Abstract
This work was designed to evaluate whether external application of nitric oxide (NO) in the form of its donor S-nitroso-N-acetylpenicillamine (SNAP) could mitigate the deleterious effects of NaCl stress on chickpea (Cicer arietinum L.) plants. SNAP (50 μM) was applied to chickpea plants grown under non-saline and saline conditions (50 and 100 mM NaCl). Salt stress inhibited growth and biomass yield, leaf relative water content (LRWC) and chlorophyll content of chickpea plants. High salinity increased electrolyte leakage, carotenoid content and the levels of osmolytes (proline, glycine betaine, soluble proteins and soluble sugars), hydrogen peroxide (H2O2) and malondialdehyde (MDA), as well as the activities of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase in chickpea plants. Expression of the representative SOD, CAT and APX genes examined was also up-regulated in chickpea plants by salt stress. On the other hand, exogenous application of NO to salinized plants enhanced the growth parameters, LRWC, photosynthetic pigment production and levels of osmolytes, as well as the activities of examined antioxidant enzymes which is correlated with up-regulation of the examined SOD, CAT and APX genes, in comparison with plants treated with NaCl only. Furthermore, electrolyte leakage, H2O2 and MDA contents showed decline in salt-stressed plants supplemented with NO as compared with those in NaCl-treated plants alone. Thus, the exogenous application of NO protected chickpea plants against salt stress-induced oxidative damage by enhancing the biosyntheses of antioxidant enzymes, thereby improving plant growth under saline stress. Taken together, our results demonstrate that NO has capability to mitigate the adverse effects of high salinity on chickpea plants by improving LRWC, photosynthetic pigment biosyntheses, osmolyte accumulation and antioxidative defense system.
Keywords: antioxidant enzymes, chickpea, gene expression, nitric oxide, osmolytes, salt stress
Introduction
Sodium chloride (NaCl) is the prevailing salt in the soil, and the higher concentration of this salt provokes two primary effects on plants, namely the osmotic and ionic effects, of which the osmotic stress minimizes the ability of plants to take up water and minerals (Khan et al., 2012). Furthermore, excessive accumulation of Na+ in the cytosol causes toxic effects on cell membranes, leading to electrolyte leakage, as well as constrains the metabolic processes in the cytosol, which ultimately reduce the physiological and biochemical activities (Ahmad, 2010; Ahmad et al., 2014; Hashem et al., 2014). Higher salt concentrations as well as prolonged exposure to NaCl stress cause oxidative stress in plants (Ahmad et al., 2012b; Rasool et al., 2013). Salt and osmotic stresses produce reactive oxygen species (ROS) that cause oxidative stress in plants (Ahmad et al., 2012b; Abdel Latef and Chaoxing, 2014). To deal with the adverse impacts of oxidative stress, plants are furnished with well-regulated antioxidant machinery that can protect biomolecules from further damages caused by the stress (Rasool et al., 2013). The ROS scavenging enzymes involve superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) (Evelin and Kapoor, 2014), which exist in different cellular compartments as isoenzymes especially in chloroplasts and mitochondria (Apel and Hirt, 2004; Ahmad et al., 2008, 2010). Accumulation of osmolytes, such as proline, glycine betaine (GB), soluble proteins and soluble sugars, is another strategy to beat osmotic stress provoked by salinity (Khan et al., 2012; Abdel Latef and Chaoxing, 2014).
Nitric oxide (NO) is an important endogenous plant bioactive signaling molecule that has a key function in various processes of plant growth and development, including seed dormancy, seed germination, primary and lateral root growth, floral transition, flowering, pollen tube growth regulation, fruit ripening, gravitropism, stomatal movements, photosynthesis, mitochondrial functionality, senescence, plant metabolism and cell death, as well as stress responses (Siddiqui et al., 2011; Manai et al., 2014; Mostofa et al., 2015). NO plays a pivotal role in stress tolerance exerted by oxidative stress (Siddiqui et al., 2011; Ahmad et al., 2012a). In the past few years, research on function of NO in salt stress tolerance has obtained much interest (Yang et al., 2011; Mostofa et al., 2015). However, the information available is sometimes contradictory, depending on the plant species, severity and duration of the salinity treatments (Begara-Morales et al., 2014; Manai et al., 2014).
Grain legumes belonging to Fabaceae family are rich in proteins and proved to be very important components of human diet (Jukanti et al., 2012). Among the grain legumes, chickpea (Cicer arietinum L.) is a very popular crop around the globe because it can supply a rich source of proteins, fats and carbohydrates for humans and animals (Rasool et al., 2015). Chickpea is differentiated as “Kabuli-type” and “desi-type” on the basis of size and color of the seeds. Kabuli-type seeds are bold with thin and white seed coat, while those of desi-type are small in size with thicker seed coat and having color ranging from brown to yellow (Khan et al., 1995; Rasool et al., 2015).
Chickpea normally grows under rainfed and irrigated conditions (Rasool et al., 2015). The soil used for the cultivation of chickpea should be free from high salinity as this crop is very sensitive to salinity stress. For instance, Kotula et al. (2015) reported that exposure of chickpea genotypes to 50 mM of NaCl stress decreased the plant growth and yield. Thus, steps are to be taken to enhance the salinity tolerance of chickpea genotypes in order to grow them on natural saline soil. Considering the vital role of NO in plant stress responses and management, the present study was designed to evaluate the influences of exogenous NO in mitigating high salinity-induced negative effects on growth and physiological attributes of chickpea plants. Additionally, the effects of exogenous NO treatment on accumulation of key osmolytes, activities of antioxidant enzymes and expression of representative antioxidant enzyme-encoding genes were examined in salt-stressed chickpea plants.
Materials and Methods
Plant Materials and Treatments
Seeds of chickpea (Cicer arietinum L.) were planted in earthen pots containing peat, perlite and sand (1:1:1, v/v/v) under glass house. Thinning was carried out to accommodate one plant per pot after 4 days of germination. Subsequently, the seedlings were grown for three more weeks under average day/night temperature of 24°C/15°C. Thereafter, 25-day-old-plants were treated with:
-
(1)
Nutrient solution alone (control) (T0): 0 mM NaCl + 0 μM SNAP
-
(2)
NO alone (T1): 0 mM NaCl + 50 μM SNAP
-
(3)
Salt stress alone (T2): 50 mM NaCl + 0 μM SNAP
-
(4)
Salt stress and NO (T3): 50 mM NaCl + 50 μM SNAP
-
(5)
Salt stress alone (T4): 100 mM NaCl + 0 μM SNAP
-
(6)
Salt stress and NO (T5): 100 mM NaCl + 50 μM SNAP
NaCl and NO were given to pots dissolved in nutrient solution every week from the first day of treatment (i.e., 25-day-old plants) up to day 45th (70-day-old plants). Collection of samples was done after 45 days of treatment. The nutrient solution is made up of (mg l-1): N 270, P 31, K 234, Ca 200, S 64, Mg 48, Fe 2.8, Mn 0.5, Cu 0.02, Zn 0.05, and Mo 0.01. 0.1 M KOH was used to adjust the pH of nutrient solution to 6.5. The experiment was laid out in randomized block design with five replicates in each treatment, and each replicate comprised five plants.
Determination of Growth Parameters
Shoot and root lengths were measured using measuring scale. Shoot dry weight (DW) was measured after the plant samples were dried at 70°C for 72 h.
Estimation of Leaf Relative Water Content and Electrolyte Leakage
Leaf relative water content (LRWC) was assayed using the method of Yamasaki and Dillenburg (1999). RWC was calculated using the following formula:
Electrolyte leakage was estimated as described previously (Dionisio-Sese and Tobita, 1998). First, the electrical conductivity (ECa) of 20 leaf disks submerged in deionized water was measured. Subsequently, the test tubes containing the leaf discs were incubated in water bath at temperature 50°C–60°C for 25 min, and the electrical conductivity (ECb) of the samples was determined. Finally, these test tubes were boiled at 100°C for 10 min, and then the electrical conductivity (ECc) was measured. The electrolytic leakage was calculated using the following formula:
Determination of the Contents of Photosynthetic Pigments
The method of Hiscox and Israelstam (1979) was used for the estimation of photosynthetic pigments using dimethyl sulphoxide (DMSO) as the extraction reagent. The absorbances at 480, 510, 645, and 663 nm were recorded by spectrophotometer (Beckman 640 D, USA), with DMSO being used as a blank.
Estimation of the Contents of Proline, GB, Soluble Proteins, and Soluble Sugars
Estimation of proline contents in fresh leaf samples was carried out as previously described by Bates et al. (1973). The absorbance was taken at 520 nm using a spectrophotometer (Beckman 640 D, USA), with toluene serving as a blank. GB contents in fresh leaf samples were measured according to Grieve and Grattan (1983). The absorbance was spectrophotometerically determined at 365 nm. GB (50–200 mg ml-1) prepared in 1N H2SO4 was used as control. Soluble protein content and soluble sugar content in fresh leaves were determined by the methods of Bradford (1976) and (Dey, 1990), respectively.
