Abstract
We aimed to establish whether the presence of hepatic steatosis influences outcome after resection of colorectal liver metastases (CLM).
Patients and methods
Patients operated between 1990 and 2014 were divided into four groups based on the degree of hepatic steatosis. The association between hepatic steatosis and outcome was analyzed, using a multivariate and a propensity score case-match analysis.
Results
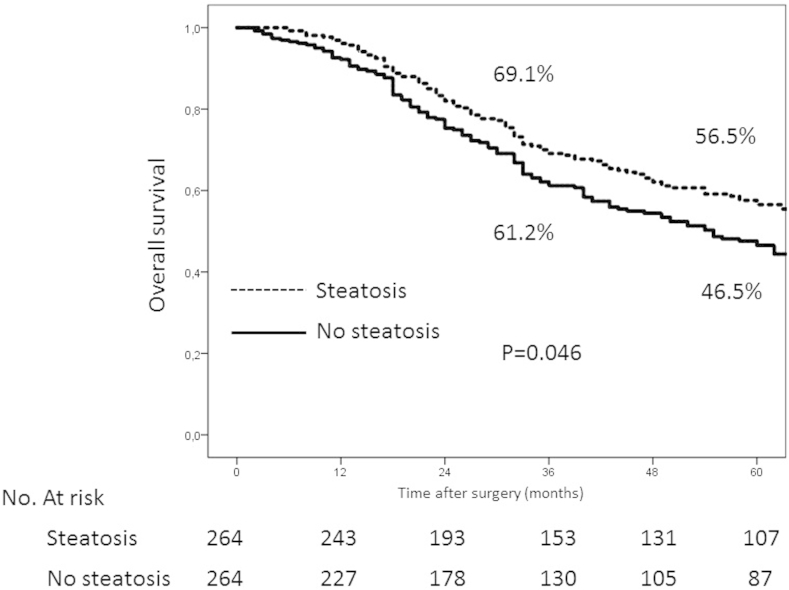
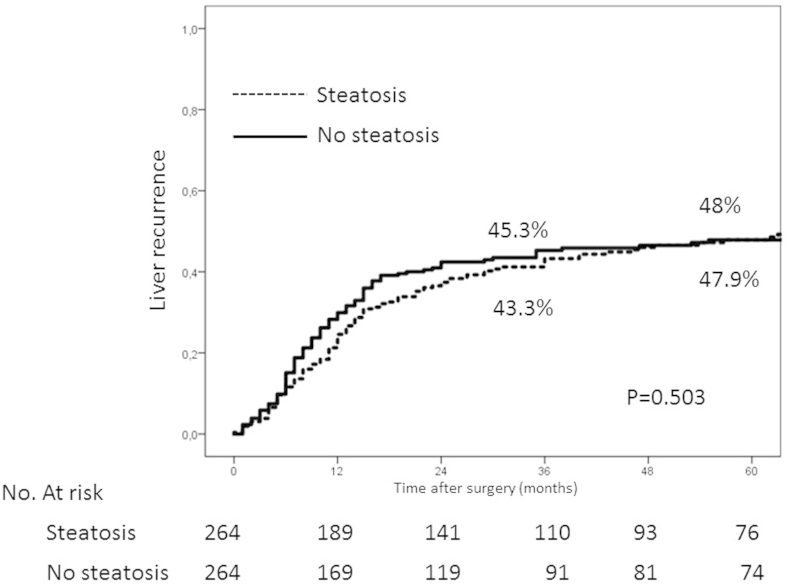
No significant differences were observed between patients with and without steatosis in either mortality or morbidity in the complete series or after matching (3.2% vs. 3.5%/p = 0.845) (32.3% vs 31.4%/p = 0.802). Five-year survival in patients with and without steatosis were 56.5% and 46.5% respectively (p = 0.046). The steatosis had a significant protective effect in the univariate analysis (HR (95% CI) = 0.78 (0.62–0.99) p = 0.048), and was close to significance in the multivariate analysis (HR (95%) = 0.81 (0.63–1.03) p = 0.089). No significant differences were seen with regard to liver recurrence.
Conclusions
The presence of steatosis does not predict short-outcome after resection of CLM, but appears to be a favorable prognostic factor for survival. This protective effect does not depend on a decrease in liver recurrence.
Introduction
Steatosis is the most common histopathological alteration of the liver. It affects more than 30% of the western population1, 2, 3 and consists of the accumulation of triglycerides inside the liver cells. The reported incidence in patients operated on for liver metastases from colorectal carcinoma (CLM) is highly variable (20–80%).4, 5, 6, 7 The etiology of liver steatosis is multifactorial but in patients with CLM it is often associated with obesity, preoperative chemotherapy (especially regimens that include irinotecan) and alcohol consumption.8, 9, 10
Some reports have described an association between steatosis and increased postoperative morbidity and mortality, particularly after major resection.11 It has also been reported that hepatic steatosis may affect the long-term results of resection of CLM.12, 13, 14 As a result of these observations, some authors5, 11, 12, 14, 15, 16 have suggested that a specific surgical strategy should be designed for patients with CLM who present steatosis.
