Abstract
Human exposure to nitrogen dioxide (NO2), an air pollutant of increasing interest in biology, results in several toxic effects to human health and also to the air microbiota. The aim of this study was to investigate the bacterial response to gaseous NO2. Two Pseudomonas fluorescens strains, namely the airborne strain MFAF76a and the clinical strain MFN1032 were exposed to 0.1, 5, or 45 ppm concentrations of NO2, and their effects on bacteria were evaluated in terms of motility, biofilm formation, antibiotic resistance, as well as expression of several chosen target genes. While 0.1 and 5 ppm of NO2did not lead to any detectable modification in the studied phenotypes of the two bacteria, several alterations were observed when the bacteria were exposed to 45 ppm of gaseous NO2. We thus chose to focus on this high concentration. NO2-exposed P. fluorescens strains showed reduced swimming motility, and decreased swarming in case of the strain MFN1032. Biofilm formed by NO2-treated airborne strain MFAF76a showed increased maximum thickness compared to non-treated cells, while NO2 had no apparent effect on the clinical MFN1032 biofilm structure. It is well known that biofilm and motility are inversely regulated by intracellular c-di-GMP level. The c-di-GMP level was however not affected in response to NO2 treatment. Finally, NO2-exposed P. fluorescens strains were found to be more resistant to ciprofloxacin and chloramphenicol. Accordingly, the resistance nodulation cell division (RND) MexEF-OprN efflux pump encoding genes were highly upregulated in the two P. fluorescens strains. Noticeably, similar phenotypes had been previously observed following a NO treatment. Interestingly, an hmp-homolog gene in P. fluorescens strains MFAF76a and MFN1032 encodes a NO dioxygenase that is involved in NO detoxification into nitrites. Its expression was upregulated in response to NO2, suggesting a possible common pathway between NO and NO2 detoxification. Taken together, our study provides evidences for the bacterial response to NO2 toxicity.
Keywords: airborne, Pseudomonas fluorescens, nitrogen dioxide, biofilm, antibiotic sensitivity, motility, air pollution
Introduction
Most world-wide cities have serious air-quality problems, which have attracted attention in the past decade. One of the most common source of air pollution is engine emissions, which include, among other toxic molecules, the nitrogen oxides (NOx; reviewed in Sher, 1998; Skalska et al., 2010). The general term NOx includes nitric oxide (NO) and nitrogen dioxide (NO2). NO in turn is able to damage bacterial cells interacting with bacterial proteins (McLean et al., 2010; Laver et al., 2013) and DNA (Tamir et al., 1996; Burney et al., 1999) either directly, or via formation of reactive nitrogen species (RNS), causing alterations in bacterial metabolism, among which respiration, and homeostasis. As a result, bacteria have developed specific NO detoxification pathways and defense mechanisms (Cruz-Ramos et al., 2002; Flatley et al., 2005; Spiro, 2007). In order to counteract the NO-mediated respiratory arrest (Husain et al., 2008), the detoxification processes are completed in several bacteria by metabolism reprogramming (Auger et al., 2011; Auger and Appanna, 2015). NO was furthermore identified as a signaling molecule, which promotes the biofilm dispersion in various bacterial strains, including Pseudomonas aeruginosa (Barraud et al., 2009; Cutruzzola and Frankenberg-Dinkel, 2015) and P. putida (Liu et al., 2012). This molecule is also known to modulate bacterial antibiotic sensitivity, protecting bacteria from a wide range of antibacterial agents (Gusarov et al., 2009; McCollister et al., 2011; van Sorge et al., 2013), such as vancomycin and daptomycin (van Sorge et al., 2013). Contrary to NO, NO2 has a low solubility in water (Augusto et al., 2002). Thence NO2 in aqueous media concerned a few reports in the microbiological context. However, in natural environments NO is unstable and quickly oxidized to form NO2 (Skalska et al., 2010), considered as a major air pollutant. Its atmospheric level is ruled by European environmental commission and World Health Organization (INERIS, 2011; Reduction of pollutant emissions from light vehicles, 2015; WHO |Ambient (outdoor) air quality health, 2015). NO2 toxicity to human health is well documented and is known to increase cardiovascular diseases (Chaloulakou et al., 2008), or to aggravate respiratory symptoms especially in children (Pershagen et al., 1995; Chauhan et al., 1998). On the opposite, the stress promoted by NO2 was poorly evaluated on bacteria.
It is increasingly evident that the air is a biotic environment, containing bacteria as one of the major compounds of primary atmosphere aerosol particles (Burrows et al., 2009b; Després et al., 2012). Mean airborne bacterial concentrations can indeed be greater than 1 104 cells m−3 (Bauer et al., 2002; Burrows et al., 2009a). Although unstable, the air microbiota is frequently constituted with members of Pseudomonas genus (Fang et al., 2007; Pearce et al., 2010; Després et al., 2012; Dybwad et al., 2012; Šantl-Temkiv et al., 2015). Among these highly versatile elements, the P. fluorescens strains are widely adaptable and distributed (Bodilis et al., 2004) in all major natural environments, including water (Bodilis et al., 2004), soil (Varivarn et al., 2013) and clouds (Ahern et al., 2007). Several P. fluorescens strains were also found to promote humans acute infections and were reported in clinical samples of immuno-compromised patients (Chapalain et al., 2008; Scales et al., 2014). All these properties make P. fluorescens a good model for further investigations of airborne bacteria.
We have investigated in previous studies the microbiota (bacteria, yeasts and fungi) of Rouen harbor terminal (France) (Morin et al., 2013). Thus, several P. fluorescens strains were isolated. Among them, the airborne P. fluorescens strain MFAF76a was characterized as a virulent strain, particularly its exoproducts against human epithelial pulmonary cells (Duclairoir Poc et al., 2014). The aim of this study is to investigate the physiological response of airborne P. fluorescens MFAF76a to NO2 as a marker of air pollution in terms of motility, biofilm formation and antibiotic resistance. This response was compared to that of the clinical strain P. fluorescens MFN1032 isolated from the sputum of a pneumonia-suffering patient (Chapalain et al., 2008). The parameters of bacterial NO2 exposure were adapted to mimic real-life air conditions. Thus, the two strains were exposed to gaseous NO2 at three concentrations: 0.1 ppm as an annual guideline value (WHO |Air quality guidelines - global update, 2005) 5 ppm as the threshold causing reversible effects on human health, and 45 ppm as a high NO2 concentration provoking irreversible effects (INERIS, 2011).
Material and methods
Strains and growth conditions
Cyan Fluorescent Protein (CFP)-labeled P. fluorescens MFN1032 and MFAF76a were used in this study. The strains and plasmids are listed in Table S1. The 729-bp cfpopt gene, encoding the CFP, was extracted from pTetONCFPopt plasmid (Sastalla et al., 2009) using PstI and Xmal enzymes (NEB, Ipswich, USA). Then CFP cassette was separated by 1% agarose gel electrophoresis and purified with QIAquick Gel Extraction Kit (Qiagen, Hilden, Allemagne). The pPSV35 vector (Rietsch et al., 2005) was digested using PstI and Xmal and purified using QIAquick PCR Purification Kit (Qiagen, Hilden, Allemagne). The CFP cassette was then cloned into the PstI and Xmal sites of the pPSV35 vector. The resulting pCFP vector was introduced into One Shot® TOP10 Chemically Competent E. coli (LMSM collection) by heat shock. After antibiotic selection of the clones (gentamycin 15 μg/mL), the transformation was confirmed by confocal laser scanning microscope (CLSM 710, ZEISS). The obtained plasmid was then extracted from E. coli using QIAprep Spin Miniprep Kit (Qiagen, Hilden, Allemagne) and introduced into P. fluorescens strains by electroporation. The transformants were selected in LB containing 15 μg/mL of gentamycin and fluorescence was assayed using CLSM.
