Abstract
Oxytocin is a potent uterotonic agent and is used clinically for induction and augmentation of labor, as well as for prevention and treatment of postpartum hemorrhage. Oxytocin increases uterine contractility by activating the oxytocin receptor (OXTR), a member of the G protein-coupled receptor family, which is prone to molecular desensitization. After oxytocin binding, the OXTR is phosphorylated by a member of the G protein-coupled receptor kinase (GRK) family, which allows for recruitment of β-arrestin, receptor internalization, and desensitization. According to previous in vitro analyses, desensitization of calcium signaling by the OXTR is mediated by GRK6. The objective of this study was to determine the role of GRK6 in mediating uterine contractility. Here, we demonstrate that uterine GRK6 levels increase in pregnancy and using a telemetry device to measure changes in uterine contractility in live mice during labor, show that mice lacking GRK6 produce a phenotype of enhanced uterine contractility during both spontaneous and oxytocin-induced labor compared with wild-type or GRK5 knockout mice. In addition, the observed enhanced contractility was associated with high rates of term stillbirth. Lastly, using a heterologous in vitro model, we show that β-arrestin recruitment to the OXTR, which is necessary for homologous OXTR desensitization, is dependent on GRK6. Our findings suggest that GRK6-mediated OXTR desensitization in labor is necessary for normal uterine contractile patterns and optimal fetal outcome.
Oxytocin is an endogenous neurohypophysial hormone released in large amounts during spontaneous labor (1). Synthetic oxytocin is used clinically for induction and augmentation of labor, and for the prevention and treatment of postpartum hemorrhage (2, 3). Due to its short serum half-life and narrow therapeutic range, continuous infusions of oxytocin are required to establish and maintain uterine contractility (4, 5). Oxytocin mediates its action through the oxytocin receptor (OXTR), which is a G protein-coupled receptor (GPCR) that undergoes rapid desensitization in the setting of agonist stimulation (6–8). Thus, prolonged oxytocin administration leads to receptor desensitization, paradoxically resulting in decreased myometrial contractility (9, 10). Diminished uterine contractility as a result of prolonged oxytocin therapy and OXTR desensitization can increase the risk for cesarean delivery due to dysfunctional labor patterns, or can increase the risk for uterine atony, resulting in postpartum hemorrhage (11, 12).
The OXTR undergoes molecular desensitization through a well-established mechanism (6). After binding of oxytocin to the OXTR, the receptor is phosphorylated by a member of the GPCR kinase (GRK) family, which then allows for the recruitment and binding of β-arrestin to the receptor. β-arrestin recruitment results in receptor internalization and uncoupling of the receptor from G proteins, thereby limiting further oxytocin signaling. After internalization, the receptor then cycles back to the plasma membrane, where it is then again available for signaling (13).
Oxytocin activation of the OXTR increases intracellular calcium, which allows for uterine smooth muscle contraction. According to recent in vitro analyses, GRK6 is the principle GRK family member mediating desensitization of calcium signaling by the OXTR (14). In addition, GRK6 has been shown to be expressed within the human myometrium at greater levels during pregnancy compared with nonpregnant myometrium (15).
The purpose of this study was to determine the role of GRK6 in mediating in vivo uterine contractility and labor phenotypes. Here, we show that uterine expression of GRK6 increases in murine pregnancy. Next, we demonstrate an enhanced uterine contractile phenotype of increased uterine contraction force and frequency in GRK6 knockout (KO) mice. This phenotype is associated with increases in stillbirth, presumably due to enhanced uterine contractility as a result of diminished OXTR desensitization. In vitro work also shows that GRK6 is required for β-arrestin recruitment to the OXTR and MAPK signaling in heterologous cells. Our findings suggest that OXTR desensitization is important in regulating normal uterine contractile patterns in labor.
Materials and Methods
Peptides, reagents, and antibodies
Oxytocin used for animal studies was obtained from Sigma-Aldrich and oxytocin used for cell culture work was obtained from Calbiochem. GeneSilencer siRNA transfection reagent was obtained from Genlantis. Antibodies were obtained as follows: GRK6, GRK5, GRK2, and OXTR were obtained from Abcam; β-arrestin-1/2 was provided by Dr Robert Lefkowitz (Duke University and Howard Hughes Medical Institute), and phosphorylated (p)-p44/42 (pERK1/2) and p44/42 (total ERK1/2) were obtained from Cell Signaling Technology. See Supplemental Table 1 for list of antibody product numbers, expected molecular weight of protein target, and dilutions used. OXTR peptide was purchased from Abcam (Supplemental Figure 1). The anti-HA-immunoprecipitation (IP) kit was obtained from Sigma-Aldrich. The HA-OXTR plasmid (in pcDNA3.1(+) vector; Invitrogen) was a gift from Dr Marc Caron (Duke University). Custom siRNA was obtained from QIAGEN. 3,3′-dithiodipropionic acid di(N-hydroxysuccinimide ester) (DSP) was used as a cross-linker in IP experiments and was purchased from Sigma-Aldrich.
Animal care, timed matings, and uterine tissue processing
All animal studies were reviewed and approved by the Duke University Institutional Animal Care and Use Committee. C57BL/6J wild-type (WT) mice were obtained from The Jackson Laboratory at 8 weeks of age and experiments performed at 10 weeks of age. GRK5 KO and GRK6 KO mice were initially provided by Dr Richard Premont (Duke University) and then bred and maintained in our mouse colony. These KO mice were created on a C57BL/6J background and have been previously described (16, 17). For all experiments, WT males were mated with WT females, GRK6 KO males were mated with GRK6 KO females, and GRK5 KO males were mated with GRK5 KO females. For maintenance of our colony, GRK6 KO males are mated with heterozygote GRK6 KO females, which produces full, live litters. When GRK6 KO males are mated with GRK6 KO females, we see marked decrease in the number of live pups born.
To determine the effect of pregnancy on uterine expression of proteins regulating GPCR desensitization, WT mice at 10 weeks of age underwent overnight timed matings. The morning that a copulatory plug was identified was considered postcoital day 0. Nonpregnant control mice were WT littermates that were not placed with a male breeder. Pregnant mice underwent humane euthanasia at postcoital days 8, 12, 13, 14, 17, 18, or postpartum day 1, at which time the pups and placental tissue was removed and the uterus was homogenized in cell lysis buffer (Cell Signaling Technology) with protease inhibitor (Sigma-Aldrich) and then prepared for SDS-PAGE and Western blotting. Nonpregnant mice underwent humane euthanasia at 11–12 weeks of age during random cycling, and the uterus was harvested and similarly processed for gel electrophoresis and Western blotting. Blots were probed with antibodies directed at GRK6, GRK5, GRK2, β-arrestin-1/2, OXTR, and pERK1/2. Total ERK1/2 served as a protein-loading control (9). Summary data presented are from 4 independent experiments using uterine tissue from 4 WT, 4 GRK6 KO, and 4 GRK5 KO mice each both nonpregnant and pregnant (postcoital d 18). The intensity of each band was determined and normalized to the intensity of the total ERK1/2 band for each experiment as previously described (9). One-way ANOVA, with Dunnett's post hoc test correction for multiple comparisons, was then used to compare the normalized mean fold change band intensities over nonpregnant band intensities for each protein of interest.
To determine whether relative uterine expression of the OXTR varied by GRK status, WT, GRK5 KO, and GRK6 KO mice underwent timed matings and were humanely euthanized on postcoital day 18. The uterus was processed in cell lysis buffer and then prepared for SDS-PAGE and Western blotting. Blots were probed for OXTR and total-ERK1/2 as a protein-loading control.
In vivo contraction studies and labor induction
In vivo contraction studies were performed as previously described with modifications as follows (18, 19). To determine whether uterine contraction patterns in response to oxytocin among nonpregnant mice were affected by GRK expression, 10-week-old WT, GRK5 KO, and GRK6 KO mice underwent surgical implantation of a DSI PysioTel PA-C10 implantable pressure transmitter telemetry device (Data Sciences International) into 1 uterine horn. Once adequate anesthesia was obtained with inhaled isoflurane, a vertical midline incision was made on the abdomen. One of the 2 uterine horns was then identified and a small incision made at the uterine fundus. The telemetry catheter was then gently guided into the uterine cavity through this incision to the midportion of the uterus and then secured in place with Vetbond tissue adhesive (3M). The telemetry transmitter was then gently placed into the abdominal cavity. Next, an ALZET 3-day miniosmotic pump (ALZET) loaded with oxytocin to deliver 3 U/d was placed in the sc tissue of the abdominal wall, and then the abdomen was closed and the mouse allowed to recover. The cage was then placed on the DSI PhysioTel Receiver (DSI) and changes in uterine pressure were recorded over 24 hours using DSI Dataquest A.R.T. software (DSI). Uterine pressure was recorded every 10 seconds (scheduled interval) with a segment duration of 5 seconds for all in vivo contraction assays.
