supporting the validity of this model to test the role of VDR in adipocytes.
Figure 1. Vdr and Fabp4 expression in adipose tissue from CN1 and CVF mice.
Quantitative PCR analysis of Vdr and Fabp4 expression in visceral white adipose tissue from 9-10 month old CN1 (control) mice and CVF mice (lacking functional VDR in adipose tissue) maintained on the high fat diet. Data were analyzed by comparative CT method, normalized to 18S and expressed relative to CN1 expression. Bars represent mean +/− standard error of 5-6 samples per genotype. Statistical difference was calculated by Student's t-test and indicated by asterisk.
Adipose-specific VDR deletion enhances post-pubertal body weight gain
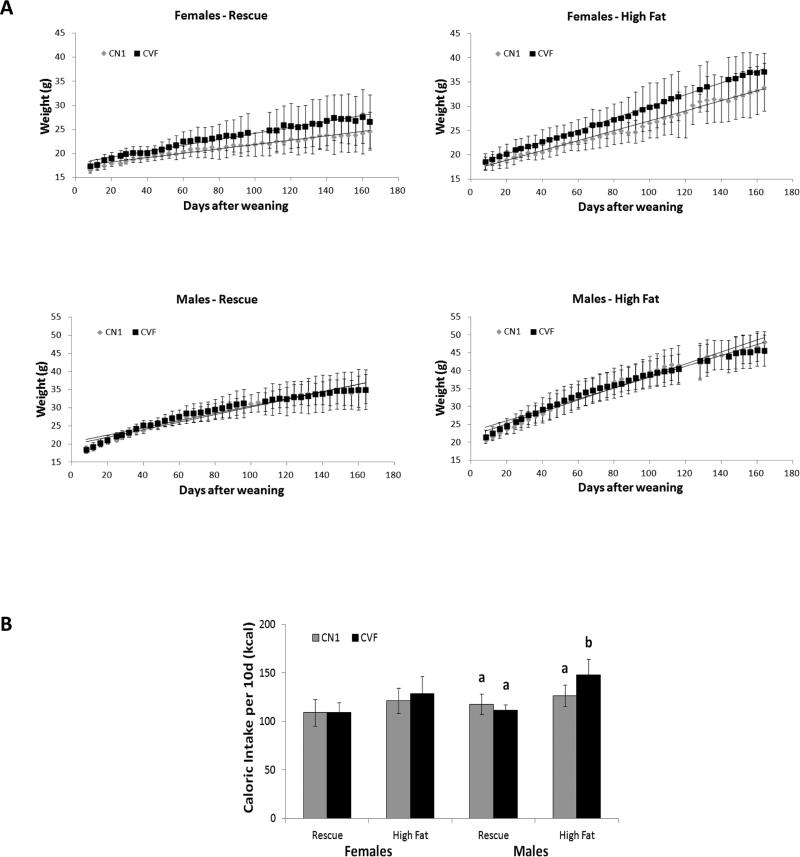
To provide insight into the role of VDR signaling in adipocytes in body weight homeostasis, we compared CVF and CN1 transgenic mice reared on either high fat diet (40% fat by energy) or rescue diet (16% fat by energy). Studies with the rescue diet were included to facilitate comparison of CVF mice to published work on the global VDRKO mice which are fed this rescue diet for optimal growth. At weaning, there were no significant differences in body weight between male or female CN1 and CVF mice (data not shown). Post-weaning body weight gain for both genotypes on the two diets, stratified by gender, is shown in Figure 2A. All animals raised on high fat diet (regardless of genotype or sex) demonstrated increased body weight gain compared to animals on the lower fat high calcium rescue diet. In addition, female CVF mice gained weight at an accelerated rate compared to CN1 mice on both high fat and rescue diets. There were no differences in weight gain patterns between male CVF and CN1 mice on either diet, indicating that the function of VDR in adipose tissue may be gender-specific. We measured food consumption over a 10 day period to determine whether the growth patterns correlated with changes in caloric intake (Figure 2B). In females, there were no significant effects of genotype on caloric intake indicating that the enhanced weight gain in CVF females was not associated with increased food consumption suggesting enhanced feed efficiency. Caloric intake was increased in CVF males relative to CN1 males on the high fat diet, although the significance of this is unclear since there was no effect of genotype on male weight gain. Future studies would be of interest to examine energy expenditure in CN1 vs. CVF males in response to high fat feeding as previous studies with global VDRKO mice have implicated VDR as a regulator of Ucp1 and energy expenditure.
Figure 2. Post-weaning body weight gain and caloric intake of CN1 and CVF mice.
A, Temporal body weight gain of CN1 and CVF mice weaned onto high calcium rescue (left) or high fat (right) diets (available ad libitum). Points and error bars represent mean +/− standard deviation of 4-12 animals per datapoint. Statistical significance at p<0.05 was determined by linear regression slope comparison of growth rates. B, Caloric intakes of CN1 and CVF mice fed high fat or rescue diets were measured over a 10 day period. Total kcals consumed was calculated and expressed as mean +/− standard deviation of 6-10 mice per group. Statistical analysis was performed via ANOVA for each gender, and significant differences (p< 0.05) between diet or genotype groups are indicated by different letters over the bars.
