Abstract
Background
Standard pancreatectomy for benign and borderline pancreatic lesions involves resecting a substantial amount of normal pancreatic parenchyma and leads to a subsequent impairment of both exocrine and endocrine pancreatic functions. A limited resection such as enucleation is the preferred option for such neoplasms. However, enucleation is associated with a high risk of postoperative complications in some cases. This study evaluated the feasibility and outcomes of performing deep enucleation with Roux-en-Y pancreaticojejunostomy reconstruction.
Methods
This study included patients who underwent pancreatic lesion enucleation from February 2010 to April 2014 in our hospital. The clinical data were collected and retrospectively analyzed.
Results
This study examined 53 patients who underwent enucleation, 33 of the procedures included deep enucleation with Roux-en-Y pancreaticojejunostomy reconstruction. There was no mortality, and the morbidity rate was 66.7% in this group. No patients developed grade C pancreatic fistulas in both group. None of the patients developed tumor recurrence or exocrine or endocrine insufficiency at a median follow-up of 25 months.
Discussion
Enucleation with Roux-en-Y pancreaticojejunostomy reconstruction is a safe and feasible procedure for the treatment of benign and borderline pancreatic neoplasms adjacent to the common pancreatic duct. This procedure can effectively mitigate the limitations of simple enucleation.
Introduction
The widespread use of high-resolution imaging and periodic medical examinations has increased the diagnosis of benign or borderline pancreatic neoplasms. Standard pancreatectomy, including pancreaticoduodenectomy and distal pancreatectomy, is no longer advocated for benign or borderline neoplasms because of its high postoperative morbidity and long-term complications, such as endocrine and exocrine insufficiency or immune disorders.1, 2, 3 Alternative surgical procedures for parenchyma-sparing pancreatectomy such as enucleation, resection of the uncinate process, duodenal-preserving resection of the pancreatic head and central pancreatectomy, have been proposed in this setting.3, 4, 5 These approaches maximally preserve healthy pancreatic parenchyma and maintain long-term, normal functional outcomes.6, 7, 8
Enucleation is the preferred choice in suitable cases because it is associated with shorter surgical time, reduced blood loss, shorter hospital stay and lower cost. Many studies have demonstrated that this tissue-sparing procedure causes minimal damage to normal pancreas and that it is a safe and effective treatment for benign and malignant lesions of the pancreas.9, 10 Conversely, the rate of postoperative fistula can reach up to 80%11, 12 in cases with lesions adjacent to the common pancreatic duct (less than 2–3 mm from the main pancreatic duct) or with irregular and extensive pancreatic wound surfaces after enucleation. In such cases, the enucleation of a pancreatic lesion alone is not feasible and may lead a surgeon to perform a central pancreatectomy or a more radical surgery. The presence of two stumps after central pancreatectomy may significantly increase the risk of morbidity for clinical postoperative pancreatic fistula (POPF)13, 14 and cause additional loss of normal pancreas parenchyma. Therefore, we developed another tissue-sparing procedure that involves enucleation with a Roux-en-Y pancreaticojejunostomy reconstruction. The aim of the present study was to evaluate the indications, operative technique, and outcomes of patients undergoing pancreatic tumor enucleations with Roux-en-Y pancreaticojejunostomy reconstruction.
Materials and methods
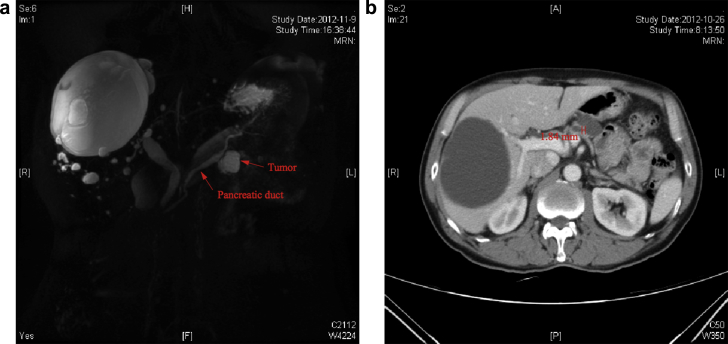
Data from patients who underwent pancreatic lesion enucleation between February 2010 and April 2014 were identified from a database that is maintained by the Department of Pancreatic and Hepatobiliary Surgery, FUSCC (Fudan University Shanghai Cancer Center). Permission from the FUSCC institutional review board was obtained prior to data review and analysis. Patient demographics, clinical presentations, preoperative evaluations, intraoperative details and pathological data were collected. Diagnoses, sites and sizes of – lesions, and distances to the MPD were evaluated by preoperative contrast-enhanced computed tomography (CT) scan or magnetic resonance imaging (MRI) in all patients. Lesion sizes and their distances to the MPD are measured by an included measuring tool of the Picture Archiving and Communication System (PACS) (detailed in Fig. 1). Preoperative mortality is defined as death in the hospital or within 30 days. The length of hospital stay is calculated from the day of surgery to the day of discharge. The presence of a pancreatic fistula is defined as any measurable volume of draining fluid on or after postoperative day 3 with an amylase content of more than 3 times the upper normal serum value according to the recommendations of the International Study Group on Pancreatic Fistula and the detailed grading system of POPF. These values are presented in Table 3.15 The presence of intra-abdominal fluid collection is identified by CT. Intra-abdominal infection is defined as intra-abdominal fluid collection with positive cultures regardless of elevations in white cell counts or repeated fever. Delayed gastric emptying is assessed according to the International Study Group of Pancreatic Surgery (ISGPS) recommendations.16
Figure 1.
