Abstract
Background
Data on prognostic implications of peri-operative blood transfusion around resection of colorectal cancer liver metastases (CRLM) are conflicting. This retrospective study assesses the association of transfusion with complications and disease-specific survival (DSS).
Methods
Major hepatectomies for CRLM from 2000 to 2010 at three institutions were included. Transfusion was analyzed based on timing and volume.
Results
Of 456 patients, 140 (30.7%) received transfusions. Transfusion was associated with extended hepatectomy (28.6 vs 18.4%; p = 0.020), tumor size (5.7 vs 4.2 cm; p < 0.001), and operative blood loss (917 vs 390 mL; p < 0.001). Transfusion was independently associated with major complications (OR 2.61; 95% CI: 1.53–4.44; p < 0.001). Transfusion at any time was not associated with DSS; however, patients who specifically received blood post-operatively had reduced DSS (37.4 vs 42.7 months; p = 0.044). Increased volume of transfusion (≥3 units) was also associated with shortened DSS (Total: 37.4 vs 41.5 months, p = 0.018; Post-operative: 27.2 vs 40.3 months, p = 0.015). On multivariate analysis, however, transfusion was not independently associated with worsened DSS, regardless of timing and volume.
Conclusion
Transfusion with major hepatectomy for colorectal cancer metastases is independently associated with increased complications but not disease-specific survival. Judicious use of transfusion per a blood utilization protocol in the peri-operative period is warranted.
Introduction
Colorectal cancer (CRC) is the third most common cancer in both men and women in the United States (US). A total of 136 830 new CRC cases were estimated to have been diagnosed in the US in 2014 with 50 310 patient deaths from disease, establishing CRC as the third-leading cause of cancer-related mortality.1 Metastases from CRC occur in approximately 50% of patients with the liver being the most common site of distant spread.2 Surgical resection of colorectal cancer liver metastases (CRLM) is a mainstay of treatment and encompasses procedures ranging from a small hepatic wedge resection to a major hepatectomy (resection of ≥3 liver segments).2, 3 Largely dependent on patient selection and the biology of disease, the 5-year survival after CRLM resection is estimated between 37 and 60%.2, 4, 5, 6 In order to optimize patient outcomes, factors associated with patient morbidity and mortality must be identified. Understanding these relationships may help guide treatment strategy to reduce recurrence and increase survival.
Peri-operative blood transfusion has been associated with decreased survival in patients with gastric adenocarcinoma, pancreatic adenocarcinoma, hepatocellular carcinoma, and lung cancer.7, 8, 9, 10, 11, 12 In resected, non-metastatic CRC, investigators have identified relationships between transfusion, earlier recurrence, and decreased survival.13, 14, 15, 16, 17, 18 In studies of metastatic CRC, 27–71% of patients undergoing hepatic resection for CRLM received blood transfusions.19, 20, 21, 22, 23, 24 These transfusions have been associated with increased post-operative morbidity.21, 24, 25 Data on the long-term prognostic implications of blood transfusion around the time of resection of CRLM are conflicting.19, 20, 21, 22, 26 Recent single institutional data have demonstrated the effectiveness of a blood utilization program in liver resection patients with favorable peri-operative results.27 The goal of this study was to assess the association of peri-operative transfusion with post-operative complications and long-term disease-specific survival (DSS) in a large, recent, multi-institutional cohort of patients who underwent major hepatectomy for CRLM.
Methods
Study population and data collection
Patients who underwent major hepatectomy, for CRLM between 2000 and 2010 were identified from a prospectively maintained surgical database at Emory University, University of Wisconsin, and University of Louisville. This study was approved by the Institutional Review Boards of all participating centers. Clinicopathologic, treatment, and follow-up data were obtained through clinical chart review. Transfusion was defined as receipt of packed red blood cells (pRBC) in the peri-operative period, either intra-operatively, post-operatively, or both. Post-operative blood transfusion was defined as receipt of pRBC in the post-operative hospital stay; this includes patients who received both intra-operative and post-operative transfusions but excludes those who received only intra-operative transfusion. Post-operative complications were defined using the Clavien-Dindo classification, as previously described; Clavien-Dindo class 3–5 complications were considered major complications.28 Clavien class 2 complications include receipt of blood transfusions and therefore comparative analyses of minor complications (Clavien 1 and 2) were not performed in this study. Survival data were gathered through review of the clinical chart and confirmed by the Social Security Death Index database. Disease-specific survival (DSS) was defined as the percentage of patients who did not die of colorectal cancer during the time period from major hepatectomy until the last clinical follow up date or date of death. For DSS analyses, 30-day mortalities, patients with less than 30-day follow-up, and patients who died of unknown cause were excluded. Primary outcomes were post-operative major complications and DSS.