The aim of this study is to analyze the effect of hepatic steatosis on the results of postoperative morbidity and mortality, survival, and liver recurrence in patients who had undergone surgery for CLM in a single-center prospective series.
Patients and methods
A prospectively compiled database including all patients operated upon for CLM between January 1990 and December 2014 was analyzed retrospectively. The data of the patients were anonymized for the purposes of this analysis. Written informed consent was considered not necessary for the study, as it is a retrospective analysis of our usual everyday work. This study was approved by the Clinical Research Ethics Committee of the University Hospital of Bellvitge. The patients were selected for surgery unless they presented unresectable extrahepatic disease and provided that the planned liver remnant was considered sufficient. The preoperative extension study was performed using multislice CT with intravenous contrast, and from 2000 onwards MRI with gadolinium was added in patients with hepatic steatosis.
Preoperative chemotherapy based on 5FU and folinic acid protocols or oxaliplatin regimens was administered to 43.9% of patients. Fewer than 5% of patients received irinotecan-based protocols.
During surgery an exploratory laparotomy and intraoperative ultrasound were performed to detect any lesions that had gone unnoticed in the preoperative study and the Pringle maneuver was used at the discretion of the surgeon. The ISGLS definition of liver failure17 was used.
After surgery, all patients were referred to the oncology department where the indication of adjuvant chemotherapy was assessed.
The surgical patients were seen every six months for a physical examination, measurement of carcinoembryonic antigen and imaging study (CT or MRI). Patients with hepatic recurrence were treated with re-resection whenever possible. The degree of liver steatosis was assessed only in the first resection specimen by a specialized pathologist, who was not aware of the anthropometric characteristics of the patients.
Definitions
Liver metastases were categorized as synchronous when diagnosed simultaneously, or within three months of the diagnosis of the primary tumor. Major resection was considered as the removal of three or more segments.
Steatosis was defined as the presence of fat vacuoles affecting more than 5% of liver cells. To assess the influence of the degree of steatosis on the results, a qualitative variable with four grades was created: no steatosis (0–5%), mild steatosis (>5% and <30%) moderate steatosis (30%–60%) and severe steatosis (>60%).
Because of the length of the study period, it was divided into three subperiods: 1990–2004, 2005–2009, and 2010–2014, each including approximately 300 patients. Tumor-free margins of less than 1 mm were considered affected.
Postoperative complications where classified according to Dindo-Clavien system.18
Statistical analysis
Qualitative variables were compared between groups using the Chi-square test, and quantitative variables using the Student t test. Survival and tumor recurrence were analyzed using the Kaplan–Meier test and the log-rank test was applied to compare survival between groups.
To avoid the bias related to the different distribution of covariates among patients with and without steatosis, a propensity score analysis was carried out to obtain a one–one match with an acceptable matching difference of up to 0.1. The covariates used in the model are specified in the results section. Once the groups were obtained, the differences in the variables were reanalyzed to confirm that the matching was adequate.
Subsequently, a multivariate analysis of predictors of postoperative mortality and morbidity was performed using the logistic regression model, and prognostic factors for survival and liver recurrence were assessed using the Cox model. The variables that were significant in the univariate analysis (p < 0.1) were included in the multivariate analysis. The results are expressed as a hazard ratio (HR) with 95% confidence intervals. The statistical analysis was performed using SPSS version 22.0 software (IBM, Armonk, New York, USA).
Results
During the study period, 1271 CLM interventions were performed in 1163 patients. After excluding patients for whom no data on the percentage of steatosis were available, patients with fibrosis and lost to follow-up, the population was reduced to 934 cases. In this population, the incidence of steatosis was 45% and the mean follow-up time was 47.05 (SD = 41.8) months. Steatosis was mild in 30.2% of patients, moderate in 10.7%, and severe in 4.2%. In the patients who received preoperative chemotherapy (44.2%), the rate of steatosis was similar to that observed in untreated patients (41.9% vs 47.7%, p = 0.078).
Postoperative mortality and morbidity
No significant differences in postoperative mortality at 90 days were observed between patients with and without steatosis (Table 1), or between patients with different degrees of steatosis (mild: 3.5%, moderate: 3%, severe: 2.6%, p = 0.931).
Table 1.
