Abstract
Introduction
Colorectal cancer is one of the most common cancers worldwide. Incidence rates of large intestine cancer indicate a role of environmental and occupational factors. The role of essential elements and their interaction with toxic metals can contribute to the explanation of a complex mechanism by which large intestine cancer develops. Bearing this in mind, determining the levels of essential and toxic elements in tissues (organs), as well as in body fluids, seems to shed light on their role in the mode of action in malignant disease.
Aim
Determination of the levels of cadmium, zinc, copper, selenium, calcium, magnesium, and iron in large intestine malignant tissue.
Material and methods
Two intraoperative intestine sections were investigated: one from the malignant tissue and the other one from the normal tissue, collected from each person with diagnosed large intestine cancer. Cadmium, zinc, copper, calcium, magnesium, and iron levels were determined with atomic absorption spectrometry, and selenium levels by spectrofluorimetric method.
Results
The levels of copper, selenium, and magnesium were higher in the malignant than in normal tissues. In addition, the zinc/copper and calcium/magnesium relationship was altered in malignant tissue, where correlations were lower compared to non-malignant tissue.
Conclusions
The results seems to demonstrate disturbed homeostasis of some essential elements. However, it is hard to confirm their involvement in the aetiology of colorectal cancer.
Keywords: large intestine, colorectal cancer, cadmium, essential elements
Introduction
Colorectal cancer is the fourth most common cancer in men and the third most common cancer in women, worldwide. The highest incidence rates are recorded in Europe, North America, and Oceania, and the lowest in South America, Africa, and Asia (e.g. about 4 cases per 100 000 population in India), although select registries in Asia (i.e. Japan, Singapore, and Israel) also record high rates. The great variation in the incidence rates of large intestine cancer observed in different parts of the world indicates a significant role of environmental factors (e.g. diet, physical activity) and so-called “western-style living” [1]. Modification of risk factors, primarily by promoting a well balanced diet, regular physical activity, and maintenance of normal body mass, can reduce cancer incidence by as much as 50–75% [2]. It has been also discussed whether occupational factors are responsible for increased risk of colon cancer. A higher risk of colorectal cancer has been reported alter alia among workers exposed to asbestos or wood dust, or those working in the textile industry. Moreover, some pesticides have also been associated with excess risk of colon cancer among pesticide applicators [3].
Large intestine cancer is the final stage of a dynamic process of dysplastic transformation, occurring most frequently in adenoma. Most colon cancers probably arise from benign neoplastic polyps [4]. In large intestine cancer, like in other cancers, early diagnosis and adequate therapeutic procedures provide a good chance of complete recovery. According to the literature data, an early diagnosis can ensure a large proportion of recovery, reaching as much as 90%. However, the chance of recovery diminishes proportionally to cancer progression. It should be noted that its detection in the most advanced state of malignancy limits 5-year survival to 5% [5].
Growing interest in concentrations of essential elements (e.g. magnesium (Mg), calcium (Ca), copper (Cu), zinc (Zn), iron (Fe), and selenium (Se)) in normal and malignant tissues and differences in their distribution have been observed for a number of years. Numerous reports stress the importance of determining serum copper and zinc levels in assessing activity and prognosis of the disease. Some studies have shown increased serum copper and decreased serum zinc levels in patients with sarcoma, lung cancer, and carcinoma of the digestive organs [6, 7]. However, the mechanisms by which the level of these elements decreases and increases in various cancerous conditions, as well as the issue of whether the altered serum copper and zinc levels are causative factors of the malignant state or its sequel, have not yet been elucidated. Nevertheless, it is obvious that in their attempts to identify possible causes of cancers and thus to facilitate their early diagnosis, researchers also try to analyse the role played by the elements in neoplastic processes.
Reports on the role of essential and toxic elements in the aetiology of large intestine cancer are rather scarce in the literature available to date. The role of copper and its metabolism in the human body has been the subject of much interest among numerous researchers. Its significant involvement in the synthesis of the connective tissue and haemoglobin, and in the normal functioning of the peripheral nervous system has already been evidenced. Tissue and plasma copper levels differ considerably depending on the place of residence, and thus on their content in the diet [8].
