Abstract
Background
Site of tumour origin, lymph node metastases and lymph node ratio (LNR) are identified as important factors determining prognosis in patients undergoing pancreaticoduodenectomy (PD). This study hypothesised that a prognostic index to predict survival could be developed through statistical modelling based on these pathological variables.
Methods
Patients who underwent PD between 2004 and 2013 were included. Univariable and multivariable (Cox regression) analyses were performed to identify predictors of survival, and a prognostic index was derived. The prognostic index was then validated using an external patient cohort.
Results
A total of 567 patients who underwent PD were used as a derivation cohort. Tumour site (p < 0.001), tumour size (p = 0.002), T-stage (p < 0.001), vascular involvement (p = 0.002), number of positive nodes (p < 0.001) and LNR (p < 0.001) were significantly associated with survival in univariable analysis. LNR (p < 0.001), tumour site (p < 0.001), T-stage (p = 0.007) remained significant predictors of survival in multivariable analysis, and were combined to derive a prognostic index. The accuracy of the prognostic index was assessed both on the original cohort, and a validation set of 194 patients from another institutional prospective database. The AUROC scores for predicting the overall survival at 3 years were 0.77 in the derivation cohort and 0.74 in the validation cohort.
Conclusion
The Pancreaticoduodenectomy Prognostic Index is a validated clinico-pathological model based on tumour site, T-stage and LNR to predict long-term survival following PD.
Introduction
Surgical resection remains the only potentially curative treatment for patients with adenocarcinomas arising from head of pancreas, ampulla, distal bile duct and ampulla. Although the median survival following pancreaticoduodenectomy (PD) has improved,1 the actual five-year survival after resection of pancreatic ductal adenocarcinoma approaches 10%.2, 3, 4, 5 For ampullary carcinoma and distal cholangiocarcinoma, 5-year survival varies between 20 and 40%6, 7 and is up to 60% for duodenal adenocarcinoma.8 Various factors, however, impact upon cancer related outcomes after PD, including tumour site, tumour size, tumour at the resection margins, lymph node metastases, and histologic grade.3, 9, 10, 11 The role of post-operative chemotherapy/radiotherapy continues to evolve and can increase survival.12, 13, 14
The presence of nodal metastases has been shown to be an indicator of poor prognosis.3, 4, 5, 15, 16 Evidence suggests that a minimum of 11–15 lymph nodes (LNs) should be examined to provide an accurate assessment of LN metastases upon which to base prognostic information, following resection for pancreatic cancer.17, 18, 19 The AJCC TNM staging system considers LN metastases as a binary outcome – they are or are not present.20 Recent studies have demonstrated that the lymph node ratio (number of metastatic lymph nodes (LNs) divided by total number of examined nodes) provides additional prognostic information over the standard reporting of LN metastasis and its role in pancreatic carcinoma is also increasingly accepted.17, 21, 22 The aim of this study was to develop a prognostic index that could be applied to the four common tumour types that require PD.
Materials and methods
This was a retrospective analysis of a prospectively maintained database of patients who underwent PD between 2004 and 2013. Patients with pancreatic ductal carcinoma, distal cholangiocarcinoma, ampullary carcinoma or duodenal carcinoma were included. Patients undergoing surgery for other pathologies were excluded. Pylorus preserving PD was the standard operation, with a Kausch Whipple procedure performed when it was deemed appropriate on oncological grounds. A standard lymphadectomy was performed to include dissection of the common hepatic artery from the splenic artery origin to the origins of the hepatic arteries. Perineural tissue and lymph nodes along the common bile duct, station 8 nodes along the hepatic artery, posterior and anterior pancreatico-duodenal nodes, nodes along the superior mesenteric vein and right lateral wall of the superior mesenteric artery were removed. The technique of pancreato-enteric anastomosis was performed at the operating surgeons discretion. Patients received adjuvant chemotherapy in line with existing practice within the United Kingdom at that time following surgery.