Bacteria were grown at 28°C under limited agitation (180 rpm) in DMB (Davis Medium Broth) minimal medium with 2.16 g/L glucose as carbon source (Duclairoir-Poc et al., 2011). Overnight cultures were diluted (A580 = 0.08) in fresh DMB and grown to the end of exponential phase (A580 = 2, 13 × 108 CFU/mL). Bacterial cultures at the end of exponential growth phase (about 3 × 107 bacteria per filter) were transferred on cellulose nitrate membrane filter (0.45 μm, pore size 0.2 μm, diameter 47 mm, Sartorius Biolab Products, Gottingen, Germany) and grown on DMB agar plates at 28°C for 4 h to obtain a single layer's bacterial population. After 4 h of incubation, the cellulose membranes containing bacteria were placed on agar one-well dishes (size 127.8 × 85.5 mm, Thermo Scientific Nunc, Roshester, USA), which were directly transferred into the gas delivery device (Figure 1).
Figure 1.
Schematic representation of NO2 gas delivery system. Bacterial NO2 exposure was done in gas phase for 2 h. Two exposure chambers (one for the NO2 exposure, the second one for the control—synthetic air exposure) were used. The gases, including NO2, N2 and O2 were mixed together to obtain pre-calculated concentrations of NO2 and maintain the O2/N2 ratio at 2/8 (v/v). NO2 concentrations, temperature and relative humidity were controlled.
Exposition to nitrogen dioxide
In order to mimic the atmospheric conditions, bacterial NO2 exposure was achieved in gas phase for 2 h, according to Ghaffari et al. (2005). The gas delivery device consisted of two sterile cylindrical Plexiglas exposure chambers (one for the NO2 exposure, the second one for the control—exposure to synthetic air). The exposure chambers were deposed in a drying oven at 28°C (Figure 1). The NO2, N2, and O2 obtained from Air Liquide GMP Europe (Mitry-Mory, France) were mixed together using digital mass flow regulators (Alicat Scientific, Inc., Tucson, USA) in order to get pre-calculated concentrations of NO2 and maintain the O2/N2 ratio at 2/8 (v/v). The resulting gas mixture and the synthetic air were routed independently to each of the exposure chamber at a constant flow rate of 2 L/min, allowing parallel treatment of bacteria originating from the same bacterial culture. After passing through the exposure chamber, the NO2 concentrations were monitored by AC32M nitrogen oxides analyzer (Environnement S.A, Poissy, France) and safely vented to a chemical hood. Temperature and relative humidity data were monitored to control reliable steady-state environmental conditions inside the exposure chambers. Three concentrations of NO2 (0.1 ppm; 5 ppm and 45 ppm) were used in this study. After exposure, bacteria were diluted to A580 = 2 in sterile saline solution and used for the subsequent experiments.
Antibiotic sensitivity assays
After NO2 exposure, bacterial sensitivity to ciprofloxacin, chloramphenicol, tobramycin and kanamycin (Sigma-Aldrich, St. Quentin Fallavier, France) was assayed. The minimum inhibitory concentration (MIC) was determined by the broth microdilution method achieved in DMB. Briefly, NO2-exposed bacteria were diluted to A580= 0.08 and added to a 96-well test plate (Nunc™, Roskilde, Denmark) containing different concentrations of antibiotics in triplicate. The test plates were incubated at 28°C for 24 h. Synthetic air- exposed bacteria were used as control. MIC was defined as the lowest antibiotic concentration that inhibited bacteria growth as determined by turbidimetry at A580.
Growth inhibition assays were achieved as previously described (van Sorge et al., 2013). Exposed bacteria were diluted in DMB supplemented by the indicated antibiotics in subinhibitory concentrations (the last antibiotic concentrations allowing bacterial growth). Bacteria were added to Bioscreen Honeycomb plates (Oy Growth Curves Ab Ltd., Helsinki, Finland) in a total volume of 200 μL of DMB (A580 = 0.08). Growth was measured every 15 min (A580) for 24 h. The NO2 effect on the bacterial antibiotic sensitivity was calculated as the percentage of bacterial growth with antibiotics after NO2 exposure on the bacterial growth with antibiotics after exposure to synthetic air, using the following formula: 100 × A580 NO2 exposed bacteria/A580 synthetic air exposed bacteria (%).
Motility assays
Swimming and swarming motility assays were performed on agar plates using DMB containing 0.2% (wt/vol) and 0.5% (wt/vol) agar, respectively, as previously described (Déziel et al., 2001). Briefly, 5 μL of NO2 or synthetic air- exposed bacteria were spotted on the surface of agar plates. The resultant diameters of swim and swarm zones were measured after 24 h of incubation at 28°C. Motilities were assayed in three independent experiments with three replicates for each experimental condition.
Biofilm monitoring by confocal laser scanning microscopy
NO2 or synthetic air- exposed bacteria were diluted in sterile saline solution to A580= 1 to avoid bacterial multiplication, and added to glass-bottom dishes (SensoPlate™, VWR, Fontenay-sous-Bois, France). After 2 h of incubation at 28°C, planktonic bacteria were removed and bacterial adhesion on glass-bottom dishes was observed using a confocal laser scanning microscope (CLSM 710, ZEISS) with an immersion objective 63 ×. After addition of DMB, the samples were incubated at 28°C for 24 h. Biofilms were rinsed with saline solution and observed using CLSM. All biofilm assays were performed in three independent experiments with two replicates for each experimental condition. The biofilm thickness and related biomass (bacterial volume, μm3/μm2) were estimated from 6 fields on 3 independent experiments using COMSTAT software (Heydorn et al., 2000).
Gene sequences identification
The non-annotated genome drafts of MFN1032 and MFAF76a were used to identify the corresponding nucleotide sequences (data not shown). Homologous sequences search in P. fluorescens annotated genomes was performed using pseudomonas genome database (http://pseudomonas.com/). The conserved nucleotide sequences were identified in P. fluorescens MFN1032 and MFAF76a using Blast+ (Stand-alone) software (v. 2.2.30, NCBI) according to Altschul et al. (1997), and are listed in Table S2.
Extraction and quantification of bis-(3′, 5′)-cyclic dimeric guanosine monophosphate (c-di-GMP)
Extraction and quantification of intracellular c-di-GMP level were performed in NO2 or synthetic air- exposed bacteria as previously described (Spangler et al., 2010; Strehmel et al., 2015). Identification and quantification of c-di-GMP was performed using three specific mass transitions from molecule ion m/z 691 to the product ions: m/z 152, m/z 135, and m/z 540. The external calibration was carried out at c-di-GMP concentrations ranging from 10 ng to 200 ng in 500 μL H2O using the internal standard cXMP (50 ng). The resulting concentrations of c-di-GMP were normalized against total protein contents of respective cultures, which was determined by the bicinchoninic acid assay (Smith et al., 1985). All experiments were performed in three replicates for each experimental condition.
Quantitative RT-PCR
Total RNA was prepared by the hot acid-phenol method (Bouffartigues et al., 2012) from NO2-exposed and not bacteria. Residual DNAs were eliminated by acid phenol treatment. The absence of DNA was confirmed by showing that PCR reactions failed without prior cDNA synthesis. RNAs were nonspecifically converted to single stranded cDNAs using the High Capacity cDNA Archive Kit (Applied Biosystems). Synthesis of cDNAs and real time PCR, allowing the quantification of mRNAs of interest were performed as previously described (Gicquel et al., 2013) using primers listed in Table S3.
Statistical analysis
All experiments were carried out several times. To assess the significance of differences between the obtained data, Mann-Whitney test or pairwise strain comparisons (t-test) were applied and quantified the significance as (*) for p < 0.05, (**) for p < 0.01 and (***) for p < 0.001.
Results and discussion
NO2 is one of the most common air pollutants, but its effects on the air microbiota is poorly studied. In order to assess the bacterial response to NO2, airborne P. fluorescens MFAF76a and clinical control MFN1032 strains were exposed to gaseous NO2 (as shown Figure 1) at 0.1, 5, or 45 ppm concentrations, and their effects on bacteria were evaluated in terms of motility, biofilm formation, antibiotic resistance, as well as expression of several chosen target genes. While 0.1 and 5 ppm of NO2 did not lead to any significant modification of the studied parameters in both the bacteria (data not shown), several alterations were observed when the bacteria were exposed to 45 ppm of gaseous NO2. We thus chose to focus on this concentration.