Statistical analyses
Univariate comparative analyses to determine associations between blood transfusion and other variables were conducted using student's t-tests and Chi-squared or Fisher's exact tests for continuous and categorical variables, respectively. A p-value < 0.05 was considered statistically significant. Binary logistic regression was used to determine relationships of clinicopathologic and treatment variables with peri-operative outcomes. Statistically significant variables from univariate binary logistic regression were included in multivariate models. Kaplan–Meier log-rank tests were used to calculate DSS. Univariate Cox regression models determined factors associated with DSS in univariate analyses. Statistically significant variables from univariate Cox analyses were included in multivariate Cox proportional hazard models of DSS. Transfusion was analyzed based on timing and volume of transfusion. All statistical analyses were conducted using SPSS Statistics 21.0 software (IBM, Armonk, NY).
Results
Patient population
Between 2000 and 2010, 456 patients underwent major hepatectomy for CRLM. Of these patients, 140 (30.7%) received a transfusion with 89 (19.5%) receiving post-operative transfusions. Average operative blood loss was 553 ± 516 mL for all patients. Demographic and clinicopathologic details of the patient population included in this study are described in Table 1. Transfusion was associated with extended hepatectomy (28.6% vs 18.4%; p = 0.020), increased tumor size (5.7 cm vs 4.2 cm; p < 0.001), and increased operative blood loss (917 mL vs 390 mL; p < 0.001). Table 1 shows the differences between the patient populations who received transfusions and those who did not.
Table 1.
Clinicopathologic features of all patients stratified by receipt of pRBC transfusion
| Variable | All Patients (n = 456) | No transfusion (n = 316) | Transfusion (n = 140) | p-value |
|---|---|---|---|---|
| Male gender* | 252 (55.3%) | 184 (58.2%) | 68 (48.6%) | 0.070 |
| Race* | ||||
| Caucasian | 373 (81.8%) | 256 (81.8%) | 117 (84.8%) | 0.414 |
| African American | 59 (12.9%) | 45 (14.4%) | 14 (10.1%) | |
| Other | 19 (4.2%) | 15 (4.7%) | 9 (1.4%) | |
| BMI** (kg/m2; mean ± SD) | 27.5 ± 5.5 | 27.6 ± 5.1 | 27.2 ± 6.3 | 0.441 |
| Age** (years; mean ± SD) | 58.6 ± 12.0 | 58.0 ± 11.6 | 59.9 ± 13.0 | 0.147 |
| ASA Class* | ||||
| 1 | 0 (0%) | 0 (0%) | 0 (0%) | 0.067 |
| 2 | 74 (16.2%) | 59 (23.4%) | 15 (13.0%) | |
| 3 | 280 (61.4%) | 185 (73.4%) | 95 (82.6%) | |
| 4 | 13 (2.9%) | 8 (3.2%) | 5 (4.3%) | |
| 5 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Splenomegaly*** | 11 (2.4%) | 7 (2.2%) | 4 (2.9%) | 0.745 |
| Pre-operative portal vein embolization* | 21 (4.6%) | 14 (4.4%) | 7 (5.0%) | 0.973 |
| Extended hepatectomy* | 98 (21.5%) | 58 (18.4%) | 40 (28.6%) | 0.020 |
| Operative blood loss** (mL) | 553 ± 516 | 390 ± 293 | 917 ± 693 | <0.001 |
| Intraoperative ablation* | 79 (17.3%) | 52 (16.5%) | 27 (19.3%) | 0.547 |
| Margin Status*** | ||||
| R0 | 400 (88.5%) | 275 (87.9%) | 125 (89.9%) | 0.475 |
| R1 | 48 (10.6%) | 36 (11.5%) | 12 (8.6%) | |
| R2 | 4 (0.9%) | 2 (0.6%) | 2 (1.4%) | |
| Number of lesions** (mean ± SD) | 1.9 ± 1.2 | 2.0 ± 1.3 | 1.8 ± 0.9 | 0.145 |
| Largest tumor size** (cm; mean ± SD) | 4.6 ± 3.2 | 4.2 ± 2.8 | 5.7 ± 3.9 | <0.001 |
| Cirrhosis*** | 1 (0.2%) | 1 (0.6%) | 0 (0%) | 1.000 |
| Sclerosis*** | 13 (2.9%) | 9 (5.0%) | 4 (4.8%) | 1.000 |
| LVI* | 17 (3.7%) | 12 (9.6%) | 5 (10.4%) | 1.000 |
| PNI*** | 9 (2.0%) | 7 (5.7%) | 2 (4.3%) | 1.000 |
| Major vascular invasion*** | 3 (0.7%) | 3 (1.9%) | 0 (0%) | 0.557 |
| Steatosis* | 135 (29.6%) | 93 (47.4%) | 42 (47.7%) | 1.000 |
| Steatohepatitis*** | 4 (0.9%) | 4 (2.3%) | 0 (0%) | 0.321 |