Characteristics of 934 patients who underwent resection for colorectal liver metastases stratified according to the presence of hepatic steatosis
| All patients n = 934 | With steatosis n = 421 | Without steatosis n = 513 | P-value | |
|---|---|---|---|---|
| Age, years, mean (SD) | 62.7 (10.5) | 62.6 (9.9) | 62.9 (11.0) | 0.644 |
| Age > 70 years, n (%) | 243 (26) | 97 (23) | 146 (28.5) | 0.052 |
| Male gender, n (%) | 643 (68.8) | 295 (70.1) | 348 (67.8) | 0.463 |
| Site of primary tumor | 0.079 | |||
| (Colon/Rectum) | 574/360 | 272/149 | 302/211 | |
| Portal vein embolization, n (%) | 68 (7.2) | 31 (7.4) | 37 (7.2) | 0.541 |
| Major liver resection, n (%) | 480 (51.3) | 211 (50.1) | 269 (52.5) | 0.116 |
| Year band, n (%) | 0.026 | |||
| 1990–2004 | 325 (34.7) | 161 (38.2) | 164 (31.9) | |
| 2005–2009 | 316 (33.8) | 146 (34.6) | 170 (33.3) | |
| 2010–2014 | 293 (31.5) | 114 (27.2) | 179 (34.8) | |
| Hilar clamping, n (%) | 730 (78.1) | 350 (83.5) | 380 (71.1) | 0.002 |
| Extra-hepatic disease, n (%) | 154 (16.4) | 59 (14) | 95 (18.5) | 0.067 |
| Preoperative chemotherapy, n (%) | 413 (44.2) | 173 (41.2) | 240 (47) | 0.078 |
| Postoperative results | ||||
| Perioperative transfusion, n (%) | 122 (13) | 54 (12.8) | 68 (13.4) | 0.802 |
| Liver failure, n (%) | 69 (7.4) | 35 (8.3) | 34 (6.6) | 0.327 |
| Hospital stay, mean (SD) | 11.08 (8.5) | 11.1 (8.8) | 11.0 (8.3) | 0.904 |
| Mortality, n (%) | 28 (3) | 14 (3.3) | 14 (2.7) | 0.595 |
| Postoperative complications, n %) | 297 (31.8) | 132 (31.4) | 165 (32.2) | 0.810 |
| Dindo-Clavien classification n (%) | 0.824 | |||
| I | 80 (26.9) | 32 (24.2) | 48 (29.1) | |
| II | 96 (32.3) | 41 (31.1) | 55 (33.3) | |
| IIIa | 66 (22.2) | 31 (23.5) | 35 (21.2) | |
| IIIb | 18 (6.1) | 10 (7.6) | 8 (4.8) | |
| IVa | 9 (3.0) | 4 (3.0) | 5 (3.0) | |
| V | 28 (9.4) | 14 (10.6) | 14 (8.5) | |
No significant differences in mortality were observed after major resection (without steatosis: 4.5%, mild: 6.2%, moderate: 5.9%, and severe: 7.1%, p = 0.867) even after hilar clamping longer than 20 min (6.2%, 6%, 2.9% and 8.3% respectively, p = 0.087).
Patients with severe steatosis had a significantly lower proportion of major resections than patients without steatosis (35.9% vs 52.5%, p = 0.036). Only 14 patients with steatosis above 60% underwent major resection. A trend was also seen towards a lower proportion of patients over 70 years (15.4% vs 28.5%, p = 0.077) and a less frequent indication of preoperative chemotherapy (31.6% vs 47%, p = 0.066). These differences were not observed with the other grades of steatosis.
No significant differences were observed in morbidity either when comparing patients with and without steatosis (Table 1), or when comparing patients with different degrees of the condition (mild: 29.4%, moderate: 39.4%, severe: 25.6%, p = 0.254). Statistically significant differences were neither observed in the distribution of complications according to Dindo-Clavien classification.
Since the two study groups were not fully comparable (Table 1) cases were matched one-to-one using the pre- and intra-operative variables displayed in Table 2. Two groups of 404 patients were obtained who were fully comparable for the variables included in the model and who did not present significant differences in postoperative outcomes.
Table 2.
Characteristics of 808 matched patients who underwent resection for colorectal liver metastases stratified according to the presence of hepatic steatosis
| With steatosis n = 404 | Without steatosis n = 404 | p | |
|---|---|---|---|
| Matching variables | |||
| Age >70 years, n (%) | 97 (24) | 93 (23) | 0.740 |
| Male gender, n (%) | 284 (70.3) | 279 (69.1) | 0.720 |
| Site of primary tumor (Colon/Rectum) | 255/149 | 247/157 | 0.562 |
| Portal vein embolization, n (%) | 30 (7.4) | 30 (7.4) | 1.000 |
| Major liver resection, n (%) | 206 (51) | 209 (51.7) | 0.833 |
| Year band, n | 0.383 | ||
| 1990–2004 | 151 | 150 | |
| 2005–2009 | 143 | 128 | |
| 2010–2014 | 110 | 126 | |
| Hilar clamping, n (%) | 336 (83.2) | 336 (83.2) | 1.000 |
| Extra-hepatic disease, n (%) | 59 (14.6) | 65 (16.1) | 0.558 |
| Preoperative chemotherapy, n (%) | 172 (42.6) | 173 (42.8) | 0.943 |
| Postoperative results | |||
| Perioperative transfusion, n (%) | 52 (12.9) | 54 (13.4) | 0.835 |
| Liver failure, n (%) | 33 (8.2) | 32 (7.9) | 0.897 |
| Hospital stay, mean (SD) | 11.2 (8.9) | 10.7 (7.9) | 0.350 |
| Mortality, n (%) | 13 (3.2) | 14 (3.5) | 0.845 |
| Postoperative complications, n (%) | 130 (32.3) | 127 (31.4) | 0.802 |
| Dindo-Clavien classification n (%) | 32 (24.6) | 37 (29.1) | 0.962 |
| I | 42 (32.3) | 39 (30.7) | |
| II | 30 (23.1) | 26 (20.5) | |
| IIIa | 9 (6.9) | 7 (5.5) | |
| IIIb | 4 (3.1) | 4 (3.1) | |
| IVa | 13 (10.0) | 14 (11.0) | |
| V | |||
Again, patients with severe steatosis had a lower proportion of major resections than patients without steatosis (35.5% vs 51.7%, p = 0.045).