Zinc is an activator of several hundred enzymes participating, among others, in RNA replication and DNA repair. Zn deficiency impairs cellular and humoral immunity, and also limits the cytokine production [9]. Zn has also been recognised as an antioxidant. An effective anti-oxidative defence depends greatly on the regular metabolism of essential elements; therefore, it is not surprising that a great body of studies have been focused on their role in the development of carcinomas and large intestine adenocarcinoma. In the experimental adenocarcinoma, selenium stimulates DNA repair [10]. The protective role of Se in cancer is supported also by clinical and epidemiological studies [11], where an inverse association between Se supplementation and the risk of colorectal cancer was observed [12].
Epidemiological studies have demonstrated an association between the risk of colon cancer and low intake of Mg. Moreover, a diet rich in Mg was found to reduce the occurrence of this cancer. Animal studies established that Mg could be a protective agent in the early stages of carcinogenesis. Nevertheless, it could also promote the growth of existing malignancies at later stages [13, 14]. It has been shown that Ca, Zn, and Cu can also reduce the risk of colon and rectum cancers [15].
Cadmium is one of the known factors responsible for the disturbed homeostasis of essential metals. According to Elinder et al., cadmium, along with nephrotoxic and carcinogenic (lung cancer) effects, induces changes in Zn, Cu, Fe, Mg, Ca, and Se interaction-based distribution, which can be manifested by the deficiency of these compounds in individual tissues, diminished haematological indices (iron, haemoglobin, haematocrit), disturbed metabolism of carbohydrates (decreased insulin secretion), and induced lipid peroxidation [16].
To be able to shed more light on the role of essential elements in their mode of action in malignant disease, the content of each element in the tumour tissue itself should be determined. Apparently the identification of the role of elements and their interaction with toxic metals (cadmium) can significantly contribute to the explanation of a complex mechanism by which inter alia large intestine cancer develops.
Aim
In view of the limited data available to date, the need to carry out studies aimed at determining the levels of metals and essential and toxic elements in tissues (organs) and body fluids seems to be well founded, so the aim of this study was to determine the levels of cadmium, zinc, copper, selenium, calcium, magnesium, and iron in intraoperative sections of large intestine malignant tissues and in normal tissues taken as the control.
Material and methods
The study was carried out in a group of 25 persons (16 men and 9 women) with diagnosed large intestine cancer (Adenocarcinoma mucinosum). Two intraoperative intestine sections, one from the malignant tissue and the other from normal tissue, located at a maximum distance from the focus of malignancy, were collected from each person. All of the tissues were examined morphologically to confirm the diagnosis. The samples were stored in polyurethane containers at –70°C until examination. None of the patients from whom the tissues were collected had previously been subjected to radiotherapy or bio-element supplementation. All of the patients were matched for age and cancer progression, had not been occupationally exposed to heavy metals, and were non-smokers. This study was approved by the Ethics Committee for Scientific Research at the Medical University in Lodz (Resolution No RNN/156/02/KE).
Cadmium levels were determined with graphite furnace absorption spectrometry (HITACHI Z8270), and zinc, copper, calcium, magnesium, and iron levels with flame atomic absorption spectrometry (GBC Avanta PM) following mineralisation. Selenium levels were determined using the spectrofluorimetric method (HITACHI F4500) [17].
The limits of detection, calculated as concentrations corresponding with the value of absorption equal to a threefold standard deviation of the signal for the lowest standard, were respectively 0.001 µg/ml for cadmium, 0.02 µg/ml for zinc, 0.02 µg/ml for copper, 0.15 µg/ml for selenium, 0.01 µg/ml for calcium, 0.01 µg/ml for magnesium, and 0.01 µg/ml for iron.
