Abstract
Background
Gallbladder adenomyomatosis (GA) is a benign gallbladder entity discovered as an asymptomatic gallbladder mass. Since gallbladder cancer is in the differential diagnosis for gallbladder masses, the ability to differentiate benign disease avoids a more extensive oncologic resection. This study sought to review imaging modalities used to diagnose GA.
Methods
PubMed and SciVerse Scopus were systematically searched using the terms: “gallbladder adenomyomatosis” and “gallbladder imaging” for articles published between January 2000 and January 2015.
Results
A total of 14 articles were reviewed in this analysis. Contemporary series report the use of ultrasound (US), computed tomography (CT) or magnetic resonance imaging (MRI) in GA imaging. Ultrasound detection of Rokitansky-Aschoff sinuses, visualized as small cystic spaces with associated “comet-tail” or “twinkling” artifact, is pathognomonic for GA. A “Pearl-Necklace” sign of small connected sinuses on MRI or “Rosary” sign on CT are additional characteristics that may assist in establishing a diagnosis.
Conclusion
Ultrasound is the most commonly used tool to investigate GA. If not diagnostic, CT or MRI are effective in attempting to differentiate a benign or malignant cholecystic mass. Characteristic signs should lead the surgeon to perform a laparoscopic cholecystectomy in symptomatic patients or manage non-operatively in asymptomatic patients.
Introduction
Within the differential of gallbladder masses belongs a spectrum of benign and malignant diseases. Adenomyomatosis of the gallbladder (GA) remains a common entity among benign gallbladder masses, diagnosed in 2%–8% of all cholecystectomies in recent studies.1, 2 Lack of familiarity surrounding the disease may lead to a more extensive operation than necessary. The condition is typically asymptomatic; though, it can present in a limited number of patients with vague abdominal pain, symptoms of epigastric distress or a picture of acute or chronic cholecystitis.3, 4 Currently, GA is most prevalent among the elderly population, with a female dominance.5 The primary mechanism leading to GA formation is hyperplasia of the gallbladder wall epithelium and the formation of intramural diverticula, recognized as Rokitansky-Aschoff sinuses (RAS). These invaginations can extend beyond the tunica muscularis of the gallbladder wall and are pathognomonic for GA.6, 7, 8
The disease presents in three different types depending on the degree of wall involvement; diffuse, segmental or fundal GA.9 Diffuse GA exhibits disseminated thickening and irregularity of the mucosa and muscularis mucosa, resulting in a cyst-like shape of the gallbladder. The segmental type, however, demonstrates a circumferential overgrowth of the gallbladder wall that leads to the formation of compartments within the gallbladder, resembling an “hourglass” appearance.10 This circumferential thickening can lead to bile stasis in one part of the gallbladder, acting as a risk factor for gallstone formation.11 Segmental GA has been reported as the most common type of GA.11, 12, 13 The last type is referred to as fundal type, which appears as an overgrowth of gallbladder fundus with bulging into the lumen, that may resemble a polyp.6
The first imaging modality that was described in the literature to visualize GA was oral cholecystography.9 “Collections of contrast medium in the dilated Rokitansky-Aschoff sinuses around the main gall-bladder shadow, strictures, septa, kinks and filling defects” were described as characteristic.14 Oral cholecystogram has been used in later studies; however, the availability of other cost-efficient alternatives with similar, if not higher, accuracy and lower risks led to its diminished use. Current studies describe alternative imaging technique for GA visualization.
The aim of this study is to review the current literature as it relates to the different GA imaging modalities in an attempt to outline a useful diagnostic approach for GA. The differential diagnosis surrounding any gallbladder abnormality includes cancer, and therefore accurate diagnosis of GA offers the patient an operative approach that is less extensive and potentially less morbid in symptomatic cases.