Impact of adipose-specific VDR deletion on abdominal white adipose tissue
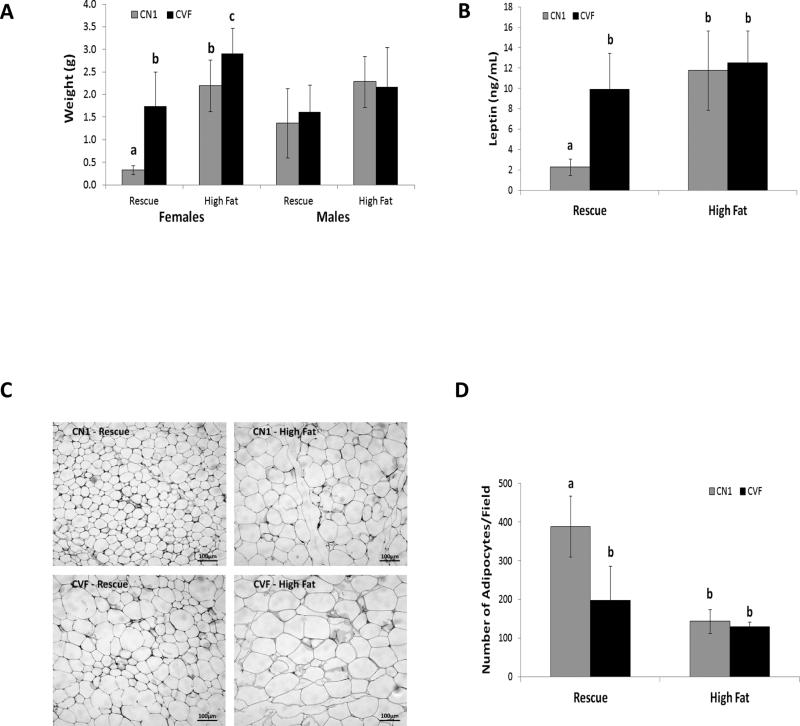
We next addressed the effect of adipose-specific VDR ablation on mass and histology of abdominal WAT, a representative visceral fat depot. In females of both genotypes, abdominal WAT mass increased in response to high fat feeding whether expressed as absolute values (Figure 3A) or as a percentage of body weight (not shown). Consistent with their accelerated body weight gain, abdominal WAT mass was significantly increased in CVF female mice compared to CN1 mice on both rescue and high fat diets. In males, WAT mass was not significantly increased in response to high fat feeding and was not affected by genotype on either diet. Consistent with the fat mass changes, serum leptin (an adipokine that regulates satiety and is positively correlated with adiposity) was significantly elevated in CVF females compared to their CN1 counterparts on the rescue diet (Figure 3B). CN1 and CVF females exhibited equivalent hyperleptinemia on the high fat diet. Genotype did not affect serum leptin in males (data not shown). Histological analysis of WAT (Figure 3C) suggested larger adipocytes in visceral fat from CVF vs CN1 females on the rescue diet but not on the high fat diet. Quantitative assessment of adipocyte size (Figure 3D) confirmed significantly fewer cells per field in CVF mice fed rescue diet and in high fat fed mice of both genotypes compared to CN1 mice fed rescue diet.
Figure 3. Characterization of visceral adipose tissue from CN1 and CVF mice.
A, Weight of abdominal adipose tissue in CN1 and CVF mice fed rescue or high fat diets. B, Serum leptin in CN1 and CVF females measured by ELISA at sacrifice. C, Histology of visceral adipose tissue from female CN1 and CVF mice was assessed on formalin-fixed, paraffin embedded, H&E stained sections. Representative images are shown from 3-7 mice per group. D, Adipocyte density was measured on H&E stained sections of visceral adipose tissue from females and expressed as number of adipocytes per field. For A, B and D, data represent mean +/− standard deviation of 3-9 mice per group (ages 7.5-10 months); statistical analysis was performed via ANOVA for each gender, and significant differences (p< 0.05) between diet or genotype groups are indicated by different letters over the bars.
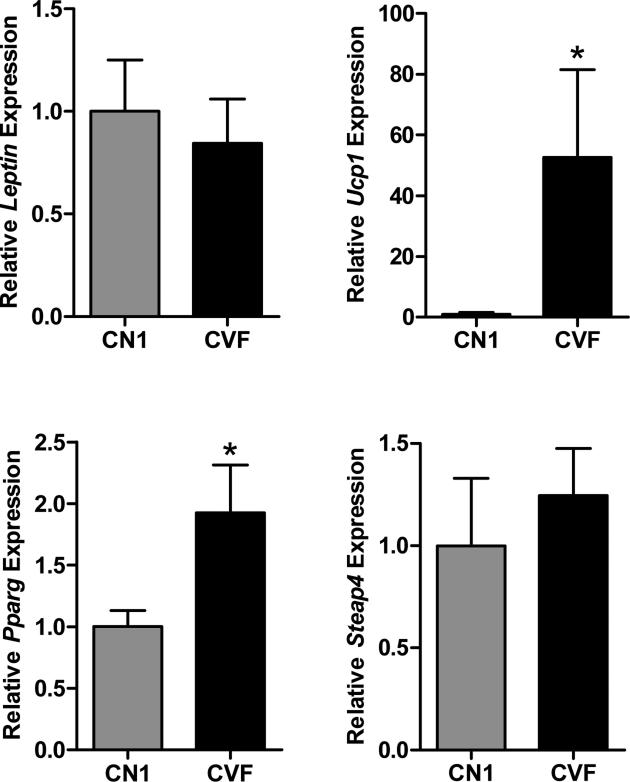
Expression of select adipogenesis-associated genes was assessed in abdominal adipose tissue from 9-10 month old CN1 and CVF mice fed the high fat diet (Figure 4). Consistent with comparable serum leptin in CVF and CN1 mice on the high fat diet, Leptin gene expression was equivalent in adipose tissue. In contrast, the brown fat identity gene Ucp1 which is repressed by Vdr (25, 26) was significantly elevated in white adipose tissue of CVF mice. The dramatic increase in Ucp1 (>40 fold higher than that of CN1 mice) is consistent with previous observations in white adipose tissue of global VDRKO mice (2). Pparg gene expression was two-fold elevated in white adipose tissue of CVF mice compared to CN1 mice, consistent with data from various model systems that VDR represses Pparg (12, 27-29). Other genes previously implicated as VDR targets relevant to adipogenesis including Steap4, Esr, Dok1 and Acaca were not altered by genotype (Figure 5 and data not shown).
