Abstract
Background
Palliative resection of stage IV pancreatic ductal adenocarcinoma (PDAC) has not shown its benefit until now. In our retrospective review, we compared the results of palliative resection to non-resection.
Methods
Between 2000 and 2009, metastasis of PDAC was confirmed in the operating room in 150 patients. 35 underwent palliative resection (resection group; R) and 115 did bypass or biopsy. 35 patients (biopsy or bypass group: NR) in the 115 patients were matched with the patients undergoing resection for tumor size and the metastasis of peritoneal seeding. Demographic, clinical, operative data and survival were analyzed.
Results
There was no significant difference of major complication (Clavien–Dindo classification 3–5) between two groups. There was no 30-day mortality in either group. More patients in R received postoperative chemotherapy (82.9% vs. 57.1%; P = 0.019). Multivariate analysis showed resection and postoperative chemotherapy as independent factor related to survival (hazard ratio, 0.44; 95% CI, 0.25–0.76; P = 0.003). Patients in R showed better survival rates compared to those in NR (P < 0.001).
Conclusion
Our study suggests resection for stage IV PDAC can be associated with increased survival. In patients of stage IV PDAC, palliative resection with chemotherapy could have some benefit in selected patients.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) has a very dismal prognosis with survival rate less than 5%.1 This poor survival results from not only its aggressive biology but also presentation at an advanced stage. There are no definitive signs or symptoms associated with the early stage and no effective screening tests.2 Currently, complete surgical resection is the only chance of cure.3 However, only 10–20% of patients with PDAC are eligible for resection at diagnosis leaving little hope for the remaining majority of patients.4, 5, 6
As for stage IV PDAC, most of metastases can be detected with the advent of higher quality of imaging and it has been considered contraindication of resection.7 If metastasis is equivocal, laparoscopic exploration can be chosen. When metastasis is confirmed, palliative chemotherapy and endoscopic stenting in case of biliary or duodenal stenosis are considered firstly in most institutions including our hospital. However, not a lot but, surgeons sometimes meet the metastasis unexpectedly after opening the abdomen in operating room (OR).
During the previous 10 years in our hospital, metastases of PDAC were confirmed in OR in 150 patients and pancreatic resection was done in 35. This study aimed to analyze the perioperative and survival data of these 35 compared to patients who didn't undergo resection in the same period.
Materials and methods
Patients
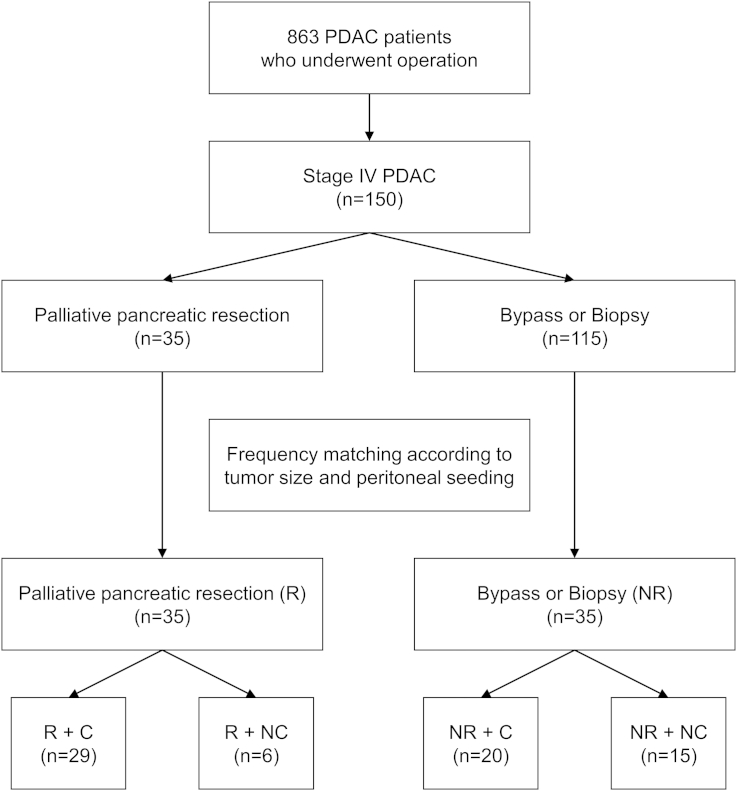
Data was retrospectively collected from our pancreas registry system and electronic medical records between January 2000 and December 2009. A total of 863 patients with PDAC underwent surgery at Asan Medical Center, Seoul, Korea. We excluded patients with intraductal papillary mucinous neoplasms, pancreatic neuroendocrine tumors, and metastatic pancreatic tumors originating from other primary neoplasms. Of 863 patients, 35 underwent palliative pancreatic resection and 115 did bypass or biopsy intraoperatively by open or laparoscopic method. Though they were all in stage IV and there's no difference of age and ASA score between 35 and 115 patients, we selected 35 tumor size- and peritoneal seeding-matched controls from the 115 patients. We used the frequency matching method to select NR group which is matched to R group with respect to particular characteristics. To be specific, there are confounding factors such as, tumor size and peritoneal seeding, between R and NR groups. Patients were classified into resection group (R) or non-resection group (NR) and postoperative chemotherapy group (C) or non-chemotherapy group (NC) (Fig. 1).
