Abstract
Background
The blood-brain barrier (BBB) formed by brain endothelial cells (ECs) interconnected by tight junctions (TJs) is essential for the homeostasis of the central nervous system (CNS). Although studies have shown the importance of various signaling molecules in BBB formation during development, little is known about the molecular basis regulating the integrity of the adult BBB.
Methods and Results
Using a mouse model with tamoxifen-inducible EC-restricted disruption of ctnnb1 (iCKO), here we show that endothelial β-catenin signaling is essential for maintaining BBB integrity and CNS homeostasis in adult. The iCKO mice developed severe seizures accompanied by neuronal injury, multiple brain petechial hemorrhages, and CNS inflammation, and all died postictal. Disruption of endothelial β-catenin induced BBB breakdown and downregulation of specific TJ proteins Claudin-1 and -3 in adult brain ECs. The clinical relevance of the data is indicated by the observation of decreased expression of Claudin-1 and nuclear β-catenin in brain ECs of hemorrhagic lesions of hemorrhagic stroke patients.
Conclusion
These results demonstrate the prerequisite role of endothelial β-catenin in maintaining the integrity of adult BBB. The results suggest that BBB dysfunction secondary to defective β-catenin transcription activity is a key pathogenic factor in hemorrhagic stroke, seizure activity and CNS inflammation.
Keywords: blood-brain barrier, cerebrovascular disorders, endothelium, hemorrhage, stroke
Introduction
The blood-brain barrier (BBB), unique to the vasculature of the central nervous system (CNS), functions as a highly restrictive barrier between the circulation and brain tissue to protect the brain from toxins, microbes, and fluctuations in the plasma compositions.1–4 The brain endothelium expresses unique transporters, specific systems for receptor-mediated and absorptive endocytosis, and detoxifying enzymes to regulate the CNS microenvironment.5 BBB disruption induces leakage of fluid and plasma protein, neuronal damage, and intra-cerebral hemorrhage6–8 and is implicated in multiple CNS diseases, such as Alzheimers, epilepsy, multiple sclerosis, and stroke.1–4 Studies have shown important roles of cross-talk between endothelial cells (ECs) and the surrounding astrocytes and pericytes in regulating BBB maturation and integrity.9–13 Pericyte deficiency increases permeability of the BBB through endothelial transcytosis10 whereas interactions between astrocytes and brain ECs solidifies the BBB integrity through activation of the sonic hedgehog signaling pathway.9 However, genetic disruption of these pathways was not sufficient to induce cerebral hemorrhaging and compromised lifespan secondary to BBB breakdown in adult mice.9–11 Thus, the signaling pathways in brain ECs regulating homeostasis of the adult BBB remain largely unknown.
As the BBB is comprised of specialized brain ECs held together by tight junctions (TJs), brain capillaries are ~50–100 times tighter than peripheral microvessels.1–3, 14 TJs are consisted of a number of proteins, including Occludin, Claudins and Junctional adhesion molecules (JAMs).15, 16 Claudins, a family of transmembrane proteins (20–27 kilodalton) with the N-terminus and the C-terminus in the cytoplasm,17 are the main constituent of the TJs, where they interact with Occludins to assemble a "zip-locked" structure and form the physical barrier in the brain EC clefts.17, 18 Among the 24 Claudins identified to date, Claudins-1, 3, 5 and -12 are expressed in brain ECs.19–23 Genetic disruption of Claudin-5 in mice results in alteration of the BBB junctional properties but not complete breakdown so that they function like a molecular sieve allowing tracer leakage from blood into brain in a size-selective manner.24 Thus, great interest arises to delineate the signaling pathways regulating the expression of these Claudins in brain ECs to understand the normal physiological function of the BBB and gain insights into pathological conditions causing loss or breakdown of the BBB and thereby develop potential novel approaches to manipulate the BBB junctional properties for drug delivery.
β-catenin is both an adherens junction (AJ) protein linking AJs to the actin cytoskeleton25 and a transcription factor central to the Wnt signaling pathway.26–28 Cytosolic β-catenin is normally phosphorylated by the Axin/GSK-3/APC complexes and degraded. Following Wnt ligand binding to Frizzled/LRP receptor complexes, GSK-3 is inactivated and β-catenin phosphorylation is inhibited leading to stabilization of β-catenin. Stabilized β-catenin is then translocated to the nucleus to form the β-catenin/LEF/TCF transcriptional complex that regulates transcription of many target genes involved in cell growth control and embryogenesis.26–28 It has been shown that Wnt/β-catenin signaling during embryonic and postnatal (up to P24) development is fundamental in mediating brain development, CNS vascularization and BBB formation and maturation.23, 29–31 However, studies show that nuclear β-catenin is rarely detected or absent in ECs of adult brain vessels,23, 32 suggesting that β-catenin signaling is not important for normal BBB function in adult. Employing a mouse model with tamoxifen-inducible EC-restricted disruption of ctnnb1 (iCKO), here we observed that endothelial β-catenin signaling is required for maintenance of the BBB integrity and CNS homeostasis in adult (over 2 months old) and transcriptional control of expression of TJ proteins Claudin-1 and -3. The adult iCKO mice developed severe seizures accompanied by neuronal injury, multiple brain petechial hemorrhages, and CNS inflammation, and all died postictal. Our data have for the first time demonstrated that constitutively active β-catenin signaling in adult brain ECs, albeit low, is essential for the BBB integrity and CNS homeostasis. Thus, development of means of activation of β-catenin transcription activity in brain ECs to enhance the adult BBB integrity may represent a novel strategy for treatment of CNS diseases such as hemorrhagic stroke and epilepsy associated with BBB dysfunction.
Methods
Mice
Tamoxifen-inducible EC-specific inactivation of ctnnb1 was achieved by cross-breeding the mice carrying the floxed ctnnb1 gene29 with End-SCL-Cre-ER(T) transgenic mice expressing the tamoxifen-inducible Cre recombinase under the control of the 5' endothelial enhancer of the stem cell leukemia locus.33 At 10–12 weeks of age, littermates of ctnnb1f//fl;Cre(+) and ctnnb1f//fl;Cre(−) mice were treated with 2mg tamoxifen/mouse/day (i.p.) for 5 consecutive days and once on the 7th day to generate WT and iCKO mice. The use of animals in preparation for this work was in compliance with the guidelines of the Animal Care and Use Committee of the University of Illinois at Chicago.
Human subjects
Archived human brain tissues from patients with spontaneous non-traumatic intracerebral hemorrhage and patients with no evidence of brain disease were used for this study. These tissues were collected at autopsies with local IRB approval from the University of Illinois at Chicago Ethics Committee.
Statistical analysis
Statistical significance was determined by one-way ANOVA with a Games-Howell post hoc analysis that calculates P values corrected for multiple comparisons. Two-group comparisons were analyzed by the unpaired two-tailed Student's t test or Mann-Whitney (nonparametric) test depending on the data distribution. Statistical analysis of the mortality study was performed with the Log –rank (Mantel-Cox) test. P < 0.05 denoted the presence of a statistically significant difference.
An expanded Materials and Methods section containing detailed description of vascular permeability measurement, primary culture of mouse brain ECs, promoter luciferase assay, transendothelial electrical resistance assay, siRNA-mediated knockdown, transmission electron microscopy, molecular analysis, histology, TUNEL staining, immunostaining is provided in the online-only Data Supplement.