To determine the effect of GRK expression on spontaneous and oxytocin-induced labor, WT, GRK5 KO, and GRK6 KO mice underwent timed matings at 10 weeks of age. On postcoital day 10, the mice were placed under anesthesia using inhaled isoflurane, a vertical midline abdominal incision was made, and the uterine horns were identified. A small incision was made at the fundus of the most accessible uterine horn, and the DSI telemetry catheter gently passed into the uterine cavity past the level of the second pup. The telemetry catheter was secured in place with Vetbond, the telemetry transmitter placed into the abdomen, the abdomen closed, and the mouse allowed to recover (18, 19). For spontaneous labor studies, the cage containing the pregnant mouse was placed on the DSI PhysioTel Receiver on postcoital day 17 and changes in uterine pressure were recorded until the morning after delivery. For oxytocin-induced labor, on postcoital day 17, the mice had an ALZET 3-day pump (Model 1003D) delivering 3 U/d of oxytocin (20) placed in the sc tissue at the nape of the neck, and then the cage was placed on the DSI PhysioTel Receiver and changes in uterine pressure were recorded using the DSI Dataquest A.R.T. software until the following morning. After delivery, all mice underwent humane euthanasia, the abdomen was opened, and the DSI telemetry device recovered and sterile processed. Five mice from each genotype, and for each experimental condition, were used.
Contraction data (changes in uterine pressure with time) for each experiment were exported from the DSI Dataquest A.R.T. software into GraphPad Prism (version 6.0; GraphPad Software). Contractile force was measured as the area under the contraction curve (AUC) using GraphPad Prism 6.0. For each experimental condition (nonpregnant oxytocin-treated, pregnant spontaneous labor, and pregnant oxytocin-induced labor) the mean AUC was calculated from each genotype and compared using one-way ANOVA with Dunnett's post hoc test correction for multiple comparisons. Contractile frequency was calculated using LabChart7 (LabChart version 7.1; ADInstruments). Contraction data (changes in uterine pressure with time) for each experiment were exported from the DSI Dataquest A.R.T. software into LabChart 7 and the contraction frequency over time was determined using the cyclic measurement function in LabChart7. A scatterplot of the contraction frequency values was then created in GraphPad Prism 6.0 and the median contraction frequency from each experimental condition (nonpregnant oxytocin-treated, pregnant spontaneous labor, and pregnant oxytocin-induced labor) was compared by genotype using a Kruskal-Wallis test with Dunn's post hoc test correction for multiple comparisons.
Placental histology
WT and GRK6 KO mice underwent timed matings and were humanely euthanized on postcoital day 18. The fetuses and placentas were removed and weighed, and then the placentas fixed in formalin. The placentas were then paraffin embedded, sectioned and mounted on slides. Placental sections were then stained with hematoxylin and eosin (H&E) or Masson's trichrome stains. Stained placental sections were then imaged at ×4 and ×10 using an Olympus Vanox photomicroscope and images taken using an Olympus DP-70 camera (Duke University PhotoPath core facility).
Cell culture
Human embryonic kidney-293 (HEK-293) cells were obtained from the Duke University Cancer Center's Cell Culture Facility and cultured in MEM (Gibco) supplemented with 10% fetal bovine serum (Sigma) and antibiotic-antimycotic (Invitrogen), in a 5% CO2 humidified atmosphere at 37°C. All cells were trypsinized with 0.05% Trypsin-EDTA (Gibco).
siRNA transfections and preparation of cell lysates for Western blotting
The sequence of the double-stranded siRNA directed against GRK6, GRK5, GRK2, and control scramble has been described previously (21). Briefly, HEK-293 cells in 6-well plates at 30%–40% confluence without antibiotics were serum-starved for 1 hour and then transfected simultaneously with 1 μg of HA-OXTR plasmid and 20 μg of GRK6, GRK5, or GRK2 siRNA, or control scramble siRNA using GeneSilencer siRNA transfection reagent (Genlantis). A final concentration of 10% serum was added 4 hours after transfection. After 72 hours of incubation, cells were serum starved for 1 hour and then stimulated with 100nM oxytocin (Calbiochem) for 2, 5, 10, 30, or 60 minutes. After stimulation, cells were lysed with cell lysis buffer (Cell Signaling Technology) and processed on an SDS-PAGE per standard Western blotting protocol. Western blottings were performed using antiphosphorylated-p44/42 (phosphorylated-ERK; Cell Signaling Technology), after which they were stripped and then reprobed for total p44/42 (Cell Signaling Technology). GRK knockdown was demonstrated by probing with the corresponding anti-GRK antibody (Abcam). The mean ± SEM intensity of the phosphorylated-ERK1/2 band at each time point was determined and expressed as a percent of maximal stimulation (phosphorylated-ERK1/2 signal at either 2 or 5 min in setting of CTL siRNA), normalized to the band density of total ERK1/2 at each time point and at each condition. The resulting curves were them compared using two-way ANOVA between cells treated with CTL siRNA and those treated with siRNA directed at a GRK, and post hoc test corrections for multiple comparison made using the Bonferroni test to compare values at each time point.
IP/β-arrestin recruitment assays
HEK-293 cells were transfected at 30%–40% confluence with 2 μg of plasmid encoding HA-OXTR and 20-μg siRNA directed against GRK2, GRK5, GRK6, or control scrambled siRNA using GeneSilencer (Genlantis) into 10-cm plates. Seventy-two hours after transfection, the media was removed and the cells were serum starved in PBS supplemented with calcium, magnesium, and HEPES for 1 hour, after which the cells were stimulated with 100nM oxytocin (Calbiochem) for 2 minutes. Fresh DSP (final concentration of 0.2 mg/mL) was then added for crosslinking and cells were incubated at room temperature for 30 minutes. The DSP reaction was quenched with 1M Tris for 15 minutes and then the media was removed and the cells washed with ice cold PBS with HEPES. Cells were lysed with RIPA buffer (Cell Signaling Technology) and the lysed cells were collected and tumbled at 4°C for 4 hours and then spun for 30 minutes. The supernatant was collected and 1000 μg of total protein was incubated with anti-HA agarose beads using an HA-IP kit (Sigma-Aldrich) per the manufacturer's instructions, and tumbled at 4°C for 4 hours. The beads were washed with cold RIPA buffer and protein bound to the beads was eluted with Laemmli buffer (Bio-Rad). The eluted protein was then processed for SDS-PAGE and Western blotting and probed with antibodies directed against β-arrestin and OXTR. Cell lysate not exposed to the IP beads was used as a protein-loading control. Summary data represents results from 5 independent experiments. One-way ANOVA, with Dunnett's post hoc test correction for multiple comparisons, was then used to compare the normalized mean fold change band intensities with bands from unstimulated cells.
Statistical analysis
All analyses were performed using GraphPad Prism 6.0 (GraphPad Software) and a corrected P < .05 (after corrections for multiple comparisons) was considered significant. Further description of the statistical tests performed can be found above following each experimental category.
Results
GRK6 KO mice exhibit a reproductive phenotype of stillbirth
There was no difference in median length of gestation between WT, GRK6 KO, and GRK5 KO mice (Table 1). There were also no significant differences in mean pup or placental weights between the 3 groups. The number of live-born pups to GRK6 KO mice was significantly lower than the number of live-born pups to either WT mice or GRK5 KO mice (25% vs 69% vs 77%, respectively; P < .001) (Table 1). The median length of gestation in our WT mice (19.3 d) was similar to that reported for C57BL/6J mice, suggesting that the presence of the uterine telemetry device did not affect the duration of pregnancy in our studies (22).
Table 1.