Magnetic Resonance Cholangiopancreatography (a) and abdominal enhanced computed tomographic scan (b) from a case with an intraductal papillary mucinous neoplasm located at the body of the pancreas
Table 3.
Main parameters for POPF grading
| Grade | A | B | C |
|---|---|---|---|
| Clinical conditions | Well | Often well | Ill appearing/bad |
| Specific treatmenta | No | Yes/no | Yes |
| US/CT(if obtained) | Negative | Negative/positive | Positive |
| Persistent drainage(after 3 weeks)b | No | Usually yes | Yes |
| Reoperation | No | No | Yes |
| Death related to POPF | No | No | Possibly yes |
| Signs of infections | No | Yes | Yes |
| Sepsis | No | No | Yes |
| Readmission | No | Yes/no | Yes/no |
US, ultrasonography; CT, computed tomographic scan; POPF, postoperative pancreatic fistula.
Partial (peripheral) or total parenteral nutrition, antibiotics, enteral nutrition, somatostatin analog and/or minimal invasive drainage.
With or without a drain in situ.
Most of surgeries were performed with an open approach, only one case performed by laparoscopic procedure was included. Enucleation was performed around the tumor capsule to ensure complete resection. In the case of an open enucleation, blunt dissection was conducted using clamps, clip closure, tying, or coagulation of small vessels. Care should be taken to avoid damaging the main blood vessels, such as the superior mesenteric, portal veins and-splenic vessels, during enucleation. The shaped pancreatic wound surface was anastomosed with a Roux-en-Y jejunal loop using a personally modified technique (detailed in Fig. 2). The pancreaticojejunostomy was sutured at full thickness using an interrupted method with 4–0 absorbable stitches. Another interrupted suture seromuscular layer of jejuna to the parenchyma of the pancreas was enhanced by the same stitches to make the intestinal wall cling to the pancreas. Two 10-mm Penrose drains and one double-cavity drain were routinely placed near the pancreatic anastomosis. The drains were routinely removed from the third postoperative day, and all were removed within a week after surgery in patients with no pancreatic fistulas or with grade A pancreatic fistulas. The drains were either kept in place or removed individually depending on the amount and color of fluid in patients with clinical POPF. In particular, if an abdominal cavity infection existed, we would change the existing drainage tube into a new or finer drainage tube and pull it out step-by-step when the amount of daily drainage fluid was less than 10 ml.
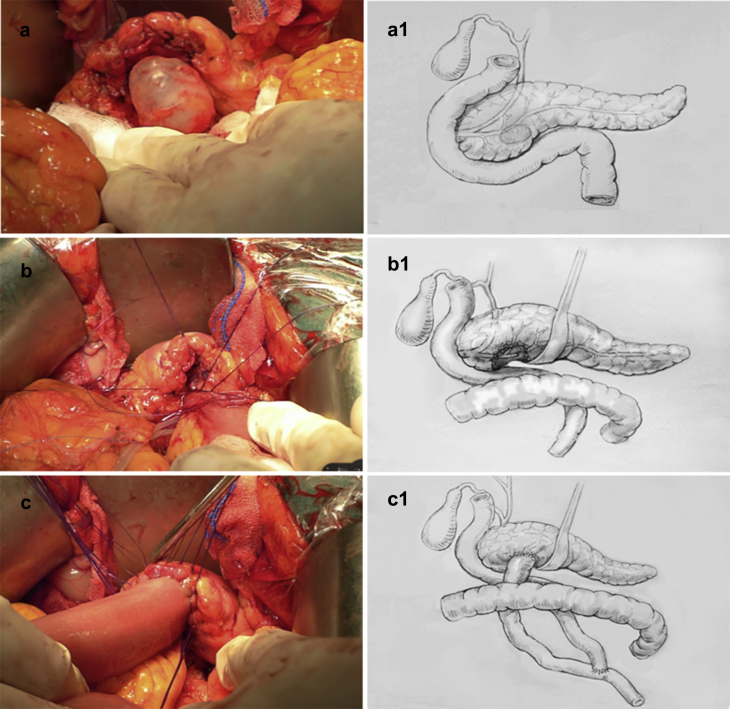
Figure 2.