Methods
A literature search of the online databases MEDLINE/PubMed and SciVerse Scopus was performed using the following terms: “gallbladder adenomyomatosis” and “gallbladder imaging”. Search results were restricted to full text articles written in the English language published between January 1st of 2000 and January 31st of 2015. Search results were limited to studies including human subjects, of adult population. Additional articles were found through the manual search of included studies' references. A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram was followed to reach the final articles included in this study. Data were extracted in a standardized spread sheet and screened for eligibility. Variables abstracted included year of publication, study location, study type, the number of patients reported with each modality, type of imaging, specific imaging characteristics and reported sensitivities and specificities. Since the main focus of the study was to describe pertinent imaging features of GA, imaging features of other gallbladder pathologies were beyond the scope of this study and thus, were not discussed.
Inclusion criteria:
-
1.
Prospective cohort studies, retrospective cohort studies, and case series with more than 5 patients published between January 1st of 2000 and January 31st of 2015.
-
2.
Studies involving only human subjects, older than 18 years.
-
3.
Studies discussing any imaging modality for gallbladder adenomyomatosis.
-
4.
Specific description of gallbladder adenomyomatosis imaging features.
Exclusion criteria:
-
1.
Pictorial essays, case reports, case series with less than 5 patients
-
2.
Studies examining pediatric age population or non-human subjects in languages other than English.
-
3.
Studies with a deficient mention of specific GA features.
Results
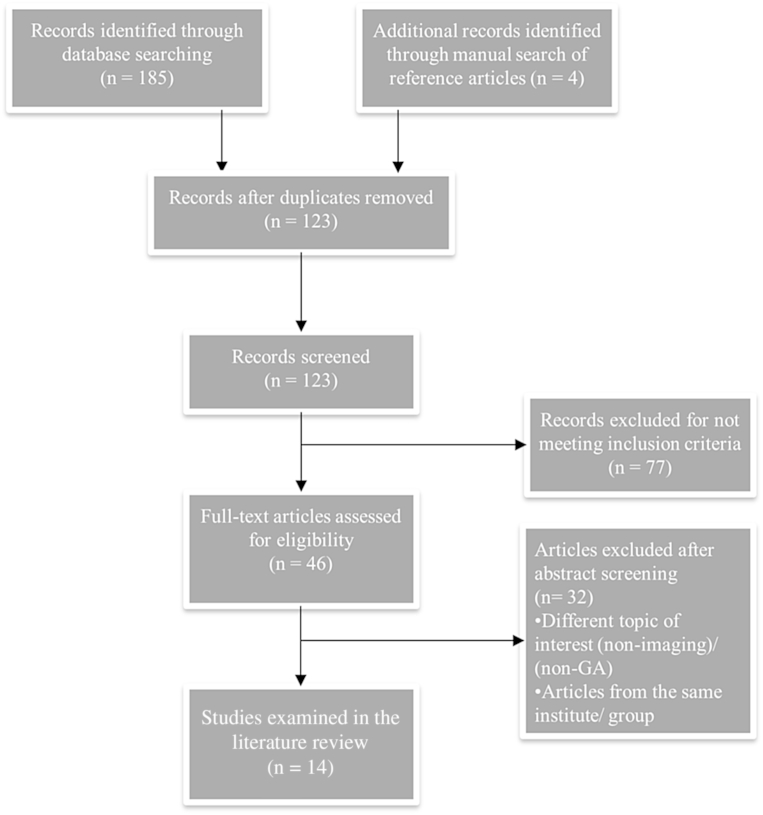
A total of 189 articles were identified; 185 articles from the database search and 4 additional articles identified through the manual search of the references. After removing the duplicates, one hundred and twenty three articles were reviewed for eligibility, of which, 14 were included in the final analysis. Fig. 1 illustrates the logic for final article selection.
Figure 1.