In the multivariate logistical regression model, age over 70 years and major resection were predictors of postoperative mortality (Table 3). The presence of steatosis was not a predictor of mortality, not even when its influence among patients older than 70 years undergoing major resection (7.4% vs 12.5%, p = 0.351) was analyzed.
Table 3.
Univariate and multivariate analysis (Logistic regression) of postoperative mortality in 934 patients who underwent resection for colorectal liver metastases
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| Hazard ratio (IC 95%) | p | Hazard ratio (IC 95%) | p | ||
| Age > 70 years, n (%) | 243 (26) | 2.95 (1.38–6.28) | 0.005 | 5.25 (2.02–13.65) | 0.001 |
| Male gender, n (%) | 643 (73.9) | 1.37 (0.57–3.25) | 0.477 | ||
| Site of primary tumor (Colon/Rectum) | 0.278 | ||||
| 643/291 | 1.58 (0.69–3.63) | ||||
| Portal vein embolization, n (%) | 68 (7.3) | 4.61 (1.86–11.2) | 0.001 | 2.89 (1.13–7.35) | 0.002 |
| Major liver resection, n (%) | 480 (51.4) | 8.09 (2.42–27.00) | 0.001 | 7.07 (2.06–24.25) | 0.002 |
| Year band, n | 0.368 | ||||
| 1990–2003 | 325 | ||||
| 2004–2009 | 316 | 0.70 (0.29–1.67) | 0.425 | ||
| 2010–2014 | 293 | 0.50 (0.18–1.33) | 0.168 | ||
| Hilar clamping, n (%) | 730 (78.2) | 1.62 (0.55–4.73) | 0.375 | ||
| Hilar clamping > 20 min, n (%) | 496 (53.1) | 1.15 (0.54–2.47) | 0.705 | ||
| Extra-hepatic disease, n (%) | 154 (16.5) | 1.39 (0.55–3.49) | 0.478 | ||
| Preoperative chemotherapy, n % | 413 (44.2) | 1.00 (0.46–2.16) | 0.993 | ||
| Perioperative transfusion, n (%) | 122 (13.1) | 3.91 (1.76–8.69) | 0.001 | ||
| Steatosis (Y/N), n | 421/513 | 1.22 (0.57–2.60) | 0.595 | ||
| Steatosis degree, n | 0.931 | ||||
| No | 512 | ||||
| >5 y <30% | 282 | 1.31 (0.57–2.99) | 0.521 | ||
| 30–60% | 100 | 1.10 (0.31–3.90) | 0.880 | ||
| >60% | 39 | 0.93 (0.12–7.32) | 0.951 | ||
Predictors of postoperative morbidity were age over 70 years (HR (95% CI) = 1.44 (1.04–1.99) p = 0.027), major resection (HR (95% CI) = 1.65 (1.22–2.24) p = 0.001), and intra-operative transfusion (HR (95% CI) = 2.53 (1.69–3.79) p = 0.0001).
Survival
After excluding postoperative mortality, the sample size was reduced to 906 patients. Five-year survival values in patients with and without steatosis were 55.1% and 45.2% respectively (p = 0.006). However, the two groups of patients were not fully comparable (Table 4). Patients with mild (p = 0.048) and moderate steatosis (p = 0.033) showed better survival than patients without steatosis, but patients with severe steatosis did not (p = 0.142).
Table 4.
