Abstract
Background
There is a clinical need for evidence-based psychotherapy response biomarkers in major depressive disorder (MDD). Based on previous studies, we hypothesized that lower 24-h urinary cortisol levels and a history of early life stress/trauma would predict an improved antidepressant response to cognitive-behavioral therapy (CBT).
Methods
50 currently depressed MDD subjects were enrolled. 24-h urine was collected and measured for cortisol levels by radioimmunoassay (RIA). Subjects were also administered early life stress/trauma measures at baseline: Global Perceived Early-Life Stress (GPELS), The Early Life Trauma Inventory (ELTI) and Klein Loss Scale (KLS). The efficacy of a twelve-week course of once-weekly CBT was evaluated by the primary outcome measure, the 24-item Hamilton Depression Rating Scale (HDRS24), at baseline and every four weeks, and the Beck Depression Inventory at baseline and weekly thereafter. 42 subjects had at least one complete follow-up visit (≥4 weeks of CBT), and 30 subjects completed the full 12-week course.
Results
Baseline 24-h urinary cortisol levels did not correlate with CBT’s antidepressant response. Higher KLS scores, a measure of early life parental loss or separation, correlated with delta HDRS24 (rs=−0.39, padjusted=0.05). Complementary general linear model analysis revealed enhanced CBT efficacy in patients with a history of early life parental loss or separation [F(1,35)=6.65, p=0.01].
Limitations
Small sample size, Treatment-naïve population.
Conclusions
Early life parental separation or loss positively correlated with CBT’s antidepressant efficacy in our sample and may warrant further study in larger clinical samples.
Keywords: Major depressive disorder, Cognitive-behavioral therapy, Parental loss, Parental separation, Hypothalamic-pituitary-adrenal axis, Cortisol
1. Introduction
Major depressive disorder (MDD) is a heterogeneous neuropsychiatric condition with the highest worldwide morbidity across all sociodemographic strata (Kessler et al., 2003; Ormel et al., 2008; Ustun et al., 2004). Standard interventions for unipolar depression include antidepressant medications, somatic therapies, and psychotherapy. Instead of a more personalized approach targeting the patient’s specific behavioral profile, history, or underlying pathophysiology, treatment selection is often based on subjective factors such as patient preference and the theoretical orientation of the treating clinician. Unfortunately, many patients do not have a beneficial antidepressant response with this approach to treatment selection. As a result, there is critical need to identify treatment response biomarkers to facilitate treatment modality selection and assess response.
The two manualized psychotherapies with the largest evidence base in MDD are interpersonal psychotherapy (IPT) and cognitive-behavioral therapy (CBT). Both psychotherapies are recommended as first-line treatments in the 2010 American Psychiatric Association’s (APA) MDD practice guideline (American Psychiatric Association., 2000). Additionally, when compared to antidepressant medications, CBT is associated with a lower risk of relapse once a patient achieves remission (Evans et al., 1992; Gloaguen et al., 1998; Simons et al., 1986; Thase et al., 1992). Although a multi-site study initially suggested that CBT was less effective in patients with severe depression (Elkin et al., 1989), a subsequent meta-analysis did not find evidence to support this claim (DeRubeis et al., 1999). As baseline illness severity does not appear to be a reliable means of predicting antidepressant efficacy, several studies have turned to depressive subtypes as potential predictors. Stewart et al. (1998) reported that atypical depression responded more robustly to cognitive therapy than other depressive subtypes. In addition, hypothalamic-pituitary-adrenal (HPA) axis dysfunction (a biometric of melancholic depression) predicted a poorer treatment response to CBT and other psychosocial interventions (Robbins et al., 1989; Thase et al., 1996). Yet, due to discrepancies in the literature (Thase and Friedman, 1999), there is currently insufficient evidence to support depressive subtypes as a reliable predictor of antidepressant response.