Delivery Outcomes After Spontaneous Labor at Term
| Characteristic | WT | GRK6 KO | GRK5 KO | P Valued |
|---|---|---|---|---|
| Gestational age delivery, da | 19.3 (17.9, 19.9) | 19.5 (18.6, 20.7) | 19.6 (19.1, 19.9) | .66 |
| Live pups, n (%)b | 29/42 (69) | 10/40 (25) | 36/47 (77) | <.001 |
| Pup birthweight, mgc | 116.7 ± 30.4 | 118.9 ± 12.2 | 119.7 ± 16.8 | .96 |
| Placental weight, mgc | 13.9 ± 1.4 | 12.7 ± 1.8 | 13.4 ± 1.0 | .36 |
Values are median (IQR).
Numerator is total number of live pups delivered, denominator is total number of pups identified at the time of delivery (n = 5 mice from each group).
Values are mean ± SD.
P value is from Kruskal-Wallis, χ2, or one-way ANOVA as appropriate.
Uterine GRK6 protein expression is up-regulated in pregnancy
To determine the effect of pregnancy on uterine expression of proteins known to regulate GPCR desensitization, WT mice underwent timed matings and the relative expression of GRK6, GRK5, GRK2, and β-arrestin1/2 were determined. By postcoital day 12, there was up-regulation of uterine GRK6 protein expression that remained elevated throughout pregnancy (Figure 1A). At term (postcoital d 18), mean uterine GRK6 protein expression was 1.5-fold increased compared with nonpregnant uterine GRK6 expression (P = .04) (Figure 1B). There was no change in uterine GRK2, GRK5, or β-arrestin1/2 expression at term (postcoital d 18) compared with levels seen within the uterus in nonpregnant mice, although there was a significant increase in uterine GRK2 expression at postcoital days 12–13 (P < .05) (Figure 1A).
Figure 1.
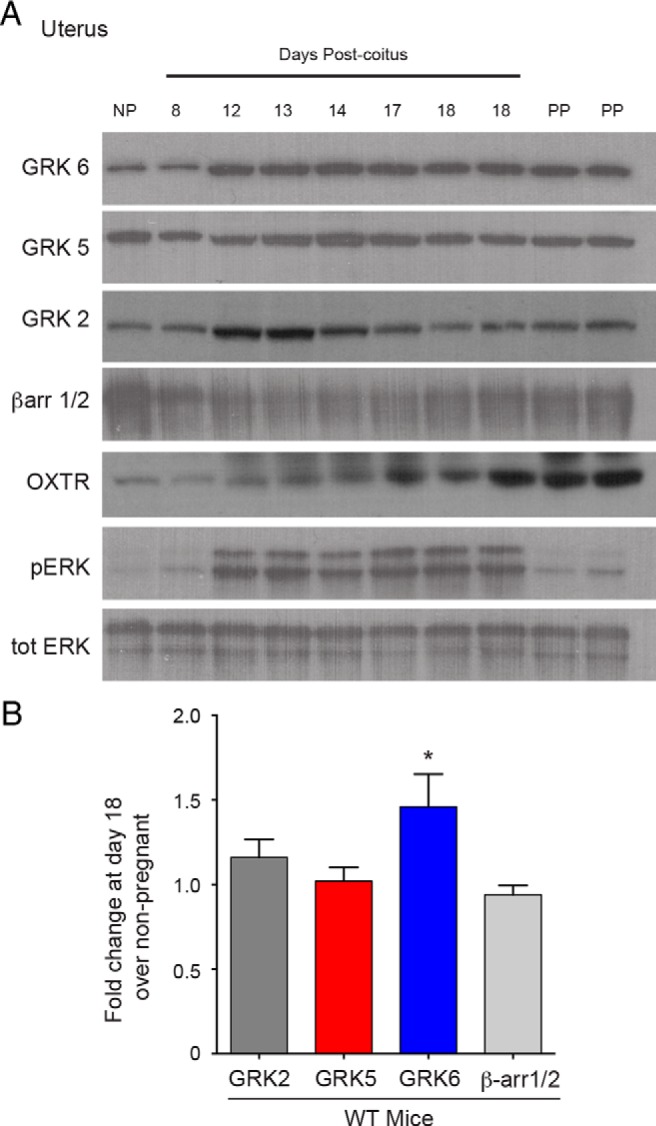
Relative changes in murine uterine proteins regulating OXTR desensitization during pregnancy. A, Representative Western blottings of changes in uterine expression of GRK, β-arrestin, OXTR, and ERK proteins during pregnancy by days postcoitus. Mean densitometry demonstrated that there was a 1.9-fold increased uterine OXTR expression and 4.6-fold increased uterine pERK expression at postcoital day 18 compared with nonpregnant (NP) (P = .003 and P = .001, respectively) B, There was a 1.5-fold increased uterine expression of GRK6 at postcoital day 18 compared with NP (*, P = .04). Relative uterine GRK2, GRK5, and βarr1/2 levels at postcoital day 18 did not significantly change compared with NP. Values are normalized to totERK as a protein-loading control. PP, postpartum.
Uterine OXTR expression increased significantly in the term (postcoital d 18) uterus compared with nonpregnant uterine tissue (1.95-fold; P = .003) (Figure 1A). Similarly, there was an enhanced phosphorylated-ERK1/2 (p-ERK) level within the term pregnant uterus compared with nonpregnant or postpartum uterus (4.6-fold; P = .001) (Figure 1A).
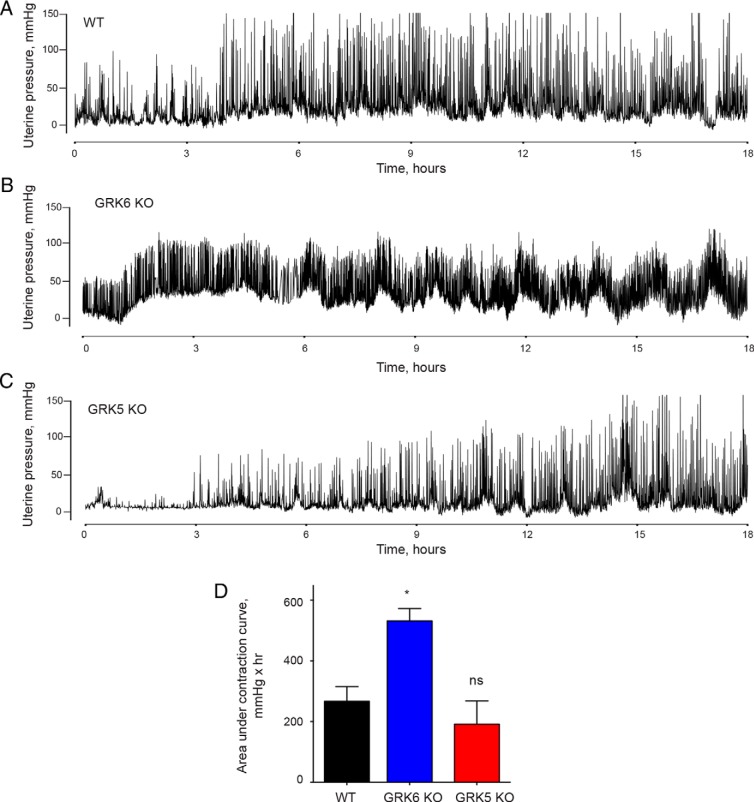
Nonpregnant mice lacking GRK6 exhibit enhanced uterine contractility in response to oxytocin
To determine the effect of myometrial GRK6 and GRK5 on uterine contractility in response to oxytocin in nonpregnant mice, WT, GRK6 KO, and GRK5 KO mice underwent surgical placement of a DSI telemetry device within the uterine cavity that measures changes in uterine pressure and treated with oxytocin (Figure 2, A–C). GRK6 KO mice exhibited greater mean uterine contraction force compared with WT mice as measured by the AUC over a 13-hour period in response to oxytocin treatment (Figure 2D and Table 2) Similarly, GRK6 KO mice demonstrated greater contraction frequency compared with WT mice in response to oxytocin stimulation (Table 2). The increase in contraction frequency among GRK6 KO mice was also associated with failure to return to baseline between contractions (Figure 2B). There was no difference in uterine contraction force seen among GRK5 KO mice compared with WT mice, although GRK5 KO mice had significantly lower median contraction frequency than WT mice with oxytocin stimulation (Figure 2D and Table 2).
Figure 2.