PRISMA flow chart demonstrating selection process of included articles. GA, Gallbladder adenomyomatosis
The most commonly utilized modalities in GA imaging include: ultrasound (US), (n = 7), computed tomography (CT), (n = 5), magnetic resonance imaging (MRI), (n = 4), and endoscopic ultrasound (EUS) (n = 5). An additional article reported the use of magnetic resonance cholangiopancreatography (MRCP) in GA visualization. Two studies published by the same group were included in this review due to the difference in patient populations examined; one of the two studies contained 45 patients examined solely by US, while the other included a smaller population of 13 patients, examined by a triple modality approach consisting of CT, MRI and US.15, 16 Table 1 shows the relative sensitivity and specificity of respective imaging techniques reported among studies.
Table 1.
Imaging modalities for gallbladder adenomyomatosis
| First author | Country | Modality | Sensitivity and specificity |
|---|---|---|---|
| Azuma, T. | Japan | EUS, US |
EUS: sensitivity: 91.7% Specificity: 87.7%. US: sensitivity: 54.2% Specificity: 53.8% |
| Bang, S. H. | South Korea | MRI, CT, US |
US: sensitivity: 73.1% Specificity: 96.3% Accuracy: 88.8% CT: sensitivity: 50.0% Specificity: 98.2% Accuracy: 82.5% MRI: sensitivity: 80.8% Specificity: 98.2% Accuracy: 92.5% |
| Ching, B. H. | USA | CT |
CT: sensitivity: 36% specificity:85.7% |
| Haradome, H. | Japan | CT, MRI, MRCP | MRCP: specificity for “Pearl Necklace” sign: 92% |
| Joo, I. | South Korea | US |
US: sensitivity: 80% Specificity: 85.7% Accuracy: 82.2% |
| Jung, S. E. | South Korea | MRI |
MRI: sensitivity: 99% Specificity: 100% |
| Yoshimitsu, K. | Japan | MRI, CT, US |
MRI: sensitivity: 93% Specificity: 93% Accuracy: 93% CT: sensitivity: 65% Specificity: 85% Accuracy: 75% US: sensitivity: 43% Specificity: 89% Accuracy: 66% |
Abbreviations: GA: gallbladder adenomyomatosis; CT: computed tomography; MRI: magnetic resonance imaging; US: ultrasound; EUS: endoscopic ultrasound; MRCP: magnetic resonance cholangiopancreatography.
Ultrasound
Ultrasound is commonly utilized in evaluating different gallbladder pathologies. It is one of the earliest modalities mentioned as a possible alternative for oral cholecystography to detect GA.7, 9, 17 Major findings on ultrasound typically include: (i) RAS visualization as small cystic spaces in the gallbladder wall (ii) presence of multiple microcystic spaces or echogenic foci, (iii) “comet-tail” or color flow ultrasound “twinkling” artifacts, and lastly, (iv) thickening of the gallbladder wall.15, 16, 18, 19, 20, 21 Ultrasound visualization of RAS is pathognomonic for the disease. On US, RAS vary widely in echogenicity, ranging from hypoechogenic to hyperechogenic and occasionally mixed echogenicity.18, 20, 22 The echogenicity depends on what accumulates inside these diverticula. Biliary content within the diverticula will appear as hypoechogenic spaces in the gallbladder wall. Sludge or stones would however, produce a hyperechogenic shadow. Thus the echogenicity pattern alone is not a reliable imaging characteristic when attempting to establish a diagnosis of GA. Furthermore, limited ultrasound identification of RAS might occur due to a lesser degree of wall thickening. Coexisting stones or intestinal gases can also overshadow the gallbladder leading to insufficient gallbladder wall visualization.