No2-mediated modifications of bacterial biofilm
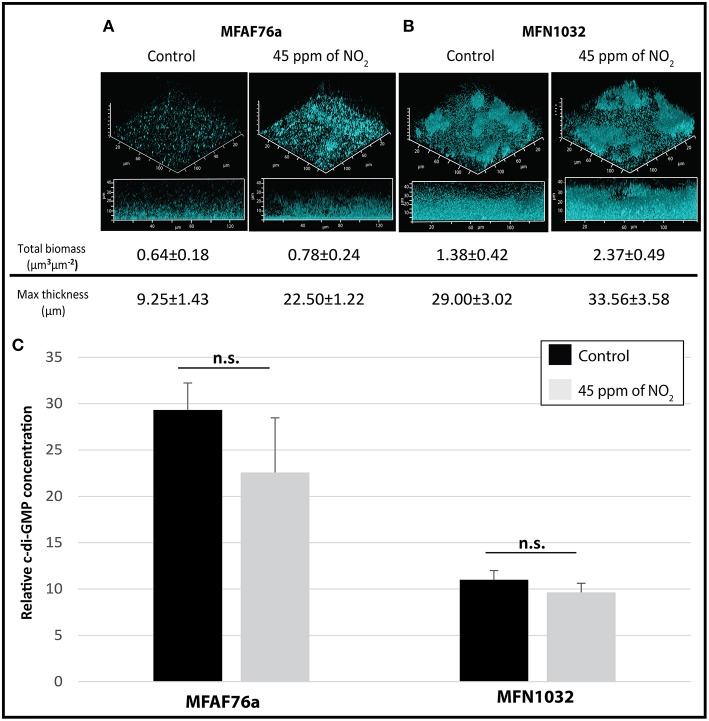
In order to test the NO2 effect on P. fluorescens biofilm, both airborne MFAF76a and clinical MFN1032 were exposed to gaseous NO2 and synthetic air and grown for 4 h in static conditions. In the control condition, the airborne strain MFAF76a produced only a poorly structured biofilm with low biomass and thickness (Figure 2A). To the best of the authors' knowledge this is the first time that the biofilm of airborne P. fluorescens strain was investigated. On the opposite, the clinical strain MFN1032 was able to form a structured mushroom-like biofilm, with about 2 and 3 fold more biomass and thickness than the airborne strain MFAF76a, respectively (Figure 2B, control). These data are consistent with previous studies showing that clinical strains can strongly adhere and form structured biofilms (Rossignol et al., 2008; Ma et al., 2009). After NO2 exposition,the airborne strain MFAF76a produced biofilms with about 3 fold increase of the maximal thickness, while the biomass was similar (Figure 2A), when compared to synthetic air treatment. These data suggest that NO2 led to induce biofilm formation in this strain. Accordingly, similar NO concentrations were previously found to promote an increase of biofilm formation in P. aeruginosa (Barraud et al., 2006), suggesting a common effect between NO and NO2 treatment. On the other hand, NO2 exposure of the clinical strain MFN1032 led to a 1.7 fold increase in biofilm production in terms of biomass, while the maximal thickness was unchanged (Figure 2B). Taken together these data suggest that the NO2-mediated biofilm modifications are strain-dependent. Since we have shown previously that airborne MFAF76a expresses a virulence activity toward A549 epithelial pulmonary cells (Duclairoir Poc et al., 2014), these data suggest that elevated concentrations of NO2 increases biofilm formation in potentially virulent airborne strain and may represent a sanitary risk.
Figure 2.
NO2 effect on P. fluorescens biofilm and intracellular c-di-GMP level. (A) Airborne MFAF76a and (B) clinical MFN1032 P. fluorescens strains were exposed in triplicate to 45 ppm of NO2. Biofilm formation was analyzed in static conditions after 24 h development using confocal laser scanning microscope. The biofilm biomass and the maximum thickness were estimated from 6 fields on 3 independent experiments using COMSTAT software. Intracellular c-di-GMP concentrations (C) were measured in triplicate by LC-MS/MS for control ( ) and 45 ppm of NO2 treated (
) and 45 ppm of NO2 treated ( ) MFAF76a and MFN1032. Obtained results are presented as average values ± SEM. Statistical significance was calculated by the non-parametric Mann-Whitney-Test. n.s., non-significant.
) MFAF76a and MFN1032. Obtained results are presented as average values ± SEM. Statistical significance was calculated by the non-parametric Mann-Whitney-Test. n.s., non-significant.
Since biofilm formation is related to increased c-di-GMP production (Ha and O'Toole, 2015), we next quantified the c-di-GMP levels after NO2 or synthetic air exposure. As shown in Figure 2C, both NO2-exposed P. fluorescens strains did not exhibit statistically significant variations of intracellular c-di-GMP concentrations. This was quite surprising since observed in our study NO2 mediated biofilm induction. NO-mediated reduction of the intracellular c-di-GMP level leading to dispersion of P. aeruginosa biofilms has been related to increase phosphodiesterases (PDEs) activity, and as a consequence to promote the switch between the biofilm and the planktonic ways of life (Petrova and Sauer, 2012; Roy et al., 2012; Li et al., 2013; Petrova et al., 2014). In this bacterium, the following PDEs, including DipA, MucR, NdbA and BdlA, are enzymes that are involved in c-di-GMP catabolism (Petrova and Sauer, 2012; Roy et al., 2012). The mRNA levels of dipA, mucR, ndbA and bdlA genes (KT186437, KT186445, KT186444 and KT186436 respectively, Table S2) were quantified by qRT-PCR experiments, in the two strains, that were both previously exposed to NO2 or synthetic air. For the two strains, NO2 exposure did not lead to any modification in gene expression (data not shown). Altogether, these data suggest that (i) NO2 may have an effect on the structure or on the biomass of the biofilm, in case of the studied airborne or clinical strains, respectively, (ii) these phenotypes would not be related to variations of the intracellular c-di-GMP levels, and (iii) NO and gaseous NO2 may have a common and concentration-dependent effect on biofilm formation.
No2 reduced bacterial motility
Since biofilm structure and production were not implemented by c-di-GMP level in our conditions, we next assayed the effects of NO2 on bacterial motility, since appendices like flagella and type IV pili are also involved in the first step of biofilm formation, i.e., adhesion (Caiazza et al., 2007; Guttenplan and Kearns, 2013).
Swimming concerns motility in a liquid medium, mediated by production and activity of flagella. As shown on Figure 3A, gaseous NO2 exposition significantly decreased the swimming motility of both strains, suggesting an impairment of the flagellum production and/or activity.
Figure 3.
NO2 decreases P. fluorescens motility. Airborne MFAF76a and clinical MFN1032 P. fluorescens strains were exposed in triplicate to 45 ppm of NO2( ). Swimming (A) and swarming (B) motilities were assayed on DMB-swim/swarm plates after 24 h incubation. The motile bacterial movement was evaluated in three independent experiments with three replicates. The data were compared with control exposed to synthetic air (
). Swimming (A) and swarming (B) motilities were assayed on DMB-swim/swarm plates after 24 h incubation. The motile bacterial movement was evaluated in three independent experiments with three replicates. The data were compared with control exposed to synthetic air ( ). Obtained results are presented as average values ± SEM. Statistical significance was calculated by the non-parametric Mann-Whitney-Test p < 0.05 (*) and < 0.001 (***).
). Obtained results are presented as average values ± SEM. Statistical significance was calculated by the non-parametric Mann-Whitney-Test p < 0.05 (*) and < 0.001 (***).