Determination of Hydrogen Peroxide (H2O2) Content and Lipid Peroxidation
H2O2 contents were estimated in dried leaf samples using the method of Velikova et al. (2000). Lipid peroxidation was assayed by quantifying the malondialdehyde (MDA) contents in fresh leaf samples using the method of Rao and Sresty (2000).
Enzyme Assays
The fresh leaf samples (0.5 g per sample) were homogenized in presence of phosphate buffer (0.1 M, pH 7.5) and ethylenediaminetetraacetic acid (EDTA, 0.5 mM). Subsequently, the samples were centrifuged at 12,000 ×g for 10 min at 4°C after the filtration. The supernatants collected served as sources for determination of SOD (EC 1.15.1.1), CAT (EC 1.11.1.6) and GR (EC 1.6.4.2) activities. For determination of APX (EC 1.11.1.11) activity, leaf samples were separately grounded in a homogenizing medium containing phosphate buffer (0.1 M, pH 7.5), 0.5 mM EDTA and 2 mM ascorbic acid (AsA).
Superoxide dismutase activity was determined by photoreduction of nitro blue tetrazolium (NBT) (Bayer and Fridovich, 1987). The absorbance was recorded at 560 nm using a spectrophotometer (Beckman 640 D, USA). One unit of SOD is the amount of protein regulating 50% photoreduction of NBT. The activity of SOD was expressed as enzyme unit (EU) mg-1 protein. For the estimation of CAT activity, the procedure of Aebi (1984) was employed. The absorbance was read at 240 nm using a spectrophotometer (Beckman 640 D, USA), and EU mg-1 protein expresses the CAT activity. APX activity was assayed using the method of Nakano and Asada (1981). The absorbance was spectrophotometerically determined at 290 nm. One unit of APX is the amount of protein used to decompose 1 μmol of substrate min-1 at 25°C, which was shown as EU mg-1 protein to express the APX activity. The method of Foyer and Halliwell (1976) was exerted for the determination of GR activity. The optical density (OD) was recorded at 340 nm using a spectrophotometer (Beckman 640 D, USA). GR activity was expressed as μmol NADPH oxidized min-1 (EU mg-1 protein).
Expression of SOD, CAT, and APX Genes
Total RNA was extracted from leaf samples using Trizol (Promega) according to the protocol of manufacturer. RNA samples were treated with DNase I (Promega) before their absorbance was read at 260 and 280 nm to determine RNA concentration and purity. The first-strand cDNA was synthesized from 5 μg RNA template using GoScriptTM Reverse Transcription System (Promega) according to the manufacturer’s instruction, with oligo (dT) 18 as a primer. Real-time quantitative PCR (RT-qPCR) was carried out using the QuantiTect SYBR Green PCR Kit (Qiagen) and Light Cycler (Model 480, Roche) with gene-specific primers designed for SOD (F: 5′-ACATTTGCTACCTCTCCCTCACCT-3′; R: 3′-TCGGGTAAGACATCGTCGGTATGT-5′), CAT (F: 5′-GGCGGTACGTTTACGATTTACGCT-3′; R: 3′- ACCTATCACGGGTCAGCACGATTT-5′) and APX (F: 5′-AAACCCAAGCTCAGAGAGCCTCAT-3′; R: 3′-TACTTCACGGTGCTTCTTGGTGGA-5′).
To standardize the results, the relative abundance of β-Actin (AB047313) reference gene (F: 5′-TGATGGTGTCAGCCACACT-3; R: 5′TGGTCTTGGCAGTCTCCATT-3) was also determined, which was then defined as 100 relative expression units (REU) and used as the internal standard. The expression level of a gene corresponds to the ratio of the copy number of cDNA of the studied gene to the copy number of β-Actin gene multiplied by 100 REU. These representative SOD, CAT and APX genes were selected as they give the highest expression levels when compared with other homologous versions (Hu et al., 2011).
Statistical Analysis
Duncan’s Multiple Range Test (DMRT) was carried out using the One-way Analysis of Variance (ANOVA). The values obtained were the means ± standard errors (SEs) of five replicates in each group. P-values ≤0.05 were considered as significant.
Results
NO Improves Growth and Biomass Yield under NaCl Stress
Exposure of chickpea plants to salinity stress resulted in a drastic decline in growth parameters expressed as shoot length, root length and shoot DW compared with untreated control (Table 1). The shoot length decreased by 18.52 and 40.58% at T2 (50 mM NaCl + 0 μM SNAP) and T4 (100 mM NaCl + 0 μM SNAP) treatments, respectively, relative to T0 control (0 mM NaCl + 0 μM SNAP). Application of NO in presence of NaCl showed an increment by 11.88% at T3 (50 mM NaCl + 50 μM SNAP) and 20.50% at T5 (100 mM NaCl + 50 μM SNAP) treatments as compared with T2 and T4, respectively. Root length was also negatively affected by NaCl stress, as T2 and T4 treatments decreased the root length by 36.90 and 59.80%, respectively, relative to T0 control. Supply of NO to NaCl-treated plants at T3 and T5 treatments boosted the root length by 12.98 and 17.85% as compared with T2 and T4, respectively. A decrease by 30.48 and 51.66% at T2 and T4, respectively, was also observed in shoot DW as compared with T0 control. However, supplementation of NO to salt-stressed plants improved the shoot DW, and the increase was 20.21% at T3 and 26.69% at T5 over T2 and T4 treatments, respectively. No significant change was observed in the examined parameters at T1 (0 mM NaCl + 50 μM SNAP) treatment compared with T0 control (Table 1).
Table 1.
Effects of NO on growth and biomass yield of chickpea plants under salt stress.
| Treatments | Shoot length (cm plant-1) | Root length (cm plant-1) | Shoot DW (g plant-1) |
|---|---|---|---|
| T0 | 40.71 ± 2.16a | 22.71 ± 1.37a | 14.73 ± 1.07a |
| T1 | 42.23 ± 2.20a | 23.55 ± 1.40a | 15.76 ± 1.11a |
| T2 | 33.17 ± 1.34c | 14.33 ± 1.05c | 10.24 ± 0.91c |
| T3 | 37.11 ± 1.70b | 16.19 ± 1.12b | 12.31 ± 0.98b |
| T4 | 24.19 ± 1.13e | 9.13 ± 0.82e | 7.12 ± 0.77e |
| T5 | 29.15 ± 1.27d | 10.76 ± 0.95d | 9.02 ± 0.85d |
Data presented are the means ± SEs (n = 5). Different letters next to the number indicate significant difference (P ≤ 0.05). T0 (control) = 0 mM NaCl + 0 μM SNAP; T1 = 0 mM NaCl + 50 μM SNAP; T2 = 50 mM NaCl + 0 μM SNAP; T3 = 50 mM NaCl + 50 μM SNAP; T4 = 100 mM NaCl + 0 μM SNAP; T5 = 100 mM NaCl + 50 μM SNAP. DW, dry weight.
Effects of NaCl and NO on LRWC and Electrolyte Leakage
LRWC was reduced by 21.54% at T2 (50 mM NaCl + 0 μM SNAP), and the maximum decrease (46.93%) in LRWC was recorded at T4 (100 mM NaCl + 0 μM SNAP) relative to T0 (0 mM NaCl + 0 μM SNAP) control (Table 2). The decrease in LRWC of salt-stressed plants was alleviated by exogenous application of NO, resulting in an enhancement in LRWC of 15.72 and 33.62% at T3 (50 mM NaCl + 50 μM SNAP) and T5 (100 mM NaCl + 50 μM SNAP), respectively, as compared with plants treated with NaCl only (T2 and T4, respectively). On the other hand, electrolyte leakage of chickpea plants increased by salt stress, and maximum elevation of 4.60-fold was recorded at T4 treatment compared with T0 control (Table 2). Exogenous application of NO reduced the electrolyte leakage in salt-stressed plants by 27.08% at T3 and 21.33% at T5 in comparison with NaCl-treated plants alone (T2 and T4, respectively). NO treatment alone (T1; 0 mM NaCl + 50 μM SNAP) had insignificant effect on LRWC and electrolyte leakage of chickpea plants as compared with T0 control (Table 2).
Table 2.
