Abstract
The metabolic fibroblast growth factors (FGFs), FGF1, FGF15/19, and FGF21 differ from classic FGFs in that they modulate energy homeostasis in response to fluctuating nutrient availability. These unique mediators of metabolism regulate a number of physiological processes which contribute to their potent pharmacological properties. Administration of pharmacological doses of these FGFs causes weight loss, increases energy expenditure, and improves carbohydrate and lipid metabolism in obese animal models. However, many questions remain regarding the precise molecular and physiological mechanisms governing the effects of individual metabolic FGFs. Here we review the metabolic actions of FGF1, FGF15/19, and FGF21 while providing insights into their pharmacological effects by examining known biological functions.
Keywords: FGF21, FGF15, FGF19, FGF1, Obesity, Metabolism
1. Metabolic FGFs
The fibroblast growth factor (FGF) family consists of 22 members involved in a myriad of functions including development, cancer, and metabolism. Classically, FGFs are considered intracrine or paracrine factors. A small subset of FGF members (FGF11-14) function intracellularly while the majority of FGFs are secreted factors [1]. Paracrine FGFs act locally and bind to a cognate FGF receptor, an interaction which is stabilized via heparan sulphate glycosaminoglycan binding. The FGF19 subfamily (FGF15/19, FGF21, FGF23), however, represent an atypical group of FGFs because they lack an affinity for heparan sulfates which allows them to freely diffuse away from their tissue of origin and serve as endocrine molecules [2]. Paracrine and endocrine FGFs signal through cell surface localized FGF receptors (FGFRs) belonging to the tyrosine kinase receptor family [1]. Upon FGF binding, an FGFR dimer forms resulting in receptor transphosphorylation and subsequent activation of downstream signaling cascades initiated through phosphorylation of FRS1/PLCγ1 or FRS2α/β [3]. Importantly, members of the FGF19 subfamily require a co-factor to initiate signaling, which in the case of FGF15/19 and FGF21, is a co-factor termed β-klotho [4–7], and in the case of FGF23, is a co-factor termed α-klotho (or Klotho) [8]. In the past decade, it was discovered that several FGFs including FGF1, FGF15/19 and FGF21, possess profound metabolic actions (Fig. 1). This review focuses on the metabolic actions of these FGFs and the development of pharmacological strategies to employ these FGFs in the treatment of metabolic disease.
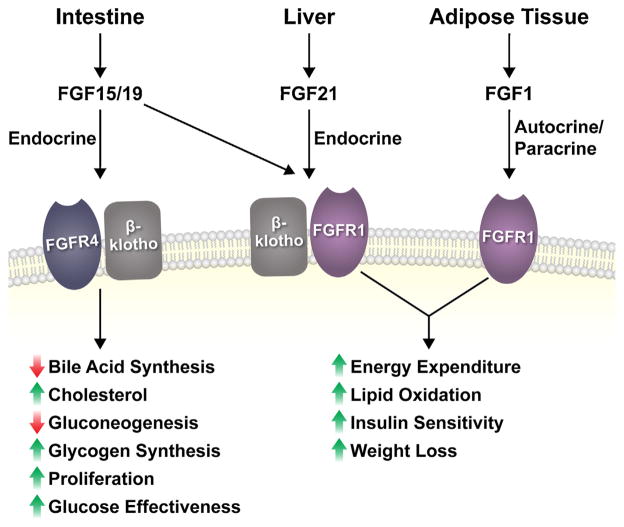
Fig. 1.
Summary of metabolic FGFs. The metabolic FGFs are produced by specific tissues and signal in either an endocrine or autocrine/paracrine fashion to target cells through FGFR4 or FGFR1, complexed with or without β-klotho, to regulate metabolism. FGF15/19 signaling through hepatic FGFR4/β-klotho regulates the indicated hepatic processes (except glucose effectiveness which occurs through central effects), while the direct cellular targets of FGF21 through FGFR1/β-klotho signaling remain unclear. Endogenous FGF1 acts in an autocrine/paracrine manner to signal through FGFR1 independent of β-klotho. Although the metabolic FGFs possess different physiological functions, they produce similar pharmacological actions in models of metabolic disease through a common FGFR1-mediated pathway.
2. Metabolic actions of FGF21
Multiple studies have demonstrated that extended administration of FGF21 to obese rodent or primate models improves glucose and lipid homeostasis, reduces adiposity, and increases weight loss without decreasing food intake [9–12] (Fig. 1). Remarkably, a single acute injection of FGF21 in either genetic- (i.e., ob/ob) or diet-induced obese mice significantly improves glucose homeostasis and insulin sensitivity [13] demonstrating therapeutic efficacy independent of a reduction in body weight. In humans, administration of an FGF21 analog, LY2405319, to obese patients significantly reduced plasma triglycerides, plasma cholesterol, plasma insulin, and body weight [14]. Therefore, FGF21 has profound effects on metabolic homeostasis which may be therapeutically targeted for the treatment of human disease.
FGF21 preferentially signals to tissues through a receptor complex consisting of FGFR1c and β-klotho (Fig. 1). While FGFR1 has a broad tissue distribution, β-klotho is expressed in a limited number of metabolic tissues including adipose tissue, liver, pancreas [15] and specific brain nuclei including the nucleus tractus solitarii (NTS), area postrema (AP), suprachiasmatic nucleus (SCN) [16] and paraventricular nucleus (PVN) [17]. Therefore, the tissue-specific effects of FGF21 are limited to those expressing β-klotho [18,19]. β-klotho functions as a scaffolding molecule forming a ternary complex with FGFR1c [5,7] and the C-terminus of FGF21, thereby allowing FGF21 to interact with FGFR1 and initiate signaling [20–22]. FGF21 activation of FGFR1 results in receptor transphosphorylation and phosphorylation of FRS2α which leads to activation of the MAPK signaling pathway and increased levels of ERK1/2 phosphorylation [4,7]. However, the precise signaling cascade(s) linking FGF21 to its metabolic actions remain unclear.
The expression and metabolic actions of FGF21 are uniquely affected by nutritional status [23] and stress of the organism [24,25]. In addition, FGF21 function is affected by other endocrine signals present in circulation in these contexts. Fgf21 mRNA expression is detectable in multiple tissues including the liver, white adipose tissue (WAT), brown adipose tissue (BAT), muscle, pancreas, and heart [26]. However, while FGF21 protein has been reported in the media from many of these cell types, circulating levels of FGF21 are derived primarily, if not exclusively, from the liver in vivo as circulating FGF21 levels are completely abolished in liver-specific FGF21 knockout mice [27]. In the following sections, we will review the major metabolic effects of FGF21 and will attempt to incorporate a physiological context for these actions.