Swarming is a complex motility that has been related to functional flagella, type IV pili and production of biosurfactants like cyclolipopeptides (Duclairoir-Poc et al., 2011) for some P. fluorescens strains, or rhamnolipids for P. aeruginosa strains (Caiazza et al., 2005). The airborne strain MFAF76a was unable to swarm in tested experimental conditions. On the opposite, MFN1032 is a swarmer clinical strain (Rossignol et al., 2008). As shown in Figure 3B, exposition to NO2 but not to synthetic air led to fully inhibit the swarming motility of this strain.
Taken together, our data show that gaseous NO2 treatment results in a decreased motility in both of the studied strains. This decrease in motility could be a consequence of a lower production of the required appendices. Alternatively it could also be due to lower appendices activity, suggesting that they could increase the attachment of the bacterium on the glass slide. This phenotype would then be consistent with the increase in biofilm maximal thickness in case of the airborne strain, and biomass in case of the clinical strain. However, to date, the switch between motility and biofilm had frequently been associated to variations in the c-di-GMP level (Ha and O'Toole, 2015), but, herein, the gaseous NO2-mediated differences in terms of biofilm structure could not be related to any c-di-GMP level variations.
Effect of no2 on mexef-oprn efflux pump expression and antibiotic resistance
To further characterize the effects of gaseous NO2 on bacterial physiology, we next assayed antibiotic resistance. Since NO, a member of RNS, was found to induce the expression of mexEF-oprN genes (Fetar et al., 2011) and modulate bacterial resistance to fluoroquinolones, chloramphenicol and aminoglycosides (Gusarov et al., 2009; McCollister et al., 2011; van Sorge et al., 2013), we investigated the effect of gaseous NO2 on these phenotypes.
In order to study the effect of NO2 on MexEF-OprN efflux pump, the transcription levels of mexE, mexF and oprN genes (KT070324, KT070321 and KT070325 for MFAF76a; KT070323, KT070322 and KT186432 for MFN1032, respectively) were compared using qRT-PCR in two P. fluorescens strains exposed or not to 45 ppm of NO2. In airborne and clinical strains, the mexE mRNA level was increased by almost 14- and 100-fold respectively; that of mexF almost 3.5- and 47-fold respectively and that of oprN almost 4.6- and 73-fold respectively (Figure 4). These data show that NO2 promoted mexEF-oprN expression, potentially causing modifications in P. fluorescens antibiotic resistance. We next tested the functionality of this pump. Since the MexEF-OprN RND efflux pump is involved in fluoroquinolone resistance, we next assayed bacterial sensitivity against ciprofloxacin by evaluating their MICs. As shown in Table 1, both the P. fluorescens strains were more resistant to this antibiotic after exposure to NO2 than to synthetic air. Chloramphenicol is a nitroaromatic antimicrobial that is a substrate for MexEF-OprN (Köhler et al., 1997; Sobel et al., 2005). Accordingly, NO2-exposed P. fluorescens strains MFAF76a and MFN1032 were about 2 fold more resistant to this antibiotic than synthetic air-treated bacteria (Table 1). Taken together, these data suggest a possible higher activity of this efflux pump in response to NO2 exposure. We next followed the growth of the NO2-exposed P. fluorescens strains in DMB medium containing ciprofloxacin or chloramphenicol at the higher antibiotic concentration leading to bacterial growth (Figure 5). Data were standardized with the control, the synthetic air treated cells growth. While ciprofloxacin had no effect on NO2-exposed bacteria, chloramphenicol at a concentration of 25 and 100 μg/mL for strain MFAF76a and MFN1032, respectively, led to an increase in growth for the two NO2-exposed P. fluorescens strains (Figure 5). Remarkably, the statistically significant increase of bacterial growth was maintained from 2 to 10 h, suggesting a possible NO2 protective effect that would be conserved for 8 h after exposure. Taken together, our data show that NO2 induced mexEF-oprN gene expression, and consequently increased the resistance to ciprofloxacin and chloramphenicol.
Figure 4.

NO2 effect on MexEF-OprN and MexXY efflux pump gene transcription. The nucleotide sequences of the mexEF-, oprN- and mexXY-homolog genes were obtained using the non-annotated genome drafts of airborne MFAF76a ( ) and clinical MFN1032 (
) and clinical MFN1032 ( ) P. fluorescens. The GenBank accession numbers of nucleotide sequences are listed in Table S2. Quantification of mRNA level was assayed using qRT-PCR on RNAs extracted from NO2- and synthetic air- exposed P. fluorescens. The PCR reactions were performed in triplicate and the standard deviations were lower than 0.15 Ct. Statistical analysis used pairwise strain comparisons (t-test) p < 0.01 (**) and < 0.001 (***). Dotted line shows the gene expression in synthetic air- exposed control.
) P. fluorescens. The GenBank accession numbers of nucleotide sequences are listed in Table S2. Quantification of mRNA level was assayed using qRT-PCR on RNAs extracted from NO2- and synthetic air- exposed P. fluorescens. The PCR reactions were performed in triplicate and the standard deviations were lower than 0.15 Ct. Statistical analysis used pairwise strain comparisons (t-test) p < 0.01 (**) and < 0.001 (***). Dotted line shows the gene expression in synthetic air- exposed control.
Table 1.
NO2 exposure increases Pseudomonas fluorescens antibiotic resistance.
| Strain | NO2 concentration | Ciprofloxacin | Chloramphenicol |
|---|---|---|---|
| (ppm) | MIC (μg/mL) | MIC (μg/mL) | |
| MFAF76a | 0 | 6.25 | 50 |
| 45 | 12.5 | >100 | |
| MFN1032 | 0 | 3.125 | 150 |
| 45 | 6.25 | 200 |
Figure 5.

NO2 protects Pseudomonas fluorescens from chloramphenicol toxicity. After 2 h exposure to 45 ppm of NO2, growth of airborne MFAF76a (A) and clinical MFN1032 (B)
P. fluorescens with ciprofloxacin ( ) and chloramphenicol (
) and chloramphenicol ( ) was assayed. Growth curves were performed with ciprofloxacin (3.125 μg/mL for MFAF76a and 1.156 μg/mL for MFN1032) and chloramphenicol (25 and 100 μg/mL respectively), and A580 was recorded at the indicated time points. The control sample was bacteria exposed to synthetic air, and grown in presence of antibiotics in indicated concentrations. The data are shown as percentages of growth relative to synthetic air-exposed control. Pooled data from three independent experiments in duplicate ± SEM are reported. Statistical significance was calculated by the non-parametric Mann-Whitney-Test p < 0.05 (*); n.s., non-significant. Dotted line shows the control (100%).
) was assayed. Growth curves were performed with ciprofloxacin (3.125 μg/mL for MFAF76a and 1.156 μg/mL for MFN1032) and chloramphenicol (25 and 100 μg/mL respectively), and A580 was recorded at the indicated time points. The control sample was bacteria exposed to synthetic air, and grown in presence of antibiotics in indicated concentrations. The data are shown as percentages of growth relative to synthetic air-exposed control. Pooled data from three independent experiments in duplicate ± SEM are reported. Statistical significance was calculated by the non-parametric Mann-Whitney-Test p < 0.05 (*); n.s., non-significant. Dotted line shows the control (100%).