The intra-laboratory quality control was based on reference material SRM 1577b – lyophilised bovine liver (National Institute of Standards & Technology, Gaithersburg, Germany), with certified measurements of the following elements [µg/g]: Cd (0.5 ±0.03), Zn (127 ±16), Cu (160 ±8), Se (0.73 ±0.06), Ca (116 ±4), Mg (601 ±28), and Fe (184 ±15). Mean discrepancies between the obtained results, compared with certified values expressed as RSD, were: Cd ±2.1%, Zn ±0.3%, Cu ±8.3%, Se ±3.5%, Ca ±6.7%, Mg ±7.2%, and Fe ±5.8%. The error of repeatability did not exceed 10% in any of the study samples.
Results
The results of the study are presented in Table I. The concentration of selenium in malignant tissue was more than two times higher than in normal tissue, and this was the highest statistically significant difference in our study. Levels of copper and magnesium were also significantly higher in malignant tissue – respectively, 170% and 180% of value determined in normal tissue. Cancerous transformation does not seem to affect zinc, calcium, cadmium, and iron levels, which were similar in malignant and non-malignant tissue.
Table I.
Cadmium (Cd), zinc (Zn), copper (Cu), selenium (Se), magnesium (Mg), calcium (Ca), and iron (Fe) concentrations (mean ± SD; µg/g wet tissue) in normal and malignant tissues of large intestine
| Element | Malignant tissue | Normal tissue |
|---|---|---|
| Cd | 0.04 ±0.03 | 0.04 ±0.02 |
| Zn | 17.50 ±5.40 | 15.35 ±6.30 |
| Cu | 1.10 ±0.57* | 0.64 ±0.39 |
| Se | 0.12 ±0.07* | 0.05 ±0.03 |
| Mg | 76.78 ±31.41* | 43.81 ±18.91 |
| Ca | 58.53 ±9.25 | 47.94 ±13.18 |
| Fe | 31.92 ±15.18 | 34.06 ±11.56 |
Statistically significant difference (p < 0.05).
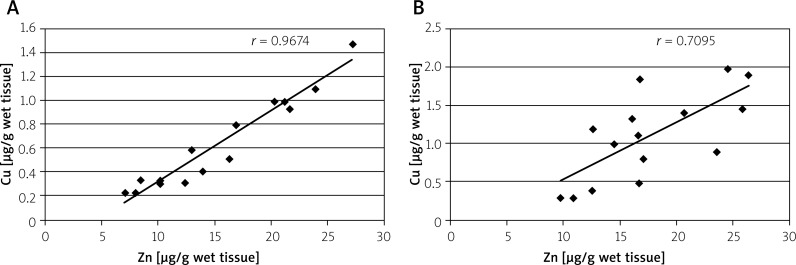
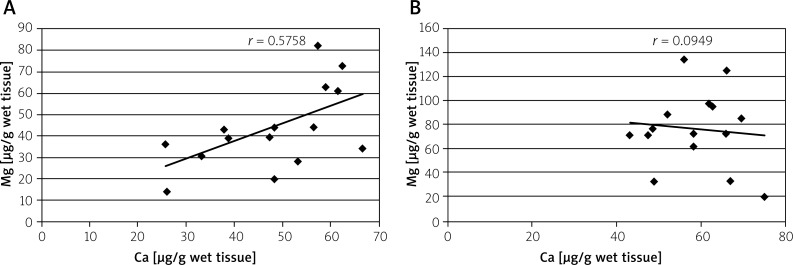
The determined values of elements were also analysed in terms of their interrelations and correlations. On the basis of adequate calculations, a high correlation (almost 1) was observed between Zn and Cu concentrations in the normal tissue, but it was disturbed in the malignant tissue, where it was much lower (Figure 1). Similarly large differences between correlations were noted between Ca and Mg concentrations. Although the correlation between these elements in normal tissue was moderate; in the malignant tissue there seemed to be no relationship at all (Figure 2).
Figure 1.