Results
Endothelial cell-specific inactivation of β-catenin induces severe seizures, brain petechial hemorrhages, and postictal death in adult iCKO mice
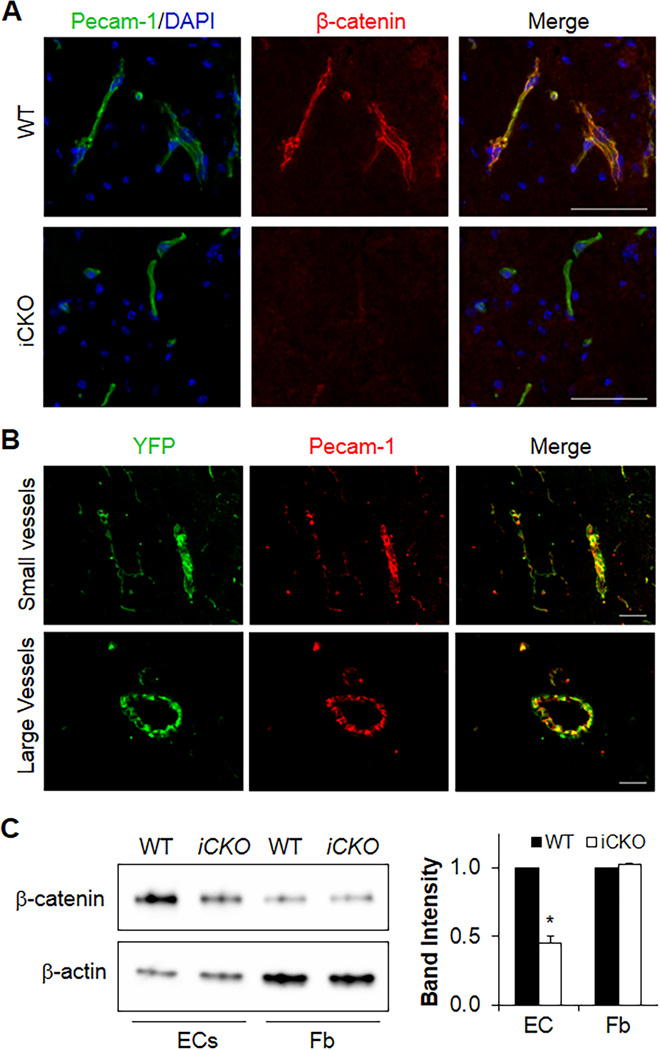
To investigate the role of β-catenin in maintaining the integrity of the adult BBB, we inactivated β-catenin in the endothelium of adult mice. Mice carrying the floxed ctnnb1 gene were bred with End-SCL-Cre-ER(T) transgenic mice. Tamoxifen treatment induced β-catenin depletion in brain ECs in adult iCKO mice but not in other brain cells (Figure 1A and Supplemental Figure 1). We also observed EC-specific depletion of β-catenin in other vascular beds including lung (Supplemental Figure 2). The specificity of EC-restricted deletion was further evaluated using the End-SCL-Cre-ER(T)/ROSA-YFP reporter mice, in which the stop sequence flanked by two loxP sites was located at the ROSA-YFP locus to disrupt YFP expression.34 Tamoxifen injection in these mice induced YFP expression only in ECs which were detected by co-immunostaining with Pecam-1 (Figure 1B). Western blotting also demonstrated decreased β-catenin protein levels in ECs but not fibroblasts isolated from iCKO brain at 7 days post-tamoxifen treatment (Figure 1C). Taken together, these data demonstrate that tamoxifen treatment induced EC-restricted disruption of β-catenin in iCKO mice.
Figure 1. Tamoxifen treatment induces deletion of β-catenin in brain ECs of adult iCKO mice.
(A) Representative micrographs of immunostaining show EC-specific deletion of β-catenin in brain of adult iCKO mice. Scale bar, 50µm. (B) The tamoxifen-inducible expression of Cre recombinase under the control of the 5' endothelial enhancer of the stem cell leukemia locus [End-SCL-Cre-ER(T)] induces EC-specific deletion of gene. Brain sections of End-SCL-Cre-ER(T)/Rosa-Stopflox/+ YFP mice were co-immunostained for YFP (green) and Pecam-1 (red). All YFP positive cells were co-immunostained with Pecam-1 demonstrating EC-specific deletion. Scale bars, 50µm. (C) Western blotting demonstrating decreased β-catenin protein levels in ECs but not fibroblasts (Fb) isolated from iCKO brain. Given that iCKO mice died between day 10 and 16 post-tamoxifen treatment, brain ECs were isolated from WT or iCKO mice at 7 days post-tamoxifen treatment when β-catenin expression was inhibited approximately 60%. After 2 days in culture, brain ECs were lysed for Western blotting. The approximately 10% contamination of non-ECs in the primary EC culture also likely contributed to the remaining β-catenin expression levels seen in ECs from iCKO brain tissue. *, P = 0.005 (2-tailed t test).
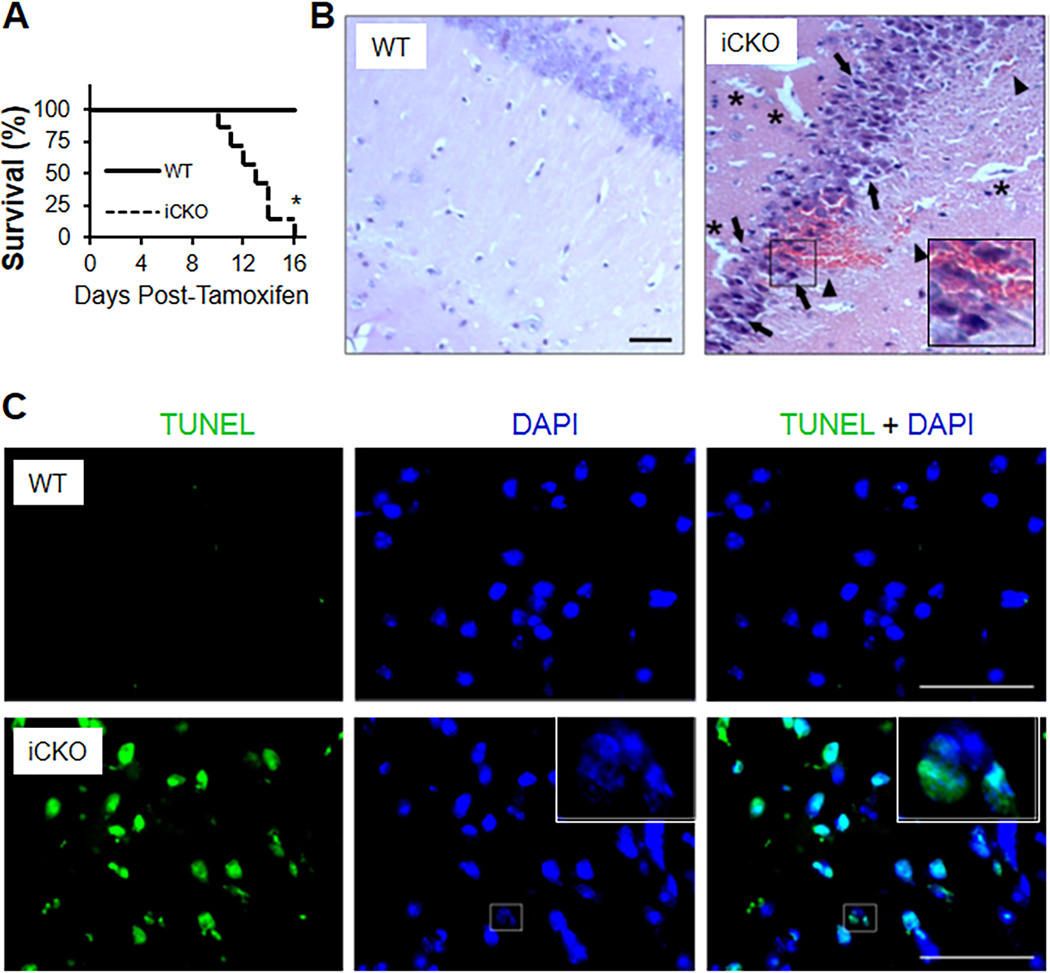
We observed that within 2 weeks post-tamoxifen, the iCKO mice developed severe neurological disorders, characterized as hypersensitivity to touch and sound, ataxia, and spontaneous seizure activity (Supplemental Movie). After suffering extreme seizure, all of iCKO mice died postictal (Figure 2A). H&E staining of brain sections from seizing mice revealed petechial hemorrhaging at multiple areas including the hippocampus and basal ganglia (Figure 2B and Supplemental Figure 3) which especially severe in the hippocampus. Perivascular edema was also evident as expansion of the interstitial space surrounding the blood vessels (Figure 2B). Edema in the brain perivascular space often induces constriction of microvessels, which may be a factor in inducing ischemia and neuronal cell death.35, 36 We also observed neuronal injury prominent at site of hemorrhage (Figure 2B) and in CA1 and CA3 regions of the pyramidal layer of the hippocampus and dentate hilus neurons in iCKO mice (Supplemental Figure 4). Neuronal injury was evident by shrinkage of cell bodies, pycnosis of nuclei, and disappearance of nucleoli. TUNEL staining also showed a great number of apoptotic nuclei in the cerebellum of iCKO mice (Figure 2C).