Figure 4. Gene expression in visceral adipose tissue from CN1 and CVF mice.
Quantitative PCR analysis of Leptin, Ucp1, Pparg and Steap4 expression in visceral white adipose tissue from CN1 and CVF mice aged 9-10 months and maintained on the high fat diet. Data were analyzed by comparative CT method, normalized to 18S and expressed relative to CN1 expression. Bars represent mean +/− standard error of 5-6 samples per genotype. Statistical difference was calculated by Student's t-test and indicated by asterisk.
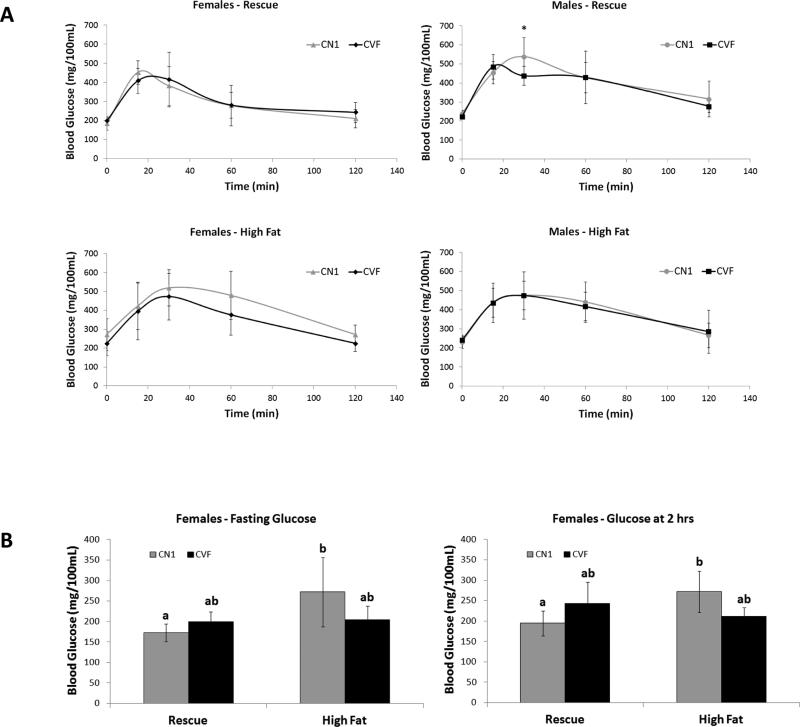
Figure 5. Glucose tolerance of CN1 and CVF mice on high fat and rescue diets.
CN1 and CVF mice fed rescue or high fat diet were fasted for 6 hours prior to ip administration of 10% glucose solution. Blood glucose was measured from tail prick at fasting and over a 2 hr period following glucose bolus. A, Blood glucose plotted over the 2 hr time course; data points represent mean +/− standard deviation of 5-9 mice per group. B, Two-way ANOVA detected significant interaction between diet and genotype for fasting and 2 hr blood glucose levels. High fat diet significantly increased glucose levels only in CN1 mice.
Adipose-specific VDR ablation does not impair glucose tolerance
Given the published literature linking high fat diets and vitamin D deficiency to obesity and metabolic syndrome (30-32) as well as reports of impaired insulin secretion in global VDRKO mice (33), glucose tolerance testing was performed to determine whether loss of VDR in adipocytes and associated increased body fat impacted systemic glucose handling. Both fasting blood glucose and clearance of an ip glucose load were measured. In response to the ip glucose load, blood glucose peaked after 15-30 minutes and returned close to baseline within 2 hours in all mice (Figure 5A). There were no significant differences in glucose peak, clearance rate or overall area under the curve between genotypes on either diet (data not shown). Fasting blood glucose was not affected by genotype, although two way analysis of variance indicated a significant diet-genotype interaction. For both fasting and 2 hr glucose data (Figure 5B), female CN1 mice exhibited increased mean blood glucose levels in response to the high fat diet, an effect not observed in CVF mice. Thus, despite the increased fat mass, CVF mice do not develop impaired glucose tolerance characteristically induced by a high fat diet. These data indicate that VDR in mature adipocytes is not required for systemic glucose handling in response to high fat diets.