| Length of stay** (days; mean ± SD) | 7 ± 22 | 5 ± 25 | 12 ± 12 | <0.001 |
| Major complication* | 96 (21.2%) | 59 (16.4%) | 26 (27.4%) | 0.022 |
| 90-day Readmission* | 72 (15.8%) | 39 (12.4%) | 33 (23.7%) | 0.004 |
| 30-day Mortality*** | 6 (1.3%) | 2 (0.6%) | 4 (2.9%) | 0.075 |
| Transfusion | 140 (30.7%) | – | – | – |
| Transfusion (total units; mean ± SD) | 1.2 ± 2.8 | – | – | – |
| Transfusion (total units) | ||||
| 0–2 | 390 (86.0%) | – | – | – |
| ≥3 | 64 (14.0%) | |||
| Post-operative transfusion | 89 (19.5%) | – | – | – |
| Post-operative transfusion (units; mean ± SD) | 0.72 ± 2.4 | – | – | – |
| Post-operative transfusion (units) | ||||
| 0–2 | 424 (93.0%) | – | – | – |
| ≥3 | 32 (7.0%) | |||
Statistical Tests: *Chi-squared test; **Independent t-test; *** Two-tailed Fisher's Exact test; Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiologists; LVI, lymphovascular invasion; PNI, perineural invasion; SD, standard deviation.
Significance of bold values indicate p <0.05.
Transfusion and post-operative outcomes
On univariate analysis, transfusion was associated with increases in major complication (27.4% vs 16.4%) and 90-day readmission (23.7% vs 12.4%) but was not associated with 30-day mortality (Table 1). Table 2 demonstrates perioperative and pathologic factors associated with major complications and 90-day readmission. When accounting for these factors in multivariate analysis, transfusion remained independently associated with increased major complications (OR 2.61; 95% CI 1.53–4.44; p < 0.001; Table 2) as well as increased 90-day readmission (OR 2.03; 95% CI 1.15–3.56; p = 0.014; Table 2).
Table 2.
Multivariate binary logistic regression analysis of risk factors for major complication and 90-day readmission
| Multivariate regression analysis of risk factors for major complication | ||||||
|---|---|---|---|---|---|---|
| Variable | Univariate |
Multivariate |
||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Male gender | 1.38 | 0.87–2.19 | 0.170 | |||
| BMI (kg/m2) | 0.99 | 0.96–1.04 | 0.977 | |||
| Age (yrs) | 1.01 | 0.99–1.03 | 0.204 | |||
| ASA 2 | Ref | Ref | Ref | Ref | Ref | Ref |
| ASA 3 | 1.56 | 0.79–3.08 | 0.197 | 1.31 | 0.65–2.66 | 0.445 |
| ASA 4 | 4.43 | 1.26–15.51 | 0.020 | 3.68 | 0.99–13.59 | 0.050 |
| Pre-operative portal vein embolization | 1.54 | 0.58–4.10 | 0.381 | |||
| Extended hepatectomy | 1.71 | 1.02–2.85 | 0.041 | 1.22 | 0.67–2.21 | 0.524 |
| Estimated blood loss (mL) | 1.00 | 0.071 | 1.00–1.00 | |||
| Number of lesions | 1.04 | 0.84–1.27 | 0.744 | |||
| Tumor size (cm) | 1.10 | 1.03–1.17 | 0.003 | 1.08 | 0.99–1.17 | 0.055 |
| Major vascular invasion | 1.67 | 0.15–18.8 | 0.677 | |||
| Transfusion | 3.08 | 1.94–4.91 | <0.001 | 2.61 | 1.53–4.44 | <0.001 |
| Multivariate regression analysis of risk factors for 90-day readmission | ||||||
|---|---|---|---|---|---|---|
| Variable | Univariate Analysis |
Multivariate Analysis |
||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Male gender | 1.53 | 0.91–2.57 | 0.11 | |||
| BMI (kg/m2) | 1.02 | 0.97–1.07 | 0.403 | |||
| Age (yrs) | 0.99 | 0.97–1.01 | 0.158 | |||
| ASA 2 | Ref | Ref | Ref | Ref | Ref | Ref |
| ASA 3 | 1.16 | 0.57–2.37 | 0.683 | 1.05 | 0.51–2.16 | 0.901 |
| ASA 4 | 6.68 | 1.89–23.66 | 0.003 | 6.09 | 1.69–21.92 | 0.006 |
| Pre-operative portal vein embolization | 0.89 | 0.25–3.10 | 0.855 | |||
| Extended hepatectomy | 0.78 | 0.41–1.49 | 0.782 | |||
| Estimated blood loss (mL) | 1.00 | 1.00–1.00 | 0.066 | |||
| Number of lesions | 0.96 | 0.76–1.23 | 0.757 | |||
| Tumor size (cm) | 1.06 | 0.98–1.13 | 0.135 | |||
| Major vascular invasion | 2.12 | 0.19–23.88 | 0.544 | |||
| Transfusion | 2.20 | 1.32–3.69 | 0.003 | 2.03 | 1.15–3.56 | 0.014 |
OR, Odds ratio; CI, confidence interval; Ref, reference.