Figure 1.
Study flow diagram shows 150 patients who were confirmed as stage IV pancreatic ductal adenocarcinoma in the operating room. 35 patients of them underwent palliative pancreatic resection (R), who were matched with 35 patients who didn't (NR) according to tumor size and peritoneal seeding. They were categorized according to chemotherapy (C)
Operation and postoperative chemotherapy
Generally, we don't perform operation for stage IV PDAC if metastasis has been preoperatively confirmed. However, all metastases in R in our study were detected in the OR and finally confirmed by frozen section intraoperatively.
This was retrospective study and we couldn't identify the accurate reason why the surgeons decided pancreatic resection at that time. However, resection was done mainly in those who seemed to be able to undergo grossly curative intended surgery such as single liver metastasis or a couple of peritoneal seeding macroscopically. Tumor location in distal pancreas rather than head also is assumed to affect the decision. In that situation, surgeon's preference and will of patients and their family seemed to be important factors.
In pancreatic resection, one of three procedures was performed: total pancreatectomy, classical Whipple's procedure or pylorus preserving pancreaticoduodenectomy, or distal pancreatectomy. With regard to liver metastasis, we attempted to remove the metastatic tumors if possible. In case of LS, surgical resection or intraoperative radiofrequency ablation was performed in R. Because the metastasis had already occurred, the operation was defined as “palliative pancreatic resection” even if all metastatic tumors were surgically removed. In NR, bypass in case of obstruction in laparotomy (gastrojejunostomy or combined biliary and gastric bypass) or biopsy only in laparoscopic exploration or in case of no obstruction in laparotomy was done. The operative time from skin incision to closure and the amount of packed red blood cells transfused during surgery and for the first 24 h postoperatively were recorded.
We also investigated whether or not to receiving postoperative chemotherapy. Standard chemotherapy for PDAC was based on 5-FU or Gemzar in our hospital during study period.
Metastasis
Metastases were classified into four categories. LS was defined as only one liver metastatic tumor detected radiologically and grossly. Multiple liver metastases (LM) included following three patterns of metastasis: LM, LM plus lung metastasis, and LM plus lung metastasis plus lymph node 16 (LN16) metastasis. Peritoneal seeding (PS) referred to following metastatic patterns including PS: PS, PS plus LS, PS plus LM, and PS plus ovarian metastasis. Others included LN16 metastasis and LN16 plus bone metastasis.
Operative and perioperative data
Operation time and transfused red blood cells were reviewed. Postoperative hospital stay and 30-day mortality in or out of hospital were also investigated. Tumor sizes were measured by computed tomography because that of NR could not be measured microscopically.
Postoperative complications were grouped into five categories according to Clavien–Dindo classification8, 9 and a postoperative pancreatic fistula (POPF) was defined by the international study group for pancreatic surgery.10, 11 According to Clavien–Dindo classification, complications requiring surgical, endoscopic, or radiologic intervention not under general anesthesia were classified as grade IIIa and those requiring general anesthesia were grade IIIb. Classification from 3 to 5 goes for major complication. In POPF, complications that can be managed by conservative care were categorized as grade A or B, and complications requiring surgical intervention as grade C. The median follow-up was 5.9 months (0.7–81.5).
Statistical analysis
Descriptive statistics were used to estimate the frequencies, means, and standard deviations of variables according to resection or not. Differences between R and NR in baseline characteristics and clinical outcomes were performed with statistical tests, independent samples t-tests for continuous variables and chi-square tests for categorical variables. Hazard ratios and 95% confidence intervals were estimated with a Cox's proportional hazard model with risk factors. Lastly, we used a two-sided, Gehan-test to compare resection and non-resection.12
P-values of less than 0.05 were considered statistically significant. All of the statistical analysis were done using Predictive Analytics Software (PASW) version 22.0 (SPSS Inc., Chicago, USA) and R software version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Table 1 shows the clinicopathological features of the patients. The two groups were similar in age, sex, body mass index, and American Society of Anaesthesiologist (ASA) score.