Adipose-specific VDR deletion has minimal effects on BAT and other metabolic organs
Weights of other metabolic organs (BAT, liver, pancreas) and bone (femur) for female mice on both diets are presented in Table 1. On the rescue diet (but not on the high fat diet) BAT, liver and femur were larger in CVF mice than CN1 mice consistent with the observed body weight differences. Pancreatic weight was not significantly altered by genotype or diet. Initial histological examination of H&E stained sections of these organs did not identify any gross abnormalities (such as fatty liver) in tissues from CVF mice.
Table 1.
Organ weights1 in female CN1 and CVF mice fed rescue or high fat diets.
| Rescue Diet | High Fat Diet | |||||
|---|---|---|---|---|---|---|
| Organ | CN1 | CVF | p-value | CN1 | CVF | p-value |
| BAT2 | 0.063 ± 0.013 | 0.152 ± 0.035 | <0.001 | 0.190 ± 0.013 | 0.218 ± 0.055 | NS |
| Liver | 1.089 ± 0.103 | 1.498 ± 0.268 | 0.009 | 1.945 ± 0.275 | 2.089 ± 0.576 | NS |
| Pancreas | 0.192 ± 0.034 | 0.189 ± 0.056 | NS | 0.150 ± 0.057 | 0.181 ± 0.047 | NS |
| Femur3 | 0.091 ± 0.005 | 0.117 ± 0.015 | 0.006 | 0.108 ± 0.020 | 0.131 ± 0.044 | NS |
Fresh organ weights (g) for female CN1 and CVF mice on rescue and high fat diets, expressed as mean +/− standard deviation. Statistical analysis was performed via t-test and p values are indicated.
Intrascapular brown adipose tissue was cleaned of adjacent white adipose tissue and muscle.
Whole bone was cleaned of muscle.
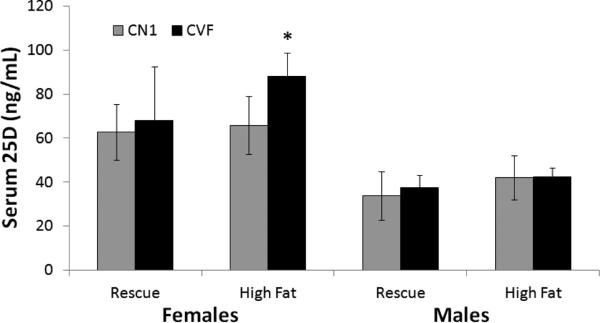
Gender and genotype affect serum 25D levels
Since some studies suggest that adipose tissue serves as a storage depot for vitamin D (34-36), it was of interest to determine whether high fat feeding or VDR deletion in adipocytes would alter circulating 25D. In female CN1 mice, serum 25D averaged approximately 60 ng/mL and was not altered by diet (Figure 6). In CVF females, serum 25D was comparable to that of CN1 females when on the rescue diet, but was significantly increased when fed the high fat diet. In males, mean serum 25D values were consistently lower than in females and were not significantly altered by diet or genotype. These data indicate that VDR in adipocytes may regulate 25D uptake or metabolism in response to high fat feeding in a gender-specific manner.
Figure 6. Circulating 25D levels in CN1 and CVF mice on high fat and rescue diets.
Serum collected at sacrifice from CN1 and CVF mice was analyzed for total 25D by ELISA. Bars represent mean +/− standard deviation of 5-7 mice per group. Statistical analysis was performed with Student's t-test and significant differences (p<0.05) between genotypes are indicated by asterisk.
Impact of adipose-specific VDR deletion on inguinal adipose tissue and mammary epithelium
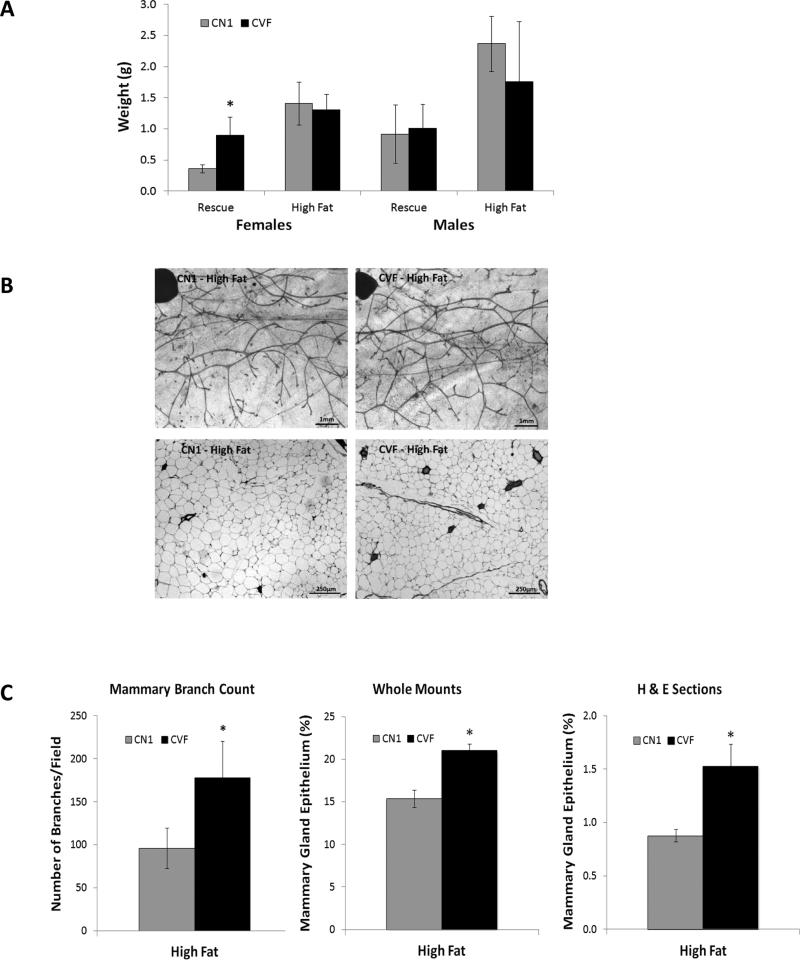
As reported above, female CVF mice demonstrated weight gain and larger visceral fat depots when fed either the rescue diet or the high fat diet compared to CN1 control mice. Since evidence suggests that visceral and subcutaneous adipose depots are metabolically distinct (37), we also examined the growth and histology of a representative subcutaneous adipose depot; the inguinal fat pad which in females comprises the mammary gland. As shown in Figure 7A, significantly heavier inguinal fat pads were evident in CVF females compared to CN1 mice when fed the rescue diet but not when fed the high fat diet. Genotype did not affect the weight of the mammary fat pad in males.