2.1. Glucose metabolism
2.1.1. Acute FGF21 administration
A single injection of recombinant FGF21 significantly lowers plasma glucose levels in genetically obese (ob/ob) and diet-induced obese (DIO) mice without inducing hypoglycemia [13]. This acute effect is dramatic, lowering plasma glucose levels by over 50% in obese mice, an effect which lasts up to 6 h [13]. Several studies have demonstrated an important role for adipose tissues in mediating the acute metabolic effects of FGF21. Mice lacking either β-klotho [19] or FGFR1 in adipose tissue [28] do not respond to acute FGF21 administration. However, it is currently unclear which type(s) of adipocytes (i.e., white, brown, or brite/beige) FGF21 signals to in order to mediate its glucose lowering effects. Moreover, the molecular signaling mechanisms for this effect have not been completely determined. At the systems level, the acute glucose lowering effects of FGF21 are primarily due to enhanced glucose disposal and whole body glycolysis, and to a lesser extent, suppression of endogenous glucose production [13]. Using hyperinsulinemic-euglycemic clamps and radioactive 2-deoxyglucose uptake, two groups independently demonstrated that acute administration of FGF21 to DIO mice increased whole-body glucose uptake, markedly increased glucose infusion rate, and trended to decrease endogenous glucose production [13,19]. Consistent with these results, acute administration of a pegylated form of FGF21 to DIO mice significantly increased the rate of glucose disposal without effecting endogenous glucose production [29]. Assessment of the relative tissue-specific uptake of glucose at the end of the clamps differed between the studies, with one group observing increased glucose uptake only in brown adipose tissue (BAT) [19], and the other group reporting increased glucose uptake in multiple tissues including white and brown adipose tissue, heart, and skeletal muscle of DIO mice treated with FGF21 [13]. Yet, both studies clearly demonstrate that brown adipose tissue, and potentially other tissues, facilitate the ability of FGF21 to acutely and potently enhance insulin sensitivity. Interestingly, FGF21 acutely enhances insulin stimulated glucose uptake in primary brown adipocytes, but not primary white adipocytes [27], suggesting direct effects. Collectively, these studies suggest that the acute insulin-sensitizing effects of FGF21 are mediated by direct FGF21 signaling to adipose tissue and may involve both non-cell autonomous and cell autonomous mechanisms to regulate glucose homeostasis.
Two recent studies reported that adiponectin levels are induced in response to FGF21 administration, and that adiponectin is critical for the acute insulin-sensitizing effects of FGF21 [30,31]. However, a more recent study did not detect any changes in adiponectin levels either in vivo or in vitro in response to FGF21 [32]. In addition, circulating adiponectin levels are not altered under physiological conditions when circulating FGF21 levels are induced [27]. And finally, administration of a bispecific antibody agonist for the FGFR1/β-klotho complex acutely increased insulin sensitivity in diet-induced obese mice lacking adiponectin [33]. It is important to note that while FGF21 primarily functions to enhance peripheral glucose disposal [13,19,29], adiponectin functions to suppress hepatic glucose production [34,35]. Therefore, the modest decrease in endogenous glucose production following acute FGF21 administration is likely attributable to adiponectin, whereas the FGF21-mediated effects on peripheral glucose disposal are adiponectin-independent and function through enhanced insulin-stimulated glucose uptake.
Physiologically, FGF21 has multiple functions, one of which is to enhance insulin sensitivity. FGF21 is induced during fasting, refeeding, and overfeeding [23,27]. The function of FGF21 during these metabolically distinct conditions is impacted by the presence or absence of other endocrine signals. During fasting when insulin is absent or reduced, elevated circulating FGF21 regulates hepatic gluconeogenesis, beta-oxidation [36] and adipose tissue lipolysis [37]. However, during refeeding when both insulin and FGF21 levels are increased in circulation, FGF21 functions to maximize nutrient uptake by enhancing insulin-stimulated glucose uptake [27] (Fig. 2). During this time, FGF21 likely serves as a metabolic switch facilitating the transition from prolonged fasting into a refed state. By enhancing insulin-stimulated nutrient uptake while still acutely regulating aspects of the fasting response, FGF21 mediates energy homeostasis when nutrient availability is fluctuating [27].
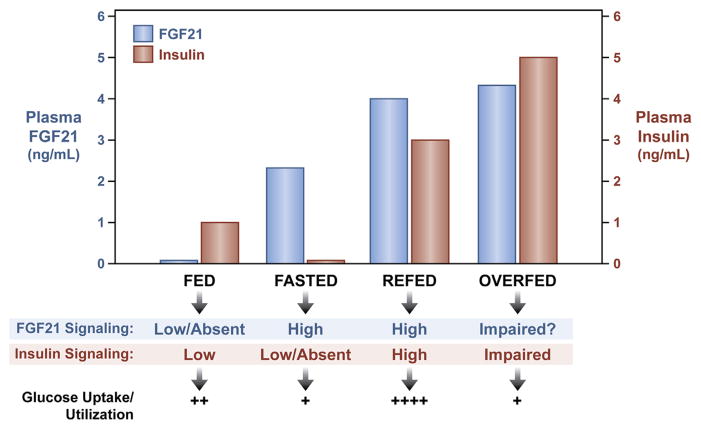
Fig. 2.
FGF21 function is altered by nutritional status. FGF21 expression is regulated by nutritional status and it may mediate its functions through signaling crosstalk with other endocrine factors like insulin. Endogenous plasma FGF21 and insulin levels under various physiological conditions are presented relative to their respective level of signaling and functional effect on glucose uptake and utilization.
In addition to refeeding, circulating FGF21 levels are elevated by overfeeding and insulin resistance [23] (Fig. 2). During overfeeding, as the nutrient load reaches excess, FGF21 may act in a compensatory manner to mitigate decreasing insulin sensitivity and facilitate insulin-stimulated disposal of excess glucose to brown adipose tissue [27] (Fig. 3). Although the transcriptional mechanisms responsible for hepatic FGF21 induction during overfeeding have not been clearly defined, the presence of the glucose activated transcription factor, ChREBP, binding element in the FGF21 promoter could function to induce both hepatic and plasma FGF21 protein levels when plasma glucose levels are abnormally elevated [38,39]. At some point, however, tissues may become “FGF21 resistant” [40], resulting in the gradual increase in plasma FGF21 levels observed with extended high fat diet feeding without an improvement of metabolic function. Importantly, while tissues of obese animals may have impaired signaling in response to physiological levels of FGF21, tissues are not completely resistant to FGF21 function. Compared to wild-type DIO mice that have impaired FGF21 signaling, DIO mice lacking circulating FGF21 have worsened glucose and insulin tolerance and increased hepatic steatosis [27] which demonstrates that FGF21 still provides some protective metabolic function under these conditions. In addition, obese animal models are still responsive to pharmacological levels of exogenous FGF21, much like insulin administration during insulin resistance. Indeed, the ED50 value for the glucose lowering effects of FGF21 in insulin resistant (and perhaps FGF21 resistant) mice, are well above physiological levels [41].
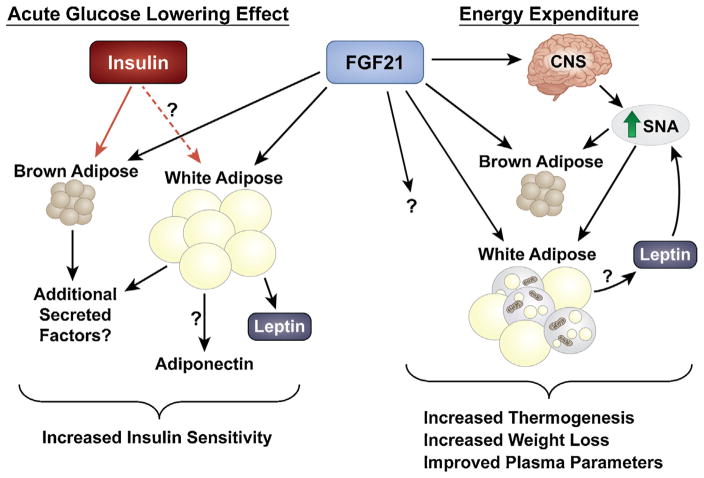
Fig. 3.