Effects of NO on leaf relative water content (LRWC), electrolyte leakage, and the contents of proline, glycine betaine (GB), total soluble proteins and total soluble sugars in leaves of chickpea plants under salt stress.
| Treatments | LRWC (%) | Electrolyte leakage (%) | Proline (μg g-1 FW) | GB (μmol g-1 FW) | Total soluble proteins (mg g-1 FW) | Total soluble sugars (mg g-1 FW) |
|---|---|---|---|---|---|---|
| T0 | 85.13 ± 2.57a | 14.17 ± 1.05d | 27.10 ± 1.69e | 2.40 ± 0.18e | 18.31 ± 0.89e | 6.20 ± 0.51e |
| T1 | 87.10 ± 2.63a | 13.21 ± 0.99d | 30.75 ± 1.79e | 2.70 ± 0.15e | 21.32 ± 1.16cd | 6.40 ± 0.54e |
| T2 | 66.79 ± 2.24bc | 25.66 ± 1.74c | 75.26 ± 2.23d | 10.71 ± 0.96d | 24.52 ± 1.26d | 7.71 ± 0.68d |
| T3 | 77.29 ± 2.48ab | 18.71 ± 121cd | 88.11 ± 3.16c | 15.35 ± 1.12c | 30.14 ± 1.44c | 7.92 ± 0.75c |
| T4 | 45.18 ± 2.57d | 65.12 ± 2.24a | 105.29 ± 3.75b | 19.22 ± 1.22b | 36.77 ± 1.72b | 8.53 ± 0.83b |
| T5 | 60.37 ± 2.14c | 51.23 ± 2.06b | 130.77 ± 3.95a | 24.72 ± 1.41a | 39.81 ± 1.95a | 9.07 ± 0.92a |
Data presented are the means ± SEs (n = 5). Different letters next to the number indicate significant difference (P ≤ 0.05). T0 (control) = 0 mM NaCl + 0 μM SNAP; T1 = 0 mM NaCl + 50 μM SNAP; T2 = 50 mM NaCl + 0 μM SNAP; T3 = 50 mM NaCl + 50 μM SNAP; T4 = 100 mM NaCl + 0 μM SNAP; T5 = 100 mM NaCl + 50 μM SNAP. FW, fresh weight.
NO Mitigates the Effects of NaCl Stress on Photosynthetic Pigment Biosyntheses
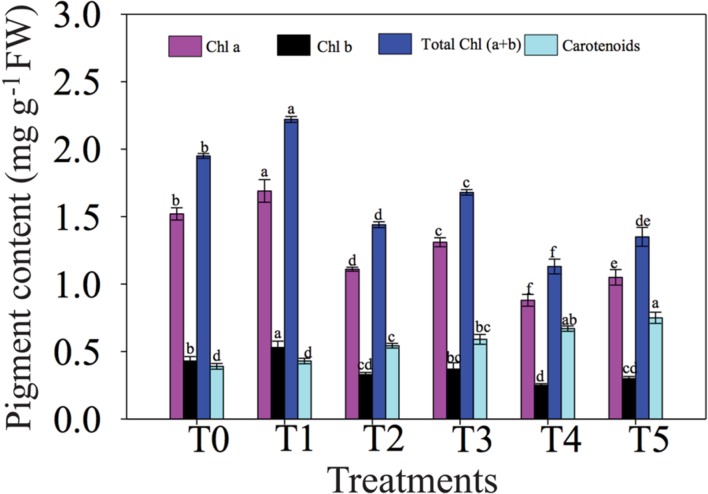
The Chl a, Chl b and total Chl contents significantly decreased by salt stress and the percent reduction in these parameters was nearly equal (≈ 42%) at T4 (100 mM NaCl + 0 μM SNAP) treatment in comparison with T0 (0 mM NaCl + 0 μM SNAP) control. The levels of carotenoids sharply increased with increasing NaCl stress intensity, and the maximum increase (71.79%) was recorded at the T4 treatment over the T0 control. Supplementation of NO elevated the contents of Chl a, Chl b and total Chl by 19.32, 20, and 19.47%, respectively, and that of carotenoids by 11.94% at T5 (100 mM NaCl + 50 μM SNAP) in NO + NaCl-treated plants over plants treated with NaCl alone (T4) (Figure 1). Exogenous NO treatment showed a positive effect on Chl biosynthesis in chickpea plants under normal conditions as control plants treated with NO (T1; 0 mM NaCl + 50 μM SNAP) showed a significant increase in Chl contents relative to untreated T0 control (Figure 1).
FIGURE 1.
Effects of NO on chlorophyll (Chl) and carotenoid contents in leaves of chickpea plants under salt stress. Data presented are the means ± SEs (n = 5). Different letters indicate significant difference (P ≤ 0.05) among the treatments. T0 (control) = 0 mM NaCl + 0 μM SNAP; T1 = 0 mM NaCl + 50 μM SNAP; T2 = 50 mM NaCl + 0 μM SNAP; T3 = 50 mM NaCl + 50 μM SNAP; T4 = 100 mM NaCl + 0 μM SNAP; T5 = 100 mM NaCl + 50 μM SNAP. FW, fresh weight.
Effects of NaCl and NO on the Contents of Proline, GB, Total Soluble Proteins and Total Soluble Sugars
NaCl triggered the induction of proline biosynthesis by 2.78-fold and 3.89-fold at T2 (50 mM NaCl + 0 μM SNAP) and T4 (100 mM NaCl + 0 μM SNAP) treatments, respectively, versus T0 (0 mM NaCl + 0 μM SNAP) control (Table 2). Exogenous application of NO induced the proline biosynthesis by 17.07% at T3 (50 mM NaCl + 50 μM SNAP) and 24.20% at T5 (100 mM NaCl + 50 μM SNAP) treatments over T2 and T4, respectively (Table 2). With regard to GB, it markedly accumulated in chickpea plants treated with NaCl alone and in combination with NO (Table 2). At concentrations T2 and T4, the accumulation of GB was 4.46- and 8.01-fold, respectively, as compared with T0 control. Supplementation of NO enhanced the accumulation of GB by 43.32 and 28.62% at T3 and T5 treatments, respectively, in comparison with T2 and T4, respectively (Table 2).
As for the soluble proteins, their total content increased by 33.91 and 100.81% at T2 and T4 treatments relative to T0 control (Table 2). An enhancement by 22.92% (T3) and 8.27% (T5) in soluble protein content was also observed in plants treated with both NaCl and NO, as compared with plants treated with NaCl alone (T2 and T4, respectively) (Table 2). In addition, chickpea seedlings treated with T2 and T4 showed elevated soluble sugar content by 24.36 and 37.58%, respectively, over the T0 control (Table 2). Application of NO further increased the soluble sugar content in T3- and T5-treated plants relative to T0-plants; however, in comparison with their respective T2- and T4-treated plants, the observed increment was not large, with only 2.72 and 6.33% (Table 2). We noticed that NO treatment (T1) alone resulted in a significant change (16.43%) in soluble protein content only in comparison with T0 control (Table 2).
Effects of NaCl and NO on H2O2 and MDA Contents
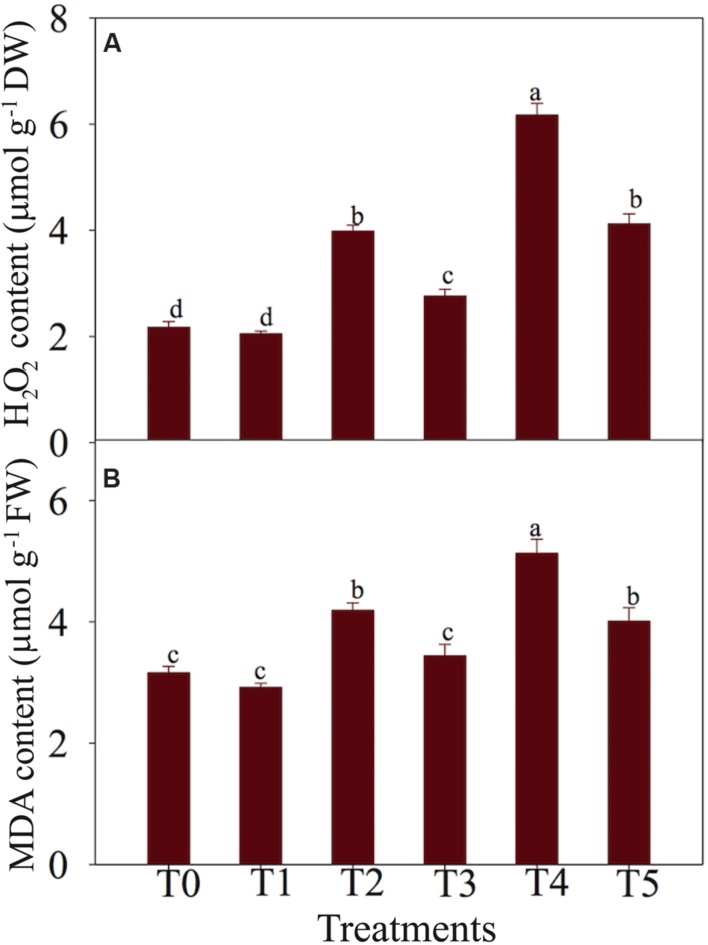
The results regarding the impacts of NaCl and NO on H2O2 and MDA contents in chickpea plants are depicted in Figures 2A,B. Increase in H2O2 contents was observed with the raise of NaCl dose applied to chickpea plants (Figure 2A). H2O2 content increased by 83.41 and 184.33% at T2 (50 mM NaCl + 0 μM SNAP) and T4 (100 mM NaCl + 0 μM SNAP), respectively, versus T0 (0 mM NaCl + 0 μM SNAP) control. Supplementation of exogenous NO to NaCl-stressed plants decreased H2O2 content by 30.65% and 33.23% in T3 (50 mM NaCl + 50 μM SNAP) and T5 (100 mM NaCl + 50 μM SNAP) treatments, respectively, as compared with plants treated with NaCl alone (T2 and T4, respectively) (Figure 2A). As for MDA, its content markedly accumulated in salt-stressed chickpea plants in the present study (Figure 2B). An increase by 32.59 and 62.34% in MDA content in T2 and T4 treatments, respectively, was recorded as compared with T0 control. Salt-treated plants supplied with NO showed a decrease by17.90% at T3 and 21.83% at T5 treatments relative to their respective T2 and T4 treatments (Figure 2B). No significant change in H2O2 and MDA contents was noted in T1 (0 mM NaCl + 50 μM SNAP)-treated plants versus T0 control (Figures 2A,B).