Table 1.
Clinicopathologic characteristics
| R group (n = 35) |
NR group (n = 35) |
P-value | |
|---|---|---|---|
| Age, mean ± SD (years) | 60.7 ± 9.1 | 58.3 ± 10.6 | 0.313 |
| Male sex, n (%) | 23 (65.7) | 25 (71.4) | 0.607 |
| BMI ≥25 kg/m2, n (%) | 9 (25.7) | 4 (11.4) | 0.222 |
| ASA score, mean ± SD | 1.94 ± 0.48 | 2.09 ± 0.45 | 0.928 |
| Presenting symptom, n (%) | |||
| None | 3 (8.6) | 2 (6.1) | 0.699 |
| Weight loss | 9 (25.7) | 9 (25.7) | 1.000 |
| Jaundice | 4 (11.4) | 9 (25.7) | 0.124 |
| Pain | 24 (68.6) | 28 (80.0) | 0.274 |
| GI trouble | 3 (8.6) | 14 (40.0) | 0.002 |
| Others | 3 (8.6) | 1 (2.9) | 0.303 |
| Tumor size, n (%) | 1.000 | ||
| ≤3 cm | 8 (22.9) | 8 (22.9) | |
| >3 cm | 27 (77.1) | 27 (77.1) | |
| Tumor location, n (%) | 0.008 | ||
| Head to neck | 13 (37.1) | 24 (68.6) | |
| Body to tail | 22 (62.9) | 11 (31.4) | |
| CA 19-9 (≥200 U/mL) (%)a | 18 (52.9) | 16 (52.8) | 0.811 |
| Metastasis, n (%) | |||
| Liver, single (LS) | 8 (22.9) | 7 (20.0) | 0.771 |
| Liver, multiple (LM) | 13 (37.1) | 17 (48.6) | 0.334 |
| Peritoneal seeding (PS) | 11 (31.4) | 11 (31.4) | 1.000 |
| Others | 3 (8.6) | 0 (0.0) | 0.077 |
R, resection; NR, non-resection; SD, standard deviation; BMI, body mass index; ASA, American Society of Anaesthesiologist; GI, gastrointestinal.
CA 19-9 level was not tested in 1 patient in R group and 9 patients in NR group.
Tumors were located in the body or tail more frequently in R than in NR (62.9% vs. 31.4%; P = 0.008). The CA 19-9 level was not significantly different between two groups. When matching the PS, there were no significant differences in other pattern of metastases.
In R, 7 patients underwent total pancreatectomy, 8 did pylorus preserving pancreaticoduodenectomy or classical Whipple's procedure, and 20 did distal pancreatectomy. In NR, 2 patients underwent single bypass, 21 did double bypass, and 12 did biopsy only. Patients in R had a longer operative time and hospital stay and required more packed red blood cells transfusions (Table 2). The rate of complications was higher in R (42.9 vs. 17.1%; P = 0.023). However, based on the Clavien–Dindo classification, There were no significant differences of major complications (classification 3–5) between two groups (P = 0.106). POPF developed in 8 out of 35 patients in R (22.9%). However, Grade C POPFs did not occur and all POPFs were resolved with just conservative management. There was no 30-day mortality in either group. More patients received postoperative chemotherapy in R than in NR (82.9% vs. 57.1%; P = 0.019).
Table 2.
Operative and perioperative data
| R group (n = 35) |
NR group (n = 35) |
P-value | |
|---|---|---|---|
| Pancreatic surgery, n (%) | |||
| TP | 7 (20.0) | ||
| PD/PPPD | 8 (22.9) | ||
| DP | 20 (57.1) | ||
| Single bypass, n (%) | 2 (5.7) | ||
| Double bypass | 21 (60.0) | ||
| Open biopsy or laparoscopic biopsy | 12 (34.3) | ||
| Operation time, minute, mean ± SD | 419.9 ± 202.9 | 174.9 ± 92.0 | <0.001 |
| pRBC transfusion unit, mean ± SD | 2.40 ± 2.96 | 0.26 ± 0.95 | <0.001 |
| Postoperative hospital day, mean ± SD | 23.5 ± 10.9 | 16.3 ± 11.8 | 0.009 |
| Complication, n (%) | 15 (42.9) | 6 (17.1) | 0.023 |
| Major complicationa, n (%) | |||
| ≥Grade IIIa | 6 (17.1) | 1 (2.9) | 0.106 |
| ≥Grade IIIb | 1 (2.9) | 1 (2.9) | 1.000 |
| POPF, n (%) | |||
| Grade A | 5 (14.3) | – | |
| Grade B | 3 (8.6) | – | |
| Grade C | 0 (0) | – | |
| 30-day mortality | 0 (0) | 0 (0) | |
| Postoperative chemotherapy, n (%) | 29 (82.9) | 20 (57.1) | 0.019 |
R, resection; NR, non-resection; TP, total pancreatectomy; PD, pancreaticoduodenectomy; PPPD, pylorus preserving pancreaticoduodenectomy; SD, standard deviation; pRBC, packed red blood cell; POPF, postoperative pancreatic fistula.