MexEF-OprN-overproducing mutants with enhanced fluoroquinolone resistance often increase bacterial susceptibility to aminoglycosides apparently owing to impairment of the MexXY system (Sobel et al., 2005; Morita et al., 2015). The effect of NO2 on tobramycin and kanamycin sensitivity was then assayed by performing MICs. As shown in Table 2, NO2 treatment led to reduce the MICs of the two tested antibiotics, suggesting that NO2 increases P. fluorescens sensitivity to aminoglycosides. Tobramycin and kanamycin, at subinhibitory concentration of 1.55 and 3.1 μg/mL respectively, were found to decrease the growth of NO2-exposed bacteria (Figure 6). This effect was observed only from 6 to 10 h of growth for MFN1032 and from 6 to 18 h of growth for MFAF76a, highlighting the time-limited NO2 effect on bacterial antibiotic sensitivity. Altogether, our data show that NO2 increases P. fluorescens sensitivity to tobramycin and kanamycin, accordingly its homolog NO is also found to increase P. aeruginosa sensitivity to tobramycin (Barraud et al., 2006). Noticeably, this phenotype is consistent with previously published data supporting decreasing resistance to aminoglycosides of MexEF-OprN-overproducing mutant (Sobel et al., 2005; Morita et al., 2015). Since this phenotype is often associated with the impairment of the MexXY-OprM efflux pump, we next assayed the effect of NO2 on the expression of the mexXY genes. As shown in Figure 4, NO2 treatment had an opposite effect on mexXY gene expression. While NO2 increased the expression of mexXY in the airborne strain MFAF76a, it drastically reduced production of mexXY mRNA in the clinical strain MFN1032. While this latter phenotype is often described in the literature (Sobel et al., 2003) as leading to increased aminoglycoside susceptibility, the overproduction of the two RND efflux pumps MexEF-OprN and MexXY-OprM is remarkable and found in very few strains, among which the multiresistant strain PA7 (Morita et al., 2015). Nevertheless, the increased expression of mexXY in the airborne strain cannot be related to the increased susceptibility to aminoglycosides, which we observed. Taken together, our data indicate that the NO2 effect on bacterial aminoglycoside resistance is complex and strain-dependent, and the up- or down- production of mexXY cannot account solely to explain the increased susceptibility to aminoglycosides of the two studied strains. Another hypothesis may arise related to the effects of NO2 on membrane properties. Indeed, the NO2 effect on P. fluorescens membrane was recently investigated, demonstrating the NO2-mediated modifications in both the membrane glycerophospholipids composition (i.e., ratio zwitterionic/anionic glycerophospholipids) and in the membrane electron-accepting properties (Kondakova, personal communication). It is thus conceivable that these membrane modifications would alter bacterial membrane permeability, facilitating the aminoglycoside entry into the bacterial cell.
Table 2.
NO2 decreases Pseudomonas fluorescens resistance to aminoglycosides.
| Strain | NO2 concentration | Kanamycin MIC | Tobramycin MIC |
|---|---|---|---|
| (ppm) | (μg/mL) | (μg/mL) | |
| MFAF76a | 0 | 8.3 | 6.2 |
| 45 | 6.2 | 3.1 | |
| MFN1032 | 0 | 20.0 | 12.5 |
| 45 | 16.7 | 8.3 |
Figure 6.

NO2 exposure affects Pseudomonas fluorescens growth with aminoglycosides. After 2 h exposure to 45 ppm of NO2, growth of airborne MFAF76a (A) and clinical MFN1032 (B) in presence of tobramycin (1.55 μg/mL;  ) and kanamycin (3.1 μg/mL;
) and kanamycin (3.1 μg/mL;  ) was tested. A580 was recorded at indicated time points. The control sample was bacteria exposed to synthetic air, and grown in presence of antibiotics in indicated concentrations. The data are presented as percentages of growth relative to air-exposed control. Pooled data from three independent experiments in duplicate ± SEM are reported. Statistical significance was calculated by the non-parametric Mann-Whitney-Test p < 0.05 (*), < 0.01 (**); n.s. non-significant. Dotted line shows the control (100%).
) was tested. A580 was recorded at indicated time points. The control sample was bacteria exposed to synthetic air, and grown in presence of antibiotics in indicated concentrations. The data are presented as percentages of growth relative to air-exposed control. Pooled data from three independent experiments in duplicate ± SEM are reported. Statistical significance was calculated by the non-parametric Mann-Whitney-Test p < 0.05 (*), < 0.01 (**); n.s. non-significant. Dotted line shows the control (100%).
No2-mediated gene expression in p. fluorescens
Remarkably, we have shown herein a link between gaseous NO2 and soluble NO treatment. Indeed, NO is found to induce the expression of mexEF-oprN genes (Fetar et al., 2011) and modulates bacterial resistance to several antibiotics (Gusarov et al., 2009; McCollister et al., 2011; van Sorge et al., 2013). Since NO2 and NO are related chemical toxic compounds, and since NO detoxification pathways have been deeply investigated, the NO2 effects on several chosen target genes were tested. The most well-studied pathway for NO detoxification is based on flavohemoglobin (FlavoHb) (Hmp for E. coli and Fhp for P. aeruginosa), which acts as an NO dioxygenase to transform NO to NO (Figure 7A) (Corker and Poole, 2003; Arai et al., 2005). After exposure to 45 ppm of NO2, the hmp mRNA levels were increased almost 25- and 23-fold in MFAF76a and in MFN1032 (respectively KR818822 and KR818823 in Table S2 and Figure 8), indicating that NO2 induces hmp expression in both P. fluorescens and suggesting a possible involvement of Hmp in NO2 detoxification. The NO2 effect on the Hmp synthesis was observed in other studies, where, to activate the Hmp-dependent detoxification pathway, NO2 was proposed to be reduced to NO (Poole et al., 1996). In Pseudomonas spp., NO2 reduction can be performed by nitrite reductase (NIR) enzymes (Figures 7B,C), including the well-studied respiratory cytochrome cd1 nitrite reductase (Figure 7B) of the denitrification pathway (Arai et al., 2005; Shiro, 2012). According to the genome draft analysis
Figure 7.

Scheme of Hmp-mediated NO detoxification and NO2 reduction pathways in Pseudomonas spp. (A) Flavohemoglobin (Hmp) is involved in NO detoxification acting as an NO dioxygenase to transform NO to NO. The NO2 reduction is performed by nitrite reductase enzymes, including the respiratory cytochrome cd1 nitrite reductase, NIR (B) and the assimilatory nitrite reductase NirBD (C). The respiratory NIR is involved in NO reduction to NO in anaerobic conditions. NirBD takes a part of the nitrate assimilatory pathway, and reduces nitrite to ammonia.
Figure 8.

Transcription of hmp is increased in response to NO2 exposure. The nucleotide sequences of the hmp-homolog gene in P. fluorescens strains were obtained using the non-annotated genome drafts of airborne P. fluorescens MFAF76a ( ) and clinical MFN1032 (
) and clinical MFN1032 ( ). The GenBank accession numbers of hmp nucleotide sequences are listed in Table S2. Quantification of mRNA level was assayed using qRT-PCR on RNAs extracted from NO and synthetic air-exposed P. fluorescens. The PCR reactions were performed in triplicate and the standard deviations were lower than 0.15 Ct. Statistical analysis used pairwise strain comparisons (t-test) p < 0.01 (**). Dotted line shows the gene expression in air-exposed control.
). The GenBank accession numbers of hmp nucleotide sequences are listed in Table S2. Quantification of mRNA level was assayed using qRT-PCR on RNAs extracted from NO and synthetic air-exposed P. fluorescens. The PCR reactions were performed in triplicate and the standard deviations were lower than 0.15 Ct. Statistical analysis used pairwise strain comparisons (t-test) p < 0.01 (**). Dotted line shows the gene expression in air-exposed control.
(data not shown), both MFAF76a and MFN1032, like the majority of P. fluorescens strains (Redondo-Nieto et al., 2013), do not possess denitrifying genes, but harbor the genes encoding for the assimilatory nitrite reductase NirBD (Figure 7C). The latter is part of the Nas assimilatory pathway (from nitrate assimilation), where nitrate is reduced to nitrite, which is then reduced to ammonia (Jeter et al., 1984; Moreno-Vivián et al., 1999). In order to test the NO2 effect on the expression of nirBD operon, the nirB mRNA level (Pfl76a_nirB -KT186428 - and Pfl1032_nirB - KT070320 -, Table S2) was compared in the NO2-exposed or non-exposed P. fluorescens strains. In both strains, the mRNA level of nirB was not modified compared to the control condition (data not shown), indicating the absence of NO2 effect on the expression of genes coding for assimilatory NIR. To the best of our knowledge, the involvement of Nas pathway in NO/NO2 detoxification was not demonstrated. Given the presence of ammonium in DMB medium (Duclairoir-Poc et al., 2011), we think that the production of supplementary ammonium through the nitrite reduction is not appropriate. However, in order to better understand the mechanism of the NO2 detoxification, the Hmp-, Nir- and Nas-mediated mechanisms should be investigated in more details.