FIGURE 2.
Effects of NO on (A) hydrogen peroxide (H2O2) content and (B) malondialdehyde (MDA) content in leaves of chickpea plants under salt stress. Data presented are the means ± SEs (n = 5). Different letters indicate significant difference (P ≤ 0.05) among the treatments. T0 (control) = 0 mM NaCl + 0 μM SNAP; T1 = 0 mM NaCl + 50 μM SNAP; T2 = 50 mM NaCl + 0 μM SNAP; T3 = 50 mM NaCl + 50 μM SNAP; T4 = 100 mM NaCl + 0 μM SNAP; T5 = 100 mM NaCl + 50 μM SNAP. DW, dry weight; FW, fresh weight.
Effects of NaCl and NO on Antioxidant Enzyme Activities
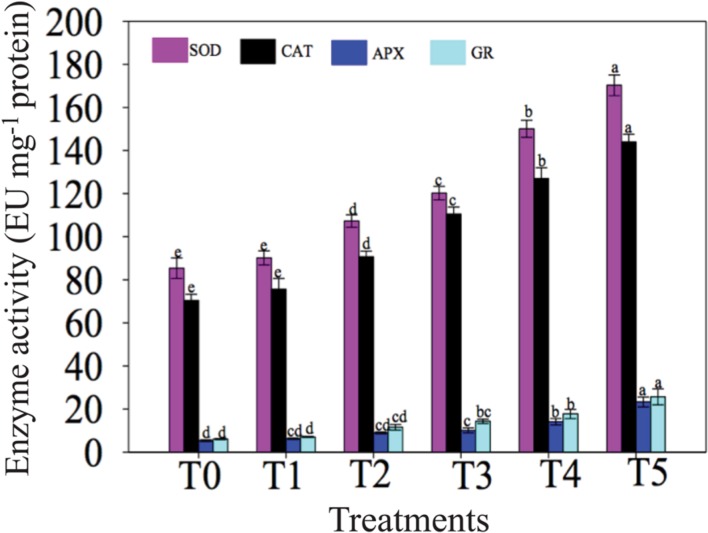
The activities of antioxidant enzymes significantly increased in response to NaCl with or without application of exogenous NO (Figure 3). Maximum salt stress-induced elevation by 75.83, 80.40, 164.73, and 191.93% in SOD, CAT, APX and GR activities, respectively, was recorded in chickpea plants of T4 (100 mM NaCl + 0 μM SNAP) treatment versus T0 (0 mM NaCl + 0 μM SNAP). Moreover, exogenous application of NO to salt-exposed plants had an additive impact on the activities of antioxidant enzymes. The highest values for SOD, CAT, APX and GR activities were noted in chickpea plants subjected to T5 (100 mM NaCl + 50 μM SNAP) treatment with the increase of 13.44, 13.39, 64.85, and 44.81%, respectively, as compared with plants treated with NaCl alone (T4 treatment). No significant alteration was observed in antioxidant enzyme activities at T1 (0 mM NaCl + 50 μM SNAP) treatment compared with T0 control (Figure 3).
FIGURE 3.
Effects of NO on activities of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) in leaves of chickpea plants under salt stress. Data presented are the means ± SEs (n = 5). Different letters indicate significant difference (P ≤ 0.05) among the treatments. T0 (control) = 0 mM NaCl + 0 μM SNAP; T1 = 0 mM NaCl + 50 μM SNAP; T2 = 50 mM NaCl + 0 μM SNAP; T3 = 50 mM NaCl + 50 μM SNAP; T4 = 100 mM NaCl + 0 μM SNAP; T5 = 100 mM NaCl + 50 μM SNAP. EU, enzyme unit.
Impacts of NaCl and NO on Transcript Levels of Genes Encoding SOD, APX, and CAT Enzymes
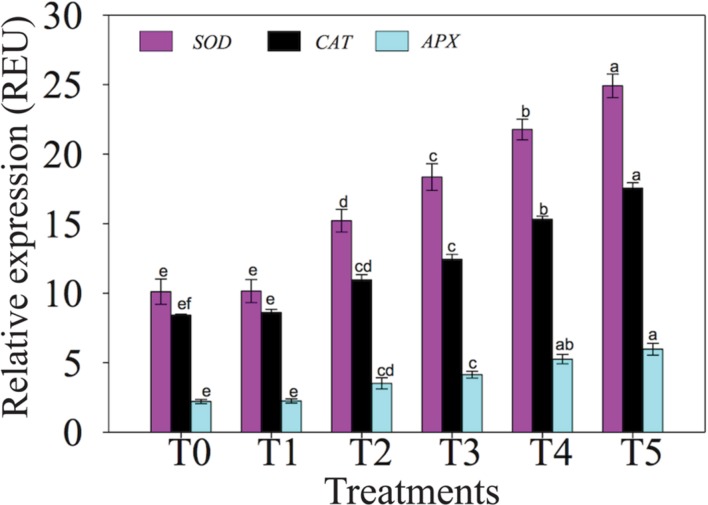
The expression of SOD, APX and CAT antioxidant enzymes-related genes in leaves of chickpea plants under high salinity in presence and absence of NO is presented in Figure 4. Expression of selected genes up-regulated under NaCl stress with or without supplementation of exogenous NO. SOD, CAT and APX genes showed up-regulation of 2.15-, 1.81-, and 2.38-fold in chickpea plants of T4 (100 mM NaCl + 0 μM SNAP) treatment, respectively, over T0 (0 mM NaCl + 0 μM SNAP) control. Moreover, supplementation of NO to NaCl-treated plants also displayed a remarkable increase in expression level of SOD (14.42%), CAT (14.63%) and APX (13.50%) in T5-treated plants versus T4-treated ones. Insignificant change in expression level of examined genes was recorded in T1 (0 mM NaCl + 50 μM SNAP)-treated chickpea plants in comparison with T0 control (Figure 4).
FIGURE 4.
Effects of NO on expression levels of selected SOD, CAT and APX genes in leaves of chickpea plants under salt stress. Data presented are the means ± SEs (n = 5). Different letters indicate significant difference (P ≤ 0.05) among the treatments. T0 (control) = 0 mM NaCl + 0 μM SNAP; T1 = 0 mM NaCl + 50 μM SNAP; T2 = 50 mM NaCl + 0 μM SNAP; T3 = 50 mM NaCl + 50 μM SNAP; T4 = 100 mM NaCl + 0 μM SNAP; T5 = 100 mM NaCl + 50 μM SNAP. REU, relative expression unit.
Discussion
Nitric oxide is an important signaling molecule involved in amelioration of growth and development of plants under various biotic and abiotic stresses (Kausar and Shahbaz, 2013; Liu et al., 2013; Esim and Atici, 2014; Manai et al., 2014). In the present study, salt stress significantly reduced the growth and biomass yield of chickpea plants (Table 1), which is in harmony with earlier reports on different crops, such as wheat (Triticum aestivum) (Kausar et al., 2013), tomato (Lycopersicon esculentum) (Abdel Latef and Chaoxing, 2011), pepper (Capsicum annuum) (Abdel Latef and Chaoxing, 2014) and rice (Oryza sativa) (Mostofa et al., 2015). Co-application of NO markedly ameliorated shoot length, root length and shoot DW of chickpea plants under high salinity (Table 1), which was in agreement with previous findings in many other crops, including wheat (Hasanuzzaman et al., 2011) and rice (Mostofa et al., 2015). Huaifu et al. (2007) reported that supplementation of NO promoted growth of plants exposed to saline conditions. NO can relax the cell wall, act on the phospholipids bilayer, increase membrane fluidness and induce cell enlargement and plant growth (Leshem and Haramaty, 1996). Dong et al. (2014) found that NO application resulted in an improvement in stem and root lengths of cotton (Gossypium hirsutum) seedlings under salt stress. They reported that NO is involved in increasing osmotic pressure of the plant cells and improving the cytoplasmic viscosity under high salinity. Zhang et al. (2004) and Wu et al. (2011) working on soybean (Glycine max) and maize (Zea mays), respectively, demonstrated that application of NO enhanced the plant growth under saline conditions, which might be due to increased activities of antioxidant enzymes. More recently, NO was shown to alleviate the effects of both biotic and abiotic stresses on plants by mediating H2O2- and salicylic acid-induced mitigation of oxidative damage through the up-regulation of the antioxidant defense (Klessig et al., 2000; Mostofa et al., 2015; Singh et al., 2015).