The entire process of performing an enucleation with Roux-en-Y pancreaticojejunostomy reconstruction. a/a1 Intraoperative view of an intraductal papillary mucinous neoplasm that was adjacent to the MPD and located in the neck of the pancreas; b/b1 The tumor had been enucleated from the parenchyma of the pancreas; the splenoportal junction and small splenic arterial and venous branches have been divided. c/c1 After enucleation of the pancreatic segment containing the tumor, the wound surface of the pancreas was sutured to achieve hemostasis with interrupted nonabsorbable Lembert sutures. The wound surface of the pancreas was anastomosed to a Roux-en-Y loop of the jejunum with a parenchyma-to-mucosa technique
A frozen section analysis of the enucleated tumor was routinely performed in all patients to confirm the nature of the lesion. The volume and amylase activity of the drainage fluid were measured on postoperative days 1, 3, 5, and 7. Somatostatin was administered c.i.v. at a dose of 250 mg per hour in the first 3 days after partial pancreatectomy and a subcutaneous injection of octreotide at a dose of 0.1 mg three times daily for another 3 days as one type of routine treatment.
Some remedial measures would be followed if these complications occurred. Postoperative infectious fluid collections were treated by CT-guided percutaneous abscess drainage(s) and appropriate i.v. antibiotics. A postpancreatectomy hemorrhage was first approached with interventional radiological techniques, such as embolization or stenting of the bleeding vessel. A second surgery was indicated in cases of failure or major bleeding.
Pancreatic neuroendocrine tumors (pNETs), solid pseudopapillary tumors (SPTs), serous cystadenomas (SCAs), mucinous cystadenomas (MCNs), – branch duct-intraductal papillary mucinous neoplasms (BD-IPMNs) and other benign lesions were defined as cured if there was no evidence of tumor upon imaging during follow-up. Insulinomas were considered cured when postoperative fasting serum glucose levels were >40 mg/dl and concomitant insulin levels were <20 μU/ml. All surgical procedures were performed by or under the supervision of senior surgeons (Professors Xianjun Yu and Quanxing Ni). Intraoperative ultrasonography (IOUS) was performed in borderline cases to study tumor morphology, to determine tumor proximity to the main pancreatic duct and/or vessels, and to rule out the presence of multifocal lesions. Patient follow-up consisted of clinical, radiological and laboratory assessments to evaluate tumor recurrence and long-term exocrine and endocrine impairment. The presence of new worsening diabetes was demonstrated by measuring serum glucose levels and by oral glucose tolerance testing. New exocrine insufficiency was defined by development of steatorrhea, weight loss in the absence of tumor recurrence and requirement for ongoing oral enzyme supplementation for more than 3 months. Endocrine dysfunction was defined as a need for new oral antidiabetic or insulin medication for more than 3 months postoperatively.
Statistical analysis
Continuous data were compared with Student's t-test or the Mann Whitney U- test. A χ2 and Fisher's exact test were used to compare categorical variables. p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS statistical software package version 17.0 (SPSS, Chicago, Illinois, USA).
Results
From February 2010 to April 2014, 1510 patients received pancreatic resections at FUSCC. A total of 33 (2.2%) patients-underwent deep enucleations with Roux-en-Y pancreaticojejunostomy reconstruction, and 20 patients received simple tumor enucleation. The patient characteristics are summarized in Table 1. According to the BMI grading criteria of the World Health Organization, 38/53 patients were in a normal weight range, and 8/53 patients were overweight. Sixteen of the patients presented with symptoms attributable to their pancreatic lesions. The remaining patients had-incidental findings of a pancreatic lesion during a health examination. CT was used for further preoperative evaluation of each of the patients. Magnetic resonance cholangiopancreatography (MRCP) was routinely performed for patients with lesions located in the head or the neck of the pancreas (41 cases) and for patients with cystic lesions (36 cases). There were no patients with preoperative evidence of metastatic disease and/or local invasion, and all cases presented benign lesions. The mean (s.d.) diameters of the evaluated neoplasms at radiological examination were 23.1 (±11.1) mm in the Roux-en-Y group and 32 (±14.6) mm in the simple enucleation group.
Table 1.
Patient characteristics
| Demographics | Enucleation with Roux-en-Y |
Enucleation |
||
|---|---|---|---|---|
| n1 = 33 | % | n2 = 20 | % | |
| Age, median (range), yr | 50 | 25–73 | 58 | 29–68 |
| Gender | ||||
| Male | 7 | 21.2 | 8 | 40 |
| Female | 26 | 78.8 | 12 | 60 |
| Symptoms at diagnosis | ||||
| Epigastric pain | 5 | 15.2 | 2 | 10 |
| Hypoglycemia | 3 | 9.1 | 0 | 0 |
| Dyspepsia | 2 | 6.1 | 0 | 0 |
| Other | 1 | 3 | 3 | 15 |
| Localization | ||||
| Head | 11 | 33.3 | 4 | 20 |
| Uncinate process | 1 | 3.1 | 1 | 5 |
| Neck | 17 | 51.5 | 7 | 35 |
| Body | 4 | 12.1 | 8 | 40 |
| Distance MPD, mean ± Std, mm | 2 | 1.6 | 7.3 | 2.6 |
| Lesion size, mean ± Std, mm | 23.1 | 11.1 | 32 | 14.6 |
| BMI | ||||
| Normal body weight (18.5–24.9) | 23 | 69.7 | 15 | 75 |
| Overweight (25–29.9) | 6 | 18.2 | 2 | 10 |
| Obesity (≥30) | 1 | 3 | 1 | 5 |
| Low weight | 3 | 9.1 | 2 | 10 |
| Pathology | ||||
| Neuroendocrine tumor | 6 | 18.2 | 5 | 25 |
| Mucinous cystic tumors | 3 | 9.1 | 0 | 0 |
| IPMN | 5 | 15.1 | 3 | 15 |
| Serous/simple cystsa | 16 | 48.5 | 10 | 50 |
| Other benign lesions | 3 | 9.1 | 2 | 10 |
Includes lymphoepithelial cysts, solid pseudopapillary neoplasms, pseudocysts and pancreatic ductal stones.