Figure 7. Mammary fat pad weights and epithelial density in CN1 and CVF mice.
A, Inguinal mammary fat pads were surgically dissected from the underlying skin and weighed. Bars represent mean +/− standard deviation of 4-9 mice per group. Statistical analysis was performed with Student's t-test and significant differences (p<0.05) between genotypes are indicated by asterisk. B, Representative images of inguinal glands from CN1 and CVF mice on high fat diets. Top, whole mounts and bottom, H&E stained sections. C, Number of branches and epithelial density was quantitated in glands from CN1 and CVF mice on high fat diets. Bars represent mean +/− standard deviation. Statistical analysis was performed with Student's t-test and significant differences (p<0.05) are indicated by asterisks.
It is well established that the mammary epithelium is heavily influenced by the surrounding adipose tissue, particularly in the context of obesity-associated breast cancer. We therefore quantitatively assessed the effect of genotype and diet on the density and branching of the mammary epithelium of CVF and CN1 female mice on both diets. As shown in Figure 7B-C, female CVF mice on the high fat diet exhibited enhanced epithelial growth as measured on whole tissue mounts and on histological sections. Quantitatively, high fat fed CVF females had significant elevations in both epithelial content and branch density compared to CN1 mice. These differences were not apparent in female mice fed the rescue diet (data not shown), suggesting that VDR acts in adipocytes to modulate signals that stimulate growth of the adjacent epithelium in response to high fat diets.
DISCUSSION
Mice with global deletion of VDR develop a lean phenotype characterized by enhanced energy expenditure, increased appetite and atrophy of visceral and subcutaneous fat pads, even when fed diets with increased energy content (2-4). In the studies described here, we clearly demonstrate that conditional deletion of Vdr in adipocytes does not recapitulate the lean phenotype observed in global VDRKO mice. When fed the same rescue diet as used for global VDRKO mice, female mice with adipose-specific Vdr deletion exhibited accelerated post-weaning body weight gain compared to control mice, culminating in larger fat depots and increased serum leptin in adulthood in the absence of significant changes in food intake. These findings are in direct contrast to the global VDRKO mice which demonstrate adipose tissue atrophy and reduced serum leptin despite compensatory increases in food intake relative to their control counterparts. Since the Fabp4 promoter utilized in CVF mice drives cre-mediated deletion during adipogenic differentiation (23), our data indicates that the lean phenotype associated with global Vdr deficiency is not due to loss of Vdr action in mature adipocytes. The adipose tissue of CVF mice fed the high fat diet was found to express substantial residual Vdr mRNA which likely reflects its presence in pre-adipocytes, mesenchymal stem cells and/or tissue infiltrating immune cells. In addition, total ablation of the vitamin D endocrine system as present in global VDRKO mice leads to abnormal insulin signaling (33), serious epidermal pathologies which lead to dehydration and infections (38-40), and enhanced overall energy expenditure (1, 3, 5)– all of which can indirectly affect adiposity. Further studies are clearly necessary to determine whether the adipose atrophy observed in global VDRKO mice results from loss of Vdr actions in non-adipose cells in fat depots or in non-adipose tissues.
Our findings are also in contrast to those of Wong et al (41) who reported that mice with transgenic expression of the human VDR under the control of the identical Fabp4 promoter exhibit accelerated weight gain – a phenotype similar to the one reported here for mice with adipose specific Vdr deletion. Currently it is difficult to explain these divergent findings, but there were notable differences in the experimental designs that may partially explain the discrepancies. Dr. Li's group utilized outbred CD1 mice, a genetic background that is more prone to weight gain than the inbred C57Bl6 mice used in our study. Furthermore, the Li group ectopically expressed a human VDR transgene in the context of the endogenous mouse VDR (rather than introduction of VDR into a null background) thus gene dosage might be a confounding factor, as might differences in target genes between human and mouse receptors. In addition, they initiated dietary intervention at an older age (2months vs 3 weeks in our study) and restricted their observations to male mice. In our study adipose specific Vdr deletion did not enhance body weight or fat mass in male mice. Clearly further research is needed to determine how gender, age, genetic background and diet alter VDR functions in adipose tissue of both humans and rodents.