RWC is adversely affected by imposition of NaCl, which leads to decease in water uptake and injury of root system (Zeng et al., 2011). In the present study, supplementation of NO had a positive impact on LRWC of chickpea plants under salt stress (Table 2), which corroborated with previous reports on other plants, such as mustard (Brassica juncea) (Zeng et al., 2011) and rice (Habib and Ashraf, 2014). Khan et al. (2012) reported that NO application helped mustard plants retain more water under salt stress. It is still unclear that how NO is able to maintain RWC in stressed plants; however, it has been reported that NO could decrease solute potential, while increasing water potential in plants under osmotic stress (Ke et al., 2013).
The reduction in Chl content of chickpea leaves observed under NaCl stress (Figure 1) might be ascribed to the destruction of Chl pigments, decreased Chl syntheses and the vulnerability of the pigment-protein complexes (Rasool et al., 2013). This decrease in Chl content might partially cause a decrease in growth and biomass yield (Figure 1; Table 1). Carotenoids have been known to play a key role in photosynthetic reaction center in which they are involved in mechanisms regulating photo protection against auto-oxidation (Yang et al., 2013; Gururani et al., 2015). The synthesis of carotenoids was noted to be increased in chickpea under salinity stress (Figure 1), perhaps because these compounds act as antioxidants to minimize the oxidative damage induced by NaCl stress. NO was found to provoke the enhancement of photosynthetic pigments in chickpea plants (Figure 1), potentially by defending the membrane of the cell organelle containing Chl against salt-induced ion toxicity (Kausar et al., 2013). The enhancement in photosynthetic pigments due to NO application has also been reported in different plant species under salt stress, including wheat (Ruan et al., 2004), tomato (Wu et al., 2010) and rice (Habib et al., 2013).
Salt-stressed chickpea leaves accumulated higher levels of H2O2 and MDA contents (Figures 2A,B), thereby increasing electrolyte leakage (Table 2), which might be due to membrane destruction caused by ROS-induced oxidative damage. Exogenous application of NO reduced electrolyte leakage and the levels of MDA and H2O2 in NaCl-treated chickpea plants (Figures 2A,B), which is in agreement with the observations of Zheng et al. (2009) and Khan et al. (2012). Therefore, application of NO could be an effective practice to protect plants against oxidative injury caused by salt stress. Jasid et al. (2008) reported that NO acts as an antioxidant and ROS scavenger, decreasing electrolyte leakage in sorghum (Sorghum bicolor). NO stimulates mitogen-activated protein kinase (MAPK) (Neill et al., 2008), which in turn activates transcription factors for induction of stress-related genes (Figure 5). Another study by Huaifu et al. (2007) also suggested that NO possesses the ability of restoring and defending the cell membrane to mitigate the damage in the cell membrane system by reducing the membrane permeability and membrane lipid peroxidation, thereby preventing electrolyte leakage.
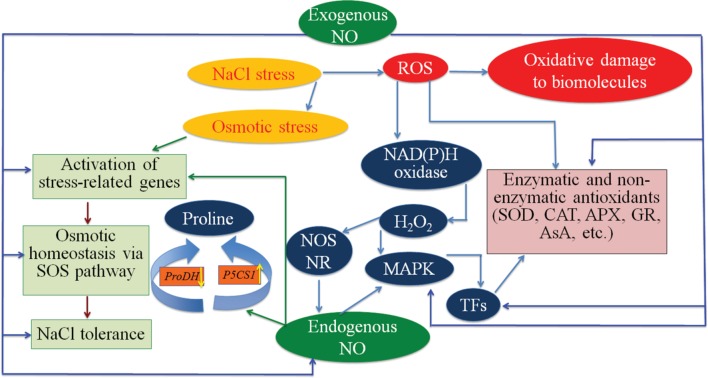
FIGURE 5.
Potential mechanisms of NaCl stress mitigation by application of exogenous NO. Excessive NaCl causes osmotic and oxidative stresses in plants. Salt stress induces ABA accumulation, which promotes H2O2 generation through NAD(P)H oxidase. Stress-induced H2O2 triggers generation of endogenous NO by activating NR (nitrate reductase) and NOS (nitric oxide synthase)-like enzymes. Exogenous application of NO to plants may enhance the biosynthesis of endogenous NO, as well as that of antioxidant enzymes through MAPK (mitogen-activated protein kinase) and other unknown signaling pathways. Exogenous NO supplementation to plants can also up-regulate genes involved in proline synthesis, such as P5CS1, and other stress-related genes responsible for NaCl tolerance, whereas it might down-regulate ProDH that is involved in proline catabolism. Exogenous NO treatment may also help balance osmotic homeostasis in plants under salt stress via the SOS (salt overly sensitive) pathway, by increasing plasma membrane H+-ATPase activity. APX, ascorbate peroxidase; AsA, ascorbic acid; CAT, catalase; GR, glutathione reductase; H2O2, hydrogen peroxide; P5CS1, δ1-pyrroline-5-carboxylate synthetase; ProDH, proline dehydrogenase; ROS, reactive oxygen species; SOD, superoxide dismutase; TFs, transcription factors.
To overcome the negative impacts of salt stress-induced osmotic stress, plants produce higher levels of osmolytes in the cytosol and other organelles (Abdel Latef and Miransari, 2014). In the present study, a similar accumulation trend of proline, GB, total soluble proteins and total soluble sugars was recorded in chickpea leaves under NaCl stress (Table 2). Increased accumulation of total soluble sugars and total soluble proteins in response to saline stress was reported by Liu et al. (2016) in Nitraria tangutorum. Proline and GB were also reported to accumulate under salt stress in B. juncea (Siddiqui et al., 2008; Khan et al., 2012), linseed (Linum usitatissimum) (Khan et al., 2010) and mulberry (Morus alba) (Ahmad et al., 2014). Proline and GB are important osmolytes that help in cell osmoregulation under salt stress (Ahmad et al., 2010, 2015). Proline is also reported to protect photosynthetic machinery, and act as energy storage under NaCl stress (Khan et al., 2013; Reddy et al., 2015). Proline has the ability to scavenge ROS and shield the cell from the oxidative damage (Ahmad et al., 2010; Khan et al., 2010; Jogaiah et al., 2013). Verdoy et al. (2006) have reported that proline accumulation enhanced the N2 fixation in Medicago truncatula plants under salt stress. GB has been reported to inhibit accumulation of ROS, protect photosynthetic machinery and activate stress-related genes (Chen and Murata, 2008). GB is also known to maintain the protein structures from damage induced by abiotic stresses (Sakamoto and Murata, 2002). Soluble proteins play a main role in osmotic adjustment under NaCl stress and can provide storage form of nitrogen (Singh et al., 1987). Increase in soluble protein content under stress may be the result of enhanced synthesis of specific stress-related proteins (Doganlar et al., 2010). Soluble sugars act as important osmolytes to maintain the cell homeostasis (Gupta and Kaur, 2005). Change in total soluble sugars under NaCl stress also involves changes in CO2 assimilation, enzyme activities and expression of certain genes (Gibson, 2005; Gupta and Kaur, 2005). Thus, application of NO to salt-stressed chickpea plants provoked a remarkable increase in levels of total soluble proteins, proline, GB and soluble sugars, perhaps to provide a better protection to plants exposed to stress. The protective nature of the osmolytes under NO treatment corroborates with the findings of Hayat et al. (2012), Khan et al. (2012), Kausar et al. (2013), and Dong et al. (2014) in various plants. Exogenous application of NO has also been known to induce the P5CS1 gene encoding δ1-pyrroline-5-carboxylate synthetase, a key enzyme involved in the proline synthesis (Zhang et al., 2008; Rejeb et al., 2014) (Figure 5). Noticeable accumulation of proline, GB, total soluble proteins and total soluble sugars due to NO application might enhance salt tolerance of cells through osmotic regulation. As a consequence, the increased osmotic pressure in the cells increased water uptake, and subsequently RWC, plant growth and biomass yield (Tables 1 and 2).