Significance of bold values indicate p <0.05.
Transfusion and survival outcomes
Three hundred eighty-eight patients were included in survival analyses. The median follow-up was 38.8 months. On univariate Cox regression, decreased DSS was associated with positive resection margins (HR 2.00; 95% CI 1.23–3.28; p = 0.006), increased number of lesions (HR 1.16; 95% CI 1.05–1.29; p = 0.003), lymphovascular invasion (HR 2.97; 95% CI 1.37–6.46; P = 0.006), perineural invasion (HR 3.07; 95% CI 1.08–8.76; p = 0.036), and major complications (HR 2.01; 95% CI 1.39–2.91; p < 0.001; Table 3).
Table 3.
Univariate cox regression analysis for reduced disease-specific survival
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| Male gender | 0.85 | 0.62–1.17 | 0.327 |
| BMI | 1.00 | 0.97–1.03 | 0.847 |
| ASA 2 | Ref | Ref | Ref |
| ASA 3 | 1.07 | 0.66–1.74 | 0.783 |
| ASA 4 | 1.81 | 0.79–4.11 | 0.159 |
| Positive margin | 2.00 | 1.23–3.28 | 0.006 |
| Number of lesions | 1.16 | 1.05–1.29 | 0.003 |
| Largest tumor size | 1.04 | 1.00–1.08 | 0.077 |
| LVI | 2.97 | 1.37–6.46 | 0.006 |
| PNI | 3.07 | 1.08–8.76 | 0.036 |
| Major vascular invasion | 3.33 | 0.46–24.3 | 0.236 |
| Major complication | 2.01 | 1.39–2.91 | <0.001 |
| Operative blood loss (mL) | 1.00 | 1.00–1.00 | 0.912 |
| Transfusion | 1.36 | 0.98–1.88 | 0.069 |
| Transfusion ≥ 3 units | 1.63 | 1.08–2.46 | 0.019 |
| Post-operative transfusion | 1.46 | 1.01–2.11 | 0.045 |
| Post-operative transfusion ≥ 3 units | 2.01 | 1.13–3.56 | 0.017 |
BMI, body mass index; ASA, American Society of Anesthesiologists; LVI, lymphovascular invasion; PNI, perineural invasion; Ref, reference.
Significance of bold values indicate p <0.05.