Correlation between zinc (Zn) and copper (Cu) concentrations in normal (A) and malignant (B) tissues of large intestine
Figure 2.
Correlation between calcium (Ca) and magnesium (Mg) concentrations in normal (A) and malignant (B) tissues of large intestine
Discussion
The majority of published literature data focus on the determination of essential elements in blood serum to identify a biomarker useful in early detection of malignant pathologies. Studies carried out to observe changes in concentrations of these elements in organs and tissues already affected by neoplastic lesions are less numerous. It is well known that the observed alterations in element concentrations in blood serum result, among others, from changes in homeostasis of essential elements in malignant tissues.
The literature data published in the last decade indicate that disturbed levels of elements, mostly zinc and copper, can play a significant role in the mechanism responsible for uncontrolled cellular growth. It is thought that Cu plays an essential role in the activation of endothelium cells by stimulating angiogenic growth factors, which results in stimulation of the proliferation process [18]. Copper involvement in the carcinogenic process may also be linked to its ability to bind to some proteins, thus stimulating their angiogenic activity. This can explain the fact that in a number of cancers even a several-fold increase in Cu level in serum and malignant tissues was observed (in our study almost twofold), whereas others do not confirm this observation, especially according to tissue levels [19, 20]. Due to long-term monitoring of serum Cu concentration, it is feasible that the absence of changes in its concentration for a long period of time indicates a very good prognosis, whereas the increase in its concentration may show the recurrence of the carcinogenic process.
Zinc plays an important role in the carcinogenic process as it takes part in each stage of cellular cycle, regulation, and expression of genes as well as in DNA synthesis. Numerous authors have shown in in vitro studies that Zn exerts anti-proliferating and pro-apoptotic effects inter alia in prostate gland cells [21]. It is also suggested that the protective effect of Zn results from its competing with other essential elements, mainly Cu and Fe. Based on the literature, it is also well known that the blood serum Zn concentration undergoes changes in the state of increased metabolic activity of the organism. A diminished Zn concentration is observed in cancers of different sites. The progression of neoplastic transformation is linked with decreasing Zn concentration in serum [22].
Numerous publications provide evidence that Se compounds are characterised by anticancer properties. Se may act in cancer prevention via numerous mechanisms and on all stages of cancer progression [11]. It has been found that Se induces apoptosis in malignant tissues [23]. An increased Se concentration in malignant tissues may point to the body's immune reaction and its attempt to induce apoptosis in aplastic cells through increasing the number of free radicals and thus enhancing oxidative stress. The mechanism by which free radicals are produced, and the results from this oxidative stress induced by selenium, is favourable to the body on the one hand, in view of anticancer effect, and unfavourable on the other as an Se toxic effect cannot be ruled out [24].
Unbalanced Mg homeostasis is often noticed in malignant cells, which accumulate this element, behaving like a magnesium trap. Moreover, high affinity of neoplastic cells for Mg was also observed in cell cultures with low Mg concentration [13].
The results of our study partly confirm observations of other researchers (Tables II and III), who found increased Cu levels in the large intestine malignant tissues. The same concerns Zn, levels of which are often only slightly departed from those found in normal tissue [6, 19, 25–28]. The observed inconsistency in element levels both in normal and malignant tissue are difficult to elucidate. It may be influenced by many factors, e.g. diet, different determination methods, and differences in the end outcomes (µg/g dry or wet tissue).
Table II.