Importantly, our studies have confirmed that Vdr represses Ucp1 (a gene normally detected only in brown adipose tissue) in white adipose tissue. Expression of Ucp1 was increased 40-fold in white adipose tissue from female CVF mice compared to CN1 controls. Mechanistically, unliganded VDR has been shown to directly inhibit expression of UCP1 in human cells via a vitamin D response element (VDRE) in the promoter-proximal region (25). The relevance of Ucp1 regulation by VDR to adiposity in vivo, however, remains to be clarified. Since similar induction of Ucp1 in adipose tissue was observed in global VDRKO mice which present with the opposite phenotype of adipose atrophy (2), further studies are necessary to determine how Ucp1 contributes to altered adiposity in response to Vdr deletion. In the setting of high fat feeding, de-repression of Ucp1 in mature adipocytes alone (as observed in our study of CVF mice) was not associated with “browning” of the tissue and was not of sufficient magnitude to suppress overall weight gain. In contrast, loss of Vdr repression of Ucp1 in multiple tissues (as in global VDRKO deletion) does significantly increase energy expenditure and thus contributes to the lean phenotype observed in this model even in the presence of high fat intake (1, 2).
Another major conclusion from this work is that the effects of adipose-specific Vdr deletion are gender-specific. We observed substantial increases in body fatness of female but not male CVF mice relative to the respective CN1 control mice. In contrast, both male and female global VDRKO mice exhibit similar lean phenotypes (3, 4). In our studies, the weight gain in response to chronic high fat feeding in CVF females was primarily attributed to increases in the visceral WAT depots which were significantly heavier than that of CN1 controls on both diets. These novel data support a role of adipocyte Vdr in regulation of weight gain and visceral adiposity and imply an interaction of Vdr signaling with female hormones that regulate these processes.
In contrast to the visceral adipose tissue, adipose-specific Vdr deletion had minimal effects on the subcutaneous (inguinal) fat pad mass in response to high fat feeding. However, CVF females exhibited significantly greater expansion of the mammary epithelium within the inguinal fat pad, indicating that Vdr in mammary adipocytes may function to regulate cross-talk with the epithelium that impacts on ductal branching. This concept is supported by data that Vdr and its metabolizing enzymes are expressed in mammary adipocytes and that pubertal mammary gland development is altered in mice with either adipose-specific or mammary epithelial-specificVdr deletion (17, 20). In addition, global VDRKO mice exhibit enhanced density of the mammary epithelium during the hormonally regulated expansion of the gland associated with puberty and pregnancy (18, 19). Collectively, these data indicate that Vdr functions in both mammary epithelial cells and in adipocytes to control glandular expansion in response to endogenous hormones and high fat diets (20). Potential Vdr-regulated mediators that are expressed in the mammary stroma and may contribute to this regulation include the estrogen synthesizing enzyme aromatase, the cytokine IL-6, and the adipokine leptin (2, 20, 42).
In conclusion, this characterization of an adipose-specific Vdr knockout mouse model has yielded some novel preliminary observations worthy of further study. These include a role for adipocyte Vdr in regulation of visceral vs subcutaneous adipose depots, metabolic responses to diets of differing fat content, and modulation of serum 25D. The observation that adipocyte Vdr signaling alters body weight and fat mass in females but not males could have implications for clinical studies of vitamin D and obesity. The finding that Vdr acts in adipocytes to modulate epithelial expansion in the mammary gland offers mechanistic insight into the links between vitamin D, obesity and breast cancer. Since our work involved disruption of the Vdr in the context of vitamin D sufficiency, additional studies to address the role of the Vdr ligand 1,25D on high fat diets, adiposity and mammary gland biology are warranted.
Highlights.