Salt stress induces the generation of huge amount of ROS, leading to the abnormalities at cellular level due to oxidation of proteins, lipids and nucleic acids (Schutzendubell and Polle, 2002; Ahmad et al., 2008, 2010; Hayat et al., 2012) (Figure 5). However, plants are capable to deal with such stressful conditions through increasing synthesis of antioxidant metabolites, including proline, and antioxidant enzymes, such as SOD, CAT, APX and GR (Schutzendubell and Polle, 2002; Ahmad et al., 2008, 2010; Hayat et al., 2012). In present study, the increase in the activities of SOD, CAT, APX and GR, as well as of proline content in chickpea plants due to salinity was observed (Figure 3; Table 2). Our results are supported by the observations reported by Hayat et al. (2012) in Solanum lycopersicum, Kausar et al. (2013) in T. aestivum and Manai et al. (2014) in S. lycopersicum. Furthermore, co-application of NO with NaCl markedly increased the activities of SOD, CAT, APX and GR in chickpea plants (Figure 3), which is in harmony with previous findings reported in mustard (Khan et al., 2012), tomato (Hayat et al., 2012; Manai et al., 2014) and in cotton (Dong et al., 2014). NO can act (i) as a direct scavenger of ROS, and (ii) antioxidant system inducer to enhance the expression of antioxidant enzyme-encoding genes (Groß et al., 2013). NO applied exogenously may also induce the synthesis of endogenous NO (Hao et al., 2008; Xiong et al., 2009; Zhao et al., 2009; Xu et al., 2010; Fan and Liu, 2012), which can function as signaling molecule or ROS scavenger under prolonged stress conditions by regulating/enhancing the activities of antioxidant enzymes (Hao et al., 2008; Xu et al., 2010; Fan and Liu, 2012) (Figure 5).
Consistent with the accumulation of antioxidant enzymes in chickpea plants under salt stress, either alone or co-applied with NO (Figure 3), the expression of representative SOD, CAT and APX genes examined was also up-regulated in treated chickpea plants (Figure 4). This result suggested that up-regulation of SOD, CAT and APX genes might enhance activities of the SOD, CAT and APX enzymes, thereby providing better protection to the cells against oxidative stress triggered by high salinity (Lu et al., 2007; Yamane et al., 2010; Hu et al., 2012). In support of our finding, several studies also reported the up-regulation of antioxidant enzyme-encoding genes under stress with or without NO treatment. For instance, Hernandez et al. (2000) have reported the enhanced expression of SOD and APX genes in a NaCl-tolerant Pisum sativum variety in comparison with the sensitive variety. Up-regulation of SOD gene expression has also been reported in other plants, including Lycopersicon esculentum (Aydin et al., 2014) and Lotus japonicus (Rubio et al., 2009) under NaCl stress. Zhang et al. (2014) showed the up-regulation of CAT and APX genes in Limonium sinense under high salinity. Menezes-Benavente et al. (2004) and Shafi et al. (2015) reported enhanced expression of APX gene in rice and Arabidopsis, respectively, under salt stress. Lin et al. (2011) noticed induced expression of APX by exogenous NO supply in sweet potato (Ipomoea batatas) under wounding stress. Therefore, it is reasonable to conclude that NO may activate the expression of antioxidant enzymes-related biosynthetic genes, which leads to accumulation of antioxidant enzymes, thereby providing better tolerance to plants under stresses (Figure 5).
Conclusion
Our study demonstrates that exogenous supply of NO is effective in mitigating salt stress in chickpea plants. Therefore, application of exogenous NO or manipulation of endogenous NO content might be promising approach for salt stress management in the era of climatic changes.
Author Contributions
PA and AA designed the experimental work. PA, AH, and EA performed the experimental work. SG carried out the statistical analysis and formatting of the manuscript. PA, AA, and L-ST wrote and revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to extend their sincere appreciation to their Institutions and acknowledge the Deanship of Scientific Research at King Saud University for funding the Research Group Project No RGP- VPP-271.
References
- Abdel Latef A. A., Chaoxing H. (2011). Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 127 228–233. 10.1016/j.scienta.2010.09.020 [DOI] [Google Scholar]
- Abdel Latef A. A., Chaoxing H. (2014). Does the inoculation with Glomus mosseae improves salt tolerance in pepper plants? J. Plant Growth Regul. 33 644–653. 10.1007/s00344-014-9414-4 [DOI] [Google Scholar]
- Abdel Latef A. A., Miransari M. (2014). “The role of arbuscular mycorrhizal fungi in alleviation of salt stress,” in Use of Microbes for the Alleviation of Soil Stresses, ed. Miransari M. (New York, NY: Springer; ), 23–38. [Google Scholar]
- Aebi H. (1984). Catalase in vitro. Method Enzymol. 105 121–126. 10.1016/S0076-6879(84)05016-3 [DOI] [PubMed] [Google Scholar]
- Ahmad P. (2010). Growth and antioxidant responses in mustard (Brassica juncea L.) plants subjected to combined effect of gibberellic acid and salinity. Arch. Agron. Soil Sci. 56 575–588. 10.1080/03650340903164231 [DOI] [Google Scholar]
- Ahmad P., Bhardwaj R., Tuteja N. (2012a). “Plant signaling under abiotic stress environment,” in Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change, eds Ahmad P., Prasad M. N. V. (New York, NY: Springer; ), 297–323. [Google Scholar]
- Ahmad P., Hakeem K. R., Kumar A., Ashraf M., Akram N. A. (2012b). Salt induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afr. J. Biotechnol. 11 2694–2703. [Google Scholar]
- Ahmad P., Hashem A., Abd-Allah E. F., Alqarawi A. A., John R., Egamberdieva D., et al. (2015). Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L) through antioxidative defense system. Front. Plant Sci. 6:868 10.3389/fpls.2015.00868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad P., Jaleel C. A., Salem M. A., Nabi G., Sharma S. (2010). Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 30 161–175. 10.3109/07388550903524243 [DOI] [PubMed] [Google Scholar]
- Ahmad P., Ozturk M., Sharma S., Gucel S. (2014). Effect of sodium carbonate-induced salinity-alkalinity on some key osmoprotectants, protein profile, antioxidant enzymes, and lipid peroxidation in two mulberry (Morus alba L.) cultivars. J. Plant Interact. 9 460–467. 10.1080/17429145.2013.855271 [DOI] [Google Scholar]
- Ahmad P., Sarwat M., Sharma S. (2008). Reactive oxygen species, antioxidants and signaling in plants. J. Plant Biol. 51 167–173. 10.1007/BF03030694 [DOI] [Google Scholar]
- Apel K., Hirt H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol. 55 373–399. 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- Aydin S., Buyuk I., Aras E. S. (2014). Expression of SOD gene and evaluating its role in stress tolerance in NaCl and PEG stressed Lycopersicon esculentum. Turk. J. Bot. 38 89–98. 10.3906/bot-1305-1 [DOI] [Google Scholar]
- Bates L., Waldren R. P., Teare J. D. (1973). Rapid determination of free proline for water stress studies. Plant Soil 39 205–207. 10.1016/j.dental.2010.07.006 [DOI] [Google Scholar]
- Bayer C., Fridovich I. (1987). Superoxide dismutase: improved assays and applicable to acrylamide gels. Anal. Biochem. 44 276–287. [DOI] [PubMed] [Google Scholar]
- Begara-Morales J. C., Sánchez-Calvo B., Chaki M., Valderrama R., Mata-Pérez C., López-Jaramillo J., et al. (2014). Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. J. Exp. Bot. 65 527–538. 10.1093/jxb/ert396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 72 248–259. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Chen T. H., Murata N. (2008). Glycinebetaine: an effective protectant against abiotic stress in plants. Trend Plant Sci. 13 499–505. 10.1016/j.tplants.2008.06.007 [DOI] [PubMed] [Google Scholar]
- Dey P. M. (1990). “Oligosaccharides,” in Methods in Plant Biochemistry: Carbohydrates, Vol. 2 ed. Dey P. M. (London: Academic Press; ), 189–218. [Google Scholar]
- Dionisio-Sese M. L., Tobita S. (1998). Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 135 1–9. 10.1016/S0168-9452(98)00025-9 [DOI] [Google Scholar]
- Doganlar Z. B., Demir K., Basak H., Gul I. (2010). Effects of salt stress on pigment and total soluble protein contents of three different tomato cultivars. Afr. J. Agric. Res. 5 2056–2065. [Google Scholar]