Due to prior reports of a positive correlation between cognitive-based psychotherapy efficacy and history of early life stress/abuse (Kuyken et al., 2015; Nemeroff et al., 2003), we similarly hypothesized that a history of early life stress would correlate with CBT’s antidepressant efficacy. Also, based on the aforementioned studies of HPA axis dysfunction/melancholic depression correlating with reduced antidepressant response to CBT (Robbins et al., 1989; Thase et al., 1996), we predicted that subjects with decreased baseline 24-h urinary cortisol (indicative of lower HPA axis activity/non-melancholic depression) would have an enhanced antidepressant response to CBT.
2. Methods
All subjects provided written informed consent prior to any research-related procedures. The Yale School of Medicine Institutional Review Board/Human Investigation Committee approved all portions of the protocol.
2.1. Study design
The methods have been described previously (Abdallah et al., 2014). In brief, medication-free currently depressed outpatients presented to the Yale Depression Research Program for initial screening and evaluation. After consenting, subjects received baseline clinical assessments from licensed psychiatric clinicians, and trained research staff completed rating scales and trauma inventories. Then, participants received structured CBT (Beck et al., 1987). The psychotherapy consisted of once-weekly 50-minute individual sessions for up to 12 weeks.
2.2. MDD subjects
18–65 year old subjects in a current major depressive episode met Diagnostic and Statistical Manual-IV-Text Revision (DSM-IV-TR) criteria for MDD. Diagnosis was determined by in-person psychiatric evaluation via Structured Clinical Interview for DSM-IV Disorders (SCID)(First et al., 1995). Exclusion criteria were as follows: a current or past diagnosis of MDD with psychotic features; active suicidal ideation; history of suicidal behavior in the preceding two years; current use of psychotropic medications; history of, or currently uncontrolled, serious medical or neurological illness; illicit substance use disorder within the preceding six months; current illicit substance use (detected by urine toxicology); history of psychiatric illness due to confirmed general medical condition(s), history of primary personality disorder, and history of psychotic spectrum illness. Pre-defined exit criteria were defined as a 25% increase over baseline Beck Depression Inventory (BDI)(Beck et al., 1961) score during weekly ratings, or an increase in passive suicidal thinking or the onset of active suicidal ideation. No subjects were terminated for clinical deterioration.
2.3. Ratings and urinary cortisol levels
At baseline, all participants completed a battery of clinician-administered psychiatric assessments including the primary outcome measure, the 24-item Hamilton Depression Rating Scale (HDRS24) (Hamilton, 1967), and the following self-administered scales: BDI, Global Perceived Early-Life Stress (GPELS) (Carpenter et al., 2004), The Early Life Trauma Inventory (ELTI) (Bremner et al., 2000), and Klein Loss Scale (KLS) (Lizardi et al., 1995). Thereafter, the HDRS24 was repeated every 4 weeks and the BDI weekly to monitor clinical response.
Patients were provided collection containers and portable refrigerated units for 24-h urine collection. Subjects were informed to flush the first specimen of the morning, noting the time, and then to continue collecting each urine sample for the next 24 h. Samples were processed for storage within 6 h after collection was complete. Urinary free cortisol concentrations were determined in duplicate in a single batch using a commercially available radioimmunoassay (RIA) kit (Coat-a-Count, DPC, Los Angeles, CA) with a within-assay variation of <10%.
2.4. Statistical analysis
Prior to model entry, the distribution of each outcome measure was examined using probability plots and Kolmogorov–Smirnov test statistics. Antidepressant response was defined as a ≥50% HDRS24 score reduction from the trial entry baseline. Paired t-test and related-samples Wilcoxon Signed Rank tests were used to examine pre- and post-treatment changes. Non-parametric (Spearman’s Rank Order) test statistics were used for correlational analyses. General linear model (GLM) repeated-measures analyses were constructed as needed. Age and sex were considered as covariates in all models. All tests were two-tailed, with a significance level set at p≤0.05. False Discovery Rate correction for multiple comparisons was used when appropriate (as indicated by padjusted).
3. Results
A total of 50 subjects were enrolled [30 women, mean age 42.6±11.4]. Of these, 42 subjects had at least one complete treatment follow-up visit (≥4 weeks of CBT), and 30 subjects completed the full 12-week course. As presented in our initial report in an intent-to-treat analysis (Abdallah et al., 2014), following 12 weeks of treatment, CBT response was associated with antidepressant efficacy on both clinician-administered and self-reported measures (p<0.001), resulting in an average 41% reduction from baseline HDRS24 scores and 38% response rate (≥50% reduction from baseline HDRS24).