Figure 2. Adult mice with EC-specific deletion of β-catenin exhibit seizures, petechial brain hemorrhages, and postictal death.
(A) Mortality rate of iCKO mice. n=8 mice/group. *, P = 0.003 [Log –rank (Mantel-Cox) test]. (B) H&E staining showing petechial hemorrhages in iCKO brain hippocampus. Asterisks indicate perivascular edema, arrowheads point to red blood cell leakage across the BBB, arrows point to necrotic neurons with pyknotic nuclei and shrunken cell bodies. (C) TUNEL staining showing massive apoptosis (green) in the iCKO cerebellum. A fragmented nucleus indicative of apoptotic cell was shown. All scale bars, 50 µm.
Endothelial β-catenin is required for the maintenance of the integrity of the adult BBB but not the pulmonary vasculature
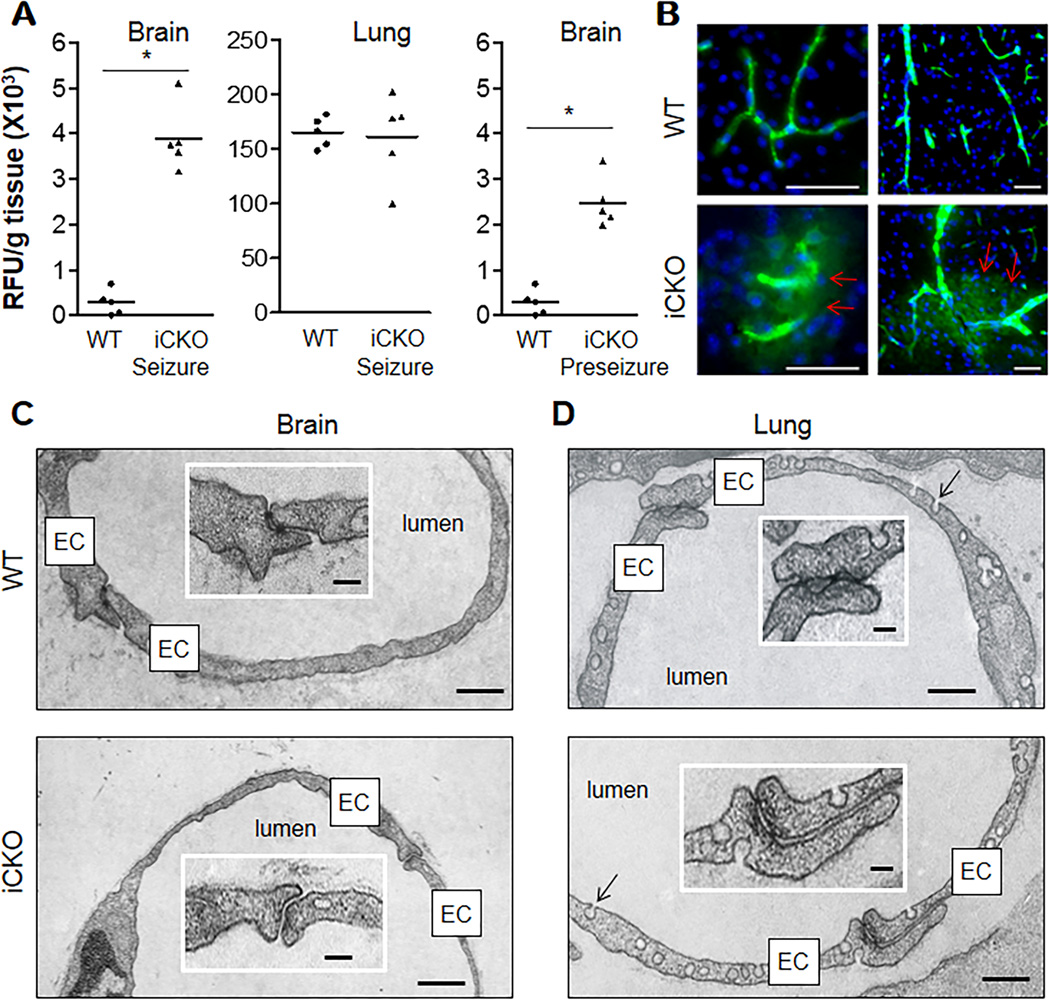
Given that β-catenin is a component of endothelial AJs,25 its loss may reduce junction stability and increase vascular permeability. To measure vascular permeability, we injected i.v. WT and iCKO mice with the FITC-conjugated bovine serum albumin (BSA) tracer. Brains from iCKO mice showed a 10-fold increase of trans-BBB albumin leakage compared to WT. Surprisingly this defect was confined to the brain as it was not seen in the lung (Figure 3A) of iCKO mice, which showed normal permeability values. Even the iCKO mice that did not display obvious neurological symptoms exhibited 6-fold albumin leakage in brain vessels compared to WT (Figure 3A), suggesting that the primary BBB defect preceded neuronal dysfunction. All brain regions examined were leaking although different regions exhibited various increases of leakiness (Supplemental Figure 5). To visualize vascular leakage, mice were perfused with FITC-conjugated BSA tracer for 5 minutes. Trans-BBB albumin leakage was seen in both small and large cerebral vessels (Figure 3B). Transmission electron microscopy showed loss of TJs of brain capillaries (Figure 3C) and post-capillary venules (Supplemental Figure 6) in adult iCKO mice. In contrast, the lung vessel junctions showed normal structure without evidence of disrupted junctions (Figure 3D and Supplemental Figure 7). These data demonstrate that loss of endothelial β-catenin selectively disrupted cerebrovascular integrity.
Figure 3. BBB dysfunction in adult mice with EC-specific inactivation of β-catenin.