Adipose-specific Vdr deletion enhanced weight gain in response to high fat diet
Effects of adipose-specific Vdr deletion were gender-specific
Ucp1 expression was increased in visceral fat of adipose specific Vdr deleted mice
Mammary gland ductal branching was enhanced by adipose-specific Vdr deletion
ACKNOWLEDGEMENTS
The authors acknowledge Dr. Namita Chatterjee for assistance with animal experiments, Samantha Robilotto and Sarah Beaudin for technical contributions and Sharon Lonergan for maintenance of the animal colonies. These studies were supported by Pre-Doctoral Fellowship W81XWH-11-1-0152 from the Department of Defense Breast Cancer Research Program to DGM and NIH grant R01CA069700 to JW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Welsh J, Zinser LN, Mianecki-Morton L, Martin J, Waltz SE, James H, et al. Age-related changes in the epithelial and stromal compartments of the mammary gland in normocalcemic mice lacking the vitamin D3 receptor. PLoS One. 2011;6(1):e16479. doi: 10.1371/journal.pone.0016479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narvaez CJ, Matthews D, Broun E, Chan M, Welsh J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology. 2009;150(2):651–61. doi: 10.1210/en.2008-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong KE, Szeto FL, Zhang W, Ye H, Kong J, Zhang Z, et al. Involvement of the vitamin D receptor in energy metabolism: regulation of uncoupling proteins. Am J Physiol Endocrinol Metab. 2009;296(4):E820–8. doi: 10.1152/ajpendo.90763.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber K, Erben RG. Differences in triglyceride and cholesterol metabolism and resistance to obesity in male and female vitamin D receptor knockout mice. J Anim Physiol Anim Nutr (Berl) 2013;97(4):675–83. doi: 10.1111/j.1439-0396.2012.01308.x. [DOI] [PubMed] [Google Scholar]
- 5.Bouillon R, Carmeliet G, Lieben L, Watanabe M, Perino A, Auwerx J, et al. Vitamin D and energy homeostasis: of mice and men. Nat Rev Endocrinol. 2014;10(2):79–87. doi: 10.1038/nrendo.2013.226. [DOI] [PubMed] [Google Scholar]
- 6.Zemel MB, Teegarden D, Loan MV, Schoeller DA, Matkovic V, Lyle RM, et al. Dairy-rich diets augment fat loss on an energy-restricted diet: a multicenter trial. Nutrients. 2009;1(1):83–100. doi: 10.3390/nu1010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teegarden D. Calcium intake and reduction in weight or fat mass. J Nutr. 2003;133(1):249s–51s. doi: 10.1093/jn/133.1.249S. [DOI] [PubMed] [Google Scholar]
- 8.Keisala T, Minasyan A, Lou YR, Zou J, Kalueff AV, Pyykko I, et al. Premature aging in vitamin D receptor mutant mice. The Journal of steroid biochemistry and molecular biology. 2009;115(3-5):91–7. doi: 10.1016/j.jsbmb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Narvaez CJ, Simmons KM, Brunton J, Salinero A, Chittur SV, Welsh JE. Induction of STEAP4 correlates with 1,25-dihydroxyvitamin D3 stimulation of adipogenesis in mesenchymal progenitor cells derived from human adipose tissue. J Cell Physiol. 2013;228(10):2024–36. doi: 10.1002/jcp.24371. [DOI] [PubMed] [Google Scholar]
- 10.Kong J, Chen Y, Zhu G, Zhao Q, Li YC. 1,25-Dihydroxyvitamin D3 upregulates leptin expression in mouse adipose tissue. J Endocrinol. 2013;216(2):265–71. doi: 10.1530/JOE-12-0344. [DOI] [PubMed] [Google Scholar]
- 11.Nimitphong H, Holick MF, Fried SK, Lee MJ. 25-hydroxyvitamin D(3) and 1,25-dihydroxyvitamin D(3) promote the differentiation of human subcutaneous preadipocytes. PLoS One. 2012;7(12):e52171. doi: 10.1371/journal.pone.0052171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong J, Li YC. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am J Physiol Endocrinol Metab. 2006;290(5):E916–24. doi: 10.1152/ajpendo.00410.2005. [DOI] [PubMed] [Google Scholar]
- 13.Barreto SC, Hopkins CA, Bhowmick M, Ray A. Extracellular matrix in obesity - cancer interactions. Horm Mol Biol Clin Investig. 2015 doi: 10.1515/hmbci-2015-0001. [DOI] [PubMed] [Google Scholar]
- 14.Boonyaratanakornkit V, Pateetin P. The Role of Ovarian Sex Steroids in Metabolic Homeostasis, Obesity, and Postmenopausal Breast Cancer: Molecular Mechanisms and Therapeutic Implications. 2015;2015:140196. doi: 10.1155/2015/140196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couldrey C, Moitra J, Vinson C, Anver M, Nagashima K, Green J. Adipose tissue: a vital in vivo role in mammary gland development but not differentiation. Dev Dyn. 2002;223(4):459–68. doi: 10.1002/dvdy.10065. [DOI] [PubMed] [Google Scholar]
- 16.Zangani D, Darcy KM, Shoemaker S, Ip MM. Adipocyte-epithelial interactions regulate the in vitro development of normal mammary epithelial cells. Exp Cell Res. 1999;247(2):399–409. doi: 10.1006/excr.1998.4373. [DOI] [PubMed] [Google Scholar]
- 17.Ching S, Kashinkunti S, Niehaus MD, Zinser GM. Mammary adipocytes bioactivate 25-hydroxyvitamin D(3) and signal via vitamin D(3) receptor, modulating mammary epithelial cell growth. J Cell Biochem. 2011;112(11):3393–405. doi: 10.1002/jcb.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zinser GM, Welsh J. Accelerated mammary gland development during pregnancy and delayed postlactational involution in vitamin D3 receptor null mice. Mol Endocrinol. 2004;18(9):2208–23. doi: 10.1210/me.2003-0469. [DOI] [PubMed] [Google Scholar]