Zinc (Zn), copper (Cu), and selenium (Se) concentrations (mean ± SD; µg/g wet tissue) in malignant and normal tissues of the large intestine according to different authors
| Authors, methods | Zn | Cu | Se | |||
|---|---|---|---|---|---|---|
| Malignant tissue | Normal tissue | Malignant tissue | Normal tissue | Malignant tissue | Normal tissue | |
| Margalioth et al., 1983 [6], AAS | 18.6 ±6.34 | 18.3 ±3.8 | 1.90 ±0.6 | 1.53 ±0.35 | – | – |
| Gregoriadis et al., 1983 [25], PIXRF | 14.3 ±2.7 | 15.2 ±3.1 | 1.70 ±0.64 | 1.34 ±0.33 | – | – |
| Drake and Sky-Peck, 1989 [19], ultramicroEDXRF | 98.2 ±18.7 | 64.1 ±20.5 | 8.9 ±3.4 | 14.1 ±4.4 | 1.26 ±0.28 | 1.53 ±0.33 |
| Witkowski et al., 1993 [26], AAS | – | – | 5.18 ±2.63 | 5.76 ±1.54 | – | – |
| Arriola et al., 1999 [29], INNA | 8.08 ±0.60 | 7.53 ±0.50 | 0.27 ±0.04 | 0.40 ±0.06 | 1.54 ±0.25 | 1.31 ±0.20 |
| Kucharzewski et al., 2003 [27], TRXRF | 14.80 ±0.82 | – | 3.87 ±0.27 | – | 0.86 ±0.19 | – |
| Daragó et al., 2005 [28], AAS | 17.44 ±5.60 | 15.54 ±6.73 | 1.09 ±0.63 | 0.64 ±0.42 | 0.12 ±0.07 | 0.05 ±0.03 |
| Milde et al., 2005 [30], AAS | 69.20 ±21.03 | – | 6.08 ±3.78 | – | 1.17 ±0.73 | – |
| Majewska et al., 2007 [4], TXRF | 14.8 ±9.63 | – | 3.55 ±2.36 | – | 0.816 ±0.557 | – |
| Lavilla et al., 2009 [31], ICP-OES, ICP-MS | 89 | 79 | 8.5 | 6.2 | 1.6 | 0.9 |
| Szewczyk et al., 2013 [7], AAS | 1.61 ±0.93* | 3.22 ±1.91* | 10.3 ±3.0* | 6.3 ±1.8* | – | – |
µg/g protein. AAS – atomic absorption spectrometry, PIXRF – photon-induced X-ray fluorescence, EDXRF – energy dispersive X-ray fluorescence, TRXRF – total reflection X-ray fluorescence, INNA – instrumental neutron activation analysis, ICP-OES – inductively coupled plasma optical emission spectrometry, ICP-MS – inductively coupled plasma mass spectrometry.
Table III.
Calcium (Ca), iron (Fe), cadmium (Cd), and magnesium (Mg) concentrations (mean ± SD; µg/g wet tissue) in malignant and normal tissues of the large intestine according to different authors
| Authors, methods | Fe | Mg | Ca | Cd | ||||
|---|---|---|---|---|---|---|---|---|
| Malignant tissue | Normal tissue | Malignant tissue | Normal tissue | Malignant tissue | Normal tissue | Malignant tissue | Normal tissue | |
| Drake and Sky-Peck, 1989 [19], ultramicroEDXRF | 129 ±41.2 | 189 ±50.5 | – | – | 393 ±195 | 591 ±253 | – | – |
| Arriola et al., 1999 [29], INNA | 120.24 ±4.09 | 131.45 ±4.21 | 20.96 ±0.82 | 19.76 ±0.73 | 9.07 ±0.83 | 11.18 ±0.93 | – | – |
| Kucharzewski et al., 2003 [32], TRXRF | 46.1 ±4.27 | – | – | – | – | – | – | – |
| Milde et al., 2005 [30], AAS | – | – | 753.59 ±310.54 | – | – | – | – | – |
| Daragó et al., 2005 [28], AAS | – | – | – | – | – | – | 0.04 ±0.02 | 0.04 ±0.02 |
| Majewska et al., 2007 [4], TXRF | 45.00 ±33.40 | – | – | – | – | – | – | – |
| Szewczyk et al., 2009 [20], colorimetrically | – | – | 6.77 ±2.4 | 6.40 ±1.7 | – | – | – | – |
| Lavilla et al., 2009 [31], ICP-OES, ICP-MS | 194 | 125 | 664 | 323 | 1462 | 459 | 0.14 | 0.23 |
Abbreviations as in Table II.