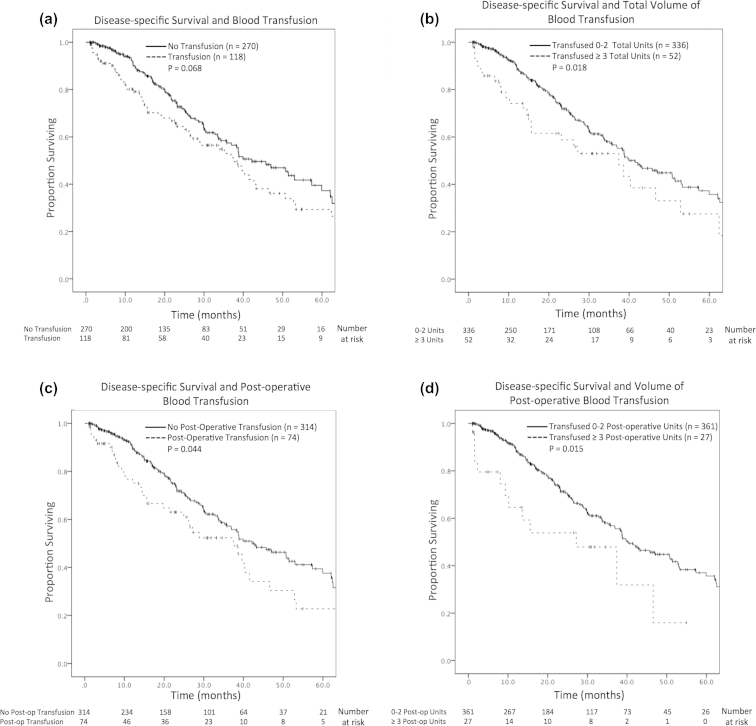
The relationship of DSS and transfusion was determined considering both the timing of the transfusion and the amount of blood transfused. There was no difference in DSS comparing patients who received blood transfusions and those who did not (37.4 months vs 42.1 months, p = 0.068; HR 1.36, CI 0.98–1.88, p = 0.069; Fig. 1a); however, receiving a total of ≥3 pRBC units at any time was associated with decreased DSS (37.4 months vs 41.5 months, p = 0.018; HR 1.63, 1.08–2.46, p = 0.019; Fig. 1b). Post-operative blood transfusion was associated with decreased DSS (37.4 months vs 42.7 months, p = 0.044; HR 1.46, 95% CI 1.01–2.11, p = 0.019; Fig. 1c), as was receiving ≥3 pRBC units post-operatively (27.2 months vs 40.3 months, p = 0.015; HR 2.01; 95% CI 1.13–3.56, p = 0.017; Fig. 1d). On multivariate analysis, however, neither the timing of blood transfusion nor the amount transfused was independently associated with reduced DSS (Table 4).
Figure 1.
Kaplan–Meier curves of disease-specific survival after major hepatectectomy for colorectal cancer liver metastases as related to transfusion of packed red blood cells. a) Receipt of transfusion. b) Receipt of ≥ 3 total units. c) Receipt of post-operative transfusion. d) Receipt of ≥ 3 post-operative units
Table 4.
Multivariate cox regression analysis of the total number of units transfused, receipt of post-operative transfusion, and number of post-operative units transfused with reduced disease-specific survival
| Multivariate cox regression analysis of the total number of units transfused and reduced disease-specific survival | |||
|---|---|---|---|
| Variable | HR | 95% CI | p-value |
| Positive margin | 1.83 | 0.87–3.82 | 0.109 |
| Number of lesions | 1.28 | 1.07–1.53 | 0.008 |
| LVI | 2.70 | 0.96–7.57 | 0.059 |
| PNI | 1.96 | 0.44–8.79 | 0.379 |
| Major complication | 1.79 | 0.94–3.40 | 0.076 |
| Transfusion ≥ 3 units | 0.39 | 0.09–1.66 | 0.202 |
| Multivariate cox regression analysis of receipt of post-operative transfusion and reduced disease-specific survival | |||
|---|---|---|---|
| Variable | HR | 95% CI | p-value |
| Positive margin | 2.02 | 0.96–4.23 | 0.064 |
| Number of lesions | 1.28 | 1.06–1.54 | 0.009 |
| LVI | 2.09 | 0.78–5.55 | 0.141 |
| PNI | 2.32 | 0.57–9.48 | 0.243 |
| Major complication | 1.92 | 1.02–3.64 | 0.044 |
| Post-operative transfusion | 1.52 | 0.70–3.28 | 0.291 |
| Multivariate cox regression analysis of number of post-operative units transfused and reduced disease-specific survival | |||
|---|---|---|---|
| Variable | HR | 95% CI | p-value |
| Positive margin | 1.76 | 0.84–3.68 | 0.136 |
| Number of lesions | 1.29 | 1.07–1.55 | 0.007 |
| LVI | 2.84 | 0.99–8.12 | 0.051 |
| PNI | 2.00 | 0.44–9.08 | 0.368 |
| Major complication | 1.84 | 0.97–3.50 | 0.063 |
| Post-operative transfusion ≥ 3 units | 0.28 | 0.04–2.18 | 0.224 |
LVI, lymphovascular invasion; PNI, perineural invasion.
Significance of bold values indicate p <0.05.
