Abstract
Background
This multicentre, randomized clinical trial assessed the safety and effectiveness of the EVARREST™ Fibrin Sealant Patch (FP) in treating parenchymal bleeding following anatomic and non-anatomic liver resections.
Methods
One hundred and two patients were stratified according to the type of hepatic resection (anatomic/non-anatomic), and randomized (1:1) after identification of an appropriate bleeding site, to FP vs Standard of Care (SoC, manual compression ± topical haemostat). The primary endpoint was haemostasis at 4 min from bleeding site identification with no re-bleeding requiring re-treatment.
Results
The FP was superior in achieving haemostasis at 4 min (96%, 48/50) to SoC (46%, 24/52; p < 0.001). Stratification for resection type showed treatment differences for primary endpoint for anatomic (24/25 FP vs 13/23 SoC; p = 0.001) and non-anatomic liver resections (24/25FP vs 11/29 SoC; p < 0.001). Adverse events related to the study procedure were reported in 40/50 patients (80%) in the FP group and 43/52 patients (83%) in the SoC group. One (2%) adverse event (infected intra-abdominal fluid collection) was possibly related to study treatment.
Conclusion
This clinical trial confirms that the FP is safe and highly effective in controlling parenchymal bleeding following hepatectomy regardless of the type of resection.
ClinicalTrials.gov NCT01993888.
Introduction
Managing intraoperative haemorrhage has been a challenging part of hepatic surgery. Although, the improvements in hepatobiliary surgery over the past two decades have resulted in low surgical mortality (<5%) and morbidity rates (4–20%) in high-volume centers, intraoperative bleeding continues to be a significant issue for hepatobiliary surgeons.1 Bleeding during hepatectomy may occur from inflow structures, branches of the hepatic veins, or from the parenchymal edge (bleeding originating in small vessels) during or immediately following hepatic division. Improvements in technique have reduced the risk of intraoperative bleeding from inflow or venous structures.2 However, bleeding following parenchymal division remains a significant issue in hepatic resection since many patients have abnormal parenchyma due to either cirrhosis, steatosis or other conditions such as sinusoidal occlusion following therapy with oxaliplatin.3, 4 Bleeding may also occur in patients with normal liver parenchyma.5
Preservation of functioning parenchyma has also become an important factor in modern hepatic surgery with an emphasis on increased utilization of multiple segmental and non-anatomic resections rather than extended resections to achieve tumour clearance and an increasing reluctance to routinely use mass inflow occlusion (Pringle manoeuvre) during parenchymal transection to minimize ischaemic damage to the future remnant.6 However segmental and non-anatomical resections can be technically challenging and can be associated with significant operative blood loss.7 These factors, in addition to increasing evidence that transfusion of blood or blood derived products can be associated with significant patient morbidity and adverse oncological outcomes,8, 9 have resulted in a contemporary focus on reducing blood loss and eliminating transfusion requirement in all forms of hepatic surgery while expanding the eligibility of patients who can be considered for hepatic resection, particularly for cancer.4
EVARREST™ Fibrin Sealant Patch (FP) is a means of treating hepatic parenchymal bleeding and was developed as an adjunctive topical haemostat to manage haemorrhage across a wide spectrum of bleeding challenges. The FP is made up of a flexible matrix of oxidized regenerative cellulose (ORC) needle punched on one side with polyglactin 910 filaments to increase the surface area that carries a biologic component composed of human thrombin and human fibrinogen. The other side of the product is non-biologic and consists of ORC. The FP works by applying the biologic side to an area of active bleeding. Activation of thrombin and fibrinogen results in the rapid development of a local clot and adherence of the FP to the bleeding surface. The FP remains in situ at the end of the surgical procedure but absorbs via hydrolysis within 8 weeks and has been assessed in two previous randomized trials of moderate and severe soft tissue bleeding in thoracic and abdominal surgery.10, 11 A third randomized trial also demonstrated that the FP was effective in controlling parenchymal bleeding following hepatectomy and that it remained effective across a variety of parenchymal types including steatotic and cirrhotic livers.12
This multicenter, prospective randomized clinical trial was designed to assess the safety and efficacy of the FP at controlling parenchymal bleeding following elective hepatectomy with specific reference to type of resection (anatomic vs non-anatomic).