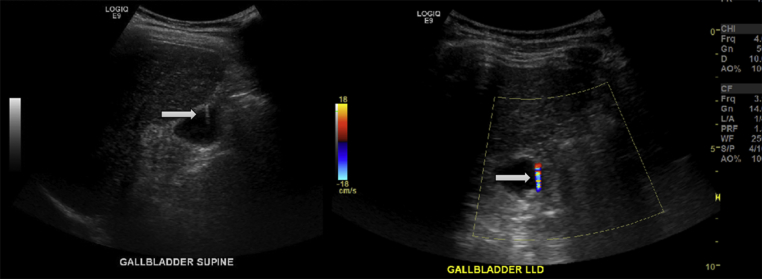
One of the commonly seen features of the disease on ultrasound is the comet-tail V-shaped artifact. This finding is highly informative and represents the acoustic signature of highly abundant cholesterol deposits in the RAS lumina.13, 15, 19, 21 The equivalent of this sign on color flow ultrasound is known as the “twinkling” artifact.20 This sign appears as a rapid alternation of blue and red signals behind stationary crystals in a strongly reflecting medium (Fig. 2).22, 23 Other signs of GA on ultrasound include the presence of intramural cystic spaces or echogenic foci, which are 80% sensitive, 85.7% specific and 82.2% accurate for the disease.16 On the other hand, gallbladder wall thickening alone is non-specific for GA and needs additional information to accurately identify the disease. When compared to gallbladder cancer, symmetrical wall thickening, intramural thickening of the outer wall and intramural echogenic foci were identified as positive predictors of adenomyomatosis; whereas irregular thickening was identified as a negative predictor of the disease.16
Figure 2.
“Comet-tail” artifact seen on ultrasound examination (left); and “twinkling” artifact seen on color flow ultrasound (right)
Xie et al. proposed contrast enhanced ultrasonography (CEUS), utilizing SonoVue contrast agent (BR1; Bracco SpA, Milan, Italy), over the conventional ultrasonography for better differentiation between gallbladder cancer and benign gallbladder pathologies including GA.19 Currently, CEUS is supported over the traditional ultrasound in differentiating benign versus malignant disease.24 Further investigations of the modality are still warranted for GA imaging.
Ultrasound use in GA is limited by its operator dependence, imaging artifacts secondary to the presence of gas or stones, and inadequate gallbladder visualization in obese patients. However, given its high availability, clinical usefulness and economic efficiency, ultrasound should be used for the initial examination as well as follow up for patients with stable GA disease and no suspicious signs of malignancy. Cross-sectional imaging should follow to investigate suspicious findings in case of unclear diagnosis.
Endoscopic ultrasound (EUS)
EUS is a minimally invasive procedure that can provide high quality images of the gallbladder. It is credited with better images of the gallbladder than ultrasound and can also be performed as an outpatient procedure.25, 26
EUS has been reported to identify GA lesions that were missed by routine abdominal ultrasound.26, 27, 28, 29 Nevertheless, EUS may mistakenly misdiagnose gallbladder cancer as GA.30 This inaccuracy may occur due to the sole presence of multiple microcysts that can also be seen in cancer.27 This mandates the presence of other GA features for a more accurate diagnosis. If the typical ultrasound findings of GA are absent, a diagnosis of cancer must be considered and further cross sectional imaging should follow.28 EUS provides an additional valuable function, which is the ability to perform EUS fine-needle aspiration of local lymph nodes; although a resectable gallbladder mass suspicious for cancer should not undergo biopsy due to the risk of seeding.
Due to the high cost of performing EUS, its relative invasiveness, and the advanced training it requires, ultrasound remains the primary screening method.