Discussion
This large, multi-institutional study examined the prognostic implications of blood transfusion around the time of major hepatectomy for CRLM. In the current study of 456 patients who underwent major hepatectomy for CRLM, 140 (30.7%) received blood transfusions. This rate of transfusion was similar to previously published rates for liver resections for this pathology (27–71%).19, 20, 21, 22, 23, 24 Transfusion was independently associated with increased post-operative morbidity including increased risk of major complications and 90-day readmission. Transfusion, regardless of timing and volume, was not independently associated with worsened DSS on multivariate analyses. These results represent outcomes generalizable to patients treated in the US, as this study considers the current practice and outcomes from 3 high-volume, academic centers over a recent 10-year period.
The literature from gastric adenocarcinoma, pancreatic adenocarcinoma, hepatocellular carcinoma, and lung cancer suggests that peri-operative transfusion is associated with earlier recurrence and decreased survival.7, 8, 9, 10, 11, 12 The mechanism behind this relationship is beyond the scope of this study but could be contributed to immunomodulation associated with blood transfusion.29 Increased immunotolerance after transfusion was first described in patients undergoing solid organ transplant where blood transfusion prior to kidney transplant was associated with longer graft survival.30 Data from subsequent studies have shown blood transfusion to be associated with differential expression and activity of cytokines, HLA class I antigens, and Fas ligand.13, 31 Some investigators propose that alterations in the immune system may lead to decreased tumor immune response leading to increased recurrence and decreased survival; however, these mechanisms are not well understood, and additional research in this area is indicated.
Results of studies of patients undergoing colectomy for non-metastatic CRC have shown blood transfusion to be associated with earlier recurrence and decreased survival.13, 14, 15, 16, 17, 18 The current study of patients with metastatic CRC differs from these studies in that an independent association between transfusion and DSS was not established. This could be related to differential biology of disease comparing patients with Stage I–III disease to those with Stage IV disease. It is possible that Stage IV disease, unlike earlier stage disease, represents a state of biologic aggressiveness that is refractory to the potential immunologic effects of transfusion. The underlying mechanism of pRBC transfusion association, or lack thereof, with survival requires further study.
Previous studies of transfusion and CRLM have yielded conflicting results when assessing recurrence and survival. Unlike the current study, some have found that transfusion during resection of CRLM is associated with earlier recurrence and decreased survival. These include studies, however, in which multivariate analyses considering other pathologic factors were not reported, and treatment regimens reflected management paradigms from the 1980s and early 1990s instead of current oncologic practice.20, 26 A recent single-center German study of 292 patients who underwent hepatic resection for CRLM reported that pRBC transfusion was independently associated with decreased recurrence-free survival but not with overall survival.22 Unlike the current study where only patients who underwent major hepatectomy were included, the German study includes all patients who underwent hepatic resection of any magnitude. Inclusion of patients with smaller, isolated metastases that did not require a major hepatectomy may suggest less aggressive disease and could account for the differences between the two studies.22 Furthermore, the incidence and need for transfusion during small non-anatomic hepatic wedge resections is also less common than for major hepatectomy, and inclusion of these patients in such studies may confound the analyses and outcomes.
Other studies, however, have yielded similar results to the current study.19, 21 In a single-institution study of 1351 patients who underwent CRLM resection from 1986 to 2001, 46% of patients underwent peri-operative RBC transfusion. Although transfusion was associated with decreased survival on univariate analysis, when considering other prognostic factors, transfusion was not independently associated with survival (HR: 1.1; 95% CI: 0.9–1.3; p = 0.37). The current study supports these results in a more recent population treated at three different institutions that required major hepatectomy.
Although transfusion was not associated with negative long-term outcomes in this study, a relationship between the immediate post-operative course and transfusion was established. Peri-operative transfusion has previously been reported to be associated with increased length of stay, increased complications, and increased 30-day mortality in studies of patients undergoing liver resections.24, 25, 32 Kooby et al. found that blood transfusion was independently associated with developing a post-operative complication.21 Similarly, the current study reported an association of transfusion with developing a major complication and with 90-day readmission that persisted in multivariate analysis when accounting for other predictive factors. Although transfusion was not related to long-term outcomes, it was associated with negative outcomes in the post-operative period.
As such, the threshold to transfuse should ideally be standardized and dictated by a transfusion protocol. Data from prospective, randomized control trials suggest that in the critically ill population, restrictive transfusion below a hemoglobin of 7 g/dL or for symptomatic anemia is equivalent and potentially superior to more liberal transfusion.33 In a recent study of patients undergoing liver resection at a single institution, a similar restrictive blood transfusion protocol was implemented. For the timeframe during which the protocol was followed, fewer patients received transfusion, and there was no increase in adverse outcomes.27 Widespread adoption of such a protocol for major hepatectomy should be considered.