Model for the pharmacological effects of FGF21. Acute pharmacological administration of FGF21 to models of metabolic disease increases insulin sensitivity whereas chronic administration increases energy expenditure, weight loss and insulin sensitivity. The acute insulin sensitizing effects of FGF21 requires functional adipose tissue(s). FGF21 enhances insulin action to increase peripheral glucose disposal, and functions to promote the secretion of adipokines including adiponectin and leptin. Extended administration of FGF21 increases energy expenditure through actions on the central nervous system (CNS) and adipose tissues. FGF21 increases sympathetic nerve activity (SNA) which stimulates brown adipose tissue thermogenesis and “browning” of white adipose tissue. Leptin also increases sympathetic nerve activity and leptin may be important for the effects of FGF21 on energy expenditure. FGF21 may also affect energy expenditure through a yet unidentified pathway. FGF21-mediated increases in energy expenditure increases weight loss and disposes of excess nutrients to improve metabolic profiles.
2.1.2. Chronic FGF21 administration
Extended administration of FGF21 increases energy expenditure and weight loss which also leads to increases in insulin sensitivity and glucose uptake [10,11] (Fig. 3). These effects on energy expenditure and weight loss are more pronounced in diet-induced models of obesity compared to genetic, leptin-deficient models (i.e., ob/ob). For example, pharmacological administration of FGF21 to DIO mice reduced body weight by ~20% when administered for 10 days, whereas ob/ob mice receiving the same dose of FGF21 for 22 days had an ~5% increase in body weight compared to 10% increase by controls [41]. Thus, in leptin-deficient mice, FGF21 reduces weight gain compared to increasing weight loss in DIO mice. It is unclear if these effects are due to loss of leptin in ob/ob mice or the gain of specific fuels/calories by high fat diet. It will be interesting to determine whether FGF21 signaling impacts leptin’s ability to increase energy expenditure [42] similar to FGF21 enhancing insulin action to mediate the acute glucose lowering effects (Fig. 3). Notably, obese humans receiving the FGF21 analog LY2405319 for 28 days had an approximate 2% decrease in body weight [14], which demonstrates that the metabolic effects of FGF21 are therapeutically applicable to human disease. While the reduction in plasma glucose levels in this human study trended lower and did not achieve statistical significance, insulin levels significantly decreased, suggesting improved insulin sensitivity [14]. Glucose tolerance, however, was not assessed in these patients.
Compared to the hyperinsulinemic-euglycemic clamp studies in rodents following acute FGF21 administration, clamp studies in DIO mice treated with recombinant FGF21 for three weeks revealed an increased glucose infusion rate, decreased basal and clamp hepatic glucose production, and increased glucose turn-over and glycogen synthesis, demonstrating enhanced insulin sensitivity and overall improved glucose handling compared to vehicle treated controls [10]. Enhanced glucose uptake determined by 2-deoxyglucose tracing at the end of these clamp studies also revealed enhanced FGF21-mediated glucose uptake into multiple tissues including white and brown adipose tissues, heart and skeletal muscle [10]. Similar clamp data pertaining to glucose flux and glucose uptake were reported for lean and DIO mice infused with recombinant FGF21 for seven days [43]. In addition to clamp studies, assessment of tissue-specific glucose uptake by [18F]-FDG/PET imaging in DIO mice revealed that brown adipose tissue, but not muscle, is a major site of glucose disposal following FGF21 administration for 2 weeks [44].
To determine which tissue(s) FGF21 directly signals upon to mediate its metabolic actions, two independent groups utilized tissue-specific FGF21 receptor knockout mice. The first group used β-klotho conditional knockout mice to remove β-klotho from the nervous system (β-klothofl/fl;Camk2a-Cre), and found that the effects of FGF21 on body weight and insulin sensitivity were lost in DIO β-klothofl/fl;Camk2a-Cre mice [45]. This study also demonstrated that administration of FGF21 directly into the brain via intracerebroventricular (ICV) injection increased sympathetic nerve activity, which functions to increase thermogenesis in adipose tissues [45]. Consistent with FGF21 acting on the nervous system, previous studies in rats demonstrated that ICV administration of FGF21 increases energy expenditure [46]. In contrast, however, a second group generated adipose-specific FGFR1 knockout mice (FGFR1fl/fl;aP2-Cre) and determined that DIO FGFR1fl/fl; aP2-Cre mice were refractory to the effects of FGF21 on energy expenditure and lowering of plasma insulin, glucose, and triglycerides [28]. In addition, the authors state, but do not show, that ablation of FGFR1 from neurons (using a Nestin-Cre) did not impair the metabolic actions of FGF21 [28]. Consistent with FGF21 acting directly on adipose tissue, FGF21 administration to lipodystrophic mice (aP2-SREBP-1c transgenic) lacking mature adipocytes did not affect body weight or glucose homeostasis [47,48]. Therefore, both adipose tissue and the central nervous system have been implicated as direct target tissues mediating the effects of FGF21 on energy expenditure (Fig. 3), but additional studies are necessary to delineate the cellular target(s) of FGF21 action.
The molecular mechanism(s) underlying the metabolic effects of extended FGF21 administration are also unclear although significant insight has been provided by a number of recent studies. The glucose lowering effect of extended FGF21 administration is independent of hepatic glucose production or improved hepatic insulin sensitivity. FGF21 effectively lowered plasma glucose levels in lean and diet-induced obese liver insulin receptor knockout mice which maintain elevated hepatic glucose production [49]. Alternatively, a number of studies have reported that extended administration of FGF21 induces a thermogenic gene expression profile in both white and brown adipose tissue [23,50] including induction of uncoupling protein 1 (UCP1), a specialized inner mitochondrial membrane protein which dissipates chemical energy as heat. These effects on gene expression also occur in vitro in differentiated brown and white adipocytes treated with FGF21 [51,52], suggesting FGF21 signals directly to these tissues to mediate a thermogenic response. The energy expending effects of FGF21, therefore, have been proposed to be mediated by adipose tissue thermogenesis. While the exact physiological context for these thermogenic effects are unclear, FGF21 has been shown to increase adipose thermogenic gene expression by increased hepatic production in neonates in response to milk [53], and being produced from adipose tissue in response to cold [52,54–56]. In addition, it was also recently determined that FGF21 is induced in rodents and humans in response to dietary protein restriction [57]. Protein restriction increases energy expenditure and decreases adiposity [57,58], both effects which require FGF21 [57].