(A) Quantification of trans-vascular leakage of FITC-BSA tracer in mouse tissues, perfused free of blood with PBS after a single bolus tracer injection. *, P = 0.0079 (Mann-Whitney 2-tailed test). Bars represent mean. (B) Brain sections of FITC-BSA-perfused mice (5 min) showing leakage of brain vessels in iCKO mice. Following FITC-BSA perfusion, the brain tissues was immediately fixed without additional PBS perfusion. Arrows indicate perivascular FITC-BSA leakage. Scale bars, 50 µm. (C) Electron microscopy analysis demonstrating open BBB junctions in brain capillaries of iCKO mice. Insert shows characteristic tight junctions in WT capillary having two "kissing" point junctions. Scale bars, 350nm, or 70nm for inset. (D) Ultrastructure of lung capillaries of iCKO mice. Lung capillaries of both WT and iCKO mice exhibited similar junction structure and characteristic enrichment with caveolae (arrows). Scale bars, 300nm, or 60nm for inset.
iCKO mice exhibit CNS inflammation
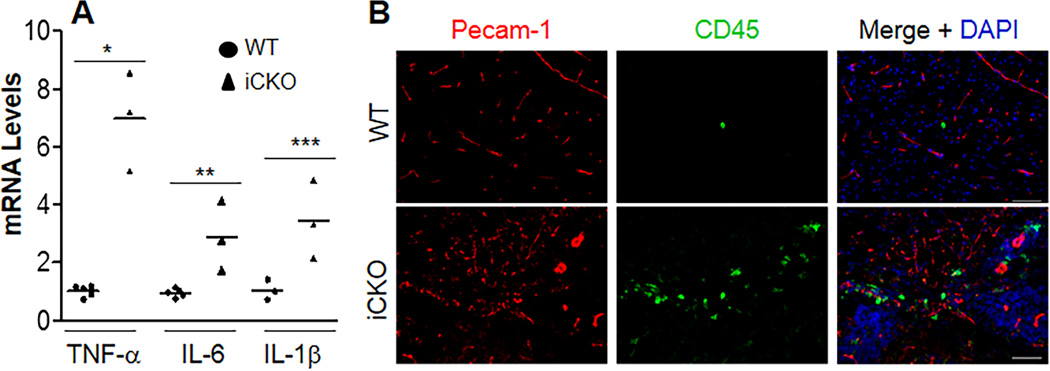
The unique microenvironment of the CNS is tightly regulated by the integrity of BBB. We next determined whether loss of BBB integrity in iCKO mice induces CNS inflammation. We observed by quantitative RT-PCR analysis a marked increase of expression of multiple pro-inflammatory cytokines in iCKO brains compared to WT (Figure 4A and Supplemental Table 1). Immunostaining also showed leukocyte infiltration in brain parenchyma of the seizing iCKO mice (Figure 4B).
Figure 4. iCKO mice exhibited prominent CNS inflammation.
(A) Quantitative RT-PCR analysis demonstrating increased expression of pro-inflammatory cytokines in brain tissues of iCKO mice with seizure. Brain tissues were collected from WT and iCKO mice with seizure. Data are expressed as mean ± SD (n=4–5/group). *, P = 0.0002; **, P = 0.009; ***, P = 0.041 (2-tailed t test). Bars represent mean. (B) Marked leukocyte infiltration in brain parenchyma of the seizing iCKO mice. Brain sections were co-immunostained for Pecam-1 (EC marker, red) and CD45 (pan-leukocyte marker, green). Scale bars, 50µm.
Diminished expression of Claudin-1 and -3 in brain ECs of iCKO mice
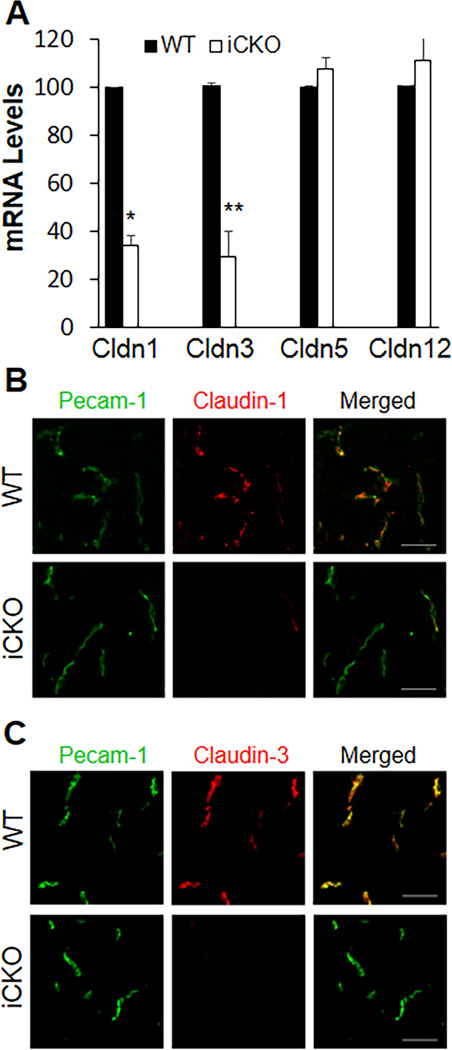
TJs comprised of a family of Claudins and Occludin as well as other junctional proteins18 are essential for the restrictiveness of the BBB in mammals. We observed 60% reduction in Claudin-1 and -3 mRNA expression in brain ECs isolated from pre-seizure iCKO mice [at 7 days post-tamoxifen treatment when β-catenin expression was partially inhibited (Figure1C)] (Figure 5A). Co-immunostaining showed a similar trend in reduced expression of Claudin-1 and -3 in brain ECs but also complete absence of these proteins in some areas (Supplemental Figure 8). iCKO mice with intense seizures invariably showed absence of Claudin-1 and -3 in brain ECs (Figure 5, B and C and Supplemental Figure 9). However, expression of Claudin-5 and -12 as well as other TJ proteins enriched in brain ECs19 were unchanged (Figure 5A and Supplemental Figure 10).
Figure 5. Diminished expression of Claudin-1 and -3 in brain ECs of iCKO mice.
(A) Quantitative RT-PCR analysis of expression of Claudin isoforms in brain ECs isolated from WT and pre-seizure iCKO mice (at 7 days post-tamoxifen). After 2 days in culture, the cells were lysed for RNA isolation. Data are expressed as mean ± SD (n=4–5/group). *, P = 0.001; **, P = 0.008 (2-tailed t test). Cldn, Claudin. (B, C) Complete loss of expression of Claudin-1 and -3 in brain ECs of seizing iCKO mice. Brain sections from WT and seizing iCKO mice were co-immunostained for Pecam-1 (EC marker) and Claudin-1 (B) or Claudin-3 (C). Scale bars, 30µm.