Conclusions
The results of our study seem to demonstrate disturbed homeostasis of some essential elements, mostly Mg and Cu. However, it is hard to confirm their involvement in the aetiology of colorectal cancer.
Acknowledgments
This study was sponsored by the Medical University of Lodz, Poland (Grant No. 503-3045-1).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Center MM, Jemal A, Smith RA, et al. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366–78. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 2.Kaleta-Stasiołek D, Kwaśniewska M, Drygas W. Selected risk factors and primary prevention of colorectal cancer [Polish] Przegl Lek. 2002;60:170–5. [PubMed] [Google Scholar]
- 3.Lee WJ, Sandler DP, Blair A, et al. Pesticide use and colorectal cancer risk in the Agricultural Health Study. Int J Cancer. 2007;121:339–346. doi: 10.1002/ijc.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majewska U, Banaś D, Braziewicz J, et al. Trace element concentration distributions in breast, lung and colon tissues. Phys Med Biol. 2007;52:3895–911. doi: 10.1088/0031-9155/52/13/016. [DOI] [PubMed] [Google Scholar]
- 5.Lutz JM, Francisci S, Mugno E, et al. Cancer prevalence in Central Europe: the EUROPREVAL Study. Ann Oncol. 2003;14:313–22. doi: 10.1093/annonc/mdg059. [DOI] [PubMed] [Google Scholar]
- 6.Margalioth EJ, Schenker JG, Chevion M. Copper and zinc levels in normal and malignant tissues. Cancer. 1983;52:868–72. doi: 10.1002/1097-0142(19830901)52:5<868::aid-cncr2820520521>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Szewczyk M, Pasternak K, Andrzejewski A, et al. Plasma and cancerous tissue concentration of Zn, Cu and Mn in patients undergoing surgical treatment for gastrointestinal cancer. J Elem. 2013;18:135–144. [Google Scholar]
- 8.Versieck J, Cornelis R. Normal levels of trace elements in human blood, plasma, or serum. Anal Chim Acta. 1980;116:217–54. [Google Scholar]
- 9.Costello LC, Franklin RB. The intermediary metabolism of the prostate: a key to understanding the pathogenesis and progression of prostate malignancy. Oncology. 2000;59:269–82. doi: 10.1159/000012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawson T. Nicotinamide and selenium stimulate the repair of DNA damage produced by N-nitrosobis (2-oxopropyl) amine. Anticancer Res. 1989;9:483–86. [PubMed] [Google Scholar]
- 11.Hu Y, McIntosh GH, Young GP. Selenium-rich foods: a promising approach to colorectal cancer prevention. Curr Pharm Biotechnol. 2012;13:165–72. doi: 10.2174/138920112798868809. [DOI] [PubMed] [Google Scholar]
- 12.Pericleous M, Mandair D, Caplin ME. Diet and supplements and their impact on colorectal cancer. J Gastrointest Oncol. 2013;4:409–23. doi: 10.3978/j.issn.2078-6891.2013.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castiglioni S, Maier JA. Magnesium and cancer: a dangerous liason. Magnes Res. 2011;24:S92–100. doi: 10.1684/mrh.2011.0285. [DOI] [PubMed] [Google Scholar]
- 14.Blaszczyk U, Duda-Chodak U. Magnesium: its role in nutrition and carcinogenesis. Rocz Panstw Zakl Hig. 2013;64:165–71. [PubMed] [Google Scholar]
- 15.Ksiądzyna D, Paradowski L. Environmental factors and the etiology of intestinal neoplasm [Polish] Gastroenterol Pol. 2004;11:369–74. [Google Scholar]