While adipose-tissue thermogenesis has been proposed to mediate the energy expending effects of FGF21, two recent studies demonstrated that UCP1 is not required for the effects of FGF21 on energy expenditure in vivo [59,60]. In addition, the importance of brown adipose tissue in mediating the effects of FGF21 has been questioned due to maintenance of FGF21 efficacy in mice following the surgical excision of intrascapular brown adipose tissue [43,44] Together, these recent data suggest that adipose-mediated thermogenesis may not be responsible for the energy expending effects of FGF21. However, UCP1 KO mice have compensatory mechanisms of thermogenesis [61], and it is possible that FGF21-mediated increases in energy expenditure involve both UCP1-dependent and -independent mechanisms within adipose tissue. In addition, the studies involving surgical removal of intrascapular brown adipose tissue do not demonstrate elimination of all brown adipose tissue, making it premature to conclude that FGF21 efficacy does not require brown adipose tissue. Finally, inducible brown adipocytes (i.e., beige/brite cells) in subcutaneous fat have high thermogenic capacity [62] and are also activated by FGF21 [23]. Therefore, additional studies are necessary to determine the mechanism(s) for the energy expending effects of FGF21.
2.2. Lipid metabolism
Administration of FGF21 to DIO mice dramatically improves hepatic and peripheral lipid metabolism including reversing hepatic steatosis, reducing adiposity, lowering plasma triglycerides, non-esterified fatty acids and cholesterol while increasing plasma β-hydroxybutarate levels [9–11] (Fig. 1). In two separate human studies, FGF21 analogs significantly improved lipid levels in patients. In the first study, the FGF21 analog LY2405319 significantly reduced plasma triglycerides, plasma LDL, and increased HDL in a dose dependent fashion [14]. The second study using a different FGF21 analog, PF-05231023, for a shorter duration of just 14 days, also possessed marked lipid lowering effects including significantly decreased triglycerides, total cholesterol and LDL cholesterol, and increased HDL cholesterol [63]. Physiologically, plasma FGF21 levels are induced and produced from the liver during fasting [27] in response to fatty acid-mediated activation of the nuclear hormone receptor peroxisome proliferator-activated receptor α (PPARα) [37,64]. Consistent with a role during fasting, FGF21 is sufficient to increase hepatic gluconeogenesis, beta-oxidation, and ketogenesis in chow fed lean mice, whereas loss of FGF21 impairs these hepatic processes during fasting [36]. This ability of FGF21 to stimulate hepatic lipid oxidation increases hepatic insulin sensitivity in obese animal models by decreasing both hepatic diacylglycerol [43] and ceramide [30] levels.
FGF21 enhances insulin action, and in models of hyperinsulinemia, FGF21 functions to enhance insulin-stimulated suppression of lipolysis [65] and hepatic gluconeogenesis [10]. Gene profiling of obese mice treated with FGF21 revealed reduced hepatic lipogenic and gluconeogenic gene expression while also reducing active levels of SREBP-1c and target genes involved in hepatic glycolysis, de novo fatty acid synthesis and triglyceride synthesis [10,11]. Therefore, the pharmacological properties of FGF21 on lipid metabolism are likely comprised of its physiological actions during fasting, refeeding and overfeeding, and may explain why humans treated with the FGF21 analog, LY2405319, showed characteristics of improved insulin sensitivity yet had elevated ketones and non-significant changes in plasma glucose levels [14].
The effect of FGF21 on hepatic metabolism, however, does not appear to be due to direct action on the liver since FGFR1 is not expressed in hepatocytes [15] and treatment of hepatocytes [17,36] or isolated perfused livers with FGF21 [36] does not recapitulate the effects observed in vivo. Instead, extrahepatic mechanisms regulate the effects on hepatic metabolism including a liver-brain axis for increasing gluconeogenesis [17], decreasing cholesterol and triglycerides [16], and a liver-to-adipose axis for regulating cholesterol levels [28] and suppressing hepatic glucose production [30,31]. In addition, it is likely that the improvements in dyslipidemia due to FGF21 treatment occurs secondary to its enhancement of insulin sensitivity and energy expenditure.
2.3. Caveats of FGF21 therapy
In contrast to the beneficial effects of exogenous FGF21, pharmacological administration or overexpression of FGF21 have been reported to cause detrimental effects including bone loss [66], impaired fertility in females [67], and altered circadian rhythm [16]. In addition, overexpression of FGF21 inhibits growth [68]. However, unlike other FGFs, FGF21 is not mitogenic [9]. Since the effects of FGF21 on fertility and circadian rhythm requires central signaling [16,67], these safety concerns may be eliminated by developing FGF21-based therapies that do not cross the blood-brain barrier. Importantly, the metabolic effects of FGF21 are retained in animal models of metabolic disease using modified variants of FGF21 that are not expected to cross the blood brain barrier [29,69,70]. However, as central FGF21 signaling is also implicated in the therapeutic effects of FGF21 [45], long-term safety studies need to be conducted with these FGF21 variants.
3. Metabolic actions of FGF15/19
FGF15 and FGF19 are mouse–human orthologues which share ~50% amino acid identity [71]. Despite this low homology, both FGF15 and FGF19 are produced from the ileum of the small intestine, enter circulation to regulate metabolism [72,73], and both similarly regulate hepatic gene expression in mice [74,75]. Therefore, FGF15 and FGF19 (FGF15/19) will be referred to interchangeably unless referring to a particular species. Unlike FGF21 which preferentially signals through FGFR1, FGF19 interacts with and activates both FGFR4 and FGFR1 in vitro and in vivo [4,6], and in both cases, requires β-klotho to mediate metabolic effects [72,76] (Fig. 1). FGF15/19 levels are induced post-prandially in epithelial cells of the small intestine [74] and are then released into portal circulation to regulate bile acid, carbohydrate and lipid metabolism (Fig. 1). The following sections will examine the multiple roles of FGF15/19 in metabolism and disease.
3.1. Bile acid metabolism
A major physiological role for FGF15/19 is the regulation of bile acid metabolism. Bile acids are synthesized from cholesterol in the liver and stored in the gall bladder [77]. Following a meal, bile acids are released from the gall bladder into the intestine where they function to emulsify lipid for absorption. Bile acid production in the liver and their reabsorption in the small intestine are crucial due to their high energetic cost of generation and their toxicity as detergents. Activation of the nuclear hormone receptor farnesoid X receptor (FXR) in the ileum by bile acids results in the transcription and production of FGF15/19 from the intestine into hepatic portal circulation [74] (Fig. 4). In mice, ileal Fgf15 mRNA levels are maximally induced 1 hour post-prandially [75], and in humans, serum FGF19 levels are increased 2–3 hours following a meal [73]. FGF15/19 subsequently binds and signals through the hepatic FGFR4/β-klotho receptor complex to suppress further production of bile acids by decreasing expression of CYP7A1, the rate-limiting enzyme regulating bile acid production [74]. Treatment of hepatocytes with FGF15/19 represses Cyp7a1 mRNA expression [74], and the effects of FGF15 on bile acid metabolism are lost in liver-specific β-klotho knockout mice [72], which demonstrate direct actions on the liver. Bile acid pools are regulated by circadian rhythm [73], and plasma FGF15 and hepatic CYP7A1 levels are negatively correlated throughout the day [72,78]. Recently the transcription factor Kruppel-like factor 15 (KLF15) was found to repress Fgf15 mRNA expression in intestinal epithelial cells thus regulating circadian expression of FGF15 and consequently CYP7A1 (Fig. 4) [78]. This study provides mechanistic insight into the regulation of FGF15 expression and the factors contributing to the changes in bile acid production throughout the day. In humans, FGF19 expression is increased by oral administration of bile acids [73] and is suppressed by bile acid sequestration [79] which demonstrates the conservation of this pathway in humans. Thus, this intestine-to-liver hormonal axis mediated by FGF15/19 serves as an important negative feedback loop to regulate bile acid homeostasis.