Claudin-1 is a transcriptional target of β-catenin signaling in brain ECs
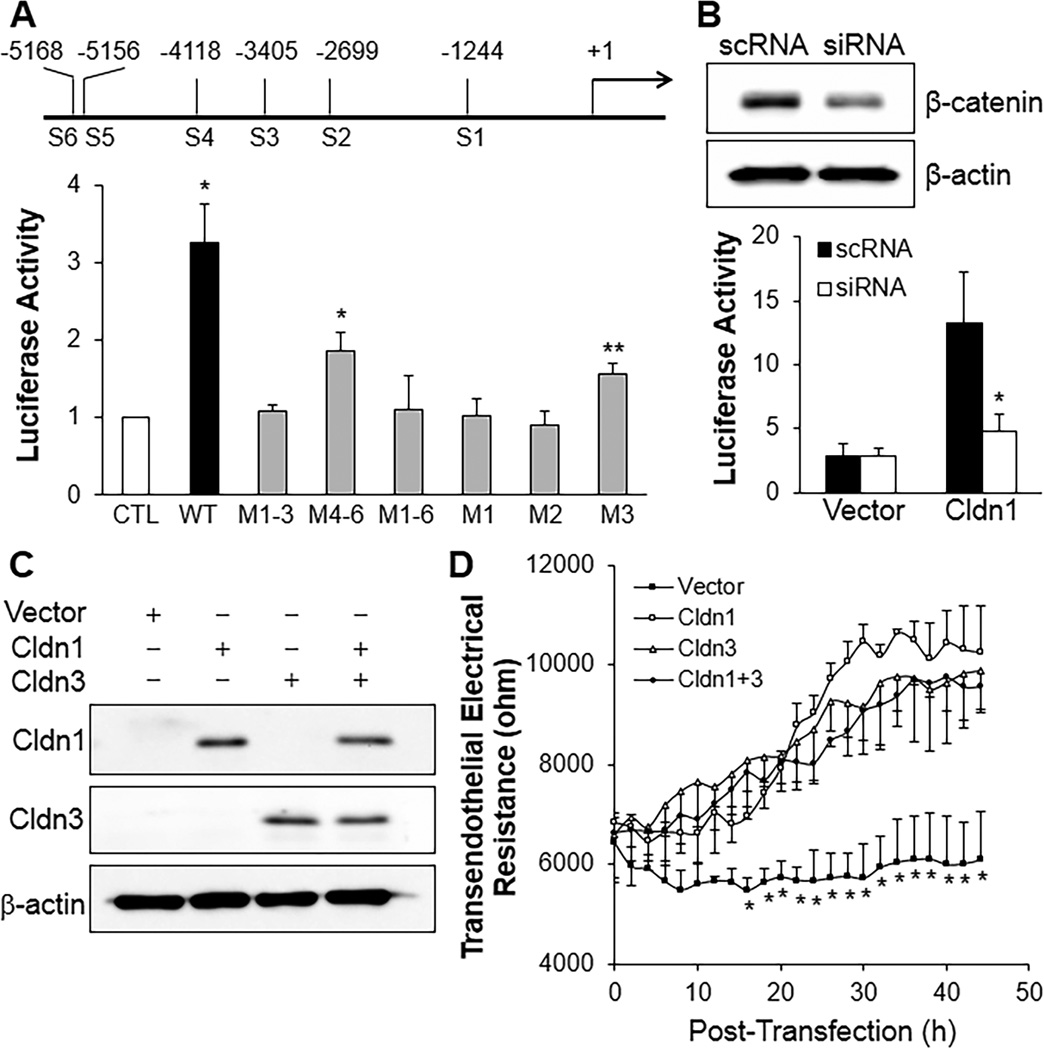
To determine whether β-catenin signaling induces transcriptional regulation of Claudin expression in brain ECs, we carried out promoter analysis of Claudin-1 using the luciferase reporter system.37 Mouse brain EC line bEnd.3 was transfected with wild-type Cldn1 promoter and constructs with mutations of putative TCF/LEF binding sites, and treated with LiCl, a GSK-3 inhibitor which induces β-catenin nuclear translocation and transcription activation.38 Mutation of all 6 binding sites or 3 sites proximal to the start site reduced luciferase expression to control levels whereas mutation of the distal 3 sites retained moderate luciferase expression (Figure 6A), suggesting the first 3 sites are necessary for transcription activation while the distal 3 sites are required for full activation. Single mutations showed the key role of the first 2 binding sites (Figure 6A). Treatment of recombinant Wnt3a had effects similar to LiCl (Supplemental Figure 11). siRNA-mediated knockdown of β-catenin abolished the LiCl-induced activation of Cldn1 promoter (Figure 6B) confirming the requirement of β-catenin in mediating activation of the Cldn1 promoter in brain ECs.
Figure 6. Claudin-1 is a transcriptional target of β-catenin signaling essential for adult BBB integrity.
(A) Luciferase reporter assay demonstrating transcriptionally active binding sites for β-catenin/TCF/LEF complex on Cldn1 promoter. bEnd.3 cells transfected with empty vector (CTL) or various constructs of Cldn1 promoter were treated with 20mM LiCl. Luciferase activity was measured at 24h post-treatment and normalized to PBS-treated cells. M1–3, mutation of sites 1–3; M1, mutation of site 1, i.e., 3. Data are expressed as mean ± SD (n=3–4/group). *, P = 0.012 versus CTL; **, P = 0.047 versus CTL (One-way ANOVA with Games-Howell multiple comparison test). (B) siRNA-mediated knockdown of β-catenin abolished LiCl-induced activation of Cldn1 promoter. Representative Western blot demonstrating siRNA-mediated knockdown of β-catenin (upper). scRNA, scrambled RNA. LiCl activation of Cldn1 promoter was dependent on β-catenin (lower). *, P = 0.015 versus scRNA (2-tailed t test). The experiment was repeated 3 times. (C, D) Forced expression of Claudin-1 or -3 in bEnd.3 cells enhanced endothelial barrier function measured by transendothelial monolayer electrical resistance. Expression of mouse Claudin-1 and -3 in plasmid DNA-transfected bEnd.3 cells was detected by Western blotting (C). Empty pcDNA3.1 vector (Vector) was used as control. Following transfection of plasmids or control vector, real-time changes of transendothelial electrical resistance were monitored (D). *, P < 0.05 versus other groups (2-tailed t test). The experiment was repeated 3 times.
To determine whether forced expression of Claudin-1 and -3 in cultured brain ECs could restore the restrictive phenotype of TJs and thereby enhance brain endothelial barrier function, we measured transendothelial electrical resistance to quantify time-dependent changes of the endothelial barrier integrity. We used the bEnd.3 cell line that expresses Claudin-5 and -12 but not Claudin-1 or Claudin-3.39 Overexpression of Claudin-1 or -3 in these cells increased the electrical resistance by 60–70% although expression of Claudin-1 and -3 together did not demonstrate a synergistic effect (Figure 6, C and D). These data suggest that diminished expression of Claudin-1 and -3 in iCKO brain ECs secondary to loss of β-catenin signaling was responsible for BBB disruption and resulting hemorrhaging, inflammation and seizure activity.