Uterine contraction responses with oxytocin infusion among nonpregnant mice. Representative contraction tracings using an ambulatory telemetry implanted in the uterus are presented from WT (A), GRK6 KO (B), and GRK5 KO (C) nonpregnant mice treated with oxytocin. D, GRK6 KO mice exhibited greater mean (±SEM) uterine contraction force as measured by AUC over the 14-hour period compared with WT mice (532 ± 40 vs 267 ± 48 mmHg × hr; *, P = .003). There was no significant difference in the mean (±SEM) contraction force generated in GRK5 KO mice compared with WT mice as measured by AUC over the same time period (192 ± 76 vs 267 ± 48 mmHg × hr; P = .43).
Table 2.
Uterine Contraction Force and Frequency by Genotype
| Contraction Characteristic | WT | GRK6 KO |
P Valuea |
GRK5 KO |
P Valueb |
|---|---|---|---|---|---|
| Nonpregnant with oxytocin infusion | |||||
| Contraction force, mmHg × hrc | 267 ± 48 | 532 ± 40 | .003 | 192 ± 76 | .43 |
| Contraction frequency/hd | 8.7 (6.0, 14.7) | 9.8 (6.3, 23.0) | <.0001 | 6.5 (4.7, 10.2) | <.0001 |
| Term pregnant, spontaneous labor | |||||
| Contraction force, mmHg × hrc | 61 ± 3.2 | 117 ± 22 | .038 | 67 ± 5.7 | .37 |
| Contraction frequency/hd | 6.6 (4.5, 8.7) | 7.3 (4.5, 15.7) | <.0001 | 6.6 (4.0, 9.8) | .69 |
| Term pregnant, oxytocin-induced labor | |||||
| Contraction force, mmHg × hrc | 187 ± 18 | 314 ± 31 | .008 | 218 ± 49 | .58 |
| Contraction frequency/hd | 9.1 (2.6, 27.4) | 14.5 (3.9, 35.8) | <.0001 | 8.6 (3.4, 29.4) | .43 |
P value comparing GRK6 KO mice with WT mice.
P value comparing GRK5 KO mice with WT mice.
Values are mean ± SD.
Values are median (10, 90%-tile).
Pregnant mice lacking GRK6 exhibit enhanced uterine contractility during spontaneous labor and oxytocin-induced labor at term
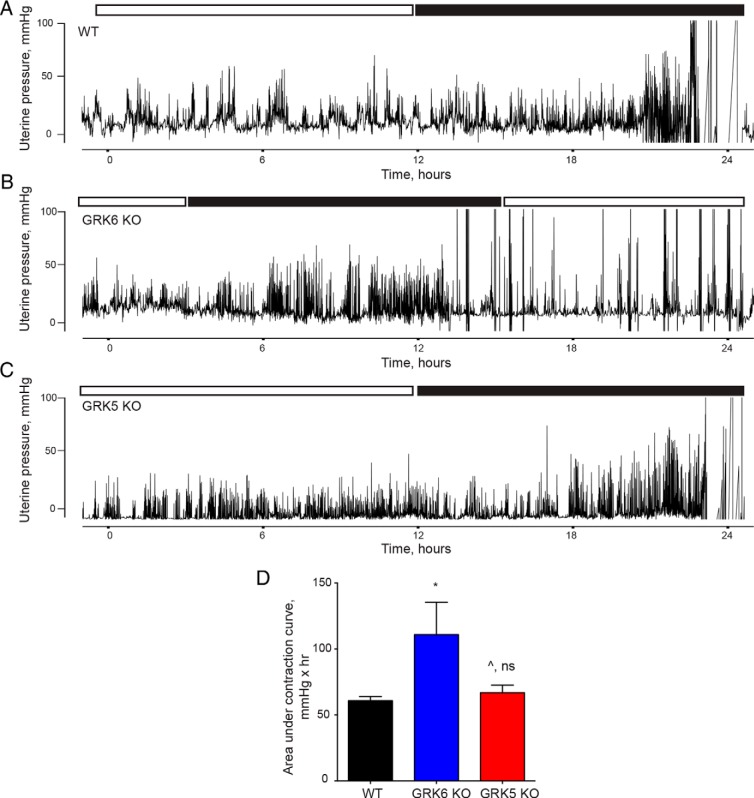
GRK6 is an important mediator of OXTR desensitization and myometrial GRK6 protein levels increase in pregnancy, thus the effect of GRK6 on uterine contractility during labor was explored. WT, GRK6 KO, and GRK5 KO mice underwent timed matings, followed by surgical implantation of a DSI telemetry device into the uterus on postcoital day 10, and then changes in uterine pressure were measured during labor. Figure 3, A–C, demonstrate representative uterine contraction tracings from WT, GRK6 KO, and GRK5 KO mice in spontaneous term labor, respectively. GRK6 KO mice exhibited a significant increase in total uterine contraction force as measured by the AUC compared with WT mice (Figure 3D and Table 2). Median uterine contraction frequency was also significantly greater among GRK6 KO mice compared with WT mice during spontaneous labor (Table 2). There was no difference in mean contraction force or median contraction frequency between GRK5 KO and WT mice during spontaneous labor at term (Figure 3D and Table 2).
Figure 3.
Uterine contraction responses during spontaneous labor at term. Representative contraction tracings using an ambulatory telemetry implanted in the uterus are presented from WT (A), GRK6 KO (B), and GRK5 KO (C) pregnant mice during spontaneous labor at term. The white and black boxes above each tracing represent the 12-hour light (white box) and 12-hour dark (black box) cycles maintained in the animal care facility. Delivery occurred during the dark cycles in all cases. D, GRK6 KO mice exhibited a significant mean (±SEM) increase in uterine contractile force compared with WT mice as measured by AUC over a 6-hour period in which delivery occurred (117 ± 22 vs 61 ± 3.2 mmHg × hr; *, P = .038). There was no difference in mean (±SEM) uterine contractile force between GRK5 KO and WT mice over a 6-hour period in which delivery occurred (67 ± 5.7 vs 61 ± 3.2 mmHg × hr; ^, P = .37).
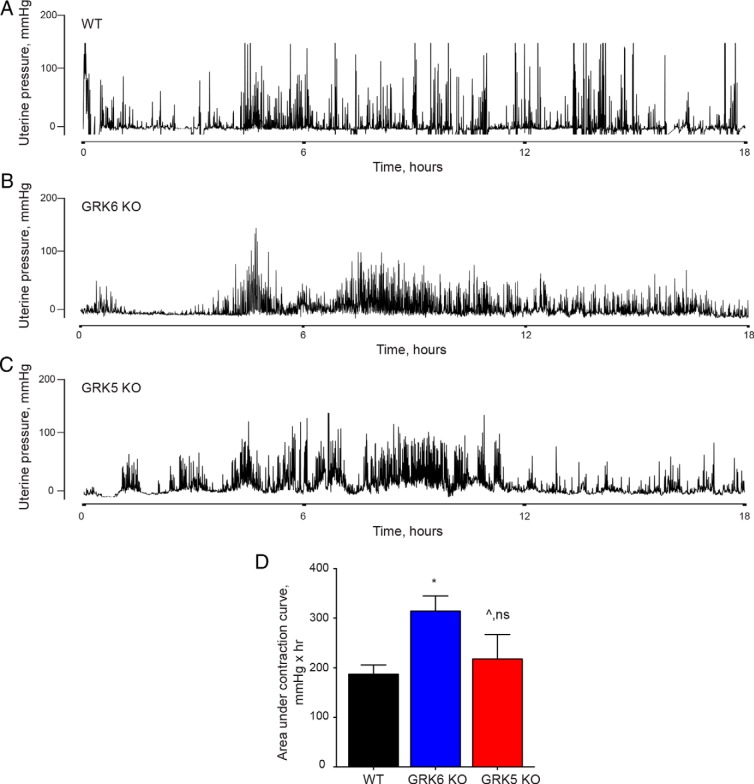
To determine the effect of myometrial GRK6 on uterine contraction patterns during induction of labor using oxytocin, WT, GRK6 KO, and GRK5 KO mice underwent timed matings and surgical implantation of a DSI telemetry device into the uterus on postcoital day 10. On postcoital day 17, an ALZET miniosmotic pump was implanted into the sc tissue that delivered oxytocin at a rate of 3U/d for the purpose of labor induction. Figure 4, A–C, demonstrate representative uterine contraction tracings from WT, GRK6 KO, and GRK5 KO mice in oxytocin-induced labor at term, respectively. GRK6 KO mice exhibited significantly increased uterine contraction force during oxytocin-induced labor compared with WT mice (Figure 4D and Table 2). Similarly, GRK6 KO mice had greater uterine contraction frequency during oxytocin-induced labor compared with WT mice (Table 2). There was no difference seen in mean contractile force or median contraction frequency during oxytocin-induced labor among GRK5 KO mice compared with WT mice (Figure 4D and Table 2). Each of the 3 genotypes, WT, GRK6 KO, and GRK5 KO mice demonstrated significantly greater uterine contractile force and frequency during oxytocin-induced labor at term compared with that seen within the same genotype during spontaneous labor at term (Figures 3D and 4D and Table 2).