Major complication involves Clavien–Dindo classification 3–5.
Table 3 shows the results of multivariate Cox proportional hazards models. Among many factors, resection with postoperative chemotherapy was statistically significant for survival rates (hazard ratio, 0.44; 95% CI, 1.03–3.15; P = 0.003). When resection and chemotherapy were included separately in the Cox proportional hazards models, they were all significant but hazard ratios were not as much as resection with postoperative chemotherapy (data was not shown).
Table 3.
Multivariate Cox proportional hazards regression analysis of factors associated with survival
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| Sex | 0.77 | 0.44–1.34 | 0.356 |
| Age ≥60 vs. <60 | 0.96 | 0.57–1.63 | 0.890 |
| Metastasis, LS | 0.94 | 0.20–4.44 | 0.935 |
| Metastasis, LM | 0.57 | 0.13–2.57 | 0.468 |
| Metastasis, PS | 0.61 | 0.14–2.73 | 0.521 |
| Resection & chemotherapy | 0.44 | 0.25–0.76 | 0.003 |
HR, hazard ratio; CI, confidence interval; LS, liver single; LM, liver multiple; PS, peritoneal seeding.
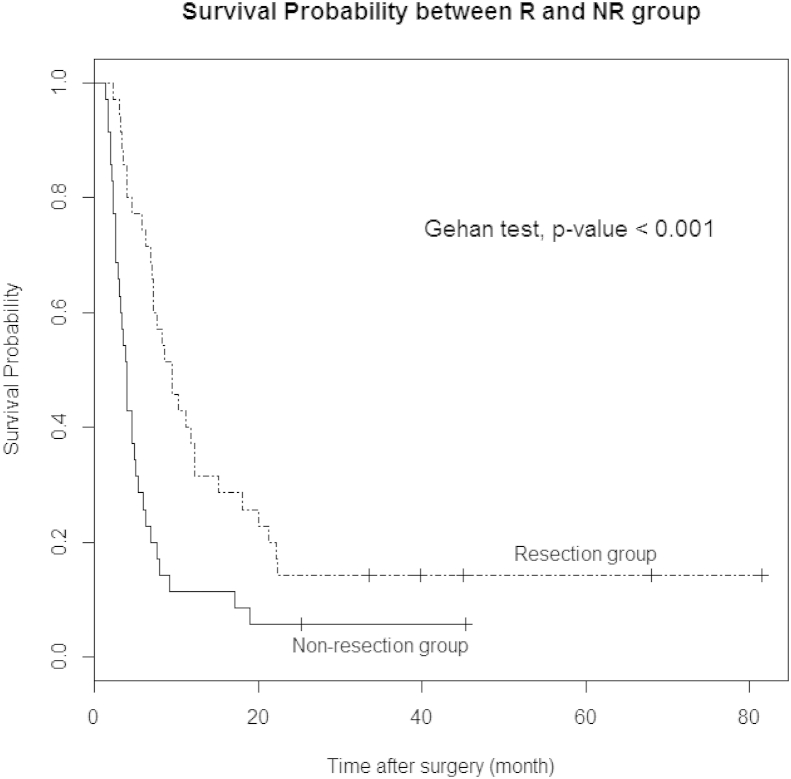
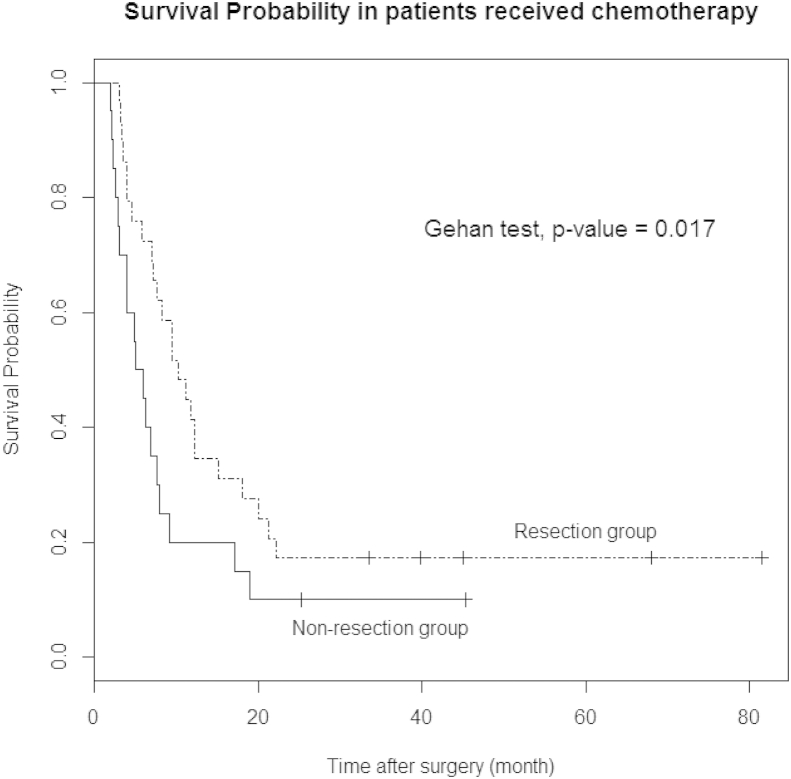
The Kaplan–Meier method with the Gehan-test was performed to compare survival differences between N and NR. There was significant difference between two groups in survival rate (P < 0.001, Fig. 2) and survival rates was better in patients who underwent palliative pancreatic resection. In patients who received chemotherapy, the survival curve for resection was also better than non-resection (P = 0.017, Fig. 3). Table 4 represents the characteristics of patients who survived more than 2 years after surgery.
Figure 2.
Survival curve shows better survival rate of patients who underwent palliative pancreatic resection (R) compared with patients who received bypass or biopsy surgery (NR)
Figure 3.
Survival comparison between resection or non-resection group in patients who received chemotherapy. Survival curve shows better survival rate of patients who underwent palliative pancreatic resection compared with patients who received bypass or biopsy surgery
Table 4.
Summary of patients who lived more than 2 years
| Patient | Sex | Age | Tumor locationa | Resection | Metastasis | CTx | Survival (month) |
|---|---|---|---|---|---|---|---|
| 1 | M | 62 | 2 | No | LM | Yes | 24.0 |
| 2 | M | 50 | 1 | No | Ps | Yes | 24.0 |
| 3 | M | 65 | 2 | DP | Ps | Yes | 33.5 |
| 4 | M | 65 | 1 | PD/PPPD | LN16 | Yes | 39.8 |
| 5 | F | 61 | 1 | TP | LS | Yes | 45.1 |
| 6 | M | 41 | 1 | No | LM | Yes | 45.4 |
| 7 | M | 47 | 2 | DP | LS | Yes | 68.0 |
| 8 | M | 47 | 2 | DP | LS | Yes | 81.5 |