The surgical variables and postoperative data of the patients who underwent enucleations with or without Roux-en-Y pancreaticojejunostomy reconstruction are shown in Table 2. The mean (s.d.) operative time was 250 ± 62 min in Roux-en-Y group and 145 ± 38 min in the simple enucleation group, and the mean (s.d.) estimated blood loss was 171 ± 146 ml and 168 ± 101 ml, respectively. There was no mortality in either of the groups, and the overall morbidity in the Roux-en-Y cohort (n = 33) was 66.7%, whereas it was only 35% in the simple enucleation group. The most common complication in the Roux-en-Y cohort was pancreatic fistula (66.7%). However, the rate of clinically important grade B pancreatic fistula was recorded as 30.3% in the Roux-en-Y cohort and as only 5% in the simple enucleation cohort. None of the patients who developed grade C POPF in either of the groups. In the Roux-en-Y cohort, one patient had delayed gastric emptying and another developed a chylous fistula on the fourth day after surgery during recovery on a liquid diet. The drains were routinely removed from the third postoperative day, and all were removed within a week after surgery in patients with either no POPF or grade A POPF. The mean (range) time to drainage tube removal-was 22.3 (13–46) days in patients with grade B POPF. None of the patients experienced postoperative bleeding or anastomotic leakage. None of the patients required additional surgery. The mean (s.d.) length of hospital stay was 16 ± 7.7 days.
Table 2.
Intraoperative data and postoperative complications
| Variable | Enucleation with Roux-en-Y |
Enucleation |
||
|---|---|---|---|---|
| n1 = 33 | % | n2 = 20 | % | |
| Duration of operation, mean ± Std, min | 250 | 62 | 145 | 38 |
| Blood loss, mean ± Std, ml | 171 | 146 | 168 | 101 |
| Days of drainage, mean ± Std, d | 13.8 | 7.8 | 6 | 3 |
| Hospital stay, mean ± Std, d | 16 | 7.7 | 11.7 | 3.7 |
| Follow up, median (range),m | 25 | 3–42 | 26 | 3–45 |
| Pancreatic fistula (PF) | ||||
| A | 12 | 36.4 | 6 | 30 |
| B | 10 | 30.3 | 1 | 5 |
| C | 0 | 0 | 0 | 0 |
| Normal | 11 | 33.3 | 13 | 65 |
| Chylous fistula | 1 | 3 | 0 | 0 |
| Delayed gastric emptying | 1 | 3 | 0 | 0 |
| Abscess | 1 | 3 | 1 | 5 |
| Morbidity | 22 | 66.7 | 7 | 35 |
Table 1 shows the final histopathological diagnoses. A total of 11 cases were diagnosed with endocrine tumors (3 cases were insulinomas and the remainder – were nonfunctioning tumors). There were also 3 patients with mucinous cystic tumors, 8 patients with branch-duct IPMNs, 24 patients with serous or simple cysts, 2 patients with SPTs and 3 patients with other benign lesions. All tumors were completely resected based on histopathological examination.
At the time of analysis, no patients had evidence of recurrence after a median follow-up of 25 (3–42) months. None of the patients developed new-onset pancreatic exocrine or endocrine insufficiency.
Discussion
The incidence of benign and borderline lesions of the pancreas has increased as the development of imaging technology and the frequency of periodic medical examinations has increased. Tissue-sparing surgeries, including enucleation, resection of the uncinate process, duodenal-preserving resection of the head of the pancreas, and middle pancreatectomy, have been proposed in these settings. Enucleation is an optimal alternative approach to standard pancreatectomy in such diseases because it maintains maximum parenchyma and organ function. However, lesions that are deeply embedded in the parenchyma of the pancreas and possible injuries of the common pancreatic duct may lead to severe fistulas in patients undergoing simple enucleation without Roux-en-Y pancreaticojejunostomy. Thus, most surgeons would prefer to perform a pancreatoduodenectomy or distal pancreatectomy in such cases. However, standard pancreatectomy is not advocated for benign and borderline lesions of the pancreas due to the high risk of postoperative exocrine and endocrine insufficiency.29, 30 Therefore, tumor enucleation may be preferred in this setting. However, in some cases, a simple tumor enucleation may be inappropriate. This study has shown that enucleation with Roux-en-Y pancreaticojejunostomy reconstruction is a safe and effective procedure for the radical treatment of both benign and borderline neoplasms of the pancreas that are either adjacent to the main pancreatic duct or deeply embedded in the parenchyma.