Computed tomography scan (CT)
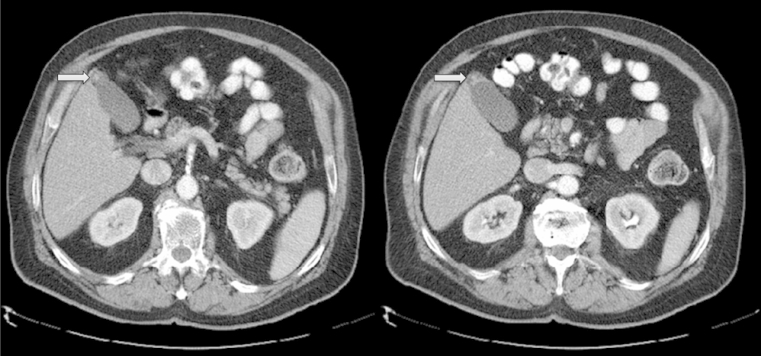
The utilization of CT to identify GA has been extensively examined. Mucosal thickening, mucosal irregularities, such as pouching in GA can be visualized on the arterial phase of a contrast enhanced CT (Fig. 3).20 Adequate wall thickness, although non-specific for the disease, is required to establish the diagnosis of GA.31 A diagnostic sign of GA on CT is the “Rosary sign”, which occurs from the combination of an unenhanced proliferative muscularis layer surrounding enhanced proliferative mucosal epithelium with intramural diverticula.15, 18, 20 The overall accuracy of CT in diagnosing the disease ranges from 61.75% to 75%; though, its ability to outline RAS is limited (38%–43%).10, 22
Figure 3.
CT scan of the abdomen showing gallbladder adenomyomatosis with fundal thickening
CT should be pursued for any findings suspicious of cancer on US. An enhancing inner wall of ≥2.6 mm thickness or inner layer hyperenhancement on portal phase CT images represents a potential malignant cause for gallbladder wall thickening. Additional findings of malignancy include outer wall thickness ≤3.4 mm, that is weakly enhancing/non-enhancing.32 Furthermore, CT is useful in differentiating the fundal subtype of GA from chronic cholecystitis as reported by Kim et al.10 Fundal GA is associated with a well-defined oval shaped contour, along with inner layer enhancement and an intralesional cystic area.
Magnetic resonance imaging (MRI)
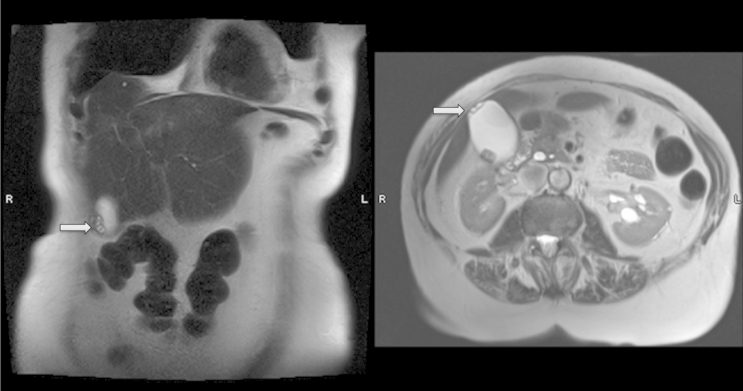
Adenomyomatosis appears on MRI as an intramural thickening of the gallbladder wall with multiple cysts (Fig. 4).15, 33, 34 A distinguished GA sign on T2-weighted MRI imaging is the “Pearl-necklace” sign, also known as “string bead sign”, and refers to multiple high-intensity cavities seen in the gallbladder wall. This pathognomonic sign carries 92%–98% specificity for GA.15, 18, 21, 22, 33, 35 However, it is only present in 70% of the patients, and becomes difficult to visualize when RAS are smaller than 3 mm, or in sinuses filled with proteinaceous fluid or small stones.33, 35 Considering that MRCP is able to identify smaller RAS as compared to MRI, the combined use of MRI and MRCP is proposed to differentiate GA from gallbladder cancer when the pearl-necklace sign cannot be demonstrated.35, 36
Figure 4.