This study is limited by its retrospective design. Interpretation of results is restricted to defining associations between transfusion and outcomes, as causality cannot be determined. Additionally, data on the pathology and treatment for the primary tumor were not available. Among the 3 participating institutions, there was no universal transfusion protocol. This may introduce a selection bias for transfusion, as the decision to transfuse was largely practitioner dependent and based on specific clinical scenarios. Regardless, this data is representative of the current practice patterns from three high volume centers and thus, is representative of current practice across the US.
Conclusions
Peri-operative blood transfusion with major hepatectomy for colorectal cancer metastases is independently associated with increased complications and 90-day readmission rates; however, transfusion was not associated with reduced disease-specific survival when accounting for other adverse prognostic factors. Judicious use of blood transfusion in the peri-operative period is warranted and should be under the direction of a transfusion protocol.
Footnotes
This study was presented at the Annual Meeting of the AHPBA, 11–15 March 2015, Miami, Florida.
Funding
This study is supported in part by the Katz Foundation.
Conflict of interest
None declared.
References
- 1.Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Smith J.J., D'Angelica M.I. Surgical management of hepatic metastases of colorectal cancer. Hematol Oncol Clin North Am. 2015;29:61–84. doi: 10.1016/j.hoc.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Jarnagin W.R., Gonen M., Fong Y., DeMatteo R.P., Ben-Porat L., Little S. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fong Y., Fortner J., Sun R.L., Brennan M.F., Blumgart L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choti M.A., Sitzmann J.V., Tiburi M.F., Sumetchotimetha W., Rangsin R., Schulick R.D. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardona K., Mastrodomenico P., D'Amico F., Shia J., Gonen M., Weiser M.R. Detailed pathologic characteristics of the primary colorectal tumor independently predict outcome after hepatectomy for metastases. Ann Surg Oncol. 2013;20:148–154. doi: 10.1245/s10434-012-2540-y. [DOI] [PubMed] [Google Scholar]
- 7.Asahara T., Katayama K., Itamoto T., Yano M., Hino H., Okamoto Y. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg. 1999;23:676–680. doi: 10.1007/pl00012367. [DOI] [PubMed] [Google Scholar]
- 8.Kwon A.H., Matsui Y., Kamiyama Y. Perioperative blood transfusion in hepatocellular carcinomas: influence of immunologic profile and recurrence free survival. Cancer. 2001;91:771–778. [PubMed] [Google Scholar]
- 9.Kneuertz P.J., Patel S.H., Chu C.K., Maithel S.K., Sarmiento J.M., Delman K.A. Effects of perioperative red blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol. 2011;18:1327–1334. doi: 10.1245/s10434-010-1476-3. [DOI] [PubMed] [Google Scholar]
- 10.Sun C., Wang Y., Yao H.S., Hu Z.Q. Allogeneic blood transfusion and the prognosis of gastric cancer patients: systematic review and meta-analysis. Int J Surg. 2014;13:102–110. doi: 10.1016/j.ijsu.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 11.Sutton J.M., Kooby D.A., Wilson G.C., Squires M.H., 3rd, Hanseman D.J., Maithel S.K. Perioperative blood transfusion is associated with decreased survival in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma: a multi-institutional study. J Gastrointest Surg. 2014;18:1575–1587. doi: 10.1007/s11605-014-2567-4. [DOI] [PubMed] [Google Scholar]
- 12.Wang T., Luo L., Huang H., Yu J., Pan C., Cai X. Perioperative blood transfusion is associated with worse clinical outcomes in resected lung cancer. Ann Thorac Surg. 2014;97:1827–1837. doi: 10.1016/j.athoracsur.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 13.Miki C., Hiro J., Ojima E., Inoue Y., Mohri Y., Kusunoki M. Perioperative allogeneic blood transfusion, the related cytokine response and long-term survival after potentially curative resection of colorectal cancer. Clin Oncol R Coll Radiol. 2006;18:60–66. doi: 10.1016/j.clon.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Burrows L., Tartter P. Effect of blood transfusions on colonic malignancy recurrent rate. Lancet. 1982;2:662. doi: 10.1016/s0140-6736(82)92764-7. [DOI] [PubMed] [Google Scholar]