Characteristics, related to long-term survival, of 906 patients who underwent resection for colorectal liver metastases, stratified according to the presence of hepatic steatosis. Postoperative 90-days mortality was excluded
| With steatosis n = 407 | Without steatosis n = 499 | p | |
|---|---|---|---|
| Age >70 years, n (%) | 90 (22.1) | 139 (27.8) | 0.046 |
| Male gender, n (%) | 283 (69.5) | 339 (67.9) | 0.606 |
| Synchronous disease, n (%) | 194 (47.7) | 317 (63.5) | 0.0001 |
| Site of primary tumor (Colon/Rectum) | 261/146 | 293/206 | 0.104 |
| Stage of primary tumor, pT (1/2/3/4) n | 5/38/287/77 | 8/46/346/9 | 0.944 |
| Nodal invasion of primary tumor, n % | 265 (65.1) | 323 (64.7) | 0.938 |
| CEA level at hepatectomy > 5 ng/mL, n (%) | 149 (42.6) unknown 57 (14%) | 204 (47.6) unknown 70 (14%) | 0.165 |
| Bilateral disease, n % | 184 (45.2) | 203 (40.8) | 0.179 |
| Portal vein embolization, n % | 25 (6.1) | 36 (7.2) | 0.444 |
| Major liver resection, n (%) | 198 (48.6) | 257 (51.6) | 0.101 |
| Intraoperative radiofrequency, n % | 40 (9.8) | 44 (8.8) | 0.602 |
| Year band, n | 0.020 | ||
| 1990–2004 | 154 | 158 | |
| 2005–2009 | 143 | 164 | |
| 2010–2014 | 110 | 177 | |
| Hilar clamping, n % | 337 (83.2) | 369 (75) | 0.003 |
| Perioperative transfusion, n % | 50 (12.3) | 62 (12.6) | 0.904 |
| Extra-hepatic disease at hepatectomy, n % | 53 (13.1) | 95 (19) | 0.015 |
| Postoperative complications, n % | 118 (29.1) | 151 (30.3) | 0.695 |
| Liver failure, n % | 24 (5.9) | 24 (4.8) | 0.467 |
| Margin invasion, n % | 74 (18.1) | 81 (16.2) | 0.548 |
| Number of liver metastases > 3, n (%) | 91 (22.3) | 107 (21,4) | 0.882 |
| Largest metastases diameter > 5 cm, n (%) | 66 (16.2) | 78 (15.6) | 0.929 |
| Preoperative chemotherapy, n% | 165 (40.5) | 236 (47.5) | 0.037 |
| Adjuvant chemotherapy, n % | 258 (74.1) unknown 15 (3.7%) | 321 (77.5) unknown 20 (4%) | 0.274 |
After a one-to-one matching of patients on the basis of the variables in Table 4, two groups of 264 fully comparable patients were obtained (Table 5). In this sample, five-year survival values for patients with and without steatosis were 56.5% and 46.5% respectively (p = 0.046) (Fig. 1 Supplementary).
Table 5.
Characteristics, related to long-term survival, of 528 matched patients who underwent resection for colorectal liver metastases, stratified according to the presence of hepatic steatosis. Postoperative 90-days mortality was excluded
| With steatosis n = 264 | Without steatosis n = 264 | p | |
|---|---|---|---|
| Age >70 years, n (%) | 57 (21.5) | 60 (22.7) | 0.753 |
| Male gender, n (%) | 181 (68.6) | 176 (66.7) | 0.642 |
| Synchronous disease | 129 (48.9) | 143 (54.2) | 0.223 |
| Site of primary tumor (Colon/Rectum) | 164/100 | 165/99 | 0.928 |
| Stage of primary tumor, pT (1/2/3/4) n | 2/22/201/39 | 4/24/186/50 | 0.441 |
| Nodal invasión of primary tumor, n % | 180 (68.2) | 180 (68,2) | 1.000 |
| CEA level at hepatectomy > 5 ng/mL, n (%) | 147 (55.7) | 143 (54,2) | 0.726 |
| Bilateral disease, n % | 117 (44.3) | 112 (42,4) | 0.661 |
| Portal vein embolization, n % | 13 (4.9) | 16 (6,1) | 0.567 |
| Major liver resection, n (%) | 136 (51.5) | 140 (53) | 0.727 |
| Intraoperative radiofrequency, n % | 26 (9.8) | 26 (9.8) | 1.000 |
| Year band, n | 121 | 123 | 0.886 |
| 1990–2004 | 87 | 82 | |
| 2005–2009 | 56 | 59 | |
| 2010–2014 | |||
| Hilar clamping, n % | 222 (84.1) | 211 (79) | 0.213 |
| Perioperative transfusion, n % | 31 (11.7) | 33 (12.5) | 0.790 |
| Extra-hepatic disease at hepatectomy, n % | 32 (12.1) | 38 (14.4) | 0.441 |
| Postoperative complications, n % | 74 (28) | 73 (27.7) | 0.923 |
| Dindo-Clavien classification n (%) | 0.849 | ||
| I | 24 (32.4) | 23 (31.5) | |
| II | 23 (31.1) | 28 (38.4) | |
| IIIa | 15 (20.3) | 14 (19.2) | |
| IIIb | 8 (10.8) | 5 (6.8) | |
| IVa | 4 (5.4) | 3 (4.1) | |
| Liver failure, n % | 17 (6.4) | 17 (6.4) | 1.000 |
| Margin invasion, n % | 41 (15.5) | 41 (15.5) | 1.000 |
| Number of liver metastases > 3, n (%) | 65 (24.6) | 62 (23.5) | 0.760 |
| Largest metastases diameter > 5 cm, n (%) | 41 (15.5) | 43 (16.3) | 0.812 |
| Preoperative chemotherapy, n % | 97 (36.7) | 102 (38.6) | 0.653 |
| Adjuvant chemotherapy, n % | 197 (74.6) | 202 (76.5) | 0.613 |
In the analysis of risk factors for survival using the Cox regression model, steatosis was only significant in the univariate analysis (HR (95% CI) = 0.76 (0.63–0.92) p = 0.006). The predictive variables were the presence of lymph node invasion, intra-operative radiofrequency, the study sub-period, the presence of extrahepatic disease, the invasion of the margin and adjuvant chemotherapy. In the same analysis carried out in the population of matched patients, steatosis had a significant protective effect in the univariate analysis (HR (95% CI) = 0.78 (0.62–0.99) p = 0.048), and was close to significance in the multivariate analysis (HR (95% CI) = 0.81 (0.63–1.03) p = 0.089). In this analysis the predictive variables were practically the same as in the study of the complete series (treatment with intra-operative radiofrequency, the presence of extrahepatic disease, the invasion of the margin and adjuvant chemotherapy) (Table 1 Supplementary).