CTx, chemotherapy; LM, liver multiple; Ps, peritoneal seeding; DP, distal pancreatectomy; PD, pancreaticoduodenectomy; PPPD, pylorus preserving pancreaticoduodenectomy; LN16, lymph node number 16; TP, total pancreatectomy; LS, liver single.
In tumor location, 1 indicates head to neck, 2 body to tail.
Discussion
Significant advances have been achieved in cancer treatment over the past several decades. In particular, improved screening technique and early detection has led to improved survival in a variety of cancers. However, as for PDAC it has been minimal, and the survival is poorer than other types of cancer even at a similar stage.
This poor outcome results largely from the absence of appropriate screening tests and resultant late diagnosis. Furthermore, it frequently recurs after resection. Various chemotherapeutic regimens, some along with radiation therapy, have failed to show significant survival benefits.13, 14, 15 Additionally, serious perioperative complications of pancreaticoduodenectomy before the mid-1980s had added to the gloomy outlook. Fortunately, during the 1980s the surgical results dramatically improved16 and curative surgical resection (R0) for PDAC improved survival rate up to about 20%.17 With decreased complications, more aggressive surgery and even palliative resection for locally advanced PDAC have been reported. These procedures are often performed when irresectability and a “point of no return” are established during surgery and resection with curative intent is likely to be an R1 or R2 resection. Several reports have shown the perioperative complication rate of R1 in experienced hands was not higher than those of bypass, and also suggested that in terms of survival and palliation, an R1 resection could be justified on acceptable condition.18, 19, 20, 21, 22 However, as for an R2 resection, its role remains more controversial.23, 24, 25, 26 A meta-analysis of palliative resection versus palliative bypass suggested planned palliative R2 resections are not justified in terms of complications and survival.4
In stage IV PDAC, there have been few studies about the role of palliative resection and until now, resection has been considered contraindicated. In fact, PDAC frequently recurs even after complete resection at the site of resection or in the liver within 2 years. This suggests the presence of micrometastasis at the time of surgery even though it was considered an R0 resection. Considering the absence of effective chemotherapy and its high recurrence rate, it is logical that stage IV PDAC has been thought to be a contraindication for resection.