- 15.Foster R.S., Jr., Costanza M.C., Foster J.C., Wanner M.C., Foster C.B. Adverse relationship between blood transfusions and survival after colectomy for colon cancer. Cancer. 1985;55:1195–1201. doi: 10.1002/1097-0142(19850315)55:6<1195::aid-cncr2820550610>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Tartter P.I. The association of perioperative blood transfusion with colorectal cancer recurrence. Ann Surg. 1992;216:633–638. doi: 10.1097/00000658-199212000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houbiers J.G., Brand A., van de Watering L.M., Hermans J., Verwey P.J., Bijnen A.B. Randomised controlled trial comparing transfusion of leucocyte-depleted or buffy-coat-depleted blood in surgery for colorectal cancer. Lancet. 1994;344:573–578. doi: 10.1016/s0140-6736(94)91965-8. [DOI] [PubMed] [Google Scholar]
- 18.Busch O.R., Hop W.C., Hoynck van Papendrecht M.A., Marquet R.L., Jeekel J. Blood transfusions and prognosis in colorectal cancer. N Engl J Med. 1993;328:1372–1376. doi: 10.1056/NEJM199305133281902. [DOI] [PubMed] [Google Scholar]
- 19.Cannon R.M., Brown R.E., St Hill C.R., Dunki-Jacobs E., Martin R.C., 2nd, McMasters K.M. Negative effects of transfused blood components after hepatectomy for metastatic colorectal cancer. Am Surg. 2013;79:35–39. [PubMed] [Google Scholar]
- 20.Rosen C.B., Nagorney D.M., Taswell H.F., Helgeson S.L., Ilstrup D.M., van Heerden J.A. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992;216:493–504. doi: 10.1097/00000658-199210000-00012. discussion 504–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kooby D.A., Stockman J., Ben-Porat L., Gonen M., Jarnagin W.R., Dematteo R.P. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869. doi: 10.1097/01.SLA.0000072371.95588.DA. discussion 869–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiergens T.S., Rentsch M., Kasparek M.S., Frenes K., Jauch K.W., Thasler W.E. Impact of perioperative allogeneic red blood cell transfusion on recurrence and overall survival after resection of colorectal liver metastases. Dis Colon Rectum. 2015;58:74–82. doi: 10.1097/DCR.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 23.Correa-Gallego C., Gonen M., Fischer M., Grant F., Kemeny N.E., Arslan-Carlon V. Perioperative complications influence recurrence and survival after resection of hepatic colorectal metastases. Ann Surg Oncol. 2013;20:2477–2484. doi: 10.1245/s10434-013-2975-9. [DOI] [PubMed] [Google Scholar]
- 24.Gruttadauria S., Saint Georges Chaumet M., Pagano D., Marsh J.W., Bartoccelli C., Cintorino D. Impact of blood transfusion on early outcome of liver resection for colorectal hepatic metastases. J Surg Oncol. 2011;103:140–147. doi: 10.1002/jso.21796. [DOI] [PubMed] [Google Scholar]
- 25.Konopke R., Kersting S., Bunk A., Dietrich J., Denz A., Gastmeier J. Colorectal liver metastasis surgery: analysis of risk factors predicting postoperative complications in relation to the extent of resection. Int J Colorectal Dis. 2009;24:687–697. doi: 10.1007/s00384-009-0669-3. [DOI] [PubMed] [Google Scholar]
- 26.Stephenson K.R., Steinberg S.M., Hughes K.S., Vetto J.T., Sugarbaker P.H., Chang A.E. Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resection of colorectal liver metastases. Ann Surg. 1988;208:679–687. doi: 10.1097/00000658-198812000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wehry J., Cannon R., Scoggins C.R., Puffer L., McMasters K.M., Martin R.C. Restrictive blood transfusion protocol in liver resection patients reduces blood transfusions with no increase in patient morbidity. Am J Surg. 2015;209:280–288. doi: 10.1016/j.amjsurg.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vamvakas E.C., Blajchman M.A. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–348. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Opelz G., Terasaki P.I. Dominant effect of transfusions on kidney graft survival. Transplantation. 1980;29:153–158. doi: 10.1097/00007890-198002000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Ghio M., Contini P., Mazzei C., Merlo A., Filaci G., Setti M. In vitro immunosuppressive activity of soluble HLA class I and Fas ligand molecules: do they play a role in autologous blood transfusion? Transfusion. 2001;41:988–996. doi: 10.1046/j.1537-2995.2001.41080988.x. [DOI] [PubMed] [Google Scholar]
- 32.Virani S., Michaelson J.S., Hutter M.M., Lancaster R.T., Warshaw A.L., Henderson W.G. Morbidity and mortality after liver resection: results of the patient safety in surgery study. J Am Coll Surg. 2007;204:1284–1292. doi: 10.1016/j.jamcollsurg.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 33.Hebert P.C., Wells G., Blajchman M.A., Marshall J., Martin C., Pagliarello G. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion requirements in critical care investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]