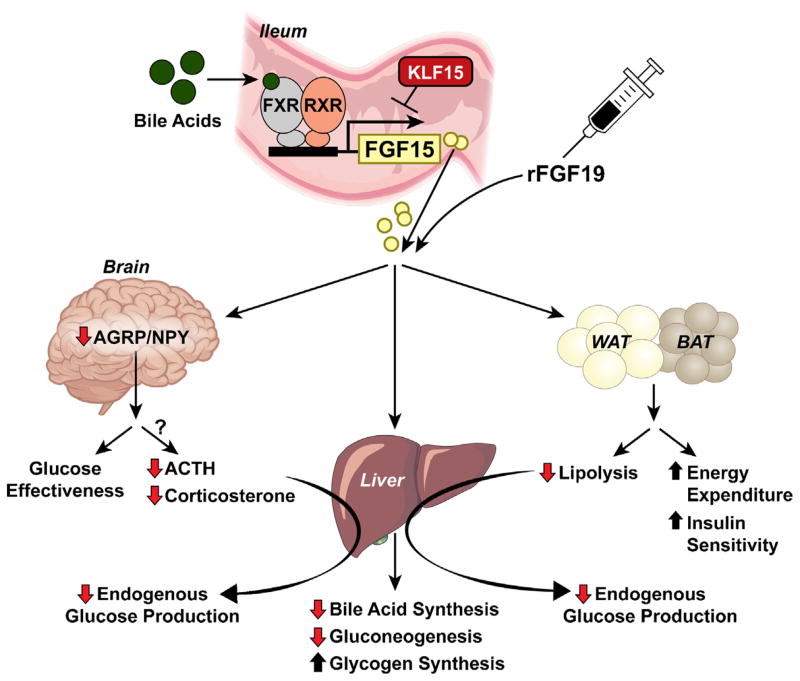
Fig. 4.
Model for the physiological and pharmacological actions of FGF15/19. FGF15 is produced from the ileum of the small intestine in response to bile acid mediated activation of FXR, whereas FGF15 expression is repressed by KLF15. Once released into circulation, or administered pharmacologically, FGF15/19 acts on multiple tissues including the liver, adipose tissues and central nervous system to elicit specific metabolic effects. FGF15/19 can act directly on the liver to decrease bile acid synthesis, decrease hepatic gluconeogenesis and increase glycogen synthesis. FGF15/19 also signals to the brain to decrease AGRP/NPY neuron activity which increases glucose effectiveness. In addition, FGF15/19 signaling in the CNS suppresses the HPA axis (decreases ACTH and corticosterone), which reduces hepatic glucose production through decreased corticosterone levels and whole body lipolysis. FGF15/19 action on white and brown adipose tissues (WAT and BAT, respectively) increases energy expenditure and insulin sensitivity.
3.2. Glucose and lipid metabolism
The effects of FGF19 on carbohydrate and lipid metabolism were first identified in mice constitutively overexpressing FGF19 [80]. These mice displayed decreased adiposity, increased energy expenditure, and improved plasma metabolite profiles [80]. Consistent with this study, peripheral administration (i.e., intraperitoneal (i.p.) or subcutaneous (s.c.) injection) of FGF19 to genetic or diet-induced obese animal models decreases body weight and adiposity while lowering circulating glucose and lipid levels [81–84]. However, unlike the effects on bile acid metabolism, the pharmacological effects of FGF19 are mediated through FGFR4-independent mechanisms in vivo [84] (Fig. 1). The ability of FGF19 to improve glucose homeostasis was retained in FGFR4 knockout mice [84], and wild-type mice receiving a mutant FGF19 variant, which specifically activated FGFR4, did not improve glucose metabolism in obese mice [85]. Since the metabolic FGFs signal through a common FGF receptor (i.e., FGFR1) [48] and since FGF19 and FGF21 both require the co-receptor β-klotho, it is plausible that the pharmacological effects of FGF19 may not represent FGF15/19 biology, but instead arise from a mimetic function of other FGFs. Interestingly, a comparison of the pharmacological effects of FGF19 and FGF21 revealed that while FGF21 mediates its effects on body weight and glucose homeostasis through FGFR1 in adipose tissue, FGF19 mediates it effects on body weight, but not glycemia, through FGFR1 in adipose tissue [28]. These data identify similarities and differences in the pharmacological mechanism of action for FGF19 and FGF21, and demonstrate the importance of adipose tissues in facilitating metabolic FGF function.
The adipose-independent effects of FGF19 on glycemia may be mediated through actions on the central nervous system (CNS). FGF15 is expressed in and functions throughout the nervous system during mouse embryogenesis [86,87]. However, Fgf15 mRNA is not detected in the adult CNS, but instead is localized to the ileum [15]. Several reports indicate a central action of FGF19 in mediating beneficial effects on metabolism. FGF19 is capable of crossing the blood brain barrier [88] and ICV injection of FGF19 into DIO rats reduced body weight, food intake, and improved glucose homeostasis; all effects which were reversible by ICV administration of a FGF-inhibitor [89]. Central FGF19 signaling reduces plasma glucose levels by regulating hepatic insulin-independent glucose disposal, a process referred to as glucose effectiveness (GE) [90]. This effect of FGF19 is mediated through suppression of AGRP/NPY neuronal activity in the arcuate nucleus [91] (Fig. 4). Additionally, FGF19 suppresses the hypothalamic-pituitary-adrenal (HPA) axis to decrease plasma ACTH and corticosterone levels to suppress hepatic glucose production and whole body lipolysis [92] (Fig. 4). Therefore, FGF19 signals to the CNS to regulate glucose homeostasis through insulin-independent glucose disposal and suppression of hepatic glucose production.
To gain insight into the physiological role of FGF15/19 in metabolism, the phenotypes of FGF15 and FGFR4 total knockout mice and liver-specific β-klotho knockout mice were examined. Similar to bile acid metabolism, the liver is an important target for the physiological effects of FGF15/19 on glucose metabolism. FGF15/19 increases hepatic protein and glycogen synthesis [93], and FGF15/19 functions to suppress hepatic gluconeogenesis through the repression of the transcriptional co-activator PGC-1α [75]. Notably, FGF15 KO mice have abnormal glucose homeostasis including elevated gluconeogenesis in the fed state [75], and both FGF15 KO [93] and liver-specific β-klotho knockout mice have elevated postprandial hepatic glycogen levels [72]. These effects of FGF15/19 are mediated independent of insulin [93] and demonstrate that FGF15/19, in addition to its pharmacological actions, functions physiologically to regulate hepatic lipid and glucose metabolism (Fig. 4).