Mean observed urinary free cortisol excretion was 34±25.6 μg/24 h. Baseline 24-h urinary cortisol levels, and ELTI and GPELS scores did not correlate with HDRS24 change (all padjusted>0.1). However, higher KLS, a measure of parental loss or separation, scores were correlated with delta HDRS24 (rs=−0.39, padjusted=0.05). KLS score did not correlate with the other assessed measures of early life stress/trauma: ELTI (rs=0.02, padjusted=0.92) and GPELS (rs=0.15, padjusted=0.37).
To further explore this association with early life parental loss or separation, subjects were stratified into two groups, i.e. KLS=0 (subjects with no history of early life parental loss or separation) or KLS≥1 (subjects with a history of early life parental loss or separation) (Table 1). We then constructed repeated-measures GLM with HDRS24 as the dependent variable, time as the within-subject factor, and KLS groups as the between-subjects factor. Consistent with the correlational analysis, there was a significant group×time interaction [F(1,35)=6.65, p=0.01), demonstrating enhanced antidepressant efficacy in patients with a history of early life parental loss or separation (Fig. 1). The time main effect was significant (p<0.001), but the group effect was not (p=0.9). Finally, in an exploratory analysis with the BDI, a higher KLS score did not significantly correlate with delta BDI (rs=−0.24, padjusted=0.45), and, after stratification by KLS status, there was not a statistically significant group×time interaction [F(1,37)=2.22, p=0.15] on a repeated-measures GLM with BDI as the dependent variable. As also observed with the HDRS24, the time main effect was significant (p<0.001), but the group effect was not (p=0.7).
Table 1.
Demographic and clinical characteristics of major depressive disorder patients stratified by a lifetime history of early life parental loss or separation (ELL).
| ELL (n=11) | No ELL (n=26) | ||
|---|---|---|---|
|
|
|||
| Mean (SD) | Mean (SD) | p-value | |
| Age, years | 44.8 (11.8) | 42.3 (10.8) | 0.55 |
| Duration of Illness, years | 23.4 (13.0) | 19.4 (13.6) | 0.48 |
| Age of Onset, years | 18.5 (7.4) | 22.8 (14.5) | 0.30 |
| Number of MDEs | 3.4 (1.8) | 2.7 (1.9) | 0.38 |
| Number of Antidepressant Trials | 1.6 (1.8) | 1.2 (1.7) | 0.58 |
| Duration of Current MDE, years | 3.7 (5.1) | 5.1 (7.8) | 0.60 |
| Education, years | 15.1 (1.9) | 14.4 (1.8) | 0.34 |
| GPELS | 5.6 (2.1) | 5.2 (2.2) | 0.69 |
| ELTI | 27.0 (14.7) | 32.6 (19.5) | 0.40 |
| HDRS24 | 32.5 (9.5) | 27.1 (6.1) | 0.11 |
| BDI | 26.9 (8.4) | 26.0 (6.9) | 0.75 |
| HAM-A | 15.8 (5.3) | 15.4 (4.9) | 0.84 |
| Total Urinary Cortisol, μg/24 h | 34.3 (19.4) | 34.6 (28.6) | 0.98 |
| n (%) | n (%) | ||
| Sex, female | 9 (81.8) | 16 (61.5) | 0.28 |
| Medication Naïve | 3 (37.5) | 9 (39.1) | 1.00 |
| Current Smoking | 1 (11.1) | 1 (4.3) | 0.49 |
| Smoking History | 8 (88.9) | 13 (56.5) | 0.12 |
| Substance Abuse History | 3 (42.9) | 4 (17.4) | 0.31 |
| Family History of MDD/Anxiety Disorder | 8 (100) | 18 (78.3) | 0.29 |
| Lifetime History of Suicide Attempt | 1 (9.1) | 0 (0) | 0.23 |
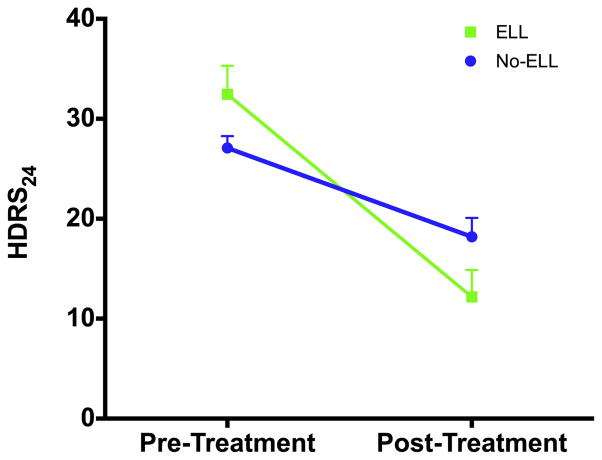
Fig. 1.