All specimens were reviewed by dedicated pancreatic specialist pathologists. The AJCC TNM system was used for staging the tumour.20 Site of tumour origin was based on macroscopic assessment of tumour location. Whenever possible, this was corroborated by histological identification of in-situ neoplasia (pan-IN for ductal adenocarcinoma, Bil-IN for cholangiocarcinoma). For the purposes of the present study, the resection margins that were consistently examined were the pancreatic neck transection margin, the superior mesenteric artery surface, proximal bile duct margin, proximal duodenal margin and distal duodenal margin. Verbeke's description23, 24 of positive resection margin (tumour found within 1 mm of the margin) was used for the patients included in the latter part of the study period.
Outcomes assessed
Median follow up of the patients included in the study was 1.36 years (range, 0.02–10.05 years). Overall survival (OS) was assessed, and reported at one and three years.
Statistical analysis
Continuous variables were categorised into four groups, prior to survival analysis, either using arbitrary cut-offs based on clinical experience (BMI and age), or by rounding the quartiles to meaningful values. The relationship between several clinico-pathological factors, and OS were then assessed using Kaplan–Meier curves, with Log–Rank tests. Factors found to be significant in univariable analysis were then analysed using a multivariable Cox regression model, with a forward stepwise entry method.
A prognostic index was then proposed based on the coefficients of Cox regression model. The Cox regression model was then converted into a PPI. The coefficients from the model (i.e. the logged hazard ratios) were rounded to the nearest 0.5, after being multiplied by two, to reduce the effect of rounding errors. Where this resulted in a negative value, a constant value (three) was added to these coefficients, in order that the score would be purely additive, and easier to use in practice. The resulting score had a potential range from 1 to 10.5. The accuracy of the prognostic index was assessed using ROC curves, both on the derivation cohort, and a validation cohort, identified from a prospectively maintained database from Leeds Teaching Hospitals (January 2008–December2013). OS at both one and three years were used as outcomes in the ROC analysis, with patients with potential follow-up shorter than this being excluded from the respective analyses.
All analyses and the statistical modelling were performed using IBM SPSS 19 (IBM Corp. Armonk, NY), by a medical statistician (JH). Missing data were excluded on a per analysis basis, and p < 0.05 was deemed to be indicative of statistical significance throughout.
Results
A total of 567 patients were identified as the derivation cohort of which 311 (55%) were male. Characteristics of patients and association with survival of the patients in the derivation cohort are provided in Table 1. There was no significant variation in the yield of lymph nodes over the study period (p = 0.116) with medians of 16 (quartiles: 13–24) in 2004, and 2115, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 in 2013. Association of lymph node variables with survival in the derivation cohort are shown in Table 2. The correlation between the number of positive nodes, and the LNR was extremely high (Spearman coefficient = 0.955). Hence, to avoid issues with multi-collinearity, only the LNR was used in subsequent analysis. No significant association was detected between the number of lymph nodes taken and OS (p = 0.592). A subgroup analysis demonstrated significant relationship between the LNR and OS in ductal adenocarcinoma and ampullary carcinoma (both p < 0.001, Supplementary Fig. 1). The relationship between LNR and OS was close to significance in cholangiocarcinoma (p = 0.059), and non-significant in duodenal carcinoma (p = 0.610), but the numbers of patients in these groups was small, giving low statistical power. The factors found to be significant in univariable analyses were then entered into multivariable Cox regression models, with OS as the outcome (Table 3). Fig. 1a–c shows the OS for components of the final model, as well as the resulting risk score (Fig. 1d).
Table 1.