Enucleation was first introduced in 1898 by Ernesto Tricomi. The procedure was gradually recognized as a safe and technically feasible approach for the treatment of benign and low malignant lesions superficial to the pancreas.10, 17 However, a lesion that is either directly adjacent to the common pancreatic duct or deeply embedded in the parenchyma is associated with a high risk of postoperative complications, such as severe pancreatic fistula.11, 12, 19 Kristin and colleagues prospectively analyzed the complication rate of enucleation with respect to lesion distance from the MPD. The authors found the complication rate in a-deep enucleation group (distance ≤3 mm) was higher than in a standard enucleation group (distance >3 mm). The most common complication was pancreatic fistulas, and this occurred in 73.3% (22/30) of patients. Additionally, 91% patients had clinical pancreatic fistulas (types B and C POPF).12 Another retrospective study including 52 patients yielded similar results in patients with pancreatic tumors at a distance of less than or equal to 2 mm from the MPD. These patients also had a 60% incidence rate of POPF after surgery, which was more than patients with tumors at a distance ≥2 mm from the MPD.19 Lu et al. reported that 7 patients who underwent enucleations of -pancreatic head tumors that were close to the common pancreatic duct had a high incidence (86%) of severe pancreatic fistula.11 Therefore, the indication for enucleation is a benign or low malignant pancreatic lesion that is located at least 3 mm from the main pancreatic duct and that is not deeply embedded in the parenchyma.4, 9, 10, 12, 17, 18, 19, 20 In our simple enucleation group, the mean (±s.d.) distance to the MDP is 7.3 ± 2.6 mm, and the clinical pancreatic fistula incidence is only 5% (1/20). However, enucleation with Roux-en-Y pancreaticojejunostomy reconstruction can significantly reduce the occurrence of related complications induced by simple enucleation. The modified procedure can expand the surgical indications of enucleation to a great extent. Moreover, this approach avoids the extended loss of normal pancreatic parenchyma. In the present study, we performed enucleation with Roux-en-Y pancreaticojejunostomy reconstruction in 33 patients with lesions that either involved the main pancreatic duct or that were located deep in the parenchyma of the pancreas.
Enucleation with Roux-en-Y pancreaticojejunostomy reconstruction is a safe and feasible procedure for selected patients with lesions that are either deeply embedded in the parenchyma of the pancreas or that are close to the MPD. In the enucleation with Roux-en-Y cohort, there was no perioperative mortality. We found that the most common complication of this procedure is a pancreatic fistula. However, no patients developed grade C POPF, and only 30.3% of the patients had grade B POPF. This rate of clinical pancreatic fistula is comparable to other limited surgeries of the pancreas, such as middle pancreatectomy.21 However, a survey of the literature shows that the occurrence (10–18%) of severe complications following middle pancreatectomy (e.g., grade C POPF, bleeding or other complications requiring re-operation) is significantly higher21, 22 than in this study. Theoretically, cases in which two stumps of the pancreas are present after middle pancreatectomy should be associated with a higher risk of POPF than cases undergoing enucleation with Roux-en-Y pancreaticojejunostomy reconstruction. Moreover, the length of time of that is required to perform an enucleation with Roux-en-Y pancreaticojejunostomy reconstruction is not longer than a middle pancreatectomy.21, 23 The mean (±s.d.) operative time was 250 ± 62 min in the present study, and this result is comparable with the reported time of middle pancreatectomy. A small amount of intraoperative blood loss is another advantage of this procedure. The mean (±s.d.) estimated blood loss was 171 ± 146 ml in this study, which was also significantly lower than the reported blood loss of middle pancreatectomy.24, 25 At a median follow-up of 25 months, none of the patient had developed tumor recurrence and exocrine or endocrine insufficiency. This novel surgical procedure provides a better quality of life and comparable oncologic safety when used for selected types of pancreatic benign or low malignant diseases. In this study, we did not compared the postoperative complications of patients who underwent enucleation without Roux-en-Y anastomosis or standard pancreatectomy, including Whipple's operation and distal pancreatectomy, at our institution as a historical control. In our previous clinical work, enucleation without Roux-en-Y anastomosis has mainly been indicated for patients with lesions situated in the shallow parts of the pancreas. When a patient presents with a lesion deeply embedded in the pancreas, we would typically perform a standard pancreatectomy or other limited surgery such as middle pancreatectomy. Therefore, unfortunately, the number of patients in this subgroup was too small for further detailed analysis. With improvement in surgical technique, the POPF rate of standard pancreatectomy, including Whipple's operation and distal pancreatectomy, at our institution has remained in the range of 10–35%.26, 27, 28 Although the short-term complications have been largely reduced in -patients undergoing standard pancreatectomy, the considerable proportion of patients with = long-term complications, such as new onset diabetes and dyspepsia, after surgery should not be ignored.29, 30