Diminished nuclear β-catenin and Claudin-1 expression in brain ECs of patients with intracerebral hemorrhage
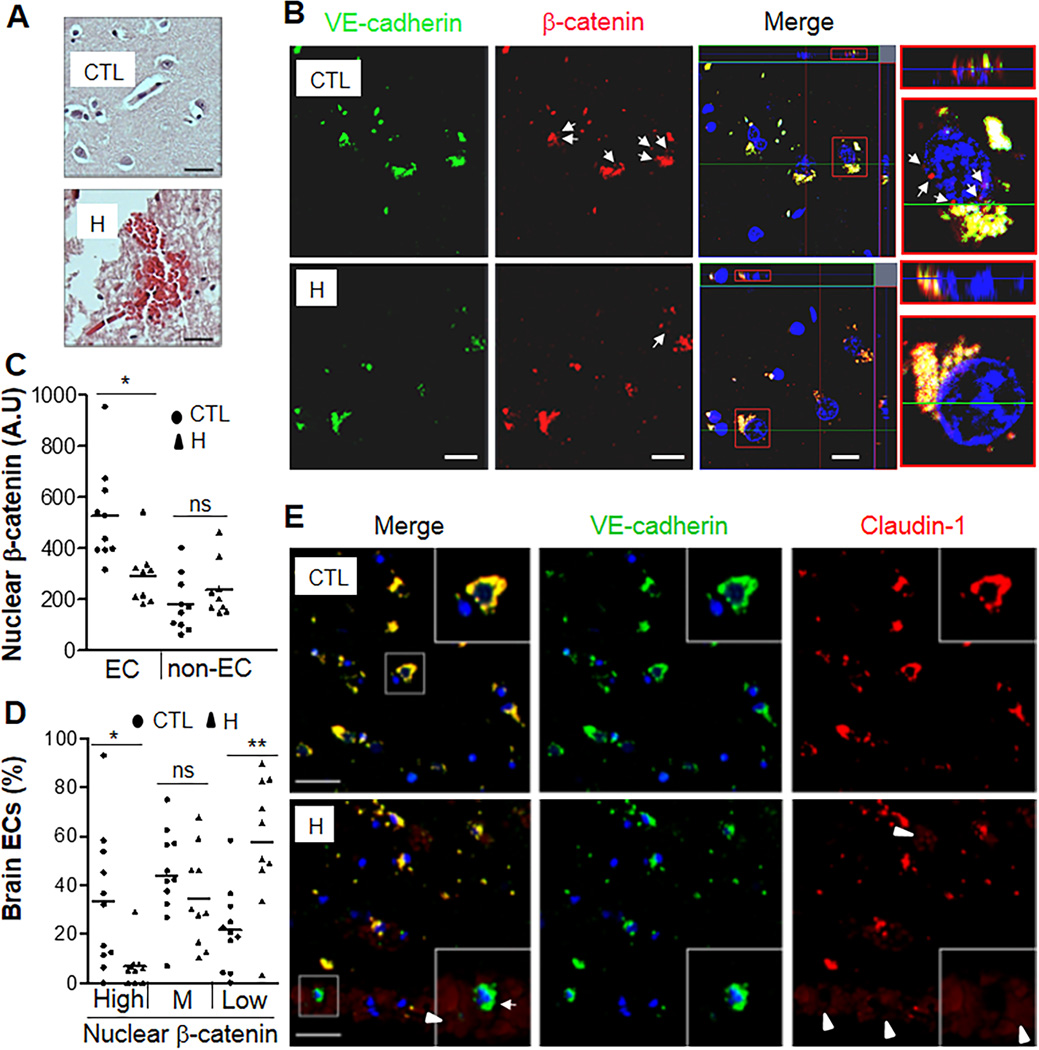
To address the possibility that defective β-catenin transcription activity and diminished Claudin-1 expression in iCKO brain ECs is relevant to the pathogenesis of hemorrhagic stroke in patients, we analyzed basal ganglia tissue collected at autopsies from patients dying of spontaneous non-traumatic intracerebral hemorrhage and other patients without evidence of brain disease (controls) (Supplemental Table 2). We observed multifocal perivascular petechial hemorrhaging within the basal ganglia of hemorrhagic patients (Figure 7A) as seen in iCKO mice (Supplemental Figure 3). On co-immunostained brain sections, tissue from hemorrhagic stroke patients showed diminished nuclear β-catenin staining, indicating defective β-catenin transcriptional activity in brain ECs but not in non-ECs (Figure 7 B–C). ~60% of the ECs from hemorrhagic patients expressed low level of nuclear β-catenin compared to ~20% of ECs from control samples. Less than 7% of ECs from hemorrhagic patients expressed high level of nuclear β-catenin compared to ~35% of ECs from control samples (Figure 7D). Accordingly, we also observed varying reductions of Claudin-1 expression in ECs from hemorrhagic patient samples, and some areas showed complete loss of Claudin-1 in brain ECs in association with localized hemorrhages (Figure 7E and Supplemental Figure 12).
Figure 7. Defective Claudin-1 expression in brain microvascular ECs of hemorrhagic stroke patients.
(A) H&E staining demonstrating petechial hemorrhages in brain sections of these patients. H, hemorrhagic stroke patients; CTL, controls. Scale bars, 30µm. (B) Representative micrographs of co-immunostaining of VE-cadherin (green) and β-catenin (red) of brain sections by confocal microscopy. Nuclei were counterstained with DAPI (blue). Arrows, nuclear expression of β-catenin in microvascular ECs. The merged image displayed the ortho view of Z-stack. The typical cells in red rectangle were blown-out and shown in XY and XZ planes. Scale bars, 10µm. (C, D) Quantification of nuclear β-catenin expression in brain microvascular ECs. The fluorescent intensity of β-catenin in nuclei was quantified using the software Zen2 from Zeiss.. Nuclear β-catenin expression in ECs but not in non-ECs of hemorrhagic tissues was markedly decreased compared to control tissues (C). **, P = 0.0037 (Mann-Whitney 2-tailed test). ns, not significant. Based on the median value of fluorescent intensity of nuclear β-catenin in ECs of control samples, three levels, High [> (median value+1/2SD)], Medium (M), and Low [< (median value-1/2SD)] were set. High level of nuclear β-catenin expression was observed in ~35% of brain ECs in control tissues whereas < 7% in hemorrhagic samples (D). ~60% brain ECs in hemorrhagic patient samples expressed only minimal (Low) nuclear β-catenin (D). *, P = 0.014; **, P = 0.0076 (Mann-Whitney 2-tailed test). ns, not significant. Bars represent mean. (E) Representative micrographs of co-immunostaining of VE-cadherin (green) and Claudin-1 (red) of brain sections from controls, and hemorrhagic stroke patients. Arrowheads, red blood cells (light red), indicating hemorrhages; Arrow, microvessel with absent Claudin-1 expression. Please note the leaked red blood cells (arrowhead) surrounding the microvessel absent Claudin-1 expression shown in the insert, indicating BBB breakdown. Scale bars, 30µm.
Discussion
We have identified the prerequisite role of β-catenin signaling in brain ECs in maintaining the integrity of the adult BBB through transcriptional control of expression of Claudin-1 and -3. Endothelial inactivation of β-catenin in adult mice results in disruption of BBB integrity. BBB breakdown in iCKO mice induces severe seizures accompanied by neuronal injury, multiple brain petechial hemorrhages, CNS inflammation, and postictal death. We also observed a marked decrease of Claudin-1 expression and diminished expression of nuclear β-catenin in brain ECs in association with localized hemorrhages in hemorrhagic stroke patients.
The BBB is comprised of specialized brain ECs and regulated by surrounding pericytes, and astrocytes.9–13 It has been shown that pericyte deficiency increases the permeability of the adult BBB to water and tracers with different molecular sizes through increased endothelial transcytosis.10 Pericytes also regulate BBB-specific gene expression in brain ECs and induce polarization of astrocyte end-feet surrounding CNS blood vessels. These data show that loss of pericytes affects adult BBB function but fails to induce BBB breakdown. Astrocytes also regulate the integrity of adult BBB through secreting Sonic hedgehog which binds Hedgehog receptors expressed in brain ECs.9 However, genetic disruption of these pathways was not sufficient to induce cerebral hemorrhaging and compromised lifespan in adult mice.9–11 Our data have shown that loss of β-catenin induces BBB breakdown which results in not only marked increase of permeability but also severe seizures, multiple brain petechial hemorrhages, and postictal death. Thus, β-catenin is the critical endogenous mediator in brain ECs maintaining the integrity of the adult BBB and CNS homeostasis. Previous studies employing the β-galactosidase reporter mice have shown that β-catenin signaling is strongly activated during brain angiogenesis and BBB formation during embryonic development but not in adult vessels,23, 32 suggesting that the role of endothelial β-catenin signaling in regulating adult BBB function is negligible. Unexpectedly, our data provide unequivocal evidence that β-catenin signaling, albeit at low levels, in adult brain ECs is essential for maintenance of the BBB integrity. Canonical Wnt factors including Wnt1, Wnt3, Wnt3a, Wnt4, and Wnt7a/b secreted by astrocytes have been implicated in BBB formation during development.29,30 Future studies are warranted to identify the Wnt factor(s) mediating the low but fundamentally important β-catenin signaling activity in adult brain ECs.