Association between clinico-pathological factors and patient survival (derivation cohort)
| N | Overall survival 1 year, 3 years |
p-Value | |
|---|---|---|---|
| Age | 0.074 | ||
| <60 | 149 (26%) | 74%, 37% | |
| 60–66 | 144 (25%) | 71%, 38% | |
| 67–74 | 174 (31%) | 66%, 30% | |
| 75+ | 100 (18%) | 65%, 32% | |
| Smoking statusa | 0.570 | ||
| Never | 135 (51%) | 72%, 39% | |
| Ex | 87 (33%) | 73%, 38% | |
| Current | 43 (16%) | 71%, 31% | |
| BMIa | 0.528 | ||
| <=25 | 157 (50%) | 73%, 32% | |
| 26–30 | 114 (36%) | 68%, 41% | |
| 31–35 | 30 (9%) | 64%, 25% | |
| >35 | 16 (5%) | 65%, 55% | |
| Tumour | <0.001b | ||
| Ductal adenocarcinoma | 279 (49%) | 63%, 23% | |
| Cholangiocarcinoma | 89 (16%) | 61%, 30% | |
| Duodenal carcinoma | 32 (6%) | 77%, 60% | |
| Ampullary carcinoma | 167 (29%) | 83%, 56% | |
| Tumour size | 0.002b | ||
| <2.0 | 85 (17%) | 74%, 50% | |
| 2.0–2.4 | 88 (18%) | 68%, 37% | |
| 2.5–3.4 | 167 (34%) | 70%, 32% | |
| 3.5+ | 148 (30%) | 63%, 25% | |
| T stage | <0.001b | ||
| 1 | 31 (6%) | 86%, 82% | |
| 2 | 60 (11%) | 88%, 74% | |
| 3 | 393 (75%) | 67%, 28% | |
| 4 | 41 (8%) | 71%, 29% | |
| R status | <0.001b | ||
| Negative | 450 (80%) | 80%, 38% | |
| Positive | 114 (20%) | 58%, 22% | |
| Vascular reconstruction | 0.002b | ||
| No | 493 (87%) | 70%, 51% | |
| Yes | 74 (13%) | 65%, 20% | |
| Adjuvant chemotherapya | 0.074 | ||
| No | 133 (48%) | 67%, 41% | |
| Yes | 147 (52%) | 82%, 40% | |
| Any pre-op comorbidity | 0.132 | ||
| No | 344 (61%) | 76%, 34% | |
| Yes | 223 (39%) | 59%, 36% | |
| Wound infection | 0.522 | ||
| No | 523 (92%) | 70%, 35% | |
| Yes | 43 (8%) | 61%, 29% | |
| Pancreatic leak | 0.097 | ||
| No | 472 (83%) | 72%, 35% | |
| Grade A | 57 (10%) | 59%, 31% | |
| Grade B | 20 (4%) | 63%, 30% | |
| Grade C | 17 (3%) | 35%, 28% | |
| Intra-abdominal collection | 0.543 | ||
| No | 534 (94%) | 70%, 35% | |
| Yes | 33 (6%) | 57%, 37% |
Survival is reported as Kaplan–Meier estimates at 1 and 3 years, and p-values are from Log-Rank tests based on all available follow-up.
Data only available post-2007.
Significant at p < 0.05.
Table 2.
Associations between LN related factors and patient survival in derivation cohort
| N | Overall survival 1 year, 3 years |
p-Value | |
|---|---|---|---|
| No. of nodes taken | 0.592 | ||
| 1–15 | 149 | 80%, 36% | |
| 16–20 | 155 | 70%, 40% | |
| 21–25 | 125 | 70%, 31% | |
| >25 | 114 | 69%, 34% | |
| No. of positive nodes | <0.001a | ||
| 0 | 148 | 84%, 62% | |
| 1–2 | 132 | 72%, 35% | |
| 3–5 | 146 | 65%, 26% | |
| >5 | 118 | 57%, 14% | |
| LN ratio | <0.001a | ||
| 0.00 | 147 | 85%, 63% | |
| 0.01–0.15 | 142 | 74%, 38% | |
| 0.16–0.25 | 114 | 59%, 18% | |
| >0.25 | 140 | 60%, 18% |
Survival is reported as Kaplan–Meier estimates at 1 and 3 years, and p-values are from Log-Rank tests based on all available follow-up.
Significant at p < 0.05.
Table 3.
Multivariable Cox regression model (derivation cohort)
| Overall survival |
|||
|---|---|---|---|
| B | HR (95% CI) | p-Value | |
| Tumour | 0.001a | ||
| Ductal adenocarcinoma | 0.0 | 1 | – |
| Cholangiocarcinoma | 0.0 | 0.98 (0.73–1.33) | 0.921 |
| Duodenal carcinoma | −1.4 | 0.25 (0.11–0.55) | 0.001a |
| Ampullary carcinoma | −0.5 | 0.61 (0.43–0.87) | 0.007a |
| T stage | 0.007a | ||
| 1 | −0.8 | 0.44 (0.19–1.06) | 0.067 |
| 2 | −0.9 | 0.41 (0.23–0.73) | 0.002a |
| 3 | 0.0 | 1 | – |
| 4 | 0.2 | 1.17 (0.72–1.88) | 0.531 |
| LNR | 1.9 | 6.79 (3.85–11.99) | <0.001a |
| Variables not in the final model | |||
| Tumour size | 0.124 | ||
| R status | 0.715 | ||
| Vascular reconstruction | 0.535 | ||
HR, hazard ratio.