3.3. Caveats of FGF19 therapy
While overexpression or administration of FGF19 improves metabolic homeostasis, it also causes hepatocellular proliferation (Fig. 1). FGF19 transgenic mice have increased hepatocyte proliferation as early as 2 months of age and develop hepatocellular carcinoma and die within 12 months of age [94]. Hepatocyte proliferation is also seen in wild-type mice injected with FGF19 for six days [94] which is mediated through a FGFR4-dependent pathway [82]. Therefore, identification of FGF19 variants which lack mitogenic activity and retain metabolic activity could be therapeutically advantageous. Indeed, a variant of FGF19 has been generated which specifically activates the FGFR1c/β-klotho complex to improve metabolic homeostasis without increasing hepatocellular proliferation like native FGF19 [95]. In addition, engineered forms of FGF19 which retain the ability to regulate bile acid metabolism but lack tumorigenicity have been reported [96,97]. However, induction of FGF19 has also been associated with the development and aggressiveness of other types of cancer including prostate [98,99] and colon cancer [100]. Therapeutic strategies to inhibit FGF19 function to reduce cancer progression have been proposed and should also be carefully evaluated. Inhibition of FGF19 in monkeys with an anti-FGF19 antibody resulted in liver toxicity and diarrhea due to impaired bile acid homeostasis [101]. Therefore, additional studies are necessary to determine whether FGF19 variants that affect metabolism also affect the progression or development of other types of cancer and vice versa.
4. Metabolic actions of FGF1
FGF1 along with FGF2 comprise the FGF1 subfamily. FGF1 signals in an autocrine/paracrine manner through interactions with FGF receptors and heparan sulfate (Fig. 1). FGF1 does not possess an N-terminal signal peptide and is therefore not classically secreted [102]. Instead, FGF1 is released from cells through mechanisms independent of the endoplasmic reticulum and Golgi processing pathways [102]. FGF1 was initially identified from pituitary and brain extracts and was identified as a mitogen for cultured fibro-blasts [102]. Fgf1 mRNA is expressed at high levels in the CNS, liver, lung and kidney [15]. Contrary to the binding properties of most FGFs, FGF1 is capable of binding both the ‘b’ and ‘c’ isoforms of FGFR1-3 and FGFR4 [1,103].
4.1. Glucose and lipid metabolism
Recent studies have identified the metabolic actions of FGF1 and proposed its therapeutic potential for treating metabolic disease. Parental delivery of recombinant FGF1 lowered blood glucose in ob/ob and DIO mice without causing hypoglycemia or effecting insulin secretion or production. Interestingly, administration of FGF1 to STZ-treated mice failed to lower plasma glucose levels, suggesting these effects are mediated through insulin sensitization. DIO wild-type mice treated with FGF1 for 3 weeks demonstrated significantly increased glucose infusion rate, enhanced insulin-stimulated glucose disposal and decreased hepatic glucose production during hyperinsulinemic-euglycemic clamps without altering circulating levels of adiponectin [104]. In vitro, however, FGF1 has mitogenic effects. To circumvent this issue, a FGF1 variant was generated which lacks mitogenic activity. This FGF1 variant lacking the first 24 amino acids on the N-terminus, termed rFGF1ΔNT, retained some binding affinity for FGFR1c and FGFR2c but not for the other FGFRs [104]. Parental delivery of rFGF1ΔNT lowered circulating glucose in ob/ob and DIO mice through FGFR1 signaling in adipose tissue as the metabolic effects were lost in DIO adipose-specific FGFR1 KO mice (FGFR1fl/fl;aP2-Cre) [104]. These effects of FGF1 are consistent with those observed following FGF19 or FGF21 administration [28]. However, unlike FGF19 and FGF21, FGF1 does not bind to nor require β-klotho for signaling [105].
Loss of function studies using FGF1 knockout mice revealed an important physiological role of FGF1 in metabolism. FGF1 null mice lack obvious abnormalities [106] despite the reported role of FGF1 as a regulator of human adipogenesis [107–110] and adipose tissue remodeling [111]. However, endogenous levels of FGF1 are induced in white adipose tissue during caloric excess and loss of FGF1 results in increased insulin resistance under conditions of high fat diet feeding [111]. Collectively, these data demonstrate that FGF1 functions physiologically to maintain metabolic homeostasis.
4.2. Caveats of FGF1 therapy
FGF1 possesses angiogenic and anti-apoptotic effects which has led to trials investigating its therapeutic potential in cases of cardiac ischemia and nerve injury [1]. Although these biological actions endow FGF1 with therapeutic potential, significant concerns remain regarding its oncogenic properties. For example, increased FGF1 expression has been detected in ovarian cancer and associated with poor survival [112] and also associated with prostate and breast cancers [113,114]. Long term studies with rFGF1ΔNT are necessary to determine its safety as a therapeutic for metabolic disease.
5. Summary/conclusions
In a manner atypical of classic FGFs, FGF15/19 and FGF21 are secreted into circulation as endocrine hormones, whereas FGF1 functions as a classical FGF in an autocrine/paracrine manner. Physiologically, each of these FGFs signals through a cognate receptor to elicit specific metabolic responses. In models of metabolic disease, administration of pharmacological doses of metabolic FGFs improves energy homeostasis through a common FGFR1 dependent pathway (Fig. 1). Importantly, the metabolic FGFs have overlapping yet distinct functions on energy homeostasis. Despite the recent explosion in studies examining the mechanisms of the pharmacological effects of metabolic FGFs, several key questions remain. For example, what is the mechanism for the energy expending effects of metabolic FGFs? In addition, which cell type(s) are directly targeted to mediate these effects? The discovery that UCP1 is not required for the effects of FGF21 on energy expenditure raises new questions about the importance of adipose tissue thermogenesis in mediating FGF21 function [59,60]. Answers to these questions and other mysteries of metabolic FGF biology will likely uncover novel therapeutic strategies for the treatment of metabolic disease.
Acknowledgments
We thank Teresa Ruggle for assistance with graphic design. This work was supported by an American Diabetes Association Junior Faculty Award 7-13-JF-49 and the National Institutes of Health (NIH) (R01DK106104) to M.J.P., and NIH F32DK102347 to K.R.M.