Figure 4.
Uterine contraction responses during oxytocin-induced labor at term. Representative contraction tracings using an ambulatory telemetry implanted in the uterus are presented from WT (A), GRK6 KO (B), and GRK5 KO (C) pregnant mice during oxytocin-induced labor at term. Increases in uterine contraction frequency and amplitude typically began approximately 4–6 hours after pump insertion. D, GRK6 KO mice demonstrated a significant mean (±SEM) increase in uterine contractile force compared with WT mice as measured by AUC over a 6-hour period in which delivery occurred (314 ± 31 vs 187 ± 18 mmHg × hr; *, P = .008). There was no difference in mean (±SEM) uterine contractile force between GRK5 KO and WT mice over a 6-hour period in which delivery occurred (218 ± 49 vs 187 ± 18 mmHg × hr; ^, P = .58).
Placental histology and uterine OXTR expression
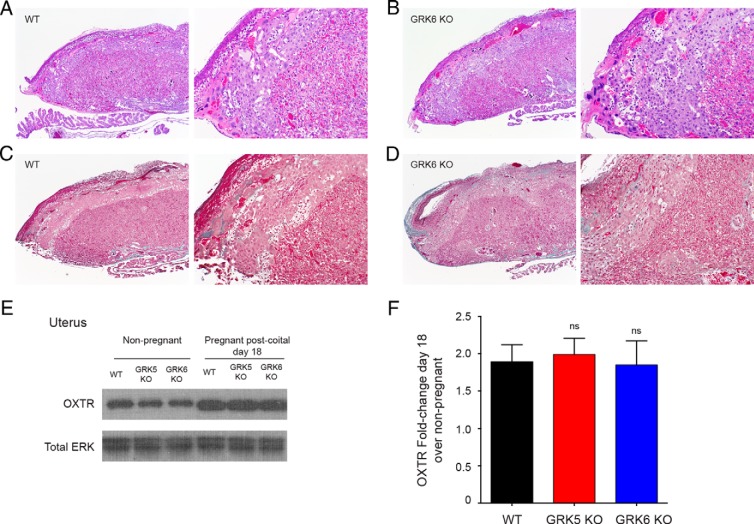
Because we saw increased rates of stillbirth among GRK6 KO mice during spontaneous labor compared with WT mice (Table 1), we analyzed placental pathology from GRK6 KO and WT mice. There were no significant differences in placental architecture seen between GRK6 KO and WT mice by H&E stain (Figure 5, A and B). Similarly, there was no difference in the degree of fibrosis seen within the placentas by Masson's trichrome stain by genotype (Figure 5, C and D).
Figure 5.
Placenta histology and relative uterine OXTR expression by genotype. Placental sections were stained with H&E (A and B) or Masson's trichrome (C and D) and for each panel, the image on the left was obtained at ×4, and the image on the right was obtained at ×10 magnification. There were no differences seen in placental architecture, including the labyrinth and spongiotrophoblast-containing junctional zones between the WT or GRK6 KO mice as noted by histology. There were also no differences seen in the extent of fibrosis (as determined by Masson's trichrome staining of collagen) within the placentas by genotype. E, Representative Western blotting demonstrating uterine OXTR expression among WT, GRK5 KO, and GRK6 KO nonpregnant and term pregnant mice. F, Mean densitometry demonstrated that there was no significant difference in mean (±SEM) fold change of uterine OXTR expression among GRK5 KO (1.99 ± 0.22-fold) or GRK6 KO (1.85 ± 0.32-fold) mice at postcoital day 18 compared with WT (1.89 ± 0.23-fold) pregnant mice at postcoital day 18 (ns, nonsignficant).
Mice lacking GRK6 exhibited enhanced uterine contractility in response to oxytocin treatment in both the nonpregnant and pregnant state. Western blotting was used to determine whether there were relative differences in uterine expression of OXTR by genotype. In the nonpregnant state, Western blotting demonstrated no differences in uterine OXTR expression among WT, GRK5 KO, and GRK6 KO mice (Figure 5E). Similarly, there was no differences seen in relative uterine OXTR expression between WT, GRK5 KO, and GRK6 KO term pregnant mice, although pregnant mice of each genotype demonstrated enhanced OXTR expression compared with nonpregnant mice (Figure 5, E and F).
GRK6 mediates β-arrestin recruitment to the OXTR and β-arrestin-mediated MAPK signaling
GRK6 is an important mediator of OXTR desensitization in response to oxytocin stimulation (14) and GRK phosphorylation of the OXTR after oxytocin binding allows for β-arrestin recruitment and GPCR desensitization (6). Mice lacking GRK6 exhibit enhanced uterine contractility in response to oxytocin, presumably due to diminished OXTR desensitization. We used co-IP to determine the effect of GRK6 expression on β-arrestin recruitment to the OXTR in response to oxytocin in heterologous cells. HEK-293 cells in the setting of CTL siRNA exhibited a 4.1-fold increased recruitment of β-arrestin to the OXTR with oxytocin stimulation compared with HEK-293 cells not treated with oxytocin (P < .0001) (Figure 6, A and B). HEK-293 cells lacking either GRK2 or GRK5 both exhibited a significant increase in β-arrestin recruitment to the OXTR with oxytocin stimulation, although were significantly diminished compared with control cells (CTL siRNA) treated with oxytocin (Figure 6, A and B). Cells lacking GRK6 did not demonstrate β-arrestin recruitment to the OXTR with oxytocin stimulation (Figure 6, A and B).
Figure 6.
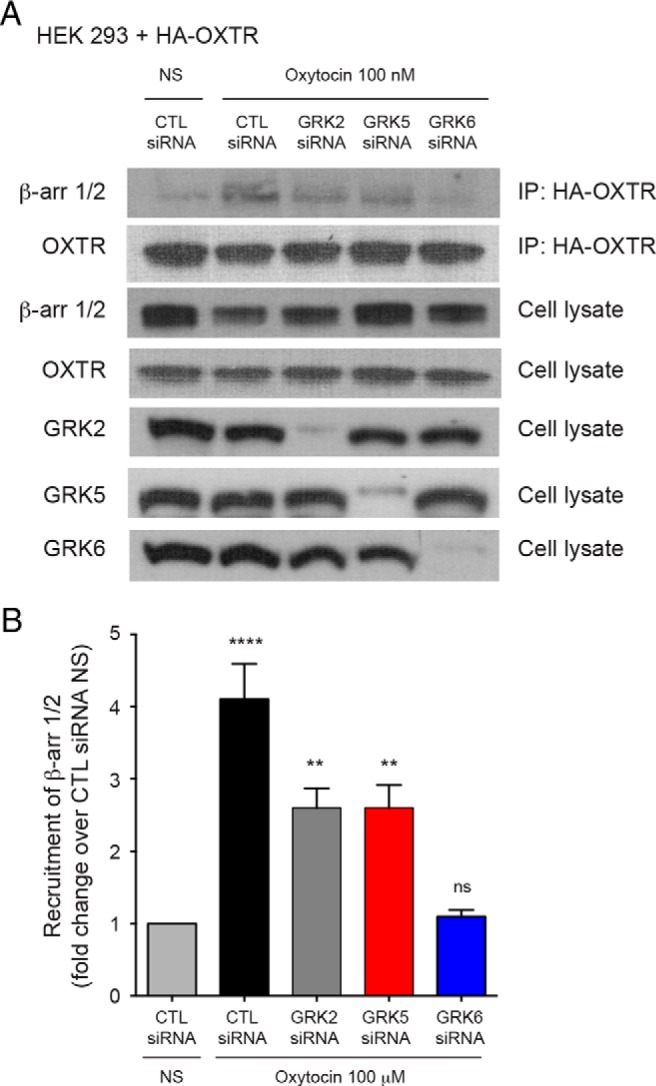
β-Arrestin recruitment to the OXTR after GRK knockdown. A and B, HEK-293 cells expressing an HA-tagged OXTR and treated with CTL siRNA, demonstrated a mean (±SEM) 4.1-fold (±0.49) increased recruitment of β-arrestin to the OXTR with oxytocin treatment compared with cells not treated with oxytocin (NS) (****, P < .0001) Similarly, there was an increase in β-arrestin recruitment to the OXTR in cells after GRK2 (2.6 ± 0.27-fold; **, P = .004) and GRK5 (2.6 ± 0.31-fold; **, P = .004) knockdown compared with cells not treated with oxytocin (NS). There was no increased β-arrestin recruitment to the OXTR with oxytocin treatment in cells after GRK6 knockdown, compared with cells not treated with oxytocin (ns). β-Arrestin recruitment to the OXTR was significantly decreased for cells stimulated with oxytocin and treated with siRNA directed against GRK2 (P = .005), GRK5 (P = .005), and GRK6 (P < .0001) compared with cells stimulated with oxytocin and treated with CTL siRNA.