B = Coefficients of the Cox regression model (logged hazard ratios).
Significant at p < 0.05.
Figure 1.
Overall survival by tumour site (a), T-stage (b), LNR (c) and the calculated Prognostic index (d)
Pancreatoduodenectomy prognostic index (PPI)
Risk score for each of the variables is given in Table 4. To calculate the PPI, the LNR should be multiplied by four, with the result added to the sub-score for the site of primary tumour and T stage (PPI = 4 × LNR + Tumour site score + T stage score). For example, a patient with T3 ampullary carcinoma and a LNR of 25% would have a PPI of: 2 + 3 + (0.25 × 4) = 6.
Table 4.
Risk score
| Factor | Score |
|---|---|
| Tumour | |
| Ductal adenocarcinoma | 3 |
| Cholangiocarcinoma | 3 |
| Duodenal carcinoma | 0 |
| Ampullary carcinoma | 2 |
| T stage | |
| 1 | 1.5 |
| 2 | 1 |
| 3 | 3 |
| 4 | 3.5 |
| LNR | 4× (individual LNR) |
The PPI was then applied to the patients in the derivation cohort, and ROC curves calculated for OS at both one and three years. This gave areas under the ROC curves (AUROCs) of 0.72 (95% CI: 0.67–0.76, p < 0.001) at one year and 0.77 (0.73–0.82 p < 0.001) at three years.
The PPI was then validated using an external cohort of 194 patients, the demographics of whom are reported in Supplementary Table 1. The derivation and validation cohorts were well matched on age and gender. However, the distributions of both primary diagnosis and T-stages differed significantly (p = 0.003, <0.001 respectively). Patients from the validation set had significantly larger tumours (p = 0.001), with significantly more nodes (p < 0.001) removed. However, the LNR (p = 0.583) and OS (p = 0.584) were similar in the two groups.
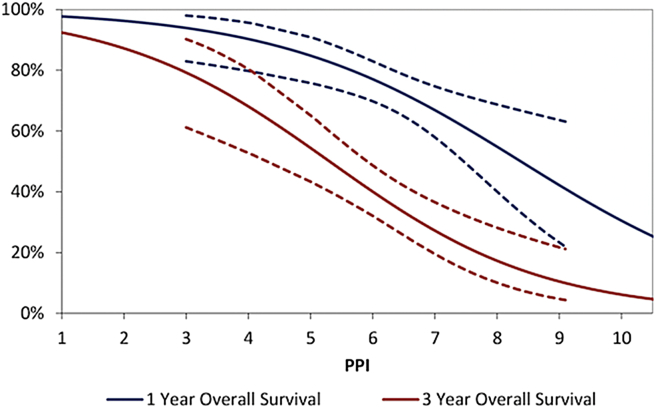
After applying the PPI to this validation cohort, the resulting AUROCs for survival were found to be significant (p < 0.001), and similar to those in the derivation cohort, with 0.66 (95% CI: 0.57–0.75) for one year OS and 0.74 (0.65–0.82) at three years. To illustrate the relationship between the PPI and patient outcomes, binary logistic regression models were produced in the validation cohort, with one and three year survival as outcomes, and the PPI as a continuous covariate. The resulting models were plotted (Fig. 2), with the PPI on the x-axis, and the predicted survival rates on the y-axis. This plot can be used to convert a patient's score into a predicted survival rate.
Figure 2.