Moreover, the procedure of enucleation with Roux-en-Y anastomosis is less affected by tumor location. Unlike middle pancreatectomy,31, 32 it is not only suitable for patients with tumors in the neck or the body of the pancreas but is also suitable for patients with tumors located in the head or uncinate process of the pancreas. In the current series, there was one uncinate process tumor, 11 tumors of the head of the pancreas, and 17 tumors of the neck of the pancreas. There were also 4 tumors of the pancreas body. The essential condition for performing this surgical procedure successfully is the preservation of enough healthy pancreas parenchyma for pancreaticojejunostomy. This strategy requires a complete and solid pancreas side-wall to support the pancreaticojejunostomy. As a result, duodenal-preserving resection of the pancreas head and central pancreatectomy may be good substitutes when there is limited healthy parenchyma remaining to support the pancreaticojejunostomy.33, 34
The lesions that are indicated for parenchyma-sparing pancreatectomy are benign and low malignant lesions of the pancreas, such as serous and mucinous cystadenoma, endocrine tumors, solid pseudo-papillary tumors, branch-duct IPMNs and other benign lesions meeting the eligibility criteria.8, 10, 11, 12, 17, 18, 20, 35 In this series, 11 cases were diagnosed with endocrine tumors, and 3 of these cases were insulinomas. The other cases were nonfunctioning tumors. There were no recurrences recorded over a median follow-up of 25 months after modified enucleation. As for endocrine tumors, Casadei and co-workers have claimed that enucleation performed in selected patients diagnosed with pancreatic endocrine tumors with diameters of less than 4 cm that are lacking malignant features is a safe and feasible procedure.18 Falconi and his partners evaluated the outcomes of a cohort of 50 patients with nonfunctioning pancreatic endocrine tumors after parenchyma-preserving resections. They found that this procedure should be indicated for patients with lesions less than 2 cm in diameter that have no worrisome features. These results are also supported by a published guideline that was proposed by Ramage et al.36 In the enucleation with Roux-en-Y cohort, of the pancreatic endocrine tumor size is 16.5 mm. With respect to other histological types of pancreatic neoplasm, benign behavior is the basic requirement for parenchyma-preserving resections. Sauvanet and colleagues assessed the feasibility and outcomes of 91 patients with presumed noninvasive IPMNs after parenchyma-preserving resections. The authors proposed that parenchyma-preserving resection was highly feasible and effective in selected patients with IPMN. Additionally, they indicated that this surgery should be avoided in cases with mural nodules larger than 5 mm and main-duct dilatation greater than 10–15 mm or in cases with the presence of an infiltrating mass.5 The nature of a lesion should be evaluated by intraoperative frozen-section examination before implementing this procedure. The completeness of resection and the status of the resection margins must be evaluated and confirmed by final histological pathology. Reoperation should be considered in cases of incomplete or R1 resection.
Enucleation with Roux-en-Y pancreaticojejunostomy reconstruction is a tissue-sparing surgery that should be given priority in selected patients with pancreatic benign or low malignant lesions. This minimally invasive surgery is not limited to the narrow definitions of laparoscopic or robotic surgery. The core idea of minimally invasive surgery is the maximum protection of related normal organs or tissues around lesions under the correct indications. Therefore, the indications for the procedure of enucleation with Roux-en-Y pancreaticojejunostomy reconstruction that we proposed include benign or low malignant pancreatic lesions that are not located at the tail of pancreas, that pose a high risk of the MPD injury (location less than 3 mm from the MPD) or that are deeply embedded in the parenchyma, causing a larger wounds after enucleation. In the present study, there was not enough data to confirm pancreatic fistula grades at different distances between pancreatic lesions and the main pancreatic duct.
In conclusion, enucleation with Roux-en-Y pancreaticojejunostomy reconstruction is a safe and feasible procedure for the treatment of benign and borderline neoplasms that are either adjacent to the common pancreatic duct or that are deeply embedded in the parenchyma. This procedure can effectively reduce the limitations of simple enucleation and can act as a supplement to existing parenchyma-preserving procedures.
Acknowledgments
The authors thank Doctor Yihui Zhang (Medical Department of China Academy of Art) and Professor Xiaoping Yu (The College of Public Art, China Academy of Art) for the creation of Fig. 2.
Footnotes
This study was supported in part by the Sino-German Center (GZ857), by the Science Foundation of Shanghai (13ZR1407500), – the Shanghai Rising Star Program (12QH1400600, 14QA1400900), – the Fudan University Young Investigator promoting program (20520133403) and – the National Science Foundation of China (Grant Nos. 81101807, -81001058, -81372649, -81372653, -81172276).
Contributor Information
Jiang Long, Email: longjiang@fudanpci.org.
Xianjun Yu, Email: yuxianjunfudan@163.com, yuxianjun@fudanpci.org.
Funding sources
This study was supported in part by the Sino-German Center (GZ857), by the Science Foundation of Shanghai (13ZR1407500), – the Shanghai Rising Star Program (12QH1400600, 14QA1400900), – the Fudan University Young Investigator promoting program (20520133403) and – the National Science Foundation of China (Grant Nos. 81101807, -81001058, -81372649, -81372653, -81172276).