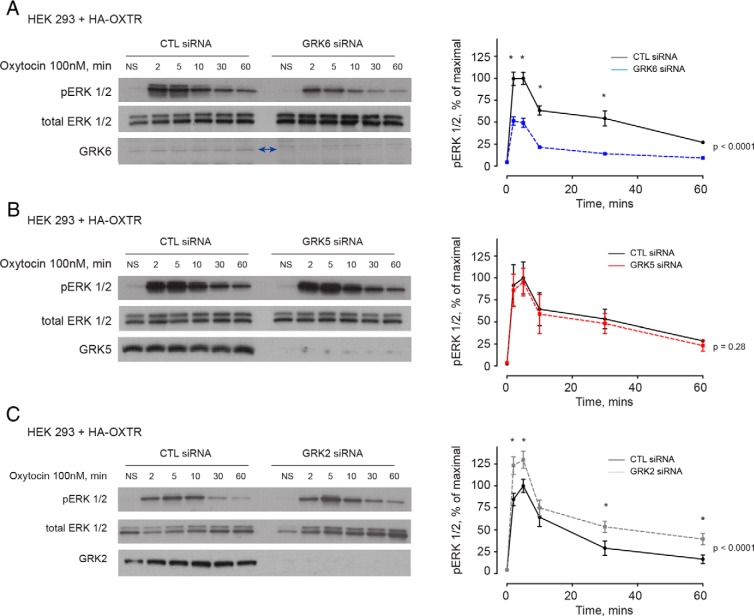
Oxytocin stimulation of the OXTR activates downstream MAPK signaling, manifested by increases in phosphorylated-ERK, through both G protein (Gq and Gi-mediated signaling) and β-arrestin-mediated signaling (9). To test the hypothesis that β-arrestin-mediated MAPK signaling is dependent on GRK interaction with the OXTR, we performed GRK loss-of-function experiments in HEK-293 cells expressing the OXTR. HEK-293 cells expressing the OXTR that were treated with siRNA directed against GRK6 demonstrated a significant reduction in phosphorylated-ERK at 2, 5, 10, and 30 minutes compared with HEK-293 cells expressing the OXTR that were treated with CTL siRNA and stimulated with oxytocin (Figure 7A). There was no difference in the amount of phosphorylated-ERK seen in HEK-293 cells expressing the OXTR that were treated with siRNA directed at GRK5 and stimulated with oxytocin compared with cells treated with CTL siRNA and also treated with oxytocin (Figure 7B). Cells expressing the OXTR that were treated with siRNA directed against GRK2 demonstrated enhanced phosphorylated-ERK signaling at 2, 5, 30 and 60 minutes in response to oxytocin compared with cells treated with CTL siRNA and stimulated with oxytocin (Figure 7C).
Figure 7.
MAPK signaling from the OXTR is mediated by GRK6. A, HEK-293 cells expressing the OXTR and treated with siRNA directed against GRK6 demonstrated a significant decrease in maximal pERK signaling at 2, 5, 10, and 30 minutes (*, P < .0001). Two-way ANOVA demonstrated that the 2 curves differed significantly (P < .0001). B, There were no differences in the pERK signal between cells treated with CTL siRNA and siRNA directed against GRK5 (P = .28). C, Cells treated with siRNA directed against GRK2 demonstrated significantly enhanced pERK expression at 2, 5, 30, and 60 minutes compared with cells treated with CTL siRNA (*, P < .0001) (C). Two-way ANOVA demonstrated that the 2 curves differed significantly (P < .0001).
Discussion
In this study, we demonstrate that 1) uterine GRK6 levels increase during murine pregnancy; 2) GRK6 loss-of-function results in enhanced uterine contractility in nonpregnant mice treated with continuous oxytocin; 3) among term pregnant mice, GRK6 loss-of-function results in enhanced uterine contractility during both spontaneous and oxytocin-induced labor and is associated with high rates of term stillbirth; 4) GRK6 is required for β-arrestin recruitment to the OXTR with oxytocin stimulation and therefore likely required for OXTR desensitization; and 5) MAPK signaling from the OXTR in response to oxytocin is dependent of GRK6 in heterologous cells. These findings are significant as they suggest that normal labor, and resulting delivery of a live born offspring, is dependent on normal uterine contractile patterns. Enhanced uterine contractility, potentially as a result of absent OXTR desensitization results in significantly greater uterine contraction force and frequency during both spontaneous and oxytocin-induced labor, which may be detrimental to fetal wellbeing during labor.
Before the work of Pierce et al, the measurement of uterine contractility was limited to the use of uterine muscle strips in tissue organ baths (18). Applying ambulatory pressure telemetry technology to the uterus has allowed for real-time measurement of changes in uterine pressure during labor that was not possible before their work. In addition to validating this technology for use in live pregnant mice in spontaneous labor at term in our study, we have also demonstrated the application of in vivo uterine pressure telemetry monitoring during oxytocin treatment in both nonpregnant and pregnant mice (19).
The OXTR, similar to other GPCRs, undergoes desensitization and internalization after agonist stimulation through a well-established mechanism (6). After agonist stimulation, GRK phosphorylation of the OXTR allows for the recruitment of β-arrestin. β-Arrestin recruitment results in receptor internalization, which uncouples the receptor from its associated G protein, interdicting further signaling. Willets et al (14) tested which members of the GRK family were responsible for desensitization to calcium signaling from the OXTR using an in vitro model of myometrial cells and demonstrated that only GRK6 was responsible for OXTR desensitization. In our study, mice lacking GRK6 demonstrated an enhanced contractile phenotype during spontaneous and oxytocin-induced labor, suggesting that prevention of OXTR desensitization due to absent GRK6 and failure of β-arrestin recruitment, results in continued down-stream calcium activation from the OXTR and enhanced contractility. In a previous study, we have shown that uterine strips obtained from mice lacking either β-arrestin1 or β-arrestin2 exhibited enhanced uterine contractility in response to oxytocin and did not desensitize (9). Together, along with the work from Willets et al (14), our data suggest that desensitization of the OXTR is dependent on GRK6 phosphorylation of the OXTR and situations in which OXTR desensitization is prevented or diminished, there is enhanced down-stream calcium signaling resulting in greater uterine contraction force and frequency (9, 14).
The clinical implications of these findings are not completely known. Prolonged oxytocin infusions are associated with paradoxical decreases in uterine contractility (9, 10, 23, 24). Decreases in uterine contractility as a result of prolonged oxytocin infusions in labor can result in adverse clinical outcomes including uterine atony, which increases the risk for postpartum hemorrhage, as well as dysfunctional labor patters that increase the risk for cesarean delivery (11, 12). Presumably, these adverse clinical events are the result of oxytocin-mediated OXTR desensitization. The findings of our current study suggest though, that some degree of OXTR desensitization is required for normal contractile patterns in labor. Oxygen delivery into the human placenta occurs between uterine contractions, such that mechanisms that enhance uterine contraction force and frequency, can affect placenta oxygen status, and therefore oxygen delivery to the fetus (25, 26, 27, 28). Using GRK6 loss-of-function in vivo models, our findings suggest that failure of OXTR desensitization leads to enhanced uterine contractility with an associated finding of stillbirth. As not all women exposed to prolonged oxytocin infusions during labor experience adverse clinical outcomes associated with OXTR desensitization, genetic predisposition, or genetic variation may explain these variable responses. Different genotypes at a single nucleotide polymorphism in GRK5 elicit variable responses in the setting of heart failure, such that one genotype enhances endogenous desensitization of the β1-adrenergic receptor, providing protection in heart failure (29). There may exist genotypes within genes regulating OXTR desensitization, including GRK6, that affect labor outcomes. Genotypes or mechanisms that further enhance OXTR desensitization above normal, physiologic levels could result in a phenotype of decreased uterine contractility manifested as postdates pregnancy, need for high doses of oxytocin in labor to maintain adequate uterine contractility, dysfunctional labor patterns, or uterine atony.