Outcome based on the risk score calculated using the Prognostic Index (dotted lines indicate 95% CI)
Discussion
This is an analysis of the clinico-pathological factors influencing OS following PD. A prognostic index based upon tumour site, tumour T stage and LNR was then developed to predict the outcomes. The index was then successfully validated upon an external patient cohort. The PI could be used in the pre- and post-operative counselling of patients and its use could be extended to research platforms. For the purpose of this study, the influence of clinic-pathological factors that influence the patient selection for PD (age, co-morbidities), post-operative morbidity, the TNM status, LNR status, the tumour type and its resectability (margin status) were assessed. The four common periampullary cancers are included and given different individual risk scores accounting to the variations in their biological behaviour.
Between these basic clinical, pathological variables and the evolving cancer genetics there are several other factors such as lymphovascular invasion and perineural invasion that are reported to influence the survival.25, 26 These variables are not studied individually as the behaviour of the primary pathology would significantly influence them and would create issues with multi-collinearity as the prognostic index is developed. In terms of considering tumour markers, CA 19.9 is sensitive in making the diagnosis of patients with pancreatic cancer who are not jaundiced but has not been consistent in assessing the overall survival following PD for periampullary cancers27 and therefore was also not included in the study.
Brennan et al.14 in the published normogram from MSKCC, included adenocarcinoma of the head, body and tail of the pancreas whereas the current proposed model can be used for all the common tumour types of the head of the pancreas. In addition, MSKCC model included the number of positive and number of negative nodes but not the LNR, which is increasingly accepted as an important prognostic indicator.14, 21, 22 While no single prognostic index can include all the possible clinic-pathological modifiable factors especially with the variations in the tumour biology, it is important to identify and include the most significant variables in a prognostic index with good predictive accuracy. The proposed Pancreaticoduodenectomy Prognostic Index has been shown to be successful in estimating the prognosis. Potential uses include counselling patients, optimizing adjuvant therapeutic options and for risk stratification in clinical trials.
One of the limitations of this study is the retrospective analysis of the database. However, both institutions that provided the patient cohorts employ prospective data collection. Also, it was not possible to assess disease-specific survival (DSS), since cause of death data were not available for a proportion of patients, despite cross checking outcomes with the national cancer registry database. However, if it is assumed that deaths from other causes are “random”, in that they are not influenced by the factors considered (which would also have been an assumption if DSS was analysed), then the relationships observed between factors and OS should be comparable to those with DSS. The number of patients with duodenal carcinoma in the study is suboptimal and the prognostic index for this specific group needs further validation in larger multi-centre studies. Adjuvant chemotherapy did not have a significant influence on outcomes in this study. There appeared to be some benefit in the first year post-surgery (82% vs. 67% survival), but by three years, survival was similar whether or not it was used (40% vs. 41%) (p = 0.074). This could be due to the inconsistency in the indications and type of chemotherapy offered (based on 5FU, Irinotecan, Gemcitabine, Capecitabine) over the years for the four subtypes of peri-pancreatic cancers. Further variation in the practice was due to difference in the time to receiving chemotherapy and the actual numbers of cycles received.
This is one of the largest studies to assess the effects upon key oncological outcomes of various clinical and pathological variables after PD. This study demonstrates the importance of the LNR as a prognostic variable amongst common tumour types and goes further to develop, and validate, a prognostic index based on a different score given for each of the factors included within the index.
Funding sources
None.
Conflicts of interest
None to declare.