Materials and methods
This was a prospective, randomized, controlled superiority study evaluating the effectiveness of the FP for adjunctive haemostasis compared with standard of care (SoC) methods to control parenchymal bleeding following elective hepatectomy. The trial was carried out in 16 centres in the United States of America, United Kingdom, Australia and New Zealand (see Acknowledgements).
Patients
Patients undergoing partial hepatectomy were recruited for the trial by investigators and local trial coordinators. Enrolment was considered if they were >18 years of age, requiring urgent or elective hepatic resection and were able to provide informed consent. Patients were excluded from enrolment if admitted for emergency trauma surgery, undergoing a liver transplant for fulminant hepatic failure, had active perihepatic sepsis, known tolerance to blood products or one of the components of the FP, were unwilling to receive blood products, were a known and current alcohol or drug abuser, were pregnant or breast feeding, or had participated in another investigational drug or device research study within the previous 30 days.
Potentially eligible patients were reviewed and enrolled following the consent process (Table 1). Screening involved a full history, physical examination, determination of full blood count, liver function tests, coagulation studies, and, if appropriate, pregnancy test and occurred within 21 days of the surgical procedure. The patient's thromboembolism risk was assessed using the Caprini score.13 Review of potential exclusion criteria and medications was undertaken on admission (Table 1). The trial was designed to align with the Consolidated Standards of Reporting Trials (CONSORT) Statement.14
Table 1.
Schedule of study events
| Procedure | Screening | Baseline | Surgical procedure | Post-surgery to discharge | 30-Day follow up | 60-Dayd follow up |
|---|---|---|---|---|---|---|
| Inclusion/exclusion | X | X | X | |||
| Informed consent | X | |||||
| Demographics | X | |||||
| Medical history | X | X | ||||
| Concomitant medications | X | X | X | X | X | |
| Physical exam | X | X | X | |||
| Complete blood counta | X | X | X | |||
| Liver function testsb | X | X | X | X | ||
| Coagulation studiesc | X | X | X | |||
| Pregnancy test (if applicable) | X | |||||
| Randomization | X | |||||
| Treatment application | X | |||||
| Intra-operative details | X | |||||
| Determination of haemostasis | X | |||||
| Bleeding & thrombotic complications | X | X | X | X | ||
| Post-operative bile leak assessment | X | |||||
| Adverse events | X | X | X | X | ||
| Operative/surgical information | X | X |
All complete blood counts included a differential.
Liver function tests included serum bilirubin, aspartate transaminase (AST), gamma glutamyl transferase (γGT), total protein and albumin concentrations.
Coagulation studies included prothrombin time (PT), partial thromboplastin time (PPT), international normalized ratio (INR), platelet count and fibrinogen determinations.
60 day follow-up could be conducted via a telephone interview.
Study procedure
The surgical procedure including vascular control, technique of parenchymal division and use of postoperative surgical drains was performed according to the surgeon's standard practice. Both the FP and ORC that constituted routine standard of care were available in the sterile field. Patient randomization occurred following the hepatic resection if the surgeon encountered an appropriate target bleeding site in the hepatic parenchyma. The appropriate target bleeding site (TBS) was defined as a bleeding site that, after 30 s of firm manual compression, demonstrated persistent bleeding that required immediate attention and where conventional methods of control such as suture, ligation or cautery were felt to be ineffective or impractical. The TBS was required to be a size that could be adequately covered by a single 10.2 cm by 10.2 cm FP with at least 1 cm overlap. Bleeding from large arteries or veins where the injured vascular wall required repair with the maintenance of vessel patency were excluded. Randomization occurred by opening the appropriate computer generated randomization envelope and a stopwatch started simultaneously.