In this study, the response of airborne P. fluorescens MFAF76a to gaseous NO2, as a marker of air pollution, was for the first time investigated and compared to the response of the clinical P. fluorescens MFN1032 strain. We show that NO2 leads to increased biofilm formation through a c-di-GMP independent mechanism, reduced motility, as well as increasing ciprofloxacin, chloramphenicol resistance and aminoglycosides susceptibility. The question is now to understand how the NO2 leads to the observed phenotypes. NO2 has some similarities with it relative NO. NO2, like NO, induced the expression of mexEF-oprN genes, encoding the RND efflux pump MexEF-OprN. Its overexpression could, among others, be involved in the observed increase of P. fluorescens resistance to ciprofloxacin and chloramphenicol. NO2 induces also bacterial biofilm formation by strain-dependent mode, without c-di-GMP production variation. Thus, the high P. fluorescens adaptability to many environments, and a possible NO2 propensity to increase some bacterial antibiotic resistance and biofilm formation may diminish the effectiveness of antibiotic therapies in highly polluted area. In addition, we show the NO2-mediated upregulation of the hmp-homolog gene in P. fluorescens, suggesting a possible common pathway between NO and NO2 detoxification. Taken together, our data show that gaseous NO2 can be perceived by airborne bacteria, leading to physiological modifications that may be relevant for human health (biofilm formation, antibiotic resistance). In the context of the worrying increase of atmospheric NO2 concentrations (Bernagaud et al., 2014), these findings are of ecological relevance, especially because of the high NO2 concentrations, found in the close vicinity of any vehicle.
Author contributions
TK contributed to the design of project, experiments, acquisition, analysis, interpretation of data, and wrote the manuscript. CC contributed in genes identification and qRT-PCR analysis. MB contributed to the transformation of P. fluorescens strains. MN and GB participated in c-di-GMP quantification. FD encouraged the study on the airborne bacteria. SC and NO participated in the design and drafted the manuscript. CDP led and coordinated the global project by conceiving the study, and participated in manuscript writing. All authors have read and approved the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Marc Feuilloley for his constructive comments and Yoan Konto-Ghiorghi for his advices in English editing and academic writing. We are grateful to Anne Groboillot for help with development of gas delivery system. We thank Audrey Garreau and Olivier Maillot for technical assistance. This study was supported by a PhD grant from the GRR SESA (Research Network of Sanitary Safety) and was financially supported by Europe, French Government (FEDER), Regional Council of Haute-Normandie, the Conseil Général de l'Eure and the Grand Evreux Agglomeration (France).
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00379
References
- Ahern H. E., Walsh K. A., Hill T. C. J., Moffett B. F. (2007). Fluorescent pseudomonads isolated from Hebridean cloud and rain water produce biosurfactants but do not cause ice nucleation. Biogeosciences 4, 115–124. 10.5194/bg-4-115-2007 [DOI] [Google Scholar]
- Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai H., Hayashi M., Kuroi A., Ishii M., Igarashi Y. (2005). Transcriptional regulation of the flavohemoglobin gene for aerobic nitric oxide detoxification by the second nitric oxide-responsive regulator of Pseudomonas aeruginosa. J. Bacteriol. 187, 3960–3968. 10.1128/JB.187.12.3960-3968.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger C., Appanna V. D. (2015). A novel ATP-generating machinery to counter nitrosative stress is mediated by substrate-level phosphorylation. Biochim. Biophys. Acta 1850, 43–50. 10.1016/j.bbagen.2014.09.028 [DOI] [PubMed] [Google Scholar]
- Auger C., Lemire J., Cecchini D., Bignucolo A., Appanna V. D. (2011). The metabolic reprogramming evoked by nitrosative stress triggers the anaerobic utilization of citrate in Pseudomonas fluorescens. PLoS ONE 6:e28469. 10.1371/journal.pone.0028469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusto O., Bonini M. G., Amanso A. M., Linares E., Santos C. C. X., De Menezes S. L. (2002). Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic. Biol. Med. 32, 841–859. 10.1016/S0891-5849(02)00786-4 [DOI] [PubMed] [Google Scholar]
- Barraud N., Hassett D. J., Hwang S.-H., Rice S. A., Kjelleberg S., Webb J. S. (2006). Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol. 188, 7344–7353. 10.1128/JB.00779-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud N., Storey M. V., Moore Z. P., Webb J. S., Rice S. A., Kjelleberg S. (2009). Nitric oxide−mediated dispersal in single− and multi−species biofilms of clinically and industrially relevant microorganisms. Microb. Biotechnol. 2, 370–378. 10.1111/j.1751-7915.2009.00098.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H., Kasper-Giebl A., Löflund M., Giebl H., Hitzenberger R., Zibuschka F., et al. (2002). The contribution of bacteria and fungal spores to the organic carbon content of cloud water, precipitation and aerosols. Atmospheric Res. 64, 109–119. 10.1016/S0169-8095(02)00084-4 [DOI] [Google Scholar]
- Bernagaud C., Burkhart J.-F., Vidal B., Lepriol T., Petit J.-F., Brunel T., et al. (2014). Observations du ratio [NO 2]/[NOx] en tunnel routier. Pollution-Atmospherique Available online at: http://lodel.irevues.inist.fr/pollution-atmospherique/index.php?id=2659 (Accessed August 18, 2015).
- Bodilis J., Calbrix R., Guérillon J., Mérieau A., Pawlak B., Orange N., et al. (2004). Phylogenetic relationships between environmental and clinical isolates of Pseudomonas fluorescens and related species deduced from 16S rRNA gene and OprF protein sequences. Syst. Appl. Microbiol. 27, 93–108. 10.1078/0723-2020-00253 [DOI] [PubMed] [Google Scholar]
- Bouffartigues E., Gicquel G., Bazire A., Bains M., Maillot M., Vieillard J., et al. (2012). Transcription of the oprF gene of Pseudomonas aeruginosa is dependent mainly on the SigX sigma factor and is sucrose induced. J. Bacteriol. 194, 4301–4311. 10.1128/JB.00509-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney S., Caulfield J. L., Niles J. C., Wishnok J. S., Tannenbaum S. R. (1999). The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat. Res. Mol. Mech. Mutagen. 424, 37–49. 10.1016/S0027-5107(99)00006-8 [DOI] [PubMed] [Google Scholar]
- Burrows S. M., Butler T., Jöckel P., Tost H., Kerkweg A., Pöschl U., et al. (2009a). Bacteria in the global atmosphere – Part 2: modeling of emissions and transport between different ecosystems. Atmos. Chem. Phys. 9, 9281–9297. 10.5194/acp-9-9281-2009 [DOI] [Google Scholar]
- Burrows S. M., Elbert W., Lawrence M. G., Pöschl U. (2009b). Bacteria in the global atmosphere – Part 1: review and synthesis of literature data for different ecosystems. Atmos. Chem. Phys. 9, 9263–9280. 10.5194/acp-9-9263-2009 [DOI] [Google Scholar]
- Caiazza N. C., Merritt J. H., Brothers K. M., O'Toole G. A. (2007). Inverse regulation of biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J. Bacteriol. 189, 3603–3612. 10.1128/JB.01685-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazza N. C., Shanks R. M. Q., O'Toole G. A. (2005). Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J. Bacteriol. 187, 7351–7361. 10.1128/JB.187.21.7351-7361.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaloulakou A., Mavroidis I., Gavriil I. (2008). Compliance with the annual NO2 air quality standard in Athens. Required NOx levels and expected health implications. Atmos. Environ. 42, 454–465. 10.1016/j.atmosenv.2007.09.067 [DOI] [Google Scholar]
- Chapalain A., Rossignol G., Lesouhaitier O., Merieau A., Gruffaz C., Guerillon J., et al. (2008). Comparative study of 7 fluorescent pseudomonad clinical isolates. Can. J. Microbiol. 54, 19–27. 10.1139/W07-110 [DOI] [PubMed] [Google Scholar]