Brenninkmeijer et al determined how human myometrial GRK levels are affected by pregnancy (15). Similar to our study, they found that GRK6 levels increased in pregnancy. Furthermore, similar to our study, they found that GRK5 levels did not change between nonpregnant and pregnant myometrium. Finally, they could not identify GRK2 in human nonpregnant myometrium, but were able to identify GRK2 in pregnant myometrial tissue. The implications of increasing GRK6 levels within the myometrium during pregnancy is unclear. It is possible that enhanced GRK6 levels with pregnancy, in concert with elevated myometrial OXTR expression, provides adequate and appropriate physiologic desensitization of the OXTR, allowing for normal uterine contraction patterns in labor. We did not identify a change in myometrial GRK5 levels with advancing gestational age, nor did GRK5 loss-of-function have an effect on uterine contractile phenotype or β-arrestin recruitment to the OXTR, consistent with the importance of GRK6 in mediating OXTR desensitization. Increases in myometrial levels of OXTR and pERK with advancing gestation have been previously demonstrated and help validate the findings that we found with changes in myometrial GRK6 expression (30–32).
Our study is limited in that we were not able to test the effects of GRK2 loss-of-function on uterine contractility during labor. Global GRK2 KO mice are lethal and uterine-specific GRK2 loss-of-function murine models were not available (33). Willets et al (14) found that GRK2 knockdown in human myometrial cells did not affect OXTR desensitization such that myometrial cells lacking GRK2 continued to desensitize to oxytocin-mediated calcium signaling, whereas cells lacking GRK6 demonstrated absent OXTR desensitization to calcium signaling. Their findings are in contrast to those of 2 other groups that used GRK2 dominant-negative constructs in HEK-293 cells to determine the effect of GRK2 on OXTR desensitization (34, 35). These 2 groups demonstrated attenuated OXTR desensitization after treatment of HEK-293 cells with dominant-negative GRK2 It is possible that overexpression of dominant-negative constructs has off-target effects that produce the observed findings or that tissue-specific effects are seen, such that mechanisms regulating OXTR desensitization within the myometrium are different than those seen in cell lines not originating from uterine smooth muscle cells. In addition, dominant-negative GRK2 that binds the OXTR but prevents phosphorylation, may prevent other GRKs from exerting their effects.
In addition to its role in mediating homologous desensitization of GPCRs, β-arrestin also has been shown to mediate down-stream intracellular signaling, independent of G protein activation, through the MAPK pathway (36–40). Downstream MAPK activation from the OXTR has been shown to be composed of a combination of G protein (Gq and Gi) and β-arrestin signaling (9). GRK6 phosphorylation of the OXTR after oxytocin stimulation is required for β-arrestin recruitment to the receptor and activation of β-arrestin-dependent, G protein-independent, down-stream signaling. Our previous work demonstrated that β-arrestin-mediated signaling accounted for approximately 50% of MAPK signaling from the OXTR (9). Similarly, here, we show with GRK6 knockdown in HEK-293 cells expressing the OXTR that approximately 50% of MAPK signaling was dependent on GRK6 and therefore β-arrestin-dependent. Similar to our in vivo contraction findings in GRK5 KO mice, where GRK5 loss-of-function did not affect the uterine contractile phenotype, GRK5 knockdown in HEK-293 cells did not affect OXTR-mediated down-stream MAPK activation. Somewhat surprisingly, GRK2 knockdown in HEK-293 cells resulted in a small, but significant increase in MAPK activation from the OXTR. In addition, in pregnant mice, we saw a transient increase in myometrial GRK2 expression in midgestation which then normalized near term. Other groups have shown that HEK-293 cells expressing either the M3 muscarinic receptor or the μ-opioid receptor, in the setting of GRK2 knockdown, that with receptor activation there is enhanced ERK1/2 signaling (41, 42). Luo et al (41) postulated that this finding in HEK-293 cells expressing the M3 muscarinic receptor was due to uninhibited G protein-dependent MAPK activation. Specific differences in the role of GRK2 at the OXTR may be due to tissue-dependent variations in OXTR action. Using primary myometrial cells, Willets et al (14) showed that GRK2 does not affect OXTR desensitization where Hasbi et al (34) and Smith et al (35) both have shown that GRK2 mediates OXTR desensitization in HEK-293 cells using dominant-negative constructs directed at GRK2. As the OXTR does not seem to desensitize via GRK2 in myometrial cells, differences seen in OXTR signaling between myometrial cells and HEK-293 cells may due to differences in receptor behavior by tissue type. Lastly, our in vitro β-arrestin recruitment data using HEK-293 cells shows that GRK2 and GRK5 both allow for some degree of β-arrestin recruitment, although GRK6 is clearly mostly responsible, demonstrating the redundancy within the GRK system.
The high rate of stillbirth in mice lacking GRK6 in our study is thought to be due to the associated enhanced contractile phenotype observed among these mice during labor. Although we documented fetal wellbeing before the onset of labor with ultrasound in only 1 pregnant GRK6 KO mouse, the pup and placental weights at delivery were similar to that seen in WT mice, suggesting that the pups survived until term and that stillbirth occurred during the labor process.
In conclusion, physiologic levels of GRK6 are important for normal uterine contractility in labor. GRK6 loss-of-function mice exhibit enhanced uterine contractile force and frequency with an associated high rate of stillbirth suggesting that failure of OXTR desensitization in labor leads to adverse fetal outcomes. GRK6 is needed for recruitment of β-arrestin to the OXTR, which allows for receptor internalization and desensitization in response to agonist binding, thus some degree of physiologic OXTR desensitization during labor is likely important for normal labor patterns.
Acknowledgments
We thank Howard Rockman for his support, guidance, and thoughtful discussions regarding this project; Reginald Williams, Cathy Bittner, and Karen Terry (Rockman laboratory, Duke University) for providing animal care support and help in setting up timed matings; and Dr Sarah England (Washington University in St. Louis) for discussions pertaining to uterine telemetry experiments and Susan Reeves of the Duke University PhotoPath core facility (Duke University Department of Pathology) for assistance with placental histology imaging.
This work was supported by the NIH Grant K08HD070872 (to C.A.G.) and the National Institutes of Health Building Interdisciplinary Research Careers in Women's Health (BIRCWH) Training Grant K12HD043446 to Duke University in support of C.A.G. as a BIRCWH Scholar. This work was also supported by The Duke University School of Medicine's Charles B. Hammond Research Fund (C.A.G.) and The Duke University Department of Obstetrics and Gynecology (Dr Haywood Brown, Chairperson).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the contraction curve
- CTL
- control
- DSP
- 3,3′-dithiodipropionic acid di(N-hydroxysuccinimide ester)
- GPCR
- G protein-coupled receptor
- GRK
- GPCR kinase
- HA
- hemagglutinin
- HEK-293
- human embryonic kidney-293
- KO
- knockout
- IP
- immunoprecipitation
- OXTR
- oxytocin receptor
- p
- phosphorylated
- siRNA
- small interferring RNA
- WT
- wild type.