Patients randomized to SoC received continuous manual pressure with or without a topical absorbable haemostat (TAH; most commonly oxidized regenerative cellulose). The total period of manual compression included the time required for randomization and the transfer of the SoC or FP from the instrument table to the surgeon and its application. Haemostasis was assessed at 4 min (primary endpoint) and 10 min from randomization and at the completion of surgery immediately prior to fascial closure (secondary endpoint).
The site and nature of the hepatic resection was prospectively stratified (anatomic vs non-anatomic) and recorded as was the area of the TBS and whether the bleeding was arterial, venous or mixed and pulsatile/non-pulsatile. The area of the transected hepatic parenchyma was measured directly.
Application of the FP and standardized surgeon training
Prior to the trial commencement, all surgeons and trial coordinators involved in this investigation attended full day teaching sessions on the use of the FP and opportunity was given to use the FP on preclinical in-vivo models of bleeding in various anatomical sites. The application technique of investigators was standardized as well as clearly defining the size and severity of the TBS required for patient randomization. Teaching videos of example TBS and correct application of the FP were also available.
Definition of postoperative bile leak
A bile leak was defined as the presence of bile containing fluid in a drain. The bilirubin concentration in the fluid was required to be at least three times higher than the upper normal serum levels in patients with a normal postoperative serum bilirubin or a 50% higher bilirubin level in fluid than serum in patients with postoperatively elevated serum bilirubin levels.
Management of protocol violations
All protocol violations were recorded and classified as major (one that may have an impact on the randomization assignment or an impact on the primary endpoint) or minor. All protocol violations were reviewed by medically qualified monitoring personnel and the study team prior to database lock.
Safety and monitoring
The protocol, consent and patient information documents were submitted by each investigator to the appropriate local Ethics Committee or Institutional Review Board and approval obtained. The study was conducted in accordance with the International Conference on Harmonization (ICH) Harmonized Tripartite Guideline for Good Clinical Practice (1996), the US Food and Drug Administration (FDA) regulations (Title 21 Code of Federal Regulations [CFR] Parts 50, 54, 56 and 312), the Declaration of Helsinki (2008), the European Union Trial Directive (2011/20/EC, May 2001) and the EU GCP Directive (2005/28/EC).
An independent Data Safety Monitoring Board (DSMB) was established and reviewed all data for any safety issues if a prospectively defined stopping criteria was met. A Clinical Events Committee (CEC) was created to adjudicate adverse events that were considered as potentially related to the TBS bleeding or were considered thrombotic events. Membership of these boards was independent with no affiliation to the trial sponsor.
Statistical methods
The intention-to-treat (ITT) set consisted of all randomized patients including those who did not complete the procedure after randomization and were considered failures. An evaluable set (or per protocol; PP) consisted of all ITT subjects with no major protocol deviations. Patients were randomized to FP or to SoC with a 1:1 allocation ratio. The sample size required was not fixed but was dependent on the data with the first planned interim data analysis at 90 patients in the ITT group.
The Whitehead triangular test15 for a binary response variable was utilized (PEST 4.4 software, Lancaster, United Kingdom) for primary response variables with a two-sided alpha of 0.05 and power 0.9. The assumed success rate in the SoC arm was 50% and 75% in the FP arm. Direct comparison of secondary effectiveness variables between the treatment and control group was undertaken using Wilcoxon Rank-Sum test and 95% confidence limits were calculated using a distribution free approach (SAS, Version 9.3, SAS, USA).