- Chauhan A. J., Krishna M. T., Frew A. J., Holgate S. T. (1998). Exposure to nitrogen dioxide (NO2) and respiratory disease risk. Rev. Environ. Health 13, 73–90. [PubMed] [Google Scholar]
- Corker H., Poole R. K. (2003). Nitric oxide formation by Escherichia coli deoendence on nitrite reductase, the NO-sensing regulator Fnr, and flavohemoglobin Hmp. J. Biol. Chem. 278, 31584–31592. 10.1074/jbc.M303282200 [DOI] [PubMed] [Google Scholar]
- Cruz-Ramos H., Crack J., Wu G., Hughes M. N., Scott C., Thomson A. J., et al. (2002). NO sensing by FNR: regulation of the Escherichia coli NO-detoxifying flavohaemoglobin, Hmp. EMBO J. 21, 3235–3244. 10.1093/emboj/cdf339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutruzzola F., Frankenberg-Dinkel N. (2015). Origin and impact of nitric oxide in Pseudomonas aeruginosa biofilms. J. Bacteriol. 198, 55–65. 10.1128/JB.00371-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després V. R., Alex Huffman J., Burrows S. M., Hoose C., Safatov A. S., Buryak G., et al. (2012). Primary biological aerosol particles in the atmosphere: a review. Tellus B 64. 10.3402/tellusb.v64i0.15598 [DOI] [Google Scholar]
- Déziel E., Comeau Y., Villemur R. (2001). Initiation of biofilm formation by Pseudomonas aeruginosa 57RP correlates with emergence of hyperpiliated and highly adherent phenotypic variants deficient in swimming, swarming, and twitching motilities. J. Bacteriol. 183, 1195–1204. 10.1128/JB.183.4.1195-1204.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclairoir-Poc C., Meylheuc T., Ngoya S., Groboillot A., Bodilis J., Taupin L., et al. (2011). Influence of growth temperature on cyclolipopeptides production and on adhesion behaviour in environmental strains of Pseudomonas fluorescens. J. Bacteriol. Parasitol. S1-002. 10.4172/2155-9597.S1-002 [DOI] [Google Scholar]
- Duclairoir Poc C., Verdon J., Groboillot A., Barreau M., Toucourou H., Mijouin L., et al. (2014). Airborne fluorescent pseudomonads?: what potential for virulence? Int. J. Curr. Microbiol. Appl. Sci. 3, 708–722. [Google Scholar]
- Dybwad M., Granum P. E., Bruheim P., Blatny J. M. (2012). Characterization of airborne bacteria at an underground subway station. Appl. Environ. Microbiol. 78, 1917–1929. 10.1128/AEM.07212-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z., Ouyang Z., Zheng H., Wang X., Hu L. (2007). Culturable airborne bacteria in outdoor environments in Beijing, China. Microb. Ecol. 54, 487–496. 10.1007/s00248-007-9216-3 [DOI] [PubMed] [Google Scholar]
- Fetar H., Gilmour C., Klinoski R., Daigle D. M., Dean C. R., Poole K. (2011). mexEF-oprN Multidrug efflux operon of Pseudomonas aeruginosa: regulation by the MexT activator in response to nitrosative stress and chloramphenicol. Antimicrob. Agents Chemother. 55, 508–514. 10.1128/AAC.00830-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatley J., Barrett J., Pullan S. T., Hughes M. N., Green J., Poole R. K. (2005). Transcriptional responses of Escherichia coli to S-nitrosoglutathione under defined chemostat conditions reveal major changes in methionine biosynthesis. J. Biol. Chem. 280, 10065–10072. 10.1074/jbc.M410393200 [DOI] [PubMed] [Google Scholar]
- Ghaffari A., Neil D. H., Ardakani A., Road J., Ghahary A., Miller C. C. (2005). A direct nitric oxide gas delivery system for bacterial and mammalian cell cultures. Nitric Oxide 12, 129–140. 10.1016/j.niox.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Gicquel G., Bouffartigues E., Bains M., Oxaran V., Rosay T., Lesouhaitier O., et al. (2013). The extra-cytoplasmic function sigma factor SigX modulates biofilm and virulence-related properties in Pseudomonas aeruginosa. PLoS ONE 8:e80407. 10.1371/journal.pone.0080407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusarov I., Shatalin K., Starodubtseva M., Nudler E. (2009). Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science 325, 1380–1384. 10.1126/science.1175439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttenplan S. B., Kearns D. B. (2013). Regulation of flagellar motility during biofilm formation. FEMS Microbiol. Rev. 37, 849–871. 10.1111/1574-6976.12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha D.-G., O'Toole G. A. (2015). c-di-GMP and its effects on biofilm formation and dispersion: a Pseudomonas aeruginosa review. Microbiol. Spectr. 3:MB-0003-2014. 10.1128/microbiolspec.MB-0003-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn A., Nielsen A. T., Hentzer M., Sternberg C., Givskov M., Ersbøll B. K., et al. (2000). Quantification of biofilm structures by the novel computer program comstat. Microbiology 146, 2395–2407. 10.1099/00221287-146-10-2395 [DOI] [PubMed] [Google Scholar]
- Husain M., Bourret T. J., McCollister B. D., Jones-Carson J., Laughlin J., Vázquez-Torres A. (2008). Nitric oxide evokes an adaptive response to oxidative stress by arresting respiration. J. Biol. Chem. 283, 7682–7689. 10.1074/jbc.M708845200 [DOI] [PubMed] [Google Scholar]
- INERIS (2011). Dioxyde d'azote: Données toxicologiques et environnementales. Portail Subst. Chim. Available online at: http://www.ineris.fr/substances/fr/substance/cas/10102-44-0/2 (Accessed September 17, 2015).
- Jeter R. M., Sias S. R., Ingraham J. L. (1984). Chromosomal location and function of genes affecting Pseudomonas aeruginosa nitrate assimilation. J. Bacteriol. 157, 673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler T., Michéa-Hamzehpour M., Henze U., Gotoh N., Curty L. K., Pechère J. C. (1997). Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol. Microbiol. 23, 345–354. 10.1046/j.1365-2958.1997.2281594.x [DOI] [PubMed] [Google Scholar]
- Laver J. R., McLean S., Bowman L. A. H., Harrison L. J., Read R. C., Poole R. K. (2013). Nitrosothiols in bacterial pathogens and pathogenesis. Antioxid. Redox Signal. 18, 309–322. 10.1089/ars.2012.4767 [DOI] [PubMed] [Google Scholar]
- Li Y., Heine S., Entian M., Sauer K., Frankenberg-Dinkel N. (2013). NO-induced biofilm dispersion in Pseudomonas aeruginosa is mediated by an MHYT domain-coupled phosphodiesterase. J. Bacteriol. 195, 3531–3542. 10.1128/JB.01156-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Huang Q., Chen W. (2012). Heterologous expression of bacterial nitric oxide synthase gene: a potential biological method to control biofilm development in the environment. Can. J. Microbiol. 58, 336–344. 10.1139/w11-141 [DOI] [PubMed] [Google Scholar]
- Ma L., Conover M., Lu H., Parsek M. R., Bayles K., Wozniak D. J. (2009). Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 5:e1000354. 10.1371/journal.ppat.1000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollister B. D., Hoffman M., Husain M., Vazquez-Torres A. (2011). Nitric oxide protects bacteria from aminoglycosides by blocking the energy-dependent phases of drug uptake?. Antimicrob. Agents Chemother. 55, 2189–2196. 10.1128/AAC.01203-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean S., Bowman L. A. H., Sanguinetti G., Read R. C., Poole R. K. (2010). Peroxynitrite toxicity in Escherichia coli K12 elicits expression of oxidative stress responses and protein nitration and nitrosylation. J. Biol. Chem. 285, 20724–20731. 10.1074/jbc.M109.085506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Vivián C., Cabello P., Martínez-Luque M., Blasco R., Castillo F. (1999). Prokaryotic nitrate reduction: molecular properties and functional distinction among bacterial nitrate reductases. J. Bacteriol. 181, 6573–6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin J.-P., Preterre D., Gouriou F., Delmas V., François A., Orange N., et al. (2013). Particules urbaines et céréalières, micro-organismes, mycotoxines et pesticides. Pollut. Atmos. Available online at: http://lodel.irevues.inist.fr/pollution-atmospherique/index.php?id=759 (Accessed January 14, 2014).