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.King J.C., Hines O.J. Predicting exocrine insufficiency following pancreatic resection. J Surg Res. 2010;164:e43–e45. doi: 10.1016/j.jss.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falconi M., Mantovani W., Crippa S., Mascetta G., Salvia R., Pederzoli P. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. 2008;95:85–91. doi: 10.1002/bjs.5652. [DOI] [PubMed] [Google Scholar]
- 3.Aranha G.V., Shoup M. Nonstandard pancreatic resections for unusual lesions. Am J Surg. 2005;189:223–228. doi: 10.1016/j.amjsurg.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Hackert T., Hinz U., Fritz S., Strobel O., Schneider L., Hartwig W. Enucleation in pancreatic surgery: indications, technique, and outcome compared to standard pancreatic resections. Langenbecks Arch Surg. 2011;396:1197–1203. doi: 10.1007/s00423-011-0801-z. [DOI] [PubMed] [Google Scholar]
- 5.Sauvanet A., Gaujoux S., Blanc B., Couvelard A., Dokmak S., Vullierme M.P. Parenchyma-sparing pancreatectomy for presumed noninvasive intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2014;260(2):364–371. doi: 10.1097/SLA.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 6.Goudard Y., Gaujoux S., Dokmak S., Cros J., Couvelard A., Palazzo M. Reappraisal of central pancreatectomy a 12-year single-center experience. JAMA Surg. 2014;149:356–363. doi: 10.1001/jamasurg.2013.4146. [DOI] [PubMed] [Google Scholar]
- 7.Xu S.B., Zhu Y.P., Zhou W., Xie K., Mou Y.P. Patients get more long-term benefit from central pancreatectomy than distal resection: a meta-analysis. Eur J Surg Oncol. 2013;39:567–574. doi: 10.1016/j.ejso.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Beger H.G., Poch B., Vasilescu C. Benign cystic neoplasm and endocrine tumours of the pancreas – when and how to operate – an overview. Int J Surg. 2014;12(6):606–614. doi: 10.1016/j.ijsu.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Zhang T., Xu J., Wang T., Liao Q., Dai M., Zhao Y. Enucleation of pancreatic lesions: indications, outcomes, and risk factors for clinical pancreatic fistula. J Gastrointest Surg. 2013;17:2099–2104. doi: 10.1007/s11605-013-2355-6. [DOI] [PubMed] [Google Scholar]
- 10.Crippa S., Bassi C., Salvia R., Falconi M., Butturini G., Pederzoli P. Enucleation of pancreatic neoplasms. Br J Surg. 2007;94:1254–1259. doi: 10.1002/bjs.5833. [DOI] [PubMed] [Google Scholar]
- 11.Lu W.J., Xu B., Gao S.L., Dong X., Zhang B., Wu Y.L. Enucleation of benign or borderline pancreatic head tumors adjacent to the common pancreatic duct. Pancreas. 2012;41:336–337. doi: 10.1097/MPA.0b013e318229b891. [DOI] [PubMed] [Google Scholar]
- 12.Heeger K., Falconi M., Partelli S., Waldmann J., Crippa S., Fendrich V. Increased rate of clinically relevant pancreatic fistula after deep enucleation of small pancreatic tumors. Langenbecks Arch Surg. 2014;399:315–321. doi: 10.1007/s00423-014-1171-0. [DOI] [PubMed] [Google Scholar]
- 13.Pratt W., Maithel S.K., Vanounou T., Callery M.P., Vollmer C.J. Postoperative pancreatic fistulas are not equivalent after proximal, distal, and central pancreatectomy. J Gastrointest Surg. 2006;10:1264–1278. doi: 10.1016/j.gassur.2006.07.011. 1278–1279. [DOI] [PubMed] [Google Scholar]
- 14.Del C.M. Are there really indications for central pancreatectomy? JAMA Surg. 2014;149:364. doi: 10.1001/jamasurg.2013.4166. [DOI] [PubMed] [Google Scholar]
- 15.Bassi C., Dervenis C., Butturini G., Fingerhut A., Yeo C., Izbicki J. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Wente M.N., Bassi C., Dervenis C., Fingerhut A., Gouma D.J., Izbicki J.R. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Cauley C.E., Pitt H.A., Ziegler K.M., Nakeeb A., Schmidt C.M., Zyromski N.J. Pancreatic enucleation: improved outcomes compared to resection. J Gastrointest Surg. 2012;16:1347–1353. doi: 10.1007/s11605-012-1893-7. [DOI] [PubMed] [Google Scholar]
- 18.Casadei R., Ricci C., Rega D., D'Ambra M., Pezzilli R., Tomassetti P. Pancreatic endocrine tumors less than 4 cm in diameter: resect or enucleate? a single-center experience. Pancreas. 2010;39:825–828. doi: 10.1097/MPA.0b013e3181cf155c. [DOI] [PubMed] [Google Scholar]
- 19.Brient C., Regenet N., Sulpice L., Brunaud L., Mucci-Hennekine S., Carrere N. Risk factors for postoperative pancreatic fistulization subsequent to enucleation. J Gastrointest Surg. 2012;16:1883–1887. doi: 10.1007/s11605-012-1971-x. [DOI] [PubMed] [Google Scholar]