Results
Patients
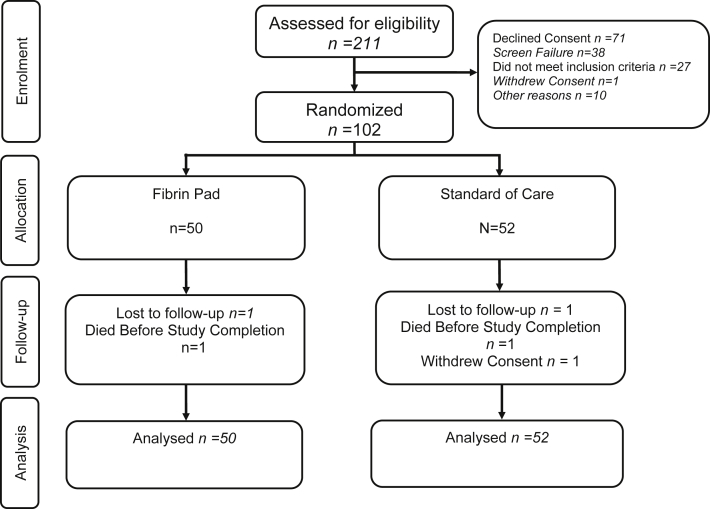
Of 211 patients screened, 102 were enrolled in the trial (50 randomized to FP treatment, 52 randomized to SoC) between October 2, 2013 and 29 August 2014. Of 109 patients not randomized, 71 declined consent and 38 were screen failures (Fig. 1). Patient demographics for the ITT set are summarized in Table 2, while patient diagnoses, operative procedures and parenchymal characteristics are summarized in Table 3. The characteristics of the TBS are also summarized in Table 3.
Figure 1.
Flow chart summarizing patient screening, enrolment and randomization14
Table 2.
Demographic details of patients in the intention to treat set (BMI = Body mass index)
| Category | Fibrin pad (n = 50) | Standard of care (n = 52) |
|---|---|---|
| Median age (range) | 64 (23–81) | 63 (36–85) |
| 18 yrs–49 yrs | 11 (22%) | 5 (10%) |
| 50 yrs–64 yrs | 15 (30%) | 23 (44%) |
| 65 yrs–74 yrs | 17 (34%) | 15 (29%) |
| ≥75 yrs | 7 (14%) | 9 (17%) |
| Gender | ||
| Male | 30 (60%) | 32 (54%) |
| Female | 20 (40%) | 20 (45%) |
| Median BMI kg/m2(range) | 27.5 (17.5–43.3) | 26.8 (15.2–42.5) |
| Underweight (<18.5 kg/m2) | 2 (4%) | 2 (4%) |
| Normal (18.5–<25 kg/m2) | 13 (26%) | 14 (27%) |
| Overweight (25–<30 kg/m2) | 19 (38%) | 16 (31%) |
| Obese (30–<40 kg/m2) | 14 (28%) | 18 (35%) |
| Morbidly obese (>40 kg/m2) | 2 (4%) | 2 (4%) |
| Venous thrombosis risk score13 | 13.0 ± 2.9 | 13.1 ± 2.8 |
Table 3.
Diagnoses, operative procedures, parenchymal type and target bleeding site (TBS) characteristics for the intention to treat set of patients
| Category | Fibrin pad (n = 50) | Standard of care (n = 52) |
|---|---|---|
| Diagnosis | ||
| Metastatic colorectal cancer | 31 (62.0%) | 34 (65%) |
| Hepatocellular carcinoma | 9 (18%) | 6 (12%) |
| Cholangiocarcinoma | 1 (2%) | 2 (4%) |
| Haemangioma | 1 (2%) | 2 (4%) |
| Transplant | 3 (6%) | 3 (6%) |
| Other | 5 (10%) | 5 (10%) |
| Duration of Surgery (median: range) | 180 min (44–491 min) | 177 min (71–385 min) |
| Operative procedures | ||
| Anatomic | 25 (50%) | 23 (44%) |
| Anatomic location | ||
| Left lobectomy | 3 (12%) | 5 (23%) |
| Left lateral lobe segment | 6 (25%) | 2 (9%) |
| Extended left lobectomy | 2 (8%) | 1 (5%) |
| Right lobectomy | 11 (46%) | 9 (41%) |
| Extended right lobectomy | 2 (8%) | 3 (14%) |
| Segmentectomy | 0 (0%) | 2 (9%) |
| Non-anatomic | 25 (50%) | 29 (56%) |
| Hepatic parenchyma | ||
| Normal | 39 (78%) | 36 (69%) |
| Abnormal | 11 (22%) | 16 (31%) |
| Cirrhotic | 6 (55%) | 3 (19%) |
| Steatotic | 3 (27%) | 8 (50%) |
| Other | 2 (18%) | 5 (31%) |
| Total transected area parenchyma | 37.5 cm2 (range 0.4–266.7 cm2) | 60 cm2 (range 0.4–240 cm2) |
| Area TBS (cm2) | 2.0 cm2 (range 0.04–57.8 cm2) | 2.0 cm2 (range 0.02–60 cm2) |
| Source of bleeding | ||
| Arterial | 7 (14%) | 7 (14%) |
| Venous | 37 (74%) | 39 (75%) |
| Mixed | 6 (12%) | 6 (12%) |
Surgical drains were placed at the end of the procedure in 35/50 (70%, median 1 drain, range 1–3 drains) of patients treated with the FP and 33/52 (64%, median 1 drain, range 1–3 drains) of patients treated with SoC.