- 19.Zinser G, Packman K, Welsh J. Vitamin D(3) receptor ablation alters mammary gland morphogenesis. Development. 2002;129(13):3067–76. doi: 10.1242/dev.129.13.3067. [DOI] [PubMed] [Google Scholar]
- 20.Johnson AL, Zinser GM, Waltz SE. Loss of vitamin D receptor signaling from the mammary epithelium or adipose tissue alters pubertal glandular development. Am J Physiol Endocrinol Metab. 2014;307(8):E674–85. doi: 10.1152/ajpendo.00200.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100(26):15712–7. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KY, Russell SJ, Ussar S, Boucher J, Vernochet C, Mori MA, et al. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes. 2013;62(3):864–74. doi: 10.2337/db12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krueger KC, Costa MJ, Du H, Feldman BJ. Characterization of Cre recombinase activity for in vivo targeting of adipocyte precursor cells. Stem Cell Reports. 2014;3(6):1147–58. doi: 10.1016/j.stemcr.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne) 2013;4:52. doi: 10.3389/fendo.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malloy PJ, Feldman BJ. Cell-autonomous regulation of brown fat identity gene UCP1 by unliganded vitamin D receptor. Mol Endocrinol. 2013;27(10):1632–42. doi: 10.1210/me.2013-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricciardi CJ, Bae J, Esposito D, Komarnytsky S, Hu P, Chen J, et al. 1,25-Dihydroxyvitamin D/vitamin D receptor suppresses brown adipocyte differentiation and mitochondrial respiration. European journal of nutrition. 2014 doi: 10.1007/s00394-014-0778-9. [DOI] [PubMed] [Google Scholar]
- 27.Woeckel VJ, Bruedigam C, Koedam M, Chiba H, van der Eerden BC, van Leeuwen JP. 1alpha,25-dihydroxyvitamin D3 and rosiglitazone synergistically enhance osteoblast-mediated mineralization. Gene. 2013;512(2):438–43. doi: 10.1016/j.gene.2012.07.051. [DOI] [PubMed] [Google Scholar]
- 28.Salamon H, Bruiners N, Lakehal K, Shi L, Ravi J, Yamaguchi KD, et al. Cutting edge: Vitamin D regulates lipid metabolism in Mycobacterium tuberculosis infection. Journal of immunology (Baltimore, Md : 1950) 2014;193(1):30–4. doi: 10.4049/jimmunol.1400736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lachinani L, Ghaedi K, Tanhaei S, Salamian A, Karamali F, Kiani-Esfahani A, et al. Characterization and Functional Assessment of Mouse PPARgamma1 Promoter. Avicenna journal of medical biotechnology. 2012;4(4):160–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad P, Kochhar A. Interplay of vitamin D and metabolic syndrome: A review. Diabetes Metab Syndr. 2015 doi: 10.1016/j.dsx.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Mutt SJ, Hypponen E, Saarnio J, Jarvelin MR, Herzig KH. Vitamin D and adipose tissue-more than storage. Front Physiol. 2014;5:228. doi: 10.3389/fphys.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson CA, Tosh AK, Belenchia AM. Vitamin D insufficiency and insulin resistance in obese adolescents. Ther Adv Endocrinol Metab. 2014;5(6):166–89. doi: 10.1177/2042018814547205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. Faseb j. 2003;17(3):509–11. doi: 10.1096/fj.02-0424fje. [DOI] [PubMed] [Google Scholar]
- 34.Lawson DE, Sedrani SH, Douglas J. Interrelationships in rats of tissue pools of cholecalciferol and 25-hydroxycholecalciferol formed in u.v. light. Biochem J. 1986;233(2):535–40. doi: 10.1042/bj2330535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawson DE, Douglas J, Lean M, Sedrani S. Estimation of vitamin D3 and 25-hydroxyvitamin D3 in muscle and adipose tissue of rats and man. Clin Chim Acta. 1986;157(2):175–81. doi: 10.1016/0009-8981(86)90223-8. [DOI] [PubMed] [Google Scholar]
- 36.Abboud M, Gordon-Thomson C, Hoy AJ, Balaban S, Rybchyn MS, Cole L, et al. Uptake of 25-hydroxyvitamin D by muscle and fat cells. The Journal of steroid biochemistry and molecular biology. 2014;144(Pt A):232–6. doi: 10.1016/j.jsbmb.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 37.Schleinitz D, Bottcher Y, Bluher M, Kovacs P. The genetics of fat distribution. Diabetologia. 2014;57(7):1276–86. doi: 10.1007/s00125-014-3214-z. [DOI] [PubMed] [Google Scholar]
- 38.Oda Y, Tu CL, Menendez A, Nguyen T, Bikle DD. Vitamin D and calcium regulation of epidermal wound healing. The Journal of steroid biochemistry and molecular biology. 2015 doi: 10.1016/j.jsbmb.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luderer HF, Nazarian RM, Zhu ED, Demay MB. Ligand-dependent actions of the vitamin D receptor are required for activation of TGF-beta signaling during the inflammatory response to cutaneous injury. Endocrinology. 2013;154(1):16–24. doi: 10.1210/en.2012-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zinser GM, Sundberg JP, Welsh J. Vitamin D(3) receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis. 2002;23(12):2103–9. doi: 10.1093/carcin/23.12.2103. [DOI] [PubMed] [Google Scholar]
- 41.Wong KE, Kong J, Zhang W, Szeto FL, Ye H, Deb DK, et al. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. The Journal of biological chemistry. 2011;286(39):33804–10. doi: 10.1074/jbc.M111.257568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan AV, Swami S, Peng L, Wang J, Moreno J, Feldman D. Tissue-selective regulation of aromatase expression by calcitriol: implications for breast cancer therapy. Endocrinology. 2010;151(1):32–42. doi: 10.1210/en.2009-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]