Currently, many institutions, including our hospital, generally perform neoadjuvant therapy for stage IV PDAC and then choose a surgical approach depending on its response. However, sometimes it is difficult to ascertain the presence of metastasis preoperatively. Metastases are often confirmed in the OR. This presents surgeons with a set of complex and difficult circumstances like this: the pancreatic mass seems not to be locally advanced and appears to be resectable, the patient's general condition is tolerable, the surgeon has a lot of experience with low complication rate, and the patient's family strongly favors resection despite having been informed of the risk and recurrence.
Though there are some case studies reported long-term survival, surgical results of stage IV PDAC have been seldom reported.27, 28, 29, 30 A few have reported that resection of main tumor and its metastatic lesions conveyed no survival gain, and resection could not be recommended7, 31, 32 while others reported its positive effect in well-selected patients by experienced surgeons.33, 34
In our study, pancreatic resections were performed in 35 patients, with or without synchronous metastasectomy. Because our institute is a high volume center in South Korea and our surgical team has accumulated experience in pancreatic surgery, we have a low surgical mortality of <1%. In present study, we had no 30-day mortality in R. There was no difference of major complication between two groups (Table 2). We could find survival benefit in R compared to NR (Fig. 2, P < 0.001).
Pancreatic resection with postoperative chemotherapy was thought as an independent factor associated with survival (Table 3). Fig. 3 also suggests that resection may have some synergistic effect with chemotherapy. Considering that patients who underwent resection received chemotherapy more (82.9% vs. 57.1%; P = 0.019), pancreatic resection can also have positive effects to lead to chemotherapy. This is similar to the result of a previous study (78% vs. 48%).19
The liver is the main metastatic site of many cancers including gastrointestinal cancer. The outcome of resection of hepatic metastases from non-colorectal primary tumors has been known to be poor.35 However, if surgeons encounter a resectable pancreatic cancer with isolated hepatic metastasis on the liver surface, resection might be chosen, especially if the patient's condition is tolerable. Though there are some studies regarding this approach, no conclusions can be drawn because of its mixed results and small number of patients.7, 32, 33, 34, 36 In our study, we resected the pancreatic mass and removed LS by surgical resection or intraoperative radiofrequency ablation. LS was not an independent factor for survival (Table 3). However, the number of LS (8 in R vs. 7 in NR) was very small. Considering the probable survival benefit of resection in our study, surface liver metastasis of PDAC can be addressed to be resected.
Though we generally do not perform resection for stage IV PDAC, 35 patients underwent surgical resection in our study. Many reasons led to the decision. Above all, the patients' general conditions were good to tolerate the surgical stress. ASA score in R was low (Table 1). Second, patients and their family had strong will to get resection regardless of metastasis and information about the risk. Third, relative low complication in our institution was an important factor for us to choose resection. Lastly, tumors were more located in pancreatic neck or body in R rather than head to neck and we might decide the resection compared to NR. Distal pancreatectomy was performed in more than half of R.
Our study has several limitations. First of all, our study could have selection bias. Though the age and ASA score had no statistical difference between R and NR, the decision of resection in R might be made based on patients' general condition and this could affect the outcomes. Furthermore, although all patients in R and NR were in stage IV and we selected 35 tumor size- and peritoneal seeding-matched controls from the 115 patients to reduce selection bias, the two groups could be in different disease phase in terms of number of metastatic liver nodule or seeding nodule. The resection decision itself might include selection bias. Second, quality of life after resection was not evaluated. Because pancreatic surgery has a relatively higher complication compared to other cancer surgeries, and the survival benefit was slight, quality of life after operation should be evaluated. Lastly, above all things it is danger to draw a positive conclusion of pancreatic resection in stage IV PDAC from a small number of R and NR in our study. Given these limitations, larger and multicenter studies should be performed.
In summary, our data couldn't suggest any recommendation but may implicate some possibility of stage IV PDAC operation. The basic premise for this aggressive approach is acceptable perioperative morbidity and mortality. In highly selected patients of stage IV PDAC, pancreatic resection and chemotherapy might have some role in survival gain.
Conflict of interest
None declared.