Treatment efficacy
The results for haemostasis at 4 and 10 min from randomization are summarized in Table 4. Additional treatment was required by 2 patients treated with the FP due to bleeding at the 4-min endpoint (1 patient) and durability of haemostasis (1 patient). For durability, the FP was haemostatic at 4 and 10 min but the surgeon felt additional treatment was required as the patient had declined blood transfusion. In the SoC group, 24 patients received additional treatment for bleeding at the 4-min endpoint, 3 patients for re-bleeding after haemostasis was achieved, 1 patient for durability of haemostasis and 1 patient because of application error.
Table 4.
Haemostatic success at 4 and 10 min from randomization (intention to treat set)
| Fibrin Pad (n = 50) | Standard of care (n = 52) | p-Value | Treatment difference | |
|---|---|---|---|---|
| Haemostasis at 4 min | ||||
| All | 48/50 (96%) | 24/52 (46%) | <0.001 | 50% |
| Anatomic | 24/25 (96%) | 13/23 (57%) | 0.001 | 39% |
| Non-anatomic | 24/25 (96%) | 11/29 (38%) | <0.001 | 58% |
| Haemostasis at 10 min | ||||
| All | 49/50 (98%) | 42/52 (81%) | 0.1243 | 17% |
| Anatomic | 25/25 (100%) | 17/23 (74%) | 0.0163 | 26% |
| Non-anatomic | 24/25 (96%) | 25/29 (86%) | 0.8559 | 10% |
In the ITT analysis the median absolute time to haemostasis was 4 min (range 4–15.9 min) in the FP group compared with 4.7 min (range 1.7–33 min) in the SoC group (p < 0.001). In one patient in the SoC group, manual compression was not maintained until the 4 min endpoint, was released early and a suture applied which was haemostatic. This was a major protocol violation and accounts for the lower value in the range for absolute time to haemostasis in this group.
Blood loss and transfusion requirements
The volume of blood lost during surgery took into account measured losses in suction and weighed surgical swabs. The median estimated blood loss was 400 ml (range 20–4000 ml) in the FP group and 350 ml (range 25–2585 ml) in the SoC group. In the intention to treat analysis 8/50 (16%) patients in the FP group and 13/52 (25%) in the SoC group required transfusion in the period between the commencement of surgery and day 60 assessment.
Post-operative course
There was no difference in median duration of surgery for patients treated with the FP or SoC (Table 3) or in total median hospital stay (6 nights, range 2–47 nights in FP group versus 7 nights, range 2–35 nights in the SoC group).
Postoperative drains and bile leak
Bile leaks occurred in 3/50 (6%) patients treated with FP and 3/52 (6%) patients treated with SoC in the safety set. A drain was placed percutaneously to treat one patient in the FP group while laparotomy and direct suture was employed in the other 2 patients. In the SoC group a percutaneous drain was used to treat one patient while no action was required in the other two patients. Intra-abdominal collections occurred in 2/50 patients (4%) in the FP group and 2/52 patients in the SoC group (4%).
Adverse events and safety
A total of 409 adverse events occurred in patients treated with the FP, with 45/50 (90%) patients experiencing at least one event of which 18 events in 12 patients were categorized as serious. There were 430 adverse events documented in 50/52 (96%) patients treated with SoC, and 25 events in 16 patients were classified as serious (Table 5).