- Dong Y. J., Jinc S. S., Liu S., Xu L. L., Kong J. (2014). Effects of exogenous nitric oxide on growth of cotton seedlings under NaCl stress. J. Soil Sci. Plant Nutr. 14 1–13. [Google Scholar]
- Esim N., Atici O. (2014). Nitric oxide improves chilling tolerance of maize by affecting apoplastic antioxidative enzymes in leaves. Plant Growth Regul. 72 29–38. 10.1007/s10725-013-9833-4 [DOI] [Google Scholar]
- Evelin H., Kapoor R. (2014). Arbuscular mycorrhizal symbiosis modulates antioxidant response in salt-stressed Trigonella foenum-graecum plants. Mycorrhiza 24 197–208. 10.1007/s00572-013-0529-4 [DOI] [PubMed] [Google Scholar]
- Fan Q. J., Liu J. H. (2012). Nitric oxide is involved in dehydration/drought tolerance in Poncirus trifoliata seedlings through regulation of antioxidant systems and stomatal response. Plant Cell Rep. 31 145–154. 10.1007/s00299-011-1148-1 [DOI] [PubMed] [Google Scholar]
- Foyer C. H., Halliwell B. (1976). The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133 21–25. 10.1007/BF00386001 [DOI] [PubMed] [Google Scholar]
- Gibson S. I. (2005). Control of plant development and gene expression by sugar signaling. Curr. Opin. Plant Biol. 8 93–102. 10.1016/j.pbi.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Grieve C. M., Grattan S. R. (1983). Rapid assay for determination of water-soluble quaternary- amino compounds. Plant Soil 70 303–307. 10.1007/BF02374789 [DOI] [Google Scholar]
- Groß F., Durner J., Gaupels F. (2013). Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci. 4:419 10.3389/fpls.2013.00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A. K., Kaur N. (2005). Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 30 761–776. 10.1007/BF02703574 [DOI] [PubMed] [Google Scholar]
- Gururani M. A., Venkatesh J., Tran L. S. P. (2015). Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 8 1304–1320. 10.1016/j.molp.2015.05.005 [DOI] [PubMed] [Google Scholar]
- Habib N., Ashraf M. (2014). Effect of exogenously applied nitric oxide on water relations and ionic composition of rice (Oryza sativa L.) plants under salt stress. Pak. J. Bot. 46 111–116. [Google Scholar]
- Habib N., Ashraf M., Shahbaz M. (2013). Effect of exogenously applied nitric oxide on some key physiological attributes of rice (Oryza sativa L.) plants under salt stress. Pak. J. Bot. 45 1563–1569. [Google Scholar]
- Hao G. P., Xing Y., Zhang J. H. (2008). Role of nitric oxide dependence on nitric oxide synthase-like activity in the water stress signaling of maize seedling. J. Integr. Plant Biol. 50 435–442. 10.1111/j.1744-7909.2008.00637.x [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M., Hossain M. A., Fujita M. (2011). Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 5 353–365. 10.1007/s11816-011-0189-9 [DOI] [PubMed] [Google Scholar]
- Hashem A., Abd_Allah E. F., El-Didamony G., Alwhibi Mona S., Egamberdieva D., Ahmad P. (2014). Alleviation of adverse impact of salinity on faba bean (Vicia faba l.) by arbuscular mycorrhizal fungi. Pak. J. Bot. 46 2003–2013. [Google Scholar]
- Hayat S., Yadav S., Wani A. S., Irfan M., Alyemini M. N., Ahmad A. (2012). Impact of sodium nitroprusside on nitrate reductase, proline and antioxidant system in Solanum lycopersicum under salinity stress. Hort. Environ. Biotechnol. 53 362–367. 10.1007/s13580-012-0481-9 [DOI] [Google Scholar]
- Hernandez J. A., Jimenez A., Mullineaux P., Sevilla F. (2000). Tolerance of pea (Pisum sativum L.) to long term salt stress is associated with induction of antioxidant defences. Plant Cell Environ. 23 853–862. 10.1046/j.1365-3040.2000.00602.x [DOI] [Google Scholar]
- Hiscox J. D., Israelstam G. F. (1979). A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 57 1332–1334. 10.1139/b79-163 [DOI] [Google Scholar]
- Hu L., Huang Z., Liu S., Fu J. (2012). Growth response and gene expression in antioxidant-related enzymes in two bermudagrass genotypes differing in salt tolerance. J. Am. Soc. Hortic. Sci. 137 134–143. [Google Scholar]
- Hu T., Li H. Y., Zhang X. Z., Luo H. J., Fu J. M. (2011). Toxic effect of NaCl on ion metabolism, antioxidative enzymes and gene expression of perennial ryegrass. Ecotoxicol. Environ. Saf. 74 2050–2056. 10.1016/j.ecoenv.2011.07.013 [DOI] [PubMed] [Google Scholar]
- Huaifu F., Shirong G., Yansheng J., Runhua Z., Juan L. (2007). Effects of exogenous nitric oxide on growth, active oxygen species metabolism, and photosynthetic characteristics in cucumber seedlings under NaCl stress. Front. Agric. China 1:308–314. 10.1007/s11703-007-0052-5 [DOI] [Google Scholar]
- Jasid S., Simontacchi M., Puntarulo S. (2008). Exposure to nitric oxide protects against oxidative damage but increases the labile iron pool in sorghum embryonic axes. J. Exp. Bot. 59 3953–3962. 10.1093/jxb/ern235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogaiah S., Govind S. R., Tran L. S. P. (2013). Systems biology-based approaches toward understanding drought tolerance in food crops. Crit. Rev. Biotechnol. 33 23–39. 10.3109/07388551.2012.659174 [DOI] [PubMed] [Google Scholar]
- Jukanti A. K., Gaur P. M., Gowda C. L. L., Chibbar R. N. (2012). Nutritional quality and health benefits of chickpea (Cicer arietinum L.): a review. Br. J. Nutr. 108(Suppl. 1), S11–S26. 10.1017/S0007114512000797 [DOI] [PubMed] [Google Scholar]
- Kausar F., Shahbaz M. (2013). Interactive effect of foliar application of nitric oxide (NO) and salinity on wheat (Triticum aestivum L.). Pak. J. Bot. 45(SI), 67–73. [Google Scholar]
- Kausar F., Shahbaz M., Ashraf M. (2013). Protective role of foliar-applied nitric oxide in Triticum aestivum under saline stress. Turk. J. Bot. 37 1155–1165. 10.3906/bot-1301-17 [DOI] [Google Scholar]
- Ke X., Cheng Z., Ma W., Gong M. (2013). Nitric oxide enhances osmoregulation of tobacco (Nicotiana tobacum L.) cultured cells under phenylethanoid glycosides (PEG) 6000 stress by regulating proline metabolism. Afr. J. Biotechnol. 12 1257–1266. [Google Scholar]
- Khan M. A., Akhtar N., Ullah I., Jaffery S. (1995). Nutritional evaluation of desi and kabuli chickpeas and their products commonly consumed in Pakistan. Int. J. Food Sci. Nutr. 46 215–223. 10.3109/09637489509012551 [DOI] [PubMed] [Google Scholar]
- Khan M. I. R., Iqbal N., Masood A., Per T. S., Khan N. A. (2013). Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 8 e26374 10.4161/psb.26374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. N., Siddiqui M. H., Mohammad F., Naeem M. (2012). Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 27 210–218. 10.1016/j.niox.2012.07.005 [DOI] [PubMed] [Google Scholar]
- Khan M. N., Siddiqui M. H., Mohammad F., Naeem M., Khan M. M. A. (2010). Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol. Plant. 32 121–132. 10.1007/s11738-009-0387-z [DOI] [Google Scholar]
- Klessig D. F., Durner J., Noad R., Navarre D. A., Wendehenne D., Kumar D., et al. (2000). Nitric oxide and salicylic acid signaling in plant defense. Proc. Nat. Acad. Sci. U.S.A. 97 8849–8855. 10.1073/pnas.97.16.8849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotula L., Khan H. A., Quealy J., Turner N. C., Vadez V., Siddique K. H., et al. (2015). Salt sensitivity in chickpea (Cicer arietinum L.): ions in reproductive tissues and yield components in contrasting genotypes. Plant Cell Environ. 38 1565–1577. 10.1111/pce.12506 [DOI] [PubMed] [Google Scholar]
- Leshem Y. Y., Haramaty E. (1996). Plant aging: the emission of NO and ethylene and effect of NO-releasing compounds on growth of pea (Pisum sativum) foliage. J. Plant Physiol. 148 258–263. 10.1016/S0176-1617(96)80251-3 [DOI] [Google Scholar]
- Lin C. C., Jih P. J., Lin H. H., Lin J. S., Chang L. L., Shen Y. H., et al. (2011). Nitric oxide activates superoxide dismutase and ascorbate peroxidase to repress the cell death induced by wounding. Plant Mol. Biol. 77 235–249. 10.1007/s11103-011-9805-x [DOI] [PubMed] [Google Scholar]
- Liu S., Dong Y. J., Xu L. L., Kong J., Bai X. Y. (2013). Roles of exogenous nitric oxide in regulating ionic equilibrium and moderating oxidative stress in cotton seedlings during salt stress. J. Soil Sci. Plant Nutr. 13 929–941. [Google Scholar]