Liver recurrence
The values of liver recurrence at five years for patients with and without steatosis were 48.1% and 44.5% (p = 0.663). No significant differences between the different grades of steatosis were observed (50.3%, 44.3% and 43.4%). After matching, no significant differences in hepatic recurrence at five years (47.9% and 48%, p = 0.503) were observed (Fig. 2 Supplementary). In the Cox regression model analysis, steatosis was not significant in the univariate analysis, either in the entire series or after matching.
Discussion
Some authors hold that hepatic steatosis has a decisive impact on the results of liver surgery and that it is an important factor in the planning of the therapeutic strategy and surgical technique in patients with liver metastases.4, 15, 16, 19 However, in the reports published so far, the impact of steatosis on postoperative morbidity and mortality after resection of CLM remains uncertain.7
In the only meta-analysis published to date,11 steatosis below 30% was associated with increased morbidity, and steatosis above 30% with increased postoperative mortality. However, certain features of the four studies included in the meta-analysis may have affected the reliability of the conclusions. In the earliest publication20 transfusion requirements and the incidence of liver failure were high, even among patients with normal livers. In the study by Kooby et al.21 matching between patients with normal liver and steatosis was performed using only three variables. Gomez et al.5 included some patients with fibrosis and some even with cirrhosis. Finally, McCormack et al.16 included only 58 patients with steatosis and various indications for liver surgery, and a significant proportion of patients had liver fibrosis or cholestasis.
To avoid limitations of this kind, our study design included only CLM patients. We excluded patients with fibrosis or cirrhosis and in the matching step we included as many of the perioperative variables that could influence the short-term results as possible. Although the long recruitment period could be considered a limitation, the fact is that the study subperiod variable did not emerge as a significant risk factor for mortality or morbidity even in the univariate analysis.
Our results indicate that mild or moderate steatosis does not represent a significant risk factor for postoperative morbidity and mortality in patients undergoing hepatectomy for CLM. However, the characteristics of patients with severe steatosis in our series suggest that the indication of hepatectomy may have been more selective in these patients. Therefore, no firm conclusions can be drawn for patients with severe steatosis. However, our results support that in selected cases (patients without associated liver fibrosis, aged less than 70, and without preoperative chemotherapy) severe steatosis should not be a contraindication for major liver surgery.
Reddy et al.22 suggest that steatohepatitis, rather than hepatic steatosis, increases postoperative morbidity. In patients operated for CLM, steatohepatitis is mainly related to the preoperative administration of chemotherapy regimens that include irinotecan;22, 23 in the absence of chemotherapy, its incidence is practically zero.16 In our study, this information was not recorded, but since very few patients received irinotecan before surgery we can assume that the incidence of steatohepatitis among our patients must be very low and is therefore unlikely to have influenced the results.
Some experimental evidence suggest that the presence of steatosis may be a negative prognostic factor for the onset and progression of CLM.24, 25 Conversely, other authors,26 observed fewer number of liver metastases in fatty livers, after the injection of rat colon cancer cells. This fact seemed to be related to a depressed angiogenesis in fatty livers.
The prognostic influence of liver steatosis in patients operated upon for CLM is also unclear.
Pathak et al.27 found no significant differences in survival in patients with and without steatosis. However, that study included only 102 patients and tumor staging data in the two groups of patients were not compared.
In a multivariate analysis published in 201312 steatosis represented a significant risk factor for hepatic recurrence, both in the full series and after performing a matching based on 13 variables. However, it should be stressed that before matching, the steatosis group presented higher rates of adverse biological characteristics, more frequent preoperative chemotherapy, and more involvement of the resection margin. This may suggest that the patients with steatosis had more aggressive tumors or were diagnosed at a more advanced stage. After matching, the two groups did not show significant differences in the 13 variables used to calculate the propensity score. However, other variables that may influence hepatic recurrence were not taken into account, such as intraoperative radiofrequency treatment and the administration of transfusions or adjuvant chemotherapy.
The influence of steatosis on survival was also analyzed in a 2013 publication based on the Livermetsurvey.13 In that study, patients who had received adjuvant chemotherapy were excluded. Steatosis was associated with improved survival (47.4% vs 43%, p = 0.0017) and this association remained in a multivariate analysis using a Cox regression model in which six tumor staging variables were included. The same authors published a new article on the subject in 201428 but in this case they analyzed steatosis patients who received adjuvant chemotherapy. They found no differences in either postoperative mortality or long-term survival. In contrast, in Vigano et al.'s study14 of 323 patients who underwent preoperative chemotherapy, patients with steatosis >30% had better survival than other patients (52.5% vs 35.2% p = 0.002). It is striking that in the multivariate analysis steatosis reached a higher level of significance than the presence of extrahepatic disease or invasion of the resection margins. None of these three studies13, 14, 28 analyzed the relationship between steatosis and hepatic recurrence.