Abbreviations
- DIO
diet induced obesity
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- UCP1
uncoupling protein 1
- KO
knockout
- CNS
central nervous system
- SNA
sympathetic nerve activity
- ICV
intracerebroventricular
- ChREBP
carbohydrate response element binding protein
- FDG/PET
fludeoxyglucose/positron emission tomography
- GE
glucose effectiveness
- BAT
brown adipose tissue
- WAT
white adipose tissue
- ob/ob
leptin deficient mice
References
- 1.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8(3):235–53. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goetz R, et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27(9):3417–28. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goetz R, Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol. 2013;14(3):166–80. doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurosu H, et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282(37):26687–95. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogawa Y, et al. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A. 2007;104(18):7432–7. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, et al. Co-receptor requirements for fibroblast growth factor-19 signaling. J Biol Chem. 2007;282(40):29069–72. doi: 10.1074/jbc.C700130200. [DOI] [PubMed] [Google Scholar]
- 7.Kharitonenkov A, et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol. 2008;215(1):1–7. doi: 10.1002/jcp.21357. [DOI] [PubMed] [Google Scholar]
- 8.Urakawa I, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–4. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 9.Kharitonenkov A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–35. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58(1):250–9. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coskun T, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149(12):6018–27. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 12.Kharitonenkov A, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148(2):774–81. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models – association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab. 2009;297(5):E1105–14. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 14.Gaich G, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18(3):333–40. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Fon Tacer K, et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24(10):2050–64. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bookout AL, et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19(9):1147–52. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang Q, et al. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes. 2014;63(12):4064–75. doi: 10.2337/db14-0541. [DOI] [PubMed] [Google Scholar]
- 18.Adams AC, et al. FGF21 requires betaklotho to act in vivo. PLoS ONE. 2012;7(11):e49977. doi: 10.1371/journal.pone.0049977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding X, et al. BetaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 2012;16(3):387–93. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Micanovic R, et al. Different roles of N- and C- termini in the functional activity of FGF21. J Cell Physiol. 2009;219(2):227–34. doi: 10.1002/jcp.21675. [DOI] [PubMed] [Google Scholar]
- 21.Yie J, et al. Understanding the physical interactions in the FGF21/FGFR/beta-Klotho complex: structural requirements and implications in FGF21 signaling. Chem Biol Drug Des. 2012;79(4):398–410. doi: 10.1111/j.1747-0285.2012.01325.x. [DOI] [PubMed] [Google Scholar]
- 22.Wu X, et al. C-terminal tail of FGF19 determines its specificity toward Klotho co-receptors. J Biol Chem. 2008;283(48):33304–9. doi: 10.1074/jbc.M803319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 2012;26(4):312–24. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Y, McKeehan WL. Stressed liver and muscle call on adipocytes with FGF21. Front Endocrinol (Lausanne) 2013;4:194. doi: 10.3389/fendo.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim KH, Lee MS. FGF21 as a stress hormone: the roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabet Metab J. 2014;38(4):245–51. doi: 10.4093/dmj.2014.38.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen BM, Mangelsdorf DJ, Kliewer SA. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol Metab. 2015;26(1):22–9. doi: 10.1016/j.tem.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markan KR, et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63(12):4057–63. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams AC, et al. The breadth of FGF21’s metabolic actions are governed by FGFR1 in adipose tissue. Mol Metab. 2012;2(1):31–7. doi: 10.1016/j.molmet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camacho RC, et al. Pegylated Fgf21 rapidly normalizes insulin-stimulated glucose utilization in diet-induced insulin resistant mice. Eur J Pharmacol. 2013;715(1–3):41–5. doi: 10.1016/j.ejphar.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 30.Holland WL, et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17(5):790–7. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Z, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17(5):779–89. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Muise ES, et al. Downstream signaling pathways in mouse adipose tissues following acute in vivo administration of fibroblast growth factor 21. PLOS ONE. 2013;8(9):e73011. doi: 10.1371/journal.pone.0073011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolumam G, et al. Sustained brown fat stimulation and insulin sensitization by a humanized bispecific antibody agonist for fibroblast growth factor receptor 1/betaKlotho complex. EBioMedicine. 2015;2(7):730–43. doi: 10.1016/j.ebiom.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Combs TP, et al. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108(12):1875–81. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–26. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 36.Potthoff MJ, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106(26):10853–8. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inagaki T, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5(6):415–25. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez J, Palou A, Pico C. Response to carbohydrate and fat refeeding in the expression of genes involved in nutrient partitioning and metabolism: striking effects on fibroblast growth factor-21 induction. Endocrinology. 2009;150(12):5341–50. doi: 10.1210/en.2009-0466. [DOI] [PubMed] [Google Scholar]
- 39.Iizuka K, Takeda J, Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett. 2009;583(17):2882–6. doi: 10.1016/j.febslet.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 40.Fisher FM, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59(11):2781–9. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hale C, et al. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology. 2012;153(1):69–80. doi: 10.1210/en.2010-1262. [DOI] [PubMed] [Google Scholar]
- 42.Rahmouni K, Haynes WG, Mark AL. Cardiovascular and sympathetic effects of leptin. Curr Hypertens Rep. 2002;4(2):119–25. doi: 10.1007/s11906-002-0036-z. [DOI] [PubMed] [Google Scholar]
- 43.Camporez JP, et al. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology. 2013;154(9):3099–109. doi: 10.1210/en.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernardo B, et al. FGF21 does not require interscapular brown adipose tissue and improves liver metabolic profile in animal models of obesity and insulin-resistance. Sci Rep. 2015;5:11382. doi: 10.1038/srep11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owen BM, et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014;20(4):670–7. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarruf DA, et al. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59(7):1817–24. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veniant MM, et al. FGF21 promotes metabolic homeostasis via white adipose and leptin in mice. PLoS ONE. 2012;7(7):e40164. doi: 10.1371/journal.pone.0040164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu AL, et al. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci Transl Med. 2011;3(113):113–26. doi: 10.1126/scitranslmed.3002669. [DOI] [PubMed] [Google Scholar]
- 49.Emanuelli B, et al. Interplay between FGF21 and insulin action in the liver regulates metabolism. J Clin Invest. 2015;125(1):458. doi: 10.1172/JCI80223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kharitonenkov A, Adams AC. Inventing new medicines: the FGF21 story. Mol Metab. 2014;3(3):221–9. doi: 10.1016/j.molmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher FM, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26(3):271–81. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee P, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19(2):302–9. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hondares E, et al. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010;11(3):206–12. doi: 10.1016/j.cmet.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chartoumpekis DV, et al. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med. 2011;17(7–8):736–40. doi: 10.2119/molmed.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hondares E, et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286(15):12983–90. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee P, et al. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes (Lond) 2014;38(2):170–6. doi: 10.1038/ijo.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laeger T, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124(9):3913–22. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hasek BE, et al. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R728–39. doi: 10.1152/ajpregu.00837.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samms RJ, et al. Discrete aspects of FGF21 in vivo pharmacology do not require UCP1. Cell Rep. 2015;11(7):991–9. doi: 10.1016/j.celrep.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 60.Veniant MM, et al. Pharmacologic effects of FGF21 are independent of the “Browning” of white adipose tissue. Cell Metab. 2015;21(5):731–8. doi: 10.1016/j.cmet.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 61.Liu X, et al. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest. 2003;111(3):399–407. doi: 10.1172/JCI15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shabalina IG, et al. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013;5(5):1196–203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 63.Dong JQ, et al. Pharmacokinetics and pharmacodynamics of PF-05231023, a novel long-acting FGF21 mimetic, in a first-in-human study. Br J Clin Pharmacol. 2015 doi: 10.1111/bcp.12676. http://dx.doi.org/10.1111/bcp.12676. [DOI] [PMC free article] [PubMed]