Early life parental loss or separation (ELL) is associated with enhanced antidepressant response to CBT. Unipolar unmedicated depressed subjects in a current major depressive episode received 12 weeks of structured weekly CBT. These subjects were stratified into those with a history of ELL (n=11) and those without ELL (“No ELL,” n=26) based on the Klein Loss Scale (KLS). There was a significant group-by-time interaction [F(1,35)=6.65, p=0.01), showing enhanced antidepressant response to CBT in ELL subjects. Group mean pre- and post-treatment scores (±SE) are displayed.
4. Discussion
4.1. Main findings and implications
In our sample of 50 currently depressed MDD outpatients who received weekly CBT for up to 12 weeks, a personal history of early life parental loss or separation was a positive predictor of anti-depressant success. Other early life stress/trauma or perceived early life stress (as detected by the ELTI or GPELS, respectively) did not correlate with CBT’s antidepressant efficacy. KLS score also did not correlate with these other measures, suggesting specificity to the loss/separation of a primary caretaker as opposed to other forms of early life stress or trauma.
Our results are partially consistent with Nemeroff et al. (2003)’s report that chronically depressed adult subjects with a history of early childhood loss, trauma or neglect had a better response to cognitive-based psychotherapy [Cognitive Behavior Analysis System of Psychotherapy (CBASP)] with and without the serotoninergic antidepressant nefazodone, compared to nefazodone alone. Similarly, in a randomized trial of adult MDD inpatients, subjects randomized to IPT and pharmacotherapy with a history of early life adversity (n=29) had a greater antidepressant response than subjects randomized to pharmacotherapy plus non-structured clinical management (n=18) (Zobel et al., 2011). Finally, a recent report of 203 unipolar depressed patients with or without a history of childhood maltreatment (sexual, physical and/or emotional abuse) receiving either 16 weeks of open-label CBT, IPT or antidepressant medication revealed that the CBT and medication groups outperformed the IPT group. However, after one year of naturalistic follow-up, previously maltreated adults demonstrated decreased time to recurrence relative to those without a history of childhood abuse (Harkness et al., 2012). Taken together, evidence-based psychotherapies (either alone or in combination with medication) appear to be critical for maximal antidepressant response in MDD patients with a significant history of early life stress/trauma.
Hypothalamic-pituitary-adrenal axis abnormalities have been identified in subjects with a history of early life stress but with some equivocal results (Tyrka et al., 2013). As an example, cerebrospinal fluid (CSF) corticotropin-releasing factor (CRF) did not distinguish healthy controls from unipolar depressed patients; instead, a history of perceived early life adversity (as ascertained by the GPELS) correlated with higher CSF CRF levels (Carpenter et al., 2004). Contrary to our initial hypothesis, baseline 24-h urinary cortisol levels did not correlate with CBT response in our sample. Thase et al. (1996) reported an inverse correlation between baseline urinary cortisol levels and CBT response, i.e. increased urinary cortisol correlating with poorer response. In that study, the negative predictive power of free urinary cortisol was primarily driven by a subset of patients with clearly elevated concentrations. Potentially limiting the ability to detect this relationship between urinary cortisol levels and CBT, our sample included only two subjects (values: 88.4 and 142.2 μg/24 h) having values that can truly be considered elevated based on prior reported normal ranges for urinary free cortisol. Although both of these subjects had an antidepressant response during the study (21 and 12 point HDRS24 decreases, respectively), it is quite possible we did not have enough patients with truly elevated urinary cortisol levels to demonstrate an effect on treatment outcome. There are other differences between these studies [as presented as Thase et al. (1996) vs. the present study] including severity of illness, inpatient vs. outpatient samples; daily three-week vs. once-weekly 12-week courses and free vs. total urinary cortisol measurements, that could also have contributed to the discrepant findings.