MRI examination of the gallbladder showing cystic spaces on coronal (left), transverse view (right)
On MRI, RAS are visualized as hypointense intramural lesions on T1-weighted images, and hyperintense on T2-weighted images.18, 36 RAS hyperintensity on T2-weighted images is attributed to the bile content within the sinuses, which yields a bright/hyperintense area.20, 22, 37 However, the presence of exceptionally thick bile or debris may lead to an inaccurate diagnosis of GA due to short T1 and T2 properties of bile.7 T2-weighted or contrast enhanced MRI imaging are able to identify RAS as small as 3 mm.20
Finally, MRI can be used to examine the patterns of gallbladder wall thickening. These patterns are based on the features of inner and outer layers, their signal intensity and presence of striations; and correlate to different pathologies.34 Among the four types recognized by Jung et al, type 3; “multiple cystic spaces of high intensity in the thickened wall whether or not layering of the thickened wall was present” is uniformly associated with GA. Type 3 pattern carries 100% sensitivity, 99% specificity and 91% positive predictive value (PPV) for GA. Other patterns correlate with different pathologies such as acute or chronic cholecystitis, or gallbladder carcinoma (Table 2).
Table 2.
MRI imaging patterns for gallbladder
| Layered pattern | Pathology associated | Sensitivity (%) | Specificity (%) | PPV (%) |
|---|---|---|---|---|
| Type 1 | Chronic cholecystitis | 93 | 97 | 95 |
| Type 2 | Acute cholecystitis | 90 | 95 | 97 |
| Type 3 | Adenomyomatosis | 100 | 99 | 91 |
| Type 4 | Gallbladder cancer | 92 | 97 | 73 |
To summarize, MRI is highly valuable for GA diagnosis. Though, the high cost of MRI, the benign nature of the disease, and the high accuracy of alternative imaging modalities, makes MRI an ideal secondary imaging tool.
Positron emission tomography (PET) scan
No studies examining PET scans in GA imaging met the inclusion criteria for this review. Few reports, consisting mostly of case reports, examined the utility of PET scan alone or in combination with 18F-labelled deoxyglucose (FDG-PET) for GA imaging.38, 39, 40 Increased uptake at the site of GA is reported. False positive results from the FDG-PET scans warrant cholecystectomy in suspicion of cancer. No evidence exists to support the routine use of FDG-PET scan in GA diagnosis.
Limitations
The present study is limited to studies published in English language; and unpublished studies or gray literature were not accounted for in the manuscript. The omission of studies published in different languages, or the unpublished literature represent a source of bias that may affect the overall conclusion. Secondly, the difference in technology and/or expertise between different countries may affect the results. Finally, no data synthesis was done due to the difference in outcomes reported across the included studies.
Conclusion
Different imaging modalities exist to assist the diagnosis of GA. Ultrasound is both, clinically and economically convenient for initial evaluation. In patients with inconclusive imaging, MRI represents an ideal secondary tool. CT can be used as a diagnostic adjunct to evaluate the gallbladder and differentiate between pathologies. EUS for GA imaging is effective in the visualization of GA, but is challenged by access, cost and advanced training required. Little evidence exists to support the role of other modalities as MRCP or PET scan. GA is a benign entity that physicians should consider and image appropriately. When imaging is diagnostic and symptoms exist, the scope of surgery dramatically changes to a standard laparoscopic cholecystectomy from a more radical approach, traditionally required in oncologic settings.
Footnotes
This work was presented at the European Society of Surgical Research, Liverpool, UK 10–13 June 2015.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.hpb.2015.09.006.
Disclosure
No relevant financial disclosures.
Funding
None received.