Table 5.
Adverse events occurring in ≥10% of patients treated with either fibrin pad (FP) or standard of care (SoC)
| System | Adverse event | Number (%) of Patients experiencing event |
|
|---|---|---|---|
| FP (n = 50) | SoC (n = 52) | ||
| Cardiac disorders | Tachycardia | 4 (8%) | 11 (21%) |
| Gastrointestinal disorders | Abdominal pain | 5 (10%) | 7 (14%) |
| Ascites | 5 (10%) | 3 (6%) | |
| Constipation | 14 (28%) | 22 (42%) | |
| Diarrhoea | 3 (6%) | 7 (14%) | |
| Nausea | 25 (50%) | 23 (44%) | |
| Vomiting | 13 (26%) | 14 (27%) | |
| General disorders and administration site conditions | Peripheral edema | 5 (10%) | 3 (6%) |
| Pyrexia | 13 (26%) | 11 (21%) | |
| Injury, poisoning and procedural complications | Procedural pain | 5 (10%) | 10 (19%) |
| Investigations | Blood lactic acid increased | 4 (8%) | 6 (12%) |
| Metabolism and nutrition disorders | Hypokalemia | 12 (24%) | 11 (21%) |
| Hypomagnesaemia | 12 (24%) | 12 (23%) | |
| Hypophosphatemia | 11 (22%) | 11 (21%) | |
| Musculoskeletal and connective tissue disorders | Musculoskeletal pain | 8 (16%) | 5 (10%) |
| Nervous system disorders | Dizziness | 6 (12%) | 6 (12%) |
| Psychiatric disorders | Confusional state | 3 (6%) | 6 (12%) |
| Insomnia | 5 (10%) | 1 (2%) | |
| Respiratory, thoracic and mediastinal disorders | Atelectasis | 3 (6%) | 9 (17%) |
| Pleural Effusion | 5 (10%) | 3 (6%) | |
| Skin and subcutaneous tissue disorders | Pruritus | 6 (12%) | 7 (14%) |
| Vascular disorders | Hypertension | 6 (12%) | 5 (10%) |
| Hypotension | 14 (28%) | 18 (35%) | |
There was one (2%) adverse event that was considered to be related to study treatment in the FP group and comprised an infected intra-abdominal fluid collection adjacent to the liver remnant and this was treated with drainage. Two patients treated with FP developed thrombotic complications. One patient presented with a subclavian vein thrombosis 23 days after surgery and one patient developed a portal vein thrombosis on day 6 following hepatectomy. In both patients the investigators assessed the relationship to the study procedure rather than the FP. Three patients treated with FP developed complications potentially related to bleeding (two haematomas and one unexplained anaemia). In all three patients, no specific interventions were required.
One patient in each treatment group died during the study. A patient treated with FP developed liver failure on day 7 post-hepatectomy that was not related to study product and died on day 23. One patient in the SoC group developed acute renal failure on post-operative day 10 and died on post-operative day 36.
Discussion
This was a multicenter, prospective, randomized, controlled superiority clinical study to evaluate the safety and effectiveness of the FP compared to standard of care in controlling hepatic parenchymal bleeding following anatomic or non-anatomic liver resection. The current investigation was part of a comprehensive assessment of the safety and effectiveness of the FP in obtaining haemostasis in a series of prospective, randomized clinical trials in mild/moderate10 and severe soft tissue11 bleeding and hepatectomy in patients with both normal and abnormal hepatic parenchyma.12
This investigation confirmed that FP is safe and effective TAH in hepatic surgery regardless of the type of resection. The FP achieved and maintained haemostasis up to wound closure in 96% of patients in comparison to 46% of standard of care patients. This was a significant difference and resulted in the trial being stopped at the first interim analysis for superior efficacy. There was an associated trend to decreased transfusion requirement in FP treated patients, although the trial was not specifically powered or structured to assess blood product requirements. In addition, the haemostatic effectiveness of the FP was maintained in patients undergoing non-anatomic (96% vs 38% in the SoC) and anatomic resections a (96% vs 57% in the SoC) and across differing parenchymal types. This is an important finding since non-anatomic resections usually leave an irregular parenchymal defect and haemostasis can be difficult to obtain.7 A previous trial also demonstrated haemostatic effectiveness in treating parenchymal bleeding in patients with abnormal (steatotic or cirrhotic) liver parenchyma.12 The results of this investigation confirm that FP contributes to the management of these patients and provides further evidence that it has a significant role to play in the management of bleeding following hepatectomy.