References
- 1. Simpson KR, James DC. Effects of oxytocin-induced uterine hyperstimulation during labor on fetal oxygen status and fetal heart rate patterns. Am J Obstet Gynecol. 2008;199:34.e1–e5. [DOI] [PubMed] [Google Scholar]
- 2. American College of Obstetricians and Gynecologists Committee on Practice Bulletins – Obstetrics. ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. 2009;114:386–397. [DOI] [PubMed] [Google Scholar]
- 3. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039–1047. [DOI] [PubMed] [Google Scholar]
- 4. Vankrieken L, Godart A, Thomas K. Oxytocin determination by radioimmunoassay. Gynecol Obstet Invest. 1983;16:180–185. [DOI] [PubMed] [Google Scholar]
- 5. Clark SL, Simpson KR, Knox GE, Garite TJ. Oxytocin: new perspectives on an old drug. Am J Obstet Gynecol. 2009;200:35 e31–36. [DOI] [PubMed] [Google Scholar]
- 6. Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-β-arrestin complexes after receptor endocytosis*. J Biol Chem. 2001;276:19452–19460. [DOI] [PubMed] [Google Scholar]
- 7. Phaneuf S, Asbóth G, Carrasco MP, et al. The desensitization of oxytocin receptors in human myometrial cells is accompanied by down-regulation of oxytocin receptor messenger RNA. J Endocrinol. 1997;154:7–18. [DOI] [PubMed] [Google Scholar]
- 8. Phaneuf S, Rodríguez Liñares B, TambyRaja RL, MacKenzie IZ, López Bernal A. Loss of myometrial oxytocin receptors during oxytocin-induced and oxytocin-augmented labour. J Reprod Fertil. 2000;120:91–97. [DOI] [PubMed] [Google Scholar]
- 9. Grotegut CA, Feng L, Mao L, Heine RP, Murtha AP, Rockman HA. β-Arrestin mediates oxytocin receptor signaling, which regulates uterine contractility and cellular migration. Am J Physiol Endocrinol Metab. 2011;300:E468–E477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crall HD, Mattison DR. Oxytocin pharmacodynamics: effect of long infusions on uterine activity. Gynecol Obstet Invest. 1991;31:17–22. [DOI] [PubMed] [Google Scholar]
- 11. Grotegut CA, Paglia MJ, Johnson LN, Thames B, James AH. Oxytocin exposure during labor among women with postpartum hemorrhage secondary to uterine atony. Am J Obstet Gynecol. 2011;204:56.e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rouse DJ, Leindecker S, Landon M, et al. The MFMU Cesarean Registry: uterine atony after primary cesarean delivery. Am J Obstet Gynecol. 2005;193:1056–1060. [DOI] [PubMed] [Google Scholar]
- 13. Conti F, Sertic S, Reversi A, Chini B. Intracellular trafficking of the human oxytocin receptor: evidence of receptor recycling via a Rab4/Rab5 “short cycle.” Am J Physiol Endocrinol Metab. 2009;296:E532–E542. [DOI] [PubMed] [Google Scholar]
- 14. Willets JM, Brighton PJ, Mistry R, Morris GE, Konje JC, Challiss RA. Regulation of oxytocin receptor responsiveness by G protein-coupled receptor kinase 6 in human myometrial smooth muscle. Mol Endocrinol. 2009;23:1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brenninkmeijer CB, Price SA, López Bernal A, Phaneuf S. Expression of G-protein-coupled receptor kinases in pregnant term and non-pregnant human myometrium. J Endocrinol. 1999;162:401–408. [DOI] [PubMed] [Google Scholar]
- 16. Gainetdinov RR, Bohn LM, Walker JK, et al. Muscarinic supersensitivity and impaired receptor desensitization in G protein-coupled receptor kinase 5-deficient mice. Neuron. 1999;24:1029–1036. [DOI] [PubMed] [Google Scholar]
- 17. Gainetdinov RR, Bohn LM, Sotnikova TD, et al. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron. 2003;38:291–303. [DOI] [PubMed] [Google Scholar]
- 18. Pierce SL, Kutschke W, Cabeza R, England SK. In vivo measurement of intrauterine pressure by telemetry: a new approach for studying parturition in mouse models. Physiol Genom. 2010;42:310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rada CC, Pierce SL, Grotegut CA, England SK. Intrauterine telemetry to measure mouse contractile pressure in vivo. J Vis Exp. 2015;(98):e52541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Imamura T, Luedke CE, Vogt SK, Muglia LJ. Oxytocin modulates the onset of murine parturition by competing ovarian and uterine effects. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1061–R1067. [DOI] [PubMed] [Google Scholar]
- 21. Kim J, Ahn S, Ren XR, et al. Functional antagonism of different G protein-coupled receptor kinases for β-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA. 2005;102:1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murray SA, Morgan JL, Kane C, et al. Mouse gestation length is genetically determined. PLoS One. 2010;5:e12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balki M, Cristian AL, Kingdom J, Carvalho JC. Oxytocin pretreatment of pregnant rat myometrium reduces the efficacy of oxytocin but not of ergonovine maleate or prostaglandin F 2 α. Reprod Sci. 2010;17:269–277. [DOI] [PubMed] [Google Scholar]
- 24. Magalhaes JK, Carvalho JC, Parkes RK, Kingdom J, Li Y, Balki M. Oxytocin pretreatment decreases oxytocin-induced myometrial contractions in pregnant rats in a concentration-dependent but not time-dependent manner. Reprod Sci. 2009;16:501–508. [DOI] [PubMed] [Google Scholar]
- 25. McNamara H, Johnson N. The effect of uterine contractions on fetal oxygen saturation. BJOG. 1995;102:644–647. [DOI] [PubMed] [Google Scholar]
- 26. Bakker PC, Kurver PH, Kuik DJ, Van Geijn HP. Elevated uterine activity increases the risk of fetal acidosis at birth. Am J Obstet Gynecol. 2007;196:313-e1–e6. [DOI] [PubMed] [Google Scholar]
- 27. Freidman EA, Sachtleben MR. Effect of oxytocin and oral prostaglandin E2 on uterine contractility and fetal heart rate patterns. Am J Obstet Gynecol. 1978;130:403–407. [DOI] [PubMed] [Google Scholar]
- 28. Lees MH, Hill JD, Ochsner AJ, 3rd, Thomas CL, Novy MJ. Maternal placental and myometrial blood flow of the rhesus monkey during uterine contractions. Am J Obstet Gynecol. 1971;110:68–81. [DOI] [PubMed] [Google Scholar]
- 29. Liggett SB, Cresci S, Kelly RJ, et al. A GRK5 polymorphism that inhibits β-adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14:510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li Y, Je HD, Malek S, Morgan KG. ERK1/2-mediated phosphorylation of myometrial caldesmon during pregnancy and labor. Am J Physiol Regul Integr Comp Physiol. 2003;284:R192–R199. [DOI] [PubMed] [Google Scholar]
- 31. Zingg HH, Laporte SA. The oxytocin receptor. Trends Endocrinol Metab. 2003;14:222–227. [DOI] [PubMed] [Google Scholar]
- 32. Ou CW, Chen ZQ, Qi S, Lye SJ. Increased expression of the rat myometrial oxytocin receptor messenger ribonucleic acid during labor requires both mechanical and hormonal signals. Biol Reprod. 1998;59:1055–1061. [DOI] [PubMed] [Google Scholar]
- 33. Matkovich SJ, Diwan A, Klanke JL, et al. Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and β-adrenergic signaling. Circ Res. 2006;99:996–1003. [DOI] [PubMed] [Google Scholar]
- 34. Hasbi A, Devost D, Laporte SA, Zingg HH. Real-time detection of interactions between the human oxytocin receptor and G protein-coupled receptor kinase-2. Mol Endocrinol. 2004;18:1277–1286. [DOI] [PubMed] [Google Scholar]
- 35. Smith MP, Ayad VJ, Mundell SJ, McArdle CA, Kelly E, López Bernal A. Internalization and desensitization of the oxytocin receptor is inhibited by dynamin and clathrin mutants in human embryonic kidney 293 cells. Mol Endocrinol. 2006;20:379–388. [DOI] [PubMed] [Google Scholar]
- 36. Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. β-Arrestin-biased agonism at the β2-adrenergic receptor. J Biol Chem. 2008;283:5669–5676. [DOI] [PubMed] [Google Scholar]
- 37. Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. [DOI] [PubMed] [Google Scholar]
- 38. Noma T, Lemaire A, Naga Prasad SV, et al. β-Arrestin-mediated β1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shenoy SK, Drake MT, Nelson CD, et al. β-Arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. [DOI] [PubMed] [Google Scholar]
- 40. Wei H, Ahn S, Shenoy SK, et al. Independent β-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA. 2003;100:10782–10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luo J, Busillo JM, Benovic JL. M3 muscarinic acetylcholine receptor-mediated signaling is regulated by distinct mechanisms. Mol Pharmacol. 2008;74:338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doll C, Pöll F, Peuker K, Loktev A, Glück L, Schulz S. Deciphering micro-opioid receptor phosphorylation and dephosphorylation in HEK293 cells. Br J Pharmacol. 2012;167:1259–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]