- Morita Y., Tomida J., Kawamura Y. (2015). Efflux-mediated fluoroquinolone resistance in the multidrug-resistant Pseudomonas aeruginosa clinical isolate PA7: identification of a novel MexS variant involved in upregulation of the mexEF-oprN multidrug efflux operon. Antimicrob. Resist. Chemother. 6, 8. 10.3389/fmicb.2015.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce D. A., Hughes K. A., Lachlan-Cope T., Harangozo S. A., Jones A. E. (2010). Biodiversity of air-borne microorganisms at Halley Station, Antarctica. Extrem. Life Extreme Cond. 14, 145–159. 10.1007/s00792-009-0293-8 [DOI] [PubMed] [Google Scholar]
- Pershagen G., Rylander E., Norberg S., Eriksson M., Nordvall S. L. (1995). Air pollution involving nitrogen dioxide exposure and wheezing bronchitis in children. Int. J. Epidemiol. 24, 1147–1153. 10.1093/ije/24.6.1147 [DOI] [PubMed] [Google Scholar]
- Petrova O. E., Cherny K. E., Sauer K. (2014). The Pseudomonas aeruginosa diguanylate cyclase GcbA, a homolog of P. fluorescens GcbA, promotes initial attachment to surfaces, but not biofilm formation, via regulation of motility. J. Bacteriol. 196, 2827–2841. 10.1128/JB.01628-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova O. E., Sauer K. (2012). PAS Domain residues and prosthetic group involved in BdlA-dependent dispersion response by Pseudomonas aeruginosa Biofilms. J. Bacteriol. 194, 5817–5828. 10.1128/JB.00780-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K., Anjum M. F., Membrillo-Hernández J., Kim S. O., Hughes M. N., Stewart V. (1996). Nitric oxide, nitrite, and Fnr regulation of hmp (flavohemoglobin) gene expression in Escherichia coli K-12. J. Bacteriol. 178, 5487–5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo-Nieto M., Barret M., Morrissey J., Germaine K., Martínez-Granero F., Barahona E., et al. (2013). Genome sequence reveals that Pseudomonas fluorescens F113 possesses a large and diverse array of systems for rhizosphere function and host interaction. BMC Genomics 14:54. 10.1186/1471-2164-14-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reduction of pollutant emissions from light vehicles (2015). Access Eur. Union Law. Available online at: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:l28186 (Accessed September 17, 2015).
- Rietsch A., Vallet-Gely I., Dove S. L., Mekalanos J. J. (2005). ExsE, a secreted regulator of type III secretion genes in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 102, 8006–8011. 10.1073/pnas.0503005102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol G., Merieau A., Guerillon J., Veron W., Lesouhaitier O., Feuilloley M. G., et al. (2008). Involvement of a phospholipase C in the hemolytic activity of a clinical strain of Pseudomonas fluorescens. BMC Microbiol. 8:189. 10.1186/1471-2180-8-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A. B., Petrova O. E., Sauer K. (2012). The phosphodiesterase DipA (PA5017) is essential for Pseudomonas aeruginosa biofilm dispersion. J. Bacteriol. 194, 2904–2915. 10.1128/JB.05346-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastalla I., Chim K., Cheung G. Y. C., Pomerantsev A. P., Leppla S. H. (2009). Codon-optimized fluorescent proteins designed for expression in low-GC Gram-positive bacteria. Appl. Environ. Microbiol. 75, 2099–2110. 10.1128/AEM.02066-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales B. S., Dickson R. P., LiPuma J. J., Huffnagle G. B. (2014). Microbiology, genomics, and clinical significance of the Pseudomonas fluorescens species complex, an unappreciated colonizer of humans. Clin. Microbiol. Rev. 27, 927–948. 10.1128/CMR.00044-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher E. (1998). Handbook of Air Pollution from Internal Combustion Engines: Pollutant Formation and Control. San Diego, MA; Chestnut Hill, MA: Academic Press. [Google Scholar]
- Shiro Y. (2012). Structure and function of bacterial nitric oxide reductases: nitric oxide reductase, anaerobic enzymes. Biochim. Biophys. Acta 1817, 1907–1913. 10.1016/j.bbabio.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Skalska K., Miller J. S., Ledakowicz S. (2010). Trends in NOx abatement: a review. Sci. Total Environ. 408, 3976–3989. 10.1016/j.scitotenv.2010.06.001 [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., et al. (1985). Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85. 10.1016/0003-2697(85)90442-7 [DOI] [PubMed] [Google Scholar]
- Sobel M. L., McKay G. A., Poole K. (2003). Contribution of the MexXY multidrug transporter to aminoglycoside resistance in Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 47, 3202–3207. 10.1128/AAC.47.10.3202-3207.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel M. L., Neshat S., Poole K. (2005). Mutations in PA2491 (mexS) Promote MexT-dependent mexEF-oprN expression and multidrug resistance in a clinical strain of Pseudomonas aeruginosa. J. Bacteriol. 187, 1246–1253. 10.1128/JB.187.4.1246-1253.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler C., Böhm A., Jenal U., Seifert R., Kaever V. (2010). A liquid chromatography-coupled tandem mass spectrometry method for quantitation of cyclic di-guanosine monophosphate. J. Microbiol. Methods 81, 226–231. 10.1016/j.mimet.2010.03.020 [DOI] [PubMed] [Google Scholar]
- Spiro S. (2007). Regulators of bacterial responses to nitric oxide. FEMS Microbiol. Rev. 31, 193–211. 10.1111/j.1574-6976.2006.00061.x [DOI] [PubMed] [Google Scholar]
- Strehmel J., Neidig A., Nusser M., Geffers R., Brenner-Weiss G., Overhage J. (2015). Sensor kinase PA4398 modulates swarming motility and biofilm formation in Pseudomonas aeruginosa PA14. Appl. Environ. Microbiol. 81, 1274–1285. 10.1128/AEM.02832-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir S., Burney S., Tannenbaum S. R. (1996). DNA damage by nitric oxide. Chem. Res. Toxicol. 9, 821–827. 10.1021/tx9600311 [DOI] [PubMed] [Google Scholar]
- Šantl-Temkiv T., Sahyoun M., Finster K., Hartmann S., Augustin-Bauditz S., Stratmann F., et al. (2015). Characterization of airborne ice-nucleation-active bacteria and bacterial fragments. Atmos. Environ. 109, 105–117. 10.1016/j.atmosenv.2015.02.060 [DOI] [Google Scholar]
- van Sorge N. M., Beasley F. C., Gusarov I., Gonzalez D. J., Köckritz-Blickwede M., von Anik, S., et al. (2013). Methicillin-resistant Staphylococcus aureus bacterial nitric oxide synthase affects antibiotic sensitivity and skin abscess development. J. Biol. Chem. 288, 6417–6426. 10.1074/jbc.M112.448738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varivarn K., Champa L. A., Silby M. W., Robleto E. A. (2013). Colonization strategies of Pseudomonas fluorescens Pf0-1: activation of soil-specific genes important for diverse and specific environments. BMC Microbiol. 13:92. 10.1186/1471-2180-13-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO |Air quality guidelines - global update (2005). WHO. Available online at: http://www.who.int/phe/health_topics/outdoorair/outdoorair_aqg/en/ (Accessed September 17, 2015).
- WHO |Ambient (outdoor) air quality health (2015). WHO. Available online at: http://www.who.int/mediacentre/factsheets/fs313/en/ (Accessed September 17, 2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.