Although this study confirmed that FP performance remained the same, regardless of resection, there are limitations to the study. First, the study cannot be blinded to the surgeon because of the difference in the TAH products. However, bias was limited due to the intraoperative randomization upon identification of the first TBS. Second, the efficacy measure was limited to only one TBS per subject to minimize the variability in assessing the primary endpoint. This makes the relevance of the other data points (e.g. blood loss, transfusions, surgery time) difficult to generalize compared to a situation if the study was designed to allow the use of FP on all bleeding sites identified during surgery. Lastly, the sample size may not be sufficient to detect a difference in clinical outcomes outside of surgical haemostatic efficacy. Despite the study design limitations, this trial was conducted in multiple high-volume institutions across many countries and considering the surgical expertise of the investigators involved in this trial, the value and generalizability of the results may support the clinical utility of FP given the large treatment difference and superior efficacy identified against current surgical standards for haemostasis.
As in previous trials, formal training of participating surgeons was undertaken to ensure that the FP was correctly applied. The importance of correct application technique is emphasized in the two haemostatic failures at 4 min in this trial, which were related to incomplete coverage of the TBS that were retreated with correct FP application. Similarly, reported treatment failures in both severe soft tissue bleeding and previous hepatectomy trials were all due to incorrect FP application or accidental dislodgement of the FP by the operating surgeon.10, 11, 12
The control group for this study design was primarily chosen by regulatory requirements of the health authorities since these are widely available products in the participating countries. This was accepted as standard by all investigators and has been used as comparison in previous trials with the FP10, 11, 12 as a reasonable, widely available and acceptable international SoC.
This investigation has confirmed that the FP is safe and effective at treating parenchymal bleeding following hepatectomy. When correctly used the FP is safe and effective in controlling problematic surgical bleeding from the liver edge in normal and abnormal parenchymal types and following anatomic or non-anatomic types of resections.
Conflicts of interest
Jonathan Batiller, Nicolas Aguirre, Jessica Shen and Richard Kocharian are employees of Ethicon. All data acquisition, analysis, interpretation and manuscript preparation occurred independently of Ethicon.
Acknowledgements
Patients were recruited from centres in the United Kingdom (Royal Infirmary of Edinburgh, Professor James Garden; St James Hospital, Leeds, Dr Ernest Hildalgo; Addenbrookes Hospital, Cambridge, Dr Emmanuel Huguet; Queen Elizabeth Hospital, Birmingham, Dr Darius Mirza), United States of America (Northwestern Memorial Hospital Chicago, Dr Talia Baker; Washington University School of Medicine, Dr William Chapman; University of Alabama, Dr Carlo Contreras; Ochsner Medical Center, Dr W. Charles Conway III; Thomas Jefferson University, Dr Cataldo Doria; University of Pittsburgh, Dr David Geller; Georgia Health Sciences University, Dr Edward Kruse; University of Chicago Centre for Advanced Medicine, Dr J. Michael Millis; Columbia-Presbyterian Center for Liver Disease and Transplantation, Dr Benjamin Samstein), New Zealand (North Shore Hospital, A Prof Jonathan Koea), and Australia (Queen Elizabeth Hospital, Woodville, SA, Prof Guy Maddern; The Alfred Hospital, Melbourne, Vic, Dr Peter Evans). The contribution of the medical staff, nursing staff, theatre staff and intensive care staff as well as the trial coordinators and trial monitors in each of these centres is gratefully acknowledged.