- 64.Badman MK, et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5(6):426–37. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Arner P, et al. FGF21 attenuates lipolysis in human adipocytes – a possible link to improved insulin sensitivity. FEBS Lett. 2008;582(12):1725–30. doi: 10.1016/j.febslet.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 66.Wei W, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci U S A. 2012;109(8):3143–8. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Owen BM, et al. FGF21 contributes to neuroendocrine control of female reproduction. Nat Med. 2013;19(9):1153–6. doi: 10.1038/nm.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inagaki T, et al. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8(1):77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu J, et al. Polyethylene glycol modified FGF21 engineered to maximize potency and minimize vacuole formation. Bioconjug Chem. 2013;24(6):915–25. doi: 10.1021/bc300603k. [DOI] [PubMed] [Google Scholar]
- 70.Smith R, et al. A novel approach to improve the function of FGF21. BioDrugs. 2013;27(2):159–66. doi: 10.1007/s40259-013-0013-x. [DOI] [PubMed] [Google Scholar]
- 71.Wright TJ, et al. Mouse FGF15 is the ortholog of human and chick FGF19, but is not uniquely required for otic induction. Dev Biol. 2004;269(1):264–75. doi: 10.1016/j.ydbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 72.Katafuchi T, et al. Detection of FGF15 in plasma by stable isotope standards and capture by anti-peptide antibodies and targeted mass spectrometry. Cell Metab. 2015;21(6):898–904. doi: 10.1016/j.cmet.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lundasen T, et al. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260(6):530–6. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 74.Inagaki T, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–25. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Potthoff MJ, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metab. 2011;13(6):729–38. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ito S, et al. Impaired negative feedback suppression of bile acid synthesis in mice lacking betaKlotho. J Clin Invest. 2005;115(8):2202–8. doi: 10.1172/JCI23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–74. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 78.Han SS, et al. Circadian control of bile acid synthesis by a KLF15-Fgf15 axis. Nat Commun. 2015;6:7231. doi: 10.1038/ncomms8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brufau G, et al. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 2010;52(4):1455–64. doi: 10.1002/hep.23831. [DOI] [PubMed] [Google Scholar]
- 80.Tomlinson E, et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143(5):1741–7. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- 81.Fu L, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145(6):2594–603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 82.Wu X, et al. Separating mitogenic metabolic activities of fibroblast growth factor 19 (FGF19) Proc Natl Acad Sci U S A. 2010;107(32):14158–63. doi: 10.1073/pnas.1009427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu X, Li Y. Role of FGF19 induced FGFR4 activation in the regulation of glucose homeostasis. Aging (Albany NY) 2009;1(12):1023–7. doi: 10.18632/aging.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu AL, et al. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLoS ONE. 2011;6(3):e17868. doi: 10.1371/journal.pone.0017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu X, et al. Selective activation of FGFR4 by an FGF19 variant does not improve glucose metabolism in ob/ob mice. Proc Natl Acad Sci U S A. 2009;106(34):14379–84. doi: 10.1073/pnas.0907812106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McWhirter JR, et al. A novel fibroblast growth factor gene expressed in the developing nervous system is a downstream target of the chimeric homeodomain oncoprotein E2A-Pbx1. Development. 1997;124(17):3221–32. doi: 10.1242/dev.124.17.3221. [DOI] [PubMed] [Google Scholar]
- 87.Gimeno L, et al. Analysis of Fgf15 expression pattern in the mouse neural tube. Brain Res Bull. 2002;57(3–4):297–9. doi: 10.1016/s0361-9230(01)00717-1. [DOI] [PubMed] [Google Scholar]
- 88.Hsuchou H, Pan W, Kastin AJ. Fibroblast growth factor 19 entry into brain. Fluids Barriers CNS. 2013;10(1):32. doi: 10.1186/2045-8118-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ryan KK, et al. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology. 2013;154(1):9–15. doi: 10.1210/en.2012-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morton GJ, et al. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest. 2013;123(11):4799–808. doi: 10.1172/JCI70710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marcelin G, et al. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol Metab. 2014;3(1):19–28. doi: 10.1016/j.molmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perry RJ, et al. FGF1 and FGF19 reverse diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat Commun. 2015;6:6980. doi: 10.1038/ncomms7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kir S, et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331(6024):1621–4. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nicholes K, et al. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am J Pathol. 2002;160(6):2295–307. doi: 10.1016/S0002-9440(10)61177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ge H, et al. Characterization of a FGF19 variant with altered receptor specificity revealed a central role for FGFR1c in the regulation of glucose metabolism. PLoS ONE. 2012;7(3):e33603. doi: 10.1371/journal.pone.0033603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou M, et al. Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Cancer Res. 2014;74(12):3306–16. doi: 10.1158/0008-5472.CAN-14-0208. [DOI] [PubMed] [Google Scholar]
- 97.Luo J, et al. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci Transl Med. 2014;6(247):247, ra100. doi: 10.1126/scitranslmed.3009098. [DOI] [PubMed] [Google Scholar]
- 98.Feng S, et al. Endocrine fibroblast growth factor FGF19 promotes prostate cancer progression. Cancer Res. 2013;73(8):2551–62. doi: 10.1158/0008-5472.CAN-12-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nagamatsu H, et al. FGF19 promotes progression of prostate cancer. Prostate. 2015;75(10):1092–101. doi: 10.1002/pros.22994. [DOI] [PubMed] [Google Scholar]
- 100.Wang H, et al. Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice. J Clin Invest. 2011;121(8):3220–32. doi: 10.1172/JCI41514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pai R, et al. Antibody-mediated inhibition of fibroblast growth factor 19 results in increased bile acids synthesis and ileal malabsorption of bile acids in cynomolgus monkeys. Toxicol Sci. 2012;126(2):446–56. doi: 10.1093/toxsci/kfs011. [DOI] [PubMed] [Google Scholar]
- 102.Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2011;149(2):121–30. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Imamura T. Physiological functions and underlying mechanisms of fibroblast growth factor (FGF) family members: recent findings and implications for their pharmacological application. Biol Pharm Bull. 2014;37(7):1081–9. doi: 10.1248/bpb.b14-00265. [DOI] [PubMed] [Google Scholar]
- 104.Suh JM, et al. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature. 2014;513(7518):436–9. doi: 10.1038/nature13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang C, et al. Differential specificity of endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in complex with KLB. PLoS ONE. 2012;7(3):e33870. doi: 10.1371/journal.pone.0033870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miller DL, et al. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol Cell Biol. 2000;20(6):2260–8. doi: 10.1128/mcb.20.6.2260-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hutley L, et al. Fibroblast growth factor 1: a key regulator of human adipogenesis. Diabetes. 2004;53(12):3097–106. doi: 10.2337/diabetes.53.12.3097. [DOI] [PubMed] [Google Scholar]
- 108.Hutley LJ, et al. A putative role for endogenous FGF-2 in FGF-1 mediated differentiation of human preadipocytes. Mol Cell Endocrinol. 2011;339(1–2):165–71. doi: 10.1016/j.mce.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 109.Newell FS, et al. Characterization of the transcriptional and functional effects of fibroblast growth factor-1 on human preadipocyte differentiation. FASEB J. 2006;20(14):2615–7. doi: 10.1096/fj.05-5710fje. [DOI] [PubMed] [Google Scholar]
- 110.Widberg CH, et al. Fibroblast growth factor receptor 1 is a key regulator of early adipogenic events in human preadipocytes. Am J Physiol Endocrinol Metab. 2009;296(1):E121–31. doi: 10.1152/ajpendo.90602.2008. [DOI] [PubMed] [Google Scholar]
- 111.Jonker JW, et al. A PPARgamma-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485(7398):391–4. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Birrer MJ, et al. Whole genome oligonucleotide-based array comparative genomic hybridization analysis identified fibroblast growth factor 1 as a prognostic marker for advanced-stage serous ovarian adenocarcinomas. J Clin Oncol. 2007;25(16):2281–7. doi: 10.1200/JCO.2006.09.0795. [DOI] [PubMed] [Google Scholar]
- 113.Finak G, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14(5):518–27. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 114.Relf M, et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57(5):963–9. [PubMed] [Google Scholar]