4.2. Study strengths and limitations
Our study has the following strengths: a well-characterized unmedicated outpatient sample, paired/within-subject design and a lengthy (12-week) CBT course administered by two highly qualified therapists trained at the Beck Institute (L.R.F. and M.K.F). Yet, there are several limitations that may hamper generalizability. First, the KLS positive sample is small (n=11); indeed, the number of these subjects was less than half the number of KLS negative subjects (n=26). Next, this sample is a relatively treatment-naïve population. Hence, these results may not be generalizable to more treatment-resistant depressed populations, who also often have a substantial loading of early life stress/trauma. Finally, without a control group, e.g. wait list, standard psychiatric treatment or another evidence-based psychotherapy for MDD, we are unable to attribute specificity to CBT in the enhanced antidepressant response of depressed subjects with personal history of early life parental loss or separation. This lack of a comparator condition, therefore, is a major limitation of our study.
4.3. Conclusions
We found currently depressed MDD subjects with a lifetime history of early life parental loss or separation had greater antidepressant response to a 12-week course of weekly individual CBT while other measures of early life stress/trauma did not. A history of early life parental loss or separation may warrant additional study in larger clinical populations to assess its validity in predicting treatment response to CBT and other evidence-based therapies for MDD.
Abbreviations
- BDI
Beck Depression Inventory
- ELL
Early Life Parental Separation or Loss (as detected by the Klein Loss Scale)
- ELTI
Early Life Trauma Inventory
- GPELS
Global Perceived Early Life Stress
- HAM-A
Hamilton Anxiety Rating Scale
- HDRS24
24-item Hamilton Depression Rating Scale
- MDE
Major Depressive Episode
References
- Abdallah CG, Niciu MJ, Fenton LR, Fasula MK, Jiang L, Black A, Rothman DL, Mason GF, Sanacora G. Decreased occipital cortical glutamate levels in response to successful cognitive-behavioral therapy and pharmacotherapy for major depressive disorder. Psychother Psychosom. 2014;83:298–307. doi: 10.1159/000361078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 2. American Psychiatric Association; Washington, D.C: 2000. [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depresion. The Guilford Press; New York: 1987. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB, Price LH. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology. 2004;29:777–784. doi: 10.1038/sj.npp.1300375. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Gelfand LA, Tang TZ, Simons AD. Medications versus cognitive behavior therapy for severely depressed outpatients: mega-analysis of four randomized comparisons. Am J Psychiatry. 1999;156:1007–1013. doi: 10.1176/ajp.156.7.1007. [DOI] [PubMed] [Google Scholar]
- Elkin I, Shea MT, Watkins JT, Imber SD, Sotsky SM, Collins JF, Glass DR, Pilkonis PA, Leber WR, Docherty JP, Fiester SJ, Parloff MB. National institute of mental health treatment of depression collaborative research program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46:971–982. doi: 10.1001/archpsyc.1989.01810110013002. discussion 983. [DOI] [PubMed] [Google Scholar]
- Evans MD, Hollon SD, DeRubeis RJ, Piasecki JM, Grove WM, Garvey MJ, Tuason VB. Differential relapse following cognitive therapy and pharmacotherapy for depression. Arch Gen Psychiatry. 1992;49:802–808. doi: 10.1001/archpsyc.1992.01820100046009. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCIDI/P. Version 2.0) New York State Psychiatric Institute Biometrics Research Department; New York: 1995. [Google Scholar]