Footnotes
This study was presented at the AUGIS Scientific Meeting, 18–19 September 2014, Brighton.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.hpb.2015.11.008.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Matsuoka L., Selby R., Genyk Y. The surgical management of pancreatic cancer. Gastroenterol Clin North Am. 2012;41:211–221. doi: 10.1016/j.gtc.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 2.House M.G., Gönen M., Jarnagin W.R., D'Angelica M., DeMatteo R.P., Fong Y. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549–1555. doi: 10.1007/s11605-007-0243-7. [DOI] [PubMed] [Google Scholar]
- 3.Winter J.M., Cameron J.L., Campbell K.A., Arnold M.A., Chang D.C., Coleman J. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210–1. [DOI] [PubMed] [Google Scholar]
- 4.Richter A., Niedergethmann M., Sturm J.W., Lorenz D., Post S., Trede M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–329. doi: 10.1007/s00268-002-6659-z. [DOI] [PubMed] [Google Scholar]
- 5.Tseng J.F., Raut C.P., Lee J.E., Pisters P.W., Vauthey J.N., Abdalla E.K. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–949. doi: 10.1016/j.gassur.2004.09.046. discussion 949–50. [DOI] [PubMed] [Google Scholar]
- 6.Lemke J., Schäfer D., Sander S., Henne-Bruns D., Kornmann M. Survival and prognostic factors in pancreatic and ampullary cancer. Anticancer Res. 2014;34:3011–3020. [PubMed] [Google Scholar]
- 7.Dickson P.V., Behrman S.W. Distal cholangiocarcinoma. Surg Clin North Am. 2014;94:325–342. doi: 10.1016/j.suc.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Jabbour S.K., Mulvihill D. Defining the role of adjuvant therapy: ampullary and duodenal adenocarcinoma. Semin Radiat Oncol. 2014;24:85–93. doi: 10.1016/j.semradonc.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Kazanjian K.K., Hines O.J., Duffy J.P., Yoon D.Y., Cortina G., Reber H.A. Improved survival following pancreaticoduodenectomy to treat adenocarcinoma of the pancreas: the influence of operative blood loss. Arch Surg. 2008;143:1166–1171. doi: 10.1001/archsurg.143.12.1166. [DOI] [PubMed] [Google Scholar]
- 10.Wagner M., Redaelli C., Lietz M., Seiler C.A., Friess H., Buchler M.W. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 11.Hatzaras I., George N., Muscarella P., Melvin W.S., Ellison E.C., Bloomston M. Predictors of survival in periampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol. 2010 Apr;17:991–997. doi: 10.1245/s10434-009-0883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neoptolemos J.P., Stocken D.D., Friess H., Bassi C., Dunn J.A., Hickey H. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 13.Neoptolemos J.P., Dunn J.A., Stocken D.D., Almond J., Link K., Beger H. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 14.Brennan M.F., Kattan M.W., Klimstra D., Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neoptolemos J.P., Stocken D.D., Dunn J.A., Almond J., Beger H.G., Pederzoli P. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–768. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim J.E., Chien M.W., Earle C.C. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger A.C., Watson J.C., Ross E.A., Hoffman J.P. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2004;70:235–240. discussion 240. [PubMed] [Google Scholar]
- 18.Slidell M.B., Chang D.C., Cameron J.L., Wolfgang C., Herman J.M., Schulick R.D. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 19.Valsangkar N.P., Bush D.M., Michaelson J.S., Ferrone C.R., Wargo J.A., Lillemoe K.D. N0/N1, PNL, or LNR? the effect of lymph node number on accurate survival prediction in pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2013;17:257–266. doi: 10.1007/s11605-012-1974-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edge S.B., Byrd D.R., Compton C., editors. AJCC cancer staging manual. 7th ed. Springer; New York: 2010. [Google Scholar]
- 21.Pawlik T.M., Gleisner A.L., Cameron J.L., Winter J.M., Assumpcao L., Lillemoe K.D. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Riediger H., Keck T., Wellner U., zur Hausen A., Adam U., Hopt U.T. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg. 2009;13:1337–1344. doi: 10.1007/s11605-009-0919-2. [DOI] [PubMed] [Google Scholar]
- 23.Verbeke C.S., Leitch D., Menon K.V., McMahon M.J., Guillou P.J., Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–1237. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]
- 24.Menon K.V., Gomez D., Smith A.M., Anthoney A., Verbeke C.S. Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP) HPB. 2009;11:18–24. doi: 10.1111/j.1477-2574.2008.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J.W., Bhandari M., Astill D.S., Wilson T.G., Kow L., Brooke-Smith M. Predicting patient survival after pancreaticoduodenectomy for malignancy: histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB. 2010;12:101–108. doi: 10.1111/j.1477-2574.2009.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S.C., Shyr Y.M., Wang S.E. Longterm survival after pancreaticoduodenectomy for periampullary adenocarcinomas. HPB. 2013;15:951–957. doi: 10.1111/hpb.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballehaninna U.K., Chamberlain R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol. 2012 Jun;3:105–119. doi: 10.3978/j.issn.2078-6891.2011.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.