- 16.Elinder CG, Kjellstrom T, Hogstedt C, et al. Cancer mortality of cadmium workers. Br J Ind Med. 1985;42:651–5. doi: 10.1136/oem.42.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watkinson JH. Fluorometric determination of selenium in biological material with 2,3-diaminonaphthalene. Anal Chem. 1966;38:92–7. doi: 10.1021/ac60233a025. [DOI] [PubMed] [Google Scholar]
- 18.Brem S, Wotoczek-Obadia MC. Regulation of angiogenesis by copper reduction and penicillamine: antagonism of cytokine and growth factor activity; 1998. Jan, pp. 24–8. AACR Special Conference: Angiogenesis and Cancer Research, Orlando, Fla. [Google Scholar]
- 19.Drake EN, Sky-Peck HH. Discriminant analysis of trace element distribution in normal and malignant human tissues. Cancer Res. 1989;49:4210–5. [PubMed] [Google Scholar]
- 20.Szewczyk M, Pasternak K, Andrzejewski A, et al. Magnesium concentration in plasma and tissues of patients undergoing surgery for stomach and large intestine cancer. J Elem. 2009;14:563–71. [Google Scholar]
- 21.Feng P, Liang JY, Li TL, et al. Zinc induces mitochondria apoptogenesis in prostate cells. Mol Urol. 2000;4:31–6. [PubMed] [Google Scholar]
- 22.Sznajda J. The clinical biochemistry in practice medicine [Polish] Warsaw: PZWL; 1983. [Google Scholar]
- 23.Behn D, Weiss-Nowak C, Kalcklosch M, et al. Studies on the distribution and characteristics of new mammalian selenium-containing proteins. Analyst. 1995;120:823–5. doi: 10.1039/an9952000823. [DOI] [PubMed] [Google Scholar]
- 24.Combs GF, Jr, Gray WP. Chemopreventive agents: selenium. Pharmacol Ther. 1998;79:179–92. doi: 10.1016/s0163-7258(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 25.Gregoriadis GC, Apostolidis NS, Romanos AN, et al. A comparative study of trace elements in normal and cancerous colorectal tissues. Cancer. 1983;52:508–19. doi: 10.1002/1097-0142(19830801)52:3<508::aid-cncr2820520322>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Witkowski K, Kozłowski A, Pardela M, et al. Level of copper in plasma and tissue of patients with esophageal and large bowel cancer [Polish] Wiad Lek. 1993;46:586–8. [PubMed] [Google Scholar]
- 27.Kucharzewski M, Braziewicz J, Majewska U, et al. Selenium, copper, and zinc concentrations in intestinal cancer tissue and in colon and rectum polyps. Biol Trace Elem Res. 2003;92:1–10. doi: 10.1385/BTER:92:1:1. [DOI] [PubMed] [Google Scholar]
- 28.Daragó A, Rzetecki T, Dziki A, et al. Biological levels of cadmium, zinc, copper and selenium in the patient with colon cancer [Polish] Bromat Chem Toksykol. 2005;38:371–6. [Google Scholar]
- 29.Arriola H, Longoria L, Quintero A, et al. INAA of trace elements in colorectal cancer patients. Biol Trace Elem Res. 1999;71-72:563–8. doi: 10.1007/BF02784244. [DOI] [PubMed] [Google Scholar]
- 30.Milde D, Altmannnova K, Vyslouzil K, et al. Trace element levels in blood serum and colon tissue in colorectal cancer. Chem Pap. 2005;59:157–60. [Google Scholar]
- 31.Lavilla I, Costas M, Miguel PS, et al. Elemental fingerprints of timorous and adjacent non-tumorous tissues from patients with colorectal cancer using ICP-MS, ICP-OES and chemometric analysis. Biometals. 2009;22:863–75. doi: 10.1007/s10534-009-9231-6. [DOI] [PubMed] [Google Scholar]
- 32.Kucharzewski M, Braziewicz J, Majewska U, et al. Iron concentrations in intestinal cancer tissue and in colon and rectum polyps. Biol Trace Elem Res. 2003;95:19–28. doi: 10.1385/BTER:95:1:19. [DOI] [PubMed] [Google Scholar]