The most important novel findings of our data are that endothelial β-catenin is essential for maintenance of the integrity of adult BBB and loss of endothelial β-catenin in adult mice induces severe seizures accompanied with neuronal injury, CNS inflammation and petechial hemorrhaging. We observed neuronal injury prominent at site of hemorrhage and in CA1 and CA3 regions of the pyramidal layer of the hippocampus and dentate hilus neurons in iCKO mice, which resembled hippocampal injury seen in patients with seizure activity.40, 41 We also observed marked increase of expression of proinflammatory cytokines and infiltration of leukocytes in iCKO mice exhibiting seizure. Our data support the concept that inflammation and extravasation of plasma solutes and fluid secondary to BBB dysfunction is a key factor mediating aberrant neuronal discharge and seizure.42 High levels of pro-inflammatory cytokines are known to have toxic CNS effects.43 Thus, our study, to the best of our knowledge, provide the first genetic evidence that β-catenin transcriptional activity in brain ECs plays a fundamental role in maintaining the homeostasis of the CNS in adulthood.
Although β-catenin is both a structural protein of endothelial AJs linking AJs to the actin cytoskeleton25 and a transcription factor central to the Wnt signaling pathway,26–28 our data show that β-catenin deficiency in ECs did not disrupt pulmonary vascular integrity at the ultrastructural level as well as permeability to tracers whereas it caused BBB breakdown in brain ECs, suggesting the essential role of β-catenin transcriptional activity in maintaining the integrity of TJs. Consistent with our observation, previous findings show that loss of β-catenin expression in ECs induces expression of other junctional proteins including plakoglobin and desmoplakin which may compensate the function of β-catenin as an AJ protein and thus maintains the integrity of AJs in the pulmonary vasculature.44 Different from lung and other vascular beds, cerebrovascular integrity is mainly dependent on the TJs. Indeed, we observed that expression of both Claudin-1 and -3 were diminished in brain ECs of adult iCKO mice. Furthermore, we have demonstrated that Claudin-1 is the transcriptional target of β-catenin signaling in brain ECs. Consistent with our observation, previous studies also identify Claudin-3 as the transcriptional target of β-catenin signaling in brain ECs.9, 23 Although both Claudin-5 and 12 are expressed in brain ECs,19 our data show that their expression are not regulated by β-catenin signaling in adult ECs. Our data also show that expression of other junctional molecules enriched in brain ECs19 are not affected in iCKO mice. Thus, our data demonstrate the critical role of β-catenin signaling in maintaining the integrity of adult BBB through transcriptional regulation of Claudin-1 and 3 expression. However, previous study using cultured mouse brain ECs has shown that β-catenin activation by Wnt3a upregulates Claudin-3 but not Claudin-1 expression.23 The discrepancy is likely owing to the culture condition of brain ECs. The extended culture of brain ECs (2 days of puromycin resistance selection followed by 1:2 passaging and grown for another 6 days) may affect the gene expression profile whereas in our protocol, we cultured brain ECs for only two days. It has been shown that prolonged culture of brain ECs results in loss of the BBB endothelial properties.45,46 Additionally, it is also possible that Claudin-1 is expressed only in the mature BBB of adult mice while minimal or absent in BBB of postnatal (up to P14) mice.23 In contrast to our observation, Claudin-5 expression is regulated by β-catenin transcriptional activity in brain ECs in vivo during postnatal development (but not in cultured brain ECs).23,32 These data suggest that brain ECs at different developmental stages may express different TJ molecules due to potential differences in interaction with surrounding pericytes and astrocytes. It has been shown that astrocyte association with the BBB is considerably reduced at postnatal day 19 and results in perivascular gliosis in adult mice.9 Thus, β-catenin signaling during embryonic and postnatal development as well as adulthood mediates the expression of a different set of TJ proteins responsible for BBB induction, maturation and maintenance of integrity, respectively.
Claudin-1 is an integral protein to TJ formation. Genetic disruption of Cldn1 results in breakdown of epithelial barrier and neonatal death (within 1 day of birth) accompanied by excessive transepidermal water loss.47 However, loss of Claudin-5 only alter the BBB junctional properties and induces tracer leakage in a size-selective manner24 but not complete breakdown, i.e., neither leakage of red blood cells nor induction of death as seen in adult ctnnb1 iCKO mice. Given that TJs are the characteristic features shared by BBB and epithelial barrier, these data suggest that Claudin-1 is essential for the integrity of BBB. We observed that expression of Claudin-1 in brain ECs of adult mice was regulated by β-caetnin signaling in a dose-dependent manner. In brain ECs collected at day 7 post-tamoxifen treatment when β-catenin expression was only partially inhibited, Claudin-1 expression was decreased about 60%. However, the permeability of BBB in these mutant mice with 60% loss of Claudin-1 was markedly increased indicating BBB dysfunction. In iCKO mice developing severe seizure, i.e. complete loss of β-catenin, Claudin-1 expression in brain ECs was completely inhibited. Consistently, the TJs and BBB were breakdown leading to petechial hemorrhaging in these mouse brain tissues. These data provide the molecular insights about the pathogenesis of intracerebral hemorrhaging associated with BBB dysfunction in patients.6, 7 We also observed decreased Claudin-1 expression in brain ECs of stroke patients with spontaneous non-traumatic intracerebral hemorrhage. Consistently, nuclear β-catenin expression was diminished in brain ECs of these patients. Given that reduced Claudin-1 expression seen in pre-seizure iCKO mice induced BBB dysfunction and transvascular protein leakage, the decreased Claudin-1 expression is a likely cause of petechial hemorrhages in these patients secondary to BBB dysfunction. Depending on the region of the brain as well as the conditions inducing BBB dysfunction, Claudin-1 expression has been shown upregulated and downregulated. For example, in the ischemia/reperfusion-induced BBB damage model48 Claudin-1 was upregulated in the hippocampus but downregulated in the cerebellum while no change in the cerebral cortex following 48h reperfusion. However, under ischemic condition, Claudin-1 was downregulated in the hippocampus but upregulated in the cerebellum while no change in the cerebral cortex. Our observation of downregulation of Claudin-1 in basal ganglia tissue of patients with hemorrhagic stroke is consistent with diminished nuclear β-catenin signaling and its role in maintaining adult BBB integrity.
In summary, we show here that endothelial β-catenin signaling is required for maintenance of the adult BBB integrity. BBB disruption secondary to impaired β-catenin transcriptional activity in ECs induced neuronal injury, CNS inflammation and petechial hemorrhages resulting in seizures and postictal death in adult mice. We also observed reduced nuclear β-catenin and Claudin-1 expression in brain vascular ECs of hemorrhagic stroke patients. Our data suggest that BBB dysfunction secondary to defective β-catenin transcription activity is a key pathogenic factor in hemorrhagic stroke, seizure activity and CNS inflammation. Thus, development of means of activation of β-catenin transcription activity in brain ECs to enhance the BBB integrity is a potential novel strategy for treatment of these CNS diseases.
Supplementary Material
Clinical Perspectives.