The authors would also like to acknowledge contributions of the following individuals for their trial support: John Albanese, Bababhai Patel, PhD, Cristina Dyogi, Cristina Seltzer, Kimberly Eason, Val Jarvis-Evans, Ellie Barnett, Justine Lee, Tania Betts, Chris McEleney, and Harish Potharaju (all Ethicon/J&J Medical associates).
Footnotes
Financial and product support provided by Ethicon Inc, Somerville, New Jersey, United States of America.
Presented at the European-African Hepatopancreaticobiliary Association. 11th International Congress, Combined with Association Surgeons Great Britain and Ireland, Manchester, England. April 22, 2015.
References
- 1.Simo K.A., Hanna E.M., Imagawa D.K., Iannitti D.A. Hemostatic agents in hepatobiliary and pancreas surgery: a review of the literature and critical evaluation of a novel carrier-bound fibrin sealant (TachoSil) ISRN Surg. 2012;1:1–13. doi: 10.5402/2012/729086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan S.T., Lo C.M., Liu C.L., Lam C.M., Yuen W.K., Yeung C. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. doi: 10.1097/00000658-199903000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vauthey J.N., Pawlik T.M., Ribero D., Wu T.T., Zorsi D., Hoff P.M. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 4.Zorzi D., Laurent A., Pawlik T.M., Lauwers G.Y., Vauthey J.N., Abdalla E.K. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 5.Alkozai E.M., Lisman T., Porte R.J. Bleeding in liver surgery: prevention and treatment. Clin Liver Dis. 2009 Feb;13:145–154. doi: 10.1016/j.cld.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Jarnagin W.R., Gonen M., Fong Y., DeMatteo R.P., Ben-Porat L., Little S. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeMatteo R.P., Palese C., Jarnagin W.R., Sun R.L., Blumgart L.H., Fong Y. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg. 2000;4:178–184. doi: 10.1016/s1091-255x(00)80054-2. [DOI] [PubMed] [Google Scholar]
- 8.Sileshi B., Achneck H., Ma L., Lawson J.H. Application of energy-based technologies and topical hemostatic agents in the management of surgical hemostasis. Vascular. 2010;18:197–204. doi: 10.2310/6670.2010.00015. [DOI] [PubMed] [Google Scholar]
- 9.Halabi W.J., Jafari M.D., Nguyen V.Q., Carmichael J.C., Mills S., Pigazzi A. Blood transfusions in colorectal cancer surgery: incidence, outcomes, and predictive factors: an American College of Surgeons National Surgical Quality Improvement Program Analysis. Am J Surg. 2013;206:1024–1033. doi: 10.1016/j.amjsurg.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Fischer C.P., Bochicchio G., Shen J., Patel B., Batiller J., Hart J.C. A prospective, randomized, controlled trial of the efficacy and safety of fibrin pad as an adjunct to control soft tissue bleeding during abdominal, retroperitoneal, pelvic, and thoracic surgery. J Am Coll Surg. 2013;217:385–393. doi: 10.1016/j.jamcollsurg.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 11.Koea J.B., Baldwin P., Shen J., Patel B., Batiller J., Arnaud A. A randomized, controlled, superiority trial to evaluate the safety and haemostatic effectiveness of fibrin pad for severe soft tissue bleeding during abdominal, retroperitoneal, pelvic and thoracic (non-cardiac) surgery. World J Surg. 2015 doi: 10.1007/s00268-015-3106-5. [DOI] [PubMed] [Google Scholar]
- 12.Koea J.B., Batiller J., Patel B., Shen J., Hammond J., Hart J. A phase III randomised, controlled, superiority trial evaluating the fibrin pad versus standard of care in controlling parenchymal bleeding during elective hepatic surgery. HPB. 2013;15:61–70. doi: 10.1111/j.1477-2574.2012.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caprini J.A. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199:S3–S10. doi: 10.1016/j.amjsurg.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Schulz K.F., Altman D.G., Moher D., CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010 Jun 1;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead J. 2nd (revised) edn. Wiley; New York: 1997. The design and analysis of sequential clinical trials. [Google Scholar]