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Lockhart A.C., Rothenberg M.L., Berlin J.D. Treatment for pancreatic cancer: current therapy and continued progress. Gastroenterology. 2005;128:1642–1654. doi: 10.1053/j.gastro.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Wagner M., Redaelli C., Lietz M., Seiler C.A., Friess H., Buchler M.W. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 4.Gillen S., Schuster T., Friess H., Kleeff J. Palliative resections versus palliative bypass procedures in pancreatic cancer–a systematic review. Am J Surg. 2012;203:496–502. doi: 10.1016/j.amjsurg.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Tempero M.A., Arnoletti J.P., Behrman S., Ben-Josef E., Benson A.B., 3rd, Berlin J.D. Pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2010;8:972–1017. doi: 10.6004/jnccn.2010.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smeenk H.G., Tran T.C., Erdmann J., van Eijck C.H., Jeekel J. Survival after surgical management of pancreatic adenocarcinoma: does curative and radical surgery truly exist? Langenbecks Arch Surg. 2005;390:94–103. doi: 10.1007/s00423-004-0476-9. [DOI] [PubMed] [Google Scholar]
- 7.Takada T., Yasuda H., Amano H., Yoshida M., Uchida T. Simultaneous hepatic resection with pancreato-duodenectomy for metastatic pancreatic head carcinoma: does it improve survival? Hepatogastroenterology. 1997;44:567–573. [PubMed] [Google Scholar]
- 8.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casadei R., Ricci C., Pezzilli R., Calculli L., D'Ambra M., Taffurelli G. Assessment of complications according to the Clavien-Dindo classification after distal pancreatectomy. JOP. 2011;12:126–130. [PubMed] [Google Scholar]
- 10.Tan W.J., Kow A.W., Liau K.H. Moving towards the New International Study Group for Pancreatic Surgery (ISGPS) definitions in pancreaticoduodenectomy: a comparison between the old and new. HPB. 2011;13:566–572. doi: 10.1111/j.1477-2574.2011.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassi C., Dervenis C., Butturini G., Fingerhut A., Yeo C., Izbicki J. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Gehan E.A. A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika. 1965;52:203–223. [PubMed] [Google Scholar]
- 13.Colucci G., Giuliani F., Gebbia V., Biglietto M., Rabitti P., Uomo G. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell'Italia Meridionale. Cancer. 2002;94:902–910. [PubMed] [Google Scholar]
- 14.Zuckerman D.S., Ryan D.P. Adjuvant therapy for pancreatic cancer: a review. Cancer. 2008;112:243–249. doi: 10.1002/cncr.23174. [DOI] [PubMed] [Google Scholar]
- 15.Dahan L., Bonnetain F., Ychou M., Mitry E., Gasmi M., Raoul J.L. Combination 5-fluorouracil, folinic acid and cisplatin (LV5FU2-CDDP) followed by gemcitabine or the reverse sequence in metastatic pancreatic cancer: final results of a randomised strategic phase III trial (FFCD 0301) Gut. 2010;59:1527–1534. doi: 10.1136/gut.2010.216135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron J.L., Pitt H.A., Yeo C.J., Lillemoe K.D., Kaufman H.S., Coleman J. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430–435. doi: 10.1097/00000658-199305010-00002. discussion 435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ujiki M.B., Talamonti M.S. Guidelines for the surgical management of pancreatic adenocarcinoma. Semin Oncol. 2007;34:311–320. doi: 10.1053/j.seminoncol.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Reinders M.E., Allema J.H., van Gulik T.M., Karsten T.M., de Wit L.T., Verbeek P.C. Outcome of microscopically nonradical, subtotal pancreaticoduodenectomy (Whipple's resection) for treatment of pancreatic head tumors. World J Surg. 1995;19:410–414. doi: 10.1007/BF00299174. discussion 414–415. [DOI] [PubMed] [Google Scholar]