- 20.Talamini M.A., Moesinger R., Yeo C.J., Poulose B., Hruban R.H., Cameron J.L. Cystadenomas of the pancreas: is enucleation an adequate operation? Ann Surg. 1998;227:896–903. doi: 10.1097/00000658-199806000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iacono C., Verlato G., Ruzzenente A., Campagnaro T., Bacchelli C., Valdegamberi A. Systematic review of central pancreatectomy and meta-analysis of central versus distal pancreatectomy. Br J Surg. 2013;100:873–885. doi: 10.1002/bjs.9136. [DOI] [PubMed] [Google Scholar]
- 22.Beger H.G., Poch B., Vasilescu C. Benign cystic neoplasm and endocrine tumours of the pancreas–when and how to operate–an overview. Int J Surg. 2014;12:606–614. doi: 10.1016/j.ijsu.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Kang C.M., Lee J.M., Kim M.W., Yoon D.S., Park J.S., Lee W.J. Experiences in central pancreatectomy. Dig Surg. 2011;28:57–62. doi: 10.1159/000322407. [DOI] [PubMed] [Google Scholar]
- 24.Brown K.M., Shoup M., Abodeely A., Hodul P., Brems J.J., Aranha G.V. Central pancreatectomy for benign pancreatic lesions. HPB. 2006;8:142–147. doi: 10.1080/13651820510037611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiNorcia J., Ahmed L., Lee M.K., Reavey P.L., Yakaitis E.A., Lee J.A. Better preservation of endocrine function after central versus distal pancreatectomy for mid-gland lesions. Surgery. 2010;148:1247–1254. doi: 10.1016/j.surg.2010.09.003. 1254–1256. [DOI] [PubMed] [Google Scholar]
- 26.Wu C.T., Xu W.Y., Liu L., Long J., Xu J., Ni Q.X. Ligamentum teres hepatis patch enhances the healing of pancreatic fistula after distal pancreatectomy. Hepatobiliary Pancreat Dis Int. 2013;12:651–655. doi: 10.1016/s1499-3872(13)60102-2. [DOI] [PubMed] [Google Scholar]
- 27.Liu C., Long J., Liu L., Xu J., Zhang B., Yu X. Pancreatic stump-closed pancreaticojejunostomy can be performed safely in normal soft pancreas cases. J Surg Res. 2012;172:e11–e17. doi: 10.1016/j.jss.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B., Xu J., Liu C., Long J., Liu L., Xu Y. Application of “papillary-like main pancreatic duct invaginated” pancreaticojejunostomy for normal soft pancreas cases. Sci Rep. 2013;3:2068. doi: 10.1038/srep02068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen N., Unnikrishnan I.R., Anjana R.M., Mohan V., Pitchumoni C.S. The complex exocrine-endocrine relationship and secondary diabetes in exocrine pancreatic disorders. J Clin Gastroenterol. 2011;45:850–861. doi: 10.1097/MCG.0b013e31822a2ae5. [DOI] [PubMed] [Google Scholar]
- 30.Sato N., Yamaguchi K., Yokohata K., Shimizu S., Morisaki T., Chijiiwa K. Short-term and long-term pancreatic exocrine and endocrine functions after pancreatectomy. Dig Dis Sci. 1998;43:2616–2621. doi: 10.1023/a:1026686824173. [DOI] [PubMed] [Google Scholar]
- 31.Dumitrascu T., Scarlat A., Ionescu M., Popescu I. Central pancreatectomy versus spleen-preserving distal pancreatectomy: a comparative analysis of early and late postoperative outcomes. Dig Surg. 2012;29:400–407. doi: 10.1159/000343927. [DOI] [PubMed] [Google Scholar]
- 32.Ocuin L.M., Sarmiento J.M., Staley C.A., Galloway J.R., Johnson C.D., Wood W.C. Comparison of central and extended left pancreatectomy for lesions of the pancreatic neck. Ann Surg Oncol. 2008;15:2096–2103. doi: 10.1245/s10434-008-9987-x. [DOI] [PubMed] [Google Scholar]
- 33.Nakao A., Fernandez-Cruz L. Pancreatic head resection with segmental duodenectomy: safety and long-term results. Ann Surg. 2007;246:923–928. doi: 10.1097/SLA.0b013e31815c2a14. 929–931. [DOI] [PubMed] [Google Scholar]
- 34.Ahn Y.J., Kim S.W., Park Y.C., Jang J.Y., Yoon Y.S., Park Y.H. Duodenal-preserving resection of the head of the pancreas and pancreatic head resection with second-portion duodenectomy for benign lesions, low-grade malignancies, and early carcinoma involving the periampullary region. Arch Surg. 2003;138:162–168. doi: 10.1001/archsurg.138.2.162. 168. [DOI] [PubMed] [Google Scholar]
- 35.Falconi M., Zerbi A., Crippa S., Balzano G., Boninsegna L., Capitanio V. Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol. 2010;17:1621–1627. doi: 10.1245/s10434-010-0949-8. [DOI] [PubMed] [Google Scholar]
- 36.Ramage J.K., Ahmed A., Ardill J., Bax N., Breen D.J., Caplin M.E. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs) Gut. 2012;61:6–32. doi: 10.1136/gutjnl-2011-300831. [DOI] [PMC free article] [PubMed] [Google Scholar]