In our series, the presence of steatosis was associated with improved survival in the univariate analysis and this association remained after a matching procedure which included 22 variables. In addition, in a multivariate study with a Cox regression model in the set of matched patients, the influence of steatosis was close to reaching statistical significance. These data suggest that steatosis is to some extent a positive prognostic factor for patients operated on for CLM, although it is probably less relevant than the presence of extrahepatic disease, the invasion of the surgical margin, or adjuvant chemotherapy. However, this protective effect cannot be attributed to an improvement in the results for liver recurrence, because steatosis did not represent a significant protective factor even in the univariate analysis.
This analysis has several limitations. Firstly, our study spans a significant amount of time, and therefore we cannot completely exclude the effect of changes in practice patterns. Secondly, the assessment of the degree of steatosis, that was graded only by a pathologist. This limitation is shared with most of the publications related to this topic.5, 12, 13, 28
No sound hypotheses have been proposed to explain the mechanism by which steatosis favors the survival of patients operated upon for CLM.13, 14 In our series, an association between steatosis and chemotherapy was not observed; therefore, despite the lack of anthropometric data, and in view of the experience of others, we believe it may be related to overweight and obesity.27 Paradoxically, obesity is often seen as a factor that favors the onset and progression of cancer;29 however, some recent evidence suggests precisely the opposite.30, 31 These observations may have important clinical implications and should be explored further in future prospective experimental and clinical studies.
Conclusions
Mild or moderate hepatic steatosis is not a negative prognostic factor for morbidity and mortality after resection of CLM. In the case of severe steatosis, however, the data are insufficient to draw conclusions regarding its impact. Therefore, caution is recommended in the indication of surgery in these patients. Our experience seems to confirm that steatosis is a favorable prognostic factor for survival, but our results do not suggest that its protective effect can be attributed to a lower incidence of liver recurrence. Moreover, its effect is probably marginal in relation to other well-known prognostic factors and so, on the basis of the evidence available, its presence does not require any modification of the standard strategy.
Acknowledgments
We thank Bernat Miguel Huguet for his invaluable help in the statistical analysis of the data.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.hpb.2015.12.002.
Conflict of interest
None to declare.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Fig. 1 Supplementary legend.
Kaplan–Meier cumulative overall survival curves for 528 matched patients after resection of colorectal liver metastases, stratified by hepatic steatosis.
Fig. 2 Supplementary legend.
Kaplan–Meier cumulative liver recurrence curves for 528 matched patients after resection of colorectal liver metastases, stratified by hepatic steatosis.
References
- 1.Browning J.D., Szczepaniak L.S., Dobbins R., Nuremberg P., Horton J.D., Cohen J.C. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. 20. [DOI] [PubMed] [Google Scholar]
- 2.Bedogni G., Bellentani S. Fatty liver: how frequent is it and why? Ann Hepatol. 2004;3:63–65. [PubMed] [Google Scholar]
- 3.Bellentani S., Saccoccio G., Masutti F., Croce L.S., Brandi G., Sasso F. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 4.Veteläinen R., van Vliet A., Gouma D.J., van Gulik T.M. Steatosis as a risk factor in liver surgery. Ann Surg. 2007;245:20–30. doi: 10.1097/01.sla.0000225113.88433.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez D., Malik H.Z., Bonney G.K., Wong V., Toogood G.J., Lodge J.P.A. Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br J Surg. 2007;94:1395–1402. doi: 10.1002/bjs.5820. [DOI] [PubMed] [Google Scholar]
- 6.van Vledder M.G., Torbenson M.S., Pawlik T.M., Boctor E.M., Hamper U.M., Olino K. The effect of steatosis on echogenicity of colorectal liver metastases on intraoperative ultrasonography. Arch Surg. 2010;145:661–667. doi: 10.1001/archsurg.2010.124. [DOI] [PubMed] [Google Scholar]
- 7.Makowiec F., Möhrle S., Neeff H., Drognitz O., Illerhaus G., Opitz O.G. Chemotherapy, liver injury, and postoperative complications in colorectal liver metastases. J Gastrointest Surg. 2011;15:153–164. doi: 10.1007/s11605-010-1368-7. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez F.G., Ritter J., Goodwin J.W., Linehan D.C., Hawkins W.G., Strasberg S.M. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845–853. doi: 10.1016/j.jamcollsurg.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Vauthey J.N., Pawlik T.M., Ribero D., Wu T.T., Zorzi D., Hoff P.M. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 10.Cauchy F., Fuks D., Zarzavadjian Le Bian A., Belghiti J., Costi R. Metabolic syndrome and non-alcoholic fatty liver disease in liver surgery: the new scourges? World J Hepatol. 2014 May 27;6:306–314. doi: 10.4254/wjh.v6.i5.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Meijer V.E., Kalish B.T., Puder M., Ijzermans J.N. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg. 2010;97:1331–1339. doi: 10.1002/bjs.7194. [DOI] [PubMed] [Google Scholar]