- Gloaguen V, Cottraux J, Cucherat M, Blackburn IM. A meta-analysis of the effects of cognitive therapy in depressed patients. J Affect Disord. 1998;49:59–72. doi: 10.1016/s0165-0327(97)00199-7. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Bagby RM, Kennedy SH. Childhood maltreatment and differential treatment response and recurrence in adult major depressive disorder. J Consult Clin Psychol. 2012;80:342–353. doi: 10.1037/a0027665. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) J Am Med Assoc. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, Lewis G, Watkins E, Brejcha C, Cardy J, Causley A, Cowderoy S, Evans A, Gradinger F, Kaur S, Lanham P, Morant N, Richards J, Shah P, Sutton H, Vicary R, Weaver A, Wilks J, Williams M, Taylor RS, Byford S. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet. 2015;20 doi: 10.1016/S0140-6736(14)62222-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Lizardi H, Klein DN, Ouimette PC, Riso LP, Anderson RL, Donaldson SK. Reports of the childhood home environment in early-onset dysthymia and episodic major depression. J Abnorm Psychol. 1995;104:132–139. doi: 10.1037//0021-843x.104.1.132. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP, Jr, Weiss PM, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A. 2003;100:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, Petukhova M, Chatterji S, Aguilar-Gaxiola S, Alonso J, Angermeyer MC, Bromet EJ, Burger H, Demyttenaere K, de Girolamo G, Haro JM, Hwang I, Karam E, Kawakami N, Lepine JP, Medina-Mora ME, Posada-Villa J, Sampson N, Scott K, Ustun TB, Von Korff M, Williams DR, Zhang M, Kessler RC. Disability and treatment of specific mental and physical disorders across the world. Br J Psychiatry. 2008;192:368–375. doi: 10.1192/bjp.bp.107.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins DR, Alessi NE, Colfer MV. Treatment of adolescents with major depression: implications of the DST and the melancholic clinical subtype. J Affect Disord. 1989;17:99–104. doi: 10.1016/0165-0327(89)90031-1. [DOI] [PubMed] [Google Scholar]
- Simons AD, Murphy GE, Levine JL, Wetzel RD. Cognitive therapy and pharmacotherapy for depression. Sustained improvement over one year. Arch Gen Psychiatry. 1986;43:43–48. doi: 10.1001/archpsyc.1986.01800010045006. [DOI] [PubMed] [Google Scholar]
- Stewart JW, Garfinkel R, Nunes EV, Donovan S, Klein DF. Atypical features and treatment response in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Clin Psychopharmacol. 1998;18:429–434. doi: 10.1097/00004714-199812000-00002. [DOI] [PubMed] [Google Scholar]
- Thase ME, Dube S, Bowler K, Howland RH, Myers JE, Friedman E, Jarrett DB. Hypothalamic-pituitary-adrenocortical activity and response to cognitive behavior therapy in unmedicated, hospitalized depressed patients. Am J Psychiatry. 1996;153:886–891. doi: 10.1176/ajp.153.7.886. [DOI] [PubMed] [Google Scholar]
- Thase ME, Friedman ES. Is psychotherapy an effective treatment for melancholia and other severe depressive states? J Affect Disord. 1999;54:1–19. doi: 10.1016/s0165-0327(99)00033-6. [DOI] [PubMed] [Google Scholar]
- Thase ME, Simons AD, McGeary J, Cahalane JF, Hughes C, Harden T, Friedman E. Relapse after cognitive behavior therapy of depression: potential implications for longer courses of treatment. Am J Psychiatry. 1992;149:1046–1052. doi: 10.1176/ajp.149.8.1046. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Burgers DE, Philip NS, Price LH, Carpenter LL. The neurobiological correlates of childhood adversity and implications for treatment. Acta Psychiatr Scand. 2013;128:434–447. doi: 10.1111/acps.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustun TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- Zobel I, Kech S, van Calker D, Dykierek P, Berger M, Schneibel R, Schramm E. Long-term effect of combined interpersonal psychotherapy and pharmacotherapy in a randomized trial of depressed patients. Acta Psychiatr Scand. 2011;123:276–282. doi: 10.1111/j.1600-0447.2010.01671.x. [DOI] [PubMed] [Google Scholar]