Blood-brain barrier (BBB) dysfunction has been implicated in a variety of CNS diseases such as Alzheimer’s disease, epilepsy, multiple sclerosis, and stroke. However, little is known about the molecular mechanisms responsible for integrity of the adult BBB. In this study, we provide the first genetic evidence that BBB disruption secondary to loss of endothelial β-catenin induces cerebral hemorrhage, seizures and death following seizure activity in adult mice. We have delineated the requisite role of β-catenin signaling in maintaining the restrictive nature of BBB and transcriptional regulation of the specific tight junction proteins Claudin-1 and -3. Our results also provide evidence of the clear association of intracerebral hemorrhage with reduced nuclear β-catenin and Claudin-1 expression in endothelial cells from hemorrhagic stroke patients. Our data suggest that BBB dysfunction secondary to defective endothelial β-catenin transcription activity is a key pathogenic factor in hemorrhagic stroke, CNS inflammation, and seizure. Thus, development of means of activation of β-catenin transcription activity in brain ECs to enhance the BBB integrity is a potential novel strategy for treatment of these CNS diseases.
Acknowledgements
We thank Drs. Yidan Zhao, Manish Mittal, and Vivian Shi of University of Illinois College of Medicine for their technical advice and discussions, David X. Zhao of Deerfield High School, IL for his technical support. Drs. Klara Valyi-Nagy and Grace Guzman of the UIC Tissue Repository for providing human autopsied tissues.
Funding Sources: This work was supported in part by NIH grants R56HL085462, R01HL123957, and R01HL125350 to Y.Y. Z and NIH grant T32HL007829 to K. T.
Footnotes
Disclosures: None.
References
- 1.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Dodelet-Devillers A, Cayrol R, van Horssen J, Haqqani AS, de Vries HE, Engelhardt B, Greenwood J, Prat A. Functions of lipid raft membrane microdomains at the blood-brain barrier. J Mol Med (Berl) 2009;87:765–774. doi: 10.1007/s00109-009-0488-6. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 4.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Abbott NJ, Romero IA. Transporting therapeutics across the blood-brain barrier. Mol Med Today. 1996;2:106–113. doi: 10.1016/1357-4310(96)88720-x. [DOI] [PubMed] [Google Scholar]
- 6.Alemany M, Stenborg A, Terent A, Sonninen P, Raininko R. Coexistence of microhemorrhages and acute spontaneous brain hemorrhage: correlation with signs of microangiopathy and clinical data. Radiology. 2006;238:240–247. doi: 10.1148/radiol.2381040551. [DOI] [PubMed] [Google Scholar]
- 7.Altmann-Schneider I, Trompet S, de Craen AJ, van Es AC, Jukema JW, Stott DJ, Sattar N, Westendorp RG, van Buchem MA, van der Grond J. Cerebral microbleeds are predictive of mortality in the elderly. Stroke. 2011;42:638–644. doi: 10.1161/STROKEAHA.110.595611. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg GA. Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1139–1151. doi: 10.1038/jcbfm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 10.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 11.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, Zlokovic BV. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 13.Tao-Cheng JH, Nagy Z, Brightman MW. Tight junctions of brain endothelium in vitro are enhanced by astroglia. J Neurosci. 1987;7:3293–3299. doi: 10.1523/JNEUROSCI.07-10-03293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gloor SM, Wachtel M, Bolliger MF, Ishihara H, Landmann R, Frei K. Molecular and cellular permeability control at the blood-brain barrier. Brain Res Brain Res Rev. 2001;36:258–264. doi: 10.1016/s0165-0173(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 15.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki Y, Okawa K, Yano T, Tsukita S, Tsukita S. Optimized proteomic analysis on gels of cell-cell adhering junctional membrane proteins. Biochemistry. 2008;47:5378–5386. doi: 10.1021/bi8002567. [DOI] [PubMed] [Google Scholar]
- 17.Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol. 2010;2:a002907. doi: 10.1101/cshperspect.a002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 19.Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One. 2010;5:e13741. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enerson BE, Drewes LR. The rat blood-brain barrier transcriptome. J Cereb Blood Flow Metab. 2006;26:959–973. doi: 10.1038/sj.jcbfm.9600249. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027:48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 22.Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim YJ, Kim KW. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–906. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- 23.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin) J Cell Biol. 1995;129:203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 28.Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, Taketo MM, Nathans J. Cannonical WNT signaling components in vascualr development and barrier formation. J Clin Invest. 2014;124:3825–3846. doi: 10.1172/JCI76431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reis M, Czupalla CJ, Ziegler N, Devraj K, Zinke J, Seidel S, Heck R, Thom S, Macas J, Bockamp E, Fruttiger M, Taketo MM, Dimmeler S, Plate KH, Liebner S. Endothelial Wnt/beta-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. J Exp Med. 2012;209:1611–1627. doi: 10.1084/jem.20111580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gothert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, Green AR, Gottgens B, Izon DJ, Begley CG. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104:1769–1777. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Sadikot RT, Adami GR, Kalinichenko VV, Pendyala S, Natarajan V, Zhao YY, Malik AB. FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J Exp Med. 2011;208:1473–1484. doi: 10.1084/jem.20102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dietrich WD, Ginsberg MD, Busto R, Watson BD, Yoshida S. Vascular aspects and hemodynamic consequences of central nervous system injury. Cent Nerv Syst Trauma. 1986;3:265–280. doi: 10.1089/cns.1986.3.265. [DOI] [PubMed] [Google Scholar]
- 36.Haseldonckx M, van Bedaf D, van de Ven M, van Reempts J, Borgers M. Vasogenic oedema and brain infarction in an experimental penumbra model. Acta Neurochir Suppl. 2000;76:105–109. doi: 10.1007/978-3-7091-6346-7_22. [DOI] [PubMed] [Google Scholar]
- 37.Mirza MK, Sun Y, Zhao YD, Potula HH, Frey RS, Vogel SM, Malik AB, Zhao YY. FoxM1 regulates re-annealing of endothelial adherens junctions through transcriptional control of beta-catenin expression. J Exp Med. 2010;207:1675–1685. doi: 10.1084/jem.20091857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 39.Brown RC, Morris AP, O'Neil RG. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007;1130:17–30. doi: 10.1016/j.brainres.2006.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazarati A, Lu X, Shinmei S, Badie-Mahdavi H, Bartfai T. Patterns of seizures, hippocampal injury and neurogenesis in three models of status epilepticus in galanin receptor type 1 (GalR1) knockout mice. Neuroscience. 2004;128:431–441. doi: 10.1016/j.neuroscience.2004.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sloviter RS. The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy. Ann Neurol. 1994;35:640–654. doi: 10.1002/ana.410350604. [DOI] [PubMed] [Google Scholar]
- 42.Marchi N, Granata T, Ghosh C, Janigro D. Blood-brain barrier dysfunction and epilepsy: pathophysiologic role and therapeutic approaches. Epilepsia. 2012;53:1877–1886. doi: 10.1111/j.1528-1167.2012.03637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang CX, Shuaib A. Involvement of inflammatory cytokines in central nervous system injury. Prog Neurobiol. 2002;67:161–172. doi: 10.1016/s0301-0082(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 44.Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, Kemler R, Dejana E. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gumbleton M, Audus KL. Progress and limitations in the use of in vitro cell cultures to serve as a permeability screen for the blood-brain barrier. J Pharm Sci. 2001;90:1681–1698. doi: 10.1002/jps.1119. [DOI] [PubMed] [Google Scholar]
- 46.Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 47.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, Threlkeld SW, Cummings EE, Juan I, Makeyev O, Besio WG, Gaitanis J, Banks WA, Sadowska GB, Stonestreet BS. Ischemia-reperfusion impairs blood-brain barrier function and alters tight junction protein expression in the ovine fetus. Neuroscience. 2012;226:89–100. doi: 10.1016/j.neuroscience.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.