- 12.Hamady Z.Z., Rees M., Welsh F.K., Toogood G.J., Prasad K.R., John T.K. Fatty liver disease as a predictor of local recurrence following resection of colorectal liver metastases. Br J Surg. 2013;100:820–826. doi: 10.1002/bjs.9057. [DOI] [PubMed] [Google Scholar]
- 13.Parkin E., O'Reilly D.A., Adam R., Kaiser G.M., Laurent C., Elias D. The effect of hepatic steatosis on survival following resection of colorectal liver metastases in patients without preoperative chemotherapy. HPB. 2013;15:463–472. doi: 10.1111/hpb.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vigano L., Capussotti L., De Rosa G., De Saussure W.O., Mentha G., Rubbia-Brandt L. Liver resection for colorectal metastases after chemotherapy: impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann Surg. 2012;258:731–742. doi: 10.1097/SLA.0b013e3182a6183e. [DOI] [PubMed] [Google Scholar]
- 15.Belghiti J., Hiramatsu K., Benoist S., Massault P.P., Sauvanet A., Farges F. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 16.McCormack L., Petrowsky H., Jochum W., Furrer K., Clavien P.A. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy. A matched case-control study. Ann Surg. 2007;245:923–930. doi: 10.1097/01.sla.0000251747.80025.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahbari N.N., Garden O.J., Padbury R., Brooke-Smith M., Crawford M., Adam R. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011 May;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal S., Daruwala Ch. Metabolic syndrome and hepatic resection: improving outcome. HPB. 2011;13:846–859. doi: 10.1111/j.1477-2574.2011.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ke Behrns, Tsiotos G.G., DeSouza N.F., Krishna M.K., Ludwig J., Nagorney D.M. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–298. doi: 10.1016/s1091-255x(98)80025-5. [DOI] [PubMed] [Google Scholar]
- 21.Kooby D.A., Fong Y., Suriawinata A., Gonen M., Allen P.J., Klimstra Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Reddy S.K., Marsh J.W., Varley P.R., Mock B.K., Chopra K.B., Geller D.A. Underlying steatohepatitis, but not simple hepatic steatosis, increases morbidity after liver resection: a case-control study. Hepatology. 2012;56:2221–2230. doi: 10.1002/hep.25935. [DOI] [PubMed] [Google Scholar]
- 23.Reissfelder C., Brand K., Sobiegalla J., Rahbari N.N., Bork U., Schirmacher P. Chemotherapy-associated liver injury and its influence on outcome after resection of colorectal liver metastases. Surgery. 2014;155:245–254. doi: 10.1016/j.surg.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 24.van der Bilt J.D.W., Kranenburg O,Borren A., van Hillegersberg R., Borel Rinkes I.H.M. Ageing and hepatic steatosis exacerbate ischemia/reperfusion-accelerated outgrowth of colorectal micrometastases. Ann Surg Oncol. 2008;15:1392–1398. doi: 10.1245/s10434-007-9758-0. [DOI] [PubMed] [Google Scholar]
- 25.VanSaun M.N., Lee K., Washington M.K., Matrisian L., Gorden D.L. High fat diet induced hepatic steatosis establishes a permissive microenvironment for colorectal metastases and promotes primary dysplasia in a murine model. AJP. 2009 July;175:355–364. doi: 10.2353/ajpath.2009.080703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karube H., Masuda H., Hayashi S., Ishii Y., Nemoto N. Fatty liver suppressed the angiogenesis in liver metastatic lesions. Hepatogastroenterology. 2000;47:1541–1545. [PubMed] [Google Scholar]
- 27.Pathak S., Tang J.M., Terlizzo M., Poston G.J., Malik H.Z. Hepatic steatosis, body mass index and long term outcome in patients undergoing hepatectomy for colorectal liver metastases. Eur J Surg Oncol. 2010;36:52–57. doi: 10.1016/j.ejso.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Parkin E., O'Reilly D.A., Adam R., Kaiser G.M., Laurent C., Elias D. Equivalent survival in patients with and without steatosis undergoing resection for colorectal liver metastases following pre-operative chemotherapy. Eur J Surg Oncol. 2014;40:1436–1444. doi: 10.1016/j.ejso.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 29.Calle E.E., Thun M.J. Obesity and cancer. Oncogene. 2004 Aug 23;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 30.Simkens L.H., Koopman M., Mol L., Veldhuis G.J., Ten Bokkel Huinink D., Muller E.W. Influence of body mass index on outcome in advanced colorectal cancer patients receiving chemotherapy with or without targeted therapy. Eur J Cancer. 2011 Nov;47:2560–2567. doi: 10.1016/j.ejca.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 31.Mathur A.K., Ghaferi A.A., Sell K., Sonnenday C.J., Englesbe M.J., Welling T.H. Influence of body mass index on complications and oncologic outcomes following hepatectomy for malignancy. J Gastrointest Surg. 2010 May;14:849–857. doi: 10.1007/s11605-010-1163-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.