- Liu W., Zhang Y., Yuan X., Xuan X., Gao Y., Yan Y. (2016). Exogenous salicylic acid improves salinity tolerance of Nitraria tangutorum. Russ. J. Plant Physiol. 63 132–142. 10.1134/S1021443716010118 [DOI] [Google Scholar]
- Lu Z., Liu D., Liu S. (2007). Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis. Plant Cell Rep. 26 1909–1917. 10.1007/s00299-007-0395-7 [DOI] [PubMed] [Google Scholar]
- Manai J., Kalai T., Gouia H., Corpas F. J. (2014). Exogenous nitric oxide (NO) ameliorates salinity-induced oxidative stress in tomato (Solanum lycopersicum) plants. J. Soil Sci. Plant Nutr. 14 433–446. [Google Scholar]
- Menezes-Benavente L., Teixeira F. K., Kamei C. L. A., Margis-Pinheiro M. (2004). Salt stress induces altered expression of genes encoding antioxidant enzymes in seedlings of a Brazilian indica rice (Oryza sativa L.). Plant Sci. 166 323–331. 10.1016/j.plantsci.2003.10.001 [DOI] [Google Scholar]
- Mostofa M. G., Fujita M., Tran L. S. P. (2015). Nitric oxide mediates hydrogen peroxide- and salicylic acid-induced salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul. 77 265–277. 10.1007/s10725-015-0061-y [DOI] [Google Scholar]
- Nakano Y., Asada K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22 867–880. [Google Scholar]
- Neill S., Barros R., Bright J., Desikan R., Hancock J., Harrison J., et al. (2008). Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 59 165–176. 10.1093/jxb/erm293 [DOI] [PubMed] [Google Scholar]
- Rao K. V. M., Sresty T. V. S. (2000). Antioxidative parameters in the seedlings of pigeon pea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci. 157 113–128. 10.1016/S0168-9452(00)00273-9 [DOI] [PubMed] [Google Scholar]
- Rasool S., Abdel Latef A. A., Ahmad P. (2015). “Chickpea: role and responses under abiotic and biotic stress,” in Legumes under Environmental Stress: Yield, Improvement and Adaptations, eds Azooz M. M., Ahmad P. (Chichester: John Wiley; ), 67–79. [Google Scholar]
- Rasool S., Ahmad A., Siddiqi T. O., Ahmad P. (2013). Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 35 1039–1050. 10.1007/s11738-012-1142-4 [DOI] [Google Scholar]
- Reddy P. S., Jogeswar G., Rasineni G. K., Maheswari M., Reddy A. R., Varshney R. K., et al. (2015). Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 94 104–113. 10.1016/j.plaphy.2015.05.014 [DOI] [PubMed] [Google Scholar]
- Rejeb K. B., Abdelly C., Savouré A. (2014). How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 80 278–284. 10.1016/j.plaphy.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Ruan H. H., Shen W. B., Xu L. L. (2004). Nitric oxide modulates the activities of plasma membrane ATPase and PPase in wheat seedling roots and promotes the salt tolerance against salt stress. Acta Bot. Sin. 46 415–422. [Google Scholar]
- Rubio M. C., Bustos-Sanmamed P., Clemente M. R., Becana M. (2009). Effects of salt stress on the expression of antioxidant genes and proteins in the model legume Lotus japonicus. New Phytol. 181 851–859. 10.1111/j.1469-8137.2008.02718.x [DOI] [PubMed] [Google Scholar]
- Sakamoto A., Murata N. (2002). The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ. 25 163–171. 10.1046/j.0016-8025.2001.00790.x [DOI] [PubMed] [Google Scholar]
- Schutzendubell A., Polle A. (2002). Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 53 1351–1365. 10.1093/jexbot/53.372.1351 [DOI] [PubMed] [Google Scholar]
- Shafi A., Chauhan R., Gill T., Swarnkar M. K., Sreenivasulu Y., Kumar S., et al. (2015). Expression of SOD and APX genes positively regulates secondary cell wall biosynthesis and promotes plant growth and yield in Arabidopsis under salt stress. Plant Mol. Biol. 87 615–631. 10.1007/s11103-015-0301-6 [DOI] [PubMed] [Google Scholar]
- Siddiqui M. H., Al-Whaibi M. H., Basalah M. O. (2011). Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 248 447–455. 10.1007/s00709-010-0206-9 [DOI] [PubMed] [Google Scholar]
- Siddiqui M. H., Khan M. N., Mohammad F., Khan M. M. A. (2008). Role of nitrogen and gibberellin (GA3) in the regulation of enzyme activities and osmoprotectant accumulation in Brassica juncea L. under salt stress. J. Agron. Crop Sci. 194 214–224. 10.1111/j.1439-037X.2008.00308.x [DOI] [Google Scholar]
- Singh A. P., Dixit G., Mishra S., Dwivedi S., Tiwari M., Mallick S., et al. (2015). Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front. Plant Sci. 6:340 10.3389/fpls.2015.00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Bracken C. A., Hasegawa P. M., Handa A. K., Buckel S., Hermodson M. A., et al. (1987). Characterization of osmotin. A thaumatin-like protein associated with osmotic adjustment in plant cells. Plant Physiol. 85 529–536. 10.1104/pp.85.2.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova V., Yordanov I., Edreva A. (2000). Oxidative stress and some antioxidant system in acid rain treated bean plants: protective role of exogenous polyamines. Plant Sci. 151 59–66. 10.1016/S0168-9452(99)00197-1 [DOI] [Google Scholar]
- Verdoy D., Coba De La Peña T., Redondo F. J., Lucas M. M., Pueyo J. J. (2006). Transgenic Medicago truncatula plants that accumulate proline display nitrogen-fixing activity with enhanced tolerance to osmotic stress. Plant Cell Environ. 29 1913–1923. 10.1111/j.1365-3040.2006.01567.x [DOI] [PubMed] [Google Scholar]
- Wu X. X., Ding H. D., Chen J. L., Zhang H. J., Zhu W. M. (2010). Attenuation of salt-induced changes in photosynthesis by exogenous nitric oxide in tomato (Lycopersicon esculentum Mill. L.) seedlings. Afr. J. Biotechnol. 9 7837–7846. [Google Scholar]
- Wu X. X., Zhu W., Zhang H., Ding H., Zhang H. J. (2011). Exogenous nitric oxide protects against salt-induced oxidative stress in the leaves from two genotypes of tomato (Lycopersicon esculentum Mill.). Acta Physiol. Plant. 33 1199–1209. 10.1007/s11738-010-0648-x [DOI] [Google Scholar]
- Xiong J., An L., Lu H., Zhu C. (2009). Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230 755–765. 10.1007/s00425-009-0984-5 [DOI] [PubMed] [Google Scholar]
- Xu Y., Sun X., Jin J., Zhou H. (2010). Protective effect of nitric oxide on light-induced oxidative damage in leaves of tall fescue. J. Plant Physiol. 167 512–518. 10.1016/j.jplph.2009.10.010 [DOI] [PubMed] [Google Scholar]
- Yamane K., Mitsuya S., Taniguchi M., Miyake H. (2010). Transcription profiles of genes encoding catalase an ascorbate peroxidase in rice leaf tissues under salinity. Plant Prod. Sci. 13 164–168. 10.1626/pps.13.164 [DOI] [Google Scholar]
- Yamasaki S., Dillenburg L. C. (1999). Measurements of leaf relative water content in Araucaria angustifolia. Rev. Bras. Fisiol. Veg. 11 69–75. [Google Scholar]
- Yang L., Bai X., Yang Y., Ahmad P., Yang Y., Hu X. (2011). Deciphering the protective role of nitric oxide against salt stress at the physiological and proteomic levels in maize. J. Proteome Res. 10 4349–4364. 10.1021/pr200333f [DOI] [PubMed] [Google Scholar]
- Yang L., Han R., Sun Y. (2013). Effects of exogenous nitric oxide on wheat exposed to enhanced ultraviolet-B radiation. Am. J. Plant Sci. 4 1285–1290. 10.4236/ajps.2013.46159 [DOI] [Google Scholar]
- Zeng C. L., Liu L., Wang B. R., Wu X. M., Zhou Y. (2011). Physiological effects of exogenous nitric oxide on Brassica juncea seedlings under NaCl stress. Biol. Plant. 55 345–348. 10.1007/s10535-011-0051-5 [DOI] [Google Scholar]
- Zhang L. P., Mehta S. K., Liu Z. P., Yang Z. M. (2008). Copper-induced proline synthesis is associated with nitric oxide generation in Chlamydomonas reinhardtii. Plant Cell Physiol. 49 411–419. 10.1093/pcp/pcn017 [DOI] [PubMed] [Google Scholar]
- Zhang X., Yin H. B., Chen S. H., He J., Guo S. L. (2014). Changes in antioxidant enzyme activity and transcript levels of related genes in Limonium sinense kuntze seedlings under NaCl stress. J. Chem. 2014 749047 10.1155/2014/749047 [DOI] [Google Scholar]
- Zhang Y. Y., Liu J., Liu Y. L. (2004). Nitric oxide alleviates growth inhibition of maize seedlings under salt stress. J. Plant Physiol. Mol. Biol. 30 455–459. [PubMed] [Google Scholar]
- Zhao M. G., Chen L., Zhang L. L., Zhang W. H. (2009). Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol. 151 755–767. 10.1104/pp.109.140996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C. F., Dong J. G., Liu F. L., Dai T. B., Liu W. C., Jing Q., et al. (2009). Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ. Exp. Bot. 67 222–227. 10.1016/j.envexpbot.2009.05.002 [DOI] [Google Scholar]