- 19.Lillemoe K.D., Cameron J.L., Yeo C.J., Sohn T.A., Nakeeb A., Sauter P.K. Pancreaticoduodenectomy. Does it have a role in the palliation of pancreatic cancer? Ann Surg. 1996;223:718–725. doi: 10.1097/00000658-199606000-00010. discussion 725–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhlmann K., de Castro S., van Heek T., Busch O., van Gulik T., Obertop H. Microscopically incomplete resection offers acceptable palliation in pancreatic cancer. Surgery. 2006;139:188–196. doi: 10.1016/j.surg.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 21.Lavu H., Mascaro A.A., Grenda D.R., Sauter P.K., Leiby B.E., Croker S.P. Margin positive pancreaticoduodenectomy is superior to palliative bypass in locally advanced pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2009;13:1937–1946. doi: 10.1007/s11605-009-1000-x. discussion 1946–1937. [DOI] [PubMed] [Google Scholar]
- 22.Raut C.P., Tseng J.F., Sun C.C., Wang H., Wolff R.A., Crane C.H. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koninger J., Wente M.N., Muller-Stich B.P., di Mola F.F., Gutt C.N., Hinz U. R2 resection in pancreatic cancer–does it make sense? Langenbecks Arch Surg. 2008;393:929–934. doi: 10.1007/s00423-008-0308-4. [DOI] [PubMed] [Google Scholar]
- 24.Bockhorn M., Cataldegirmen G., Kutup A., Marx A., Burdelski C., Vashist J.K. Crossing the Rubicon: when pancreatic resection with curative intent ends in an R2 status. Impact of “desmoplastic pseudo-pancreatitis” and anatomical site of irresectability. Ann Surg Oncol. 2009;16:1212–1221. doi: 10.1245/s10434-009-0363-2. [DOI] [PubMed] [Google Scholar]
- 25.Tachezy M., Bockhorn M., Gebauer F., Vashist Y.K., Kaifi J.T., Izbicki J.R. Bypass surgery versus intentionally incomplete resection in palliation of pancreatic cancer: is resection the lesser evil? J Gastrointest Surg. 2011;15:829–835. doi: 10.1007/s11605-011-1469-y. [DOI] [PubMed] [Google Scholar]
- 26.Schniewind B., Bestmann B., Kurdow R., Tepel J., Henne-Bruns D., Faendrich F. Bypass surgery versus palliative pancreaticoduodenectomy in patients with advanced ductal adenocarcinoma of the pancreatic head, with an emphasis on quality of life analyses. Ann Surg Oncol. 2006;13:1403–1411. doi: 10.1245/s10434-006-9172-z. [DOI] [PubMed] [Google Scholar]
- 27.Ko K., Fujioka S., Kato K., Machiki Y., Hashimoto M., Fujii K. Resection of liver metastasis after a pancreatoduodenectomy for pancreatic cancer: a case report. Hepatogastroenterology. 2001;48:375–377. [PubMed] [Google Scholar]
- 28.Ibusuki M., Hiraoka T., Kanemitsu K., Takamori H., Tsuji T. Complete remission of pancreatic cancer after multiple resections of locally pancreatic recurrent sites and liver metastasis: report of a case. Surg Today. 2008;38:563–566. doi: 10.1007/s00595-007-3642-1. [DOI] [PubMed] [Google Scholar]
- 29.Shimada K., Kosuge T., Yamamoto J., Yamasaki S., Sakamoto M. Successful outcome after resection of pancreatic cancer with a solitary hepatic metastasis. Hepatogastroenterology. 2004;51:603–605. [PubMed] [Google Scholar]
- 30.Lemke J., Barth T.F., Juchems M., Kapapa T., Henne-Bruns D., Kornmann M. Long-term survival following resection of brain metastases from pancreatic cancer. Anticancer Res. 2011;31:4599–4603. [PubMed] [Google Scholar]
- 31.Dunschede F., Will L., von Langsdorf C., Mohler M., Galle P.R., Otto G. Treatment of metachronous and simultaneous liver metastases of pancreatic cancer. Eur Surg Res. 2010;44:209–213. doi: 10.1159/000313532. [DOI] [PubMed] [Google Scholar]
- 32.Gleisner A.L., Assumpcao L., Cameron J.L., Wolfgang C.L., Choti M.A., Herman J.M. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer. 2007;110:2484–2492. doi: 10.1002/cncr.23074. [DOI] [PubMed] [Google Scholar]
- 33.Seelig S.K., Burkert B., Chromik A.M., Tannapfel A., Uhl W., Seelig M.H. Pancreatic resections for advanced M1-pancreatic carcinoma: the value of synchronous metastasectomy. HPB Surg. 2010;2010 doi: 10.1155/2010/579672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh A., Singh T., Chaudhary A. Synchronous resection of solitary liver metastases with pancreaticoduodenectomy. JOP. 2010;11:434–438. [PubMed] [Google Scholar]
- 35.Yedibela S., Gohl J., Graz V., Pfaffenberger M.K., Merkel S., Hohenberger W. Changes in indication and results after resection of hepatic metastases from noncolorectal primary tumors: a single-institutional review. Ann Surg Oncol. 2005;12:778–785. doi: 10.1245/ASO.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 36.Klempnauer J., Ridder G.J., Piso P., Pichlmayr R. Is liver resection in metastases of exocrine pancreatic carcinoma justified? Chirurg. 1996;67:366–370. [PubMed] [Google Scholar]