Conflicts of interest
None to declare.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Kim J.H., Jeong I.H., Han J.H., Hwang J.C., Yoo B.M., Kim M.W. Clinical/pathological analysis of gallbladder adenomyomatosis; type and pathogenesis. Hepatogastroenterology. 2010;57:420–425. [PubMed] [Google Scholar]
- 2.Ootani T., Shirai Y., Tsukada K., Muto T. Relationship between gallbladder carcinoma and the segmental type of adenomyomatosis of the gallbladder. Cancer. 1992;69:2647–2652. doi: 10.1002/1097-0142(19920601)69:11<2647::aid-cncr2820691105>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Erdas E., Licheri S., Pulix N., Lai M.L., Pisano G., Pomata M. Adenomyomatosis of the gallbladder. Personal experience and analysis of the literature. Chir Ital. 2002;54:673–684. [PubMed] [Google Scholar]
- 4.Jacobs L.A., DeMeester T.R., Eggleston J.C., Margulies S.I., Zuidema G.D. Hyperplastic cholecystoses. Arch Surg. 1972;104:193–194. doi: 10.1001/archsurg.1972.04180020073014. [DOI] [PubMed] [Google Scholar]
- 5.Ram M.D., Midha D. Adenomyomatosis of the gallbladder. Surgery. 1975;78:224–229. [PubMed] [Google Scholar]
- 6.Levy A.D., Murakata L.A., Rohrmann C.A., Jr. Gallbladder carcinoma: radiologic-pathologic correlation. Radiographics. 2001;21:295–314. doi: 10.1148/radiographics.21.2.g01mr16295. questionnaire, 549–555. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimitsu K., Honda H., Aibe H., Shinozaki K., Kuroiwa T., Irie H. Radiologic diagnosis of adenomyomatosis of the gallbladder: comparative study among MRI, helical CT, and transabdominal US. J Comput Assist Tomogr. 2001;25:843–850. doi: 10.1097/00004728-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Owen C.C., Bilhartz L.E. Gallbladder polyps, cholesterolosis, adenomyomatosis, and acute acalculous cholecystitis. Seminars Gastrointest Dis. 2003;14:178–188. [PubMed] [Google Scholar]
- 9.Berk R.N., van der Vegt J.H., Lichtenstein J.E. The hyperplastic cholecystoses: cholesterolosis and adenomyomatosis. Radiology. 1983;146:593–601. doi: 10.1148/radiology.146.3.6402801. [DOI] [PubMed] [Google Scholar]
- 10.Kim B.S., Oh J.Y., Nam K.J., Cho J.H., Kwon H.J., Yoon S.K. Focal thickening at the fundus of the gallbladder: computed tomography differentiation of fundal type adenomyomatosis and localized chronic cholecystitis. Gut Liver. 2014;8:219–223. doi: 10.5009/gnl.2014.8.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimura A., Shirai Y., Hatakeyama K. Segmental adenomyomatosis of the gallbladder predisposes to cholecystolithiasis. J Hepatobiliary Pancreat Surg. 2004;11:342–347. doi: 10.1007/s00534-004-0911-x. [DOI] [PubMed] [Google Scholar]
- 12.Kasahara Y., Sonobe N., Tomiyoshi H., Imano M., Nakatani M., Urata T. Adenomyomatosis of the gallbladder: a clinical survey of 30 surgically treated patients. Nihon Geka Hokan. 1992;61:190–198. [PubMed] [Google Scholar]
- 13.Boscak A.R., Al-Hawary M., Ramsburgh S.R. Best cases from the AFIP: adenomyomatosis of the gallbladder. Radiographics. 2006;26:941–946. doi: 10.1148/rg.263055180. [DOI] [PubMed] [Google Scholar]
- 14.Colquhoun J. Adenomyomatosis of the gall-bladder (intramural diverticulosis) Br J Radiol. 1961;34:101–112. doi: 10.1259/0007-1285-34-398-101. [DOI] [PubMed] [Google Scholar]
- 15.Bang S.H., Lee J.Y., Woo H., Joo I., Lee E.S., Han J.K. Differentiating between adenomyomatosis and gallbladder cancer: revisiting a comparative study of high-resolution ultrasound, multidetector CT, and MR imaging. Korean J Radiol. 2014;15:226–234. doi: 10.3348/kjr.2014.15.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joo I., Lee J.Y., Kim J.H., Kim S.J., Kim M.A., Han J.K. Differentiation of adenomyomatosis of the gallbladder from early-stage, wall-thickening-type gallbladder cancer using high-resolution ultrasound. Eur Radiol. 2013;23:730–738. doi: 10.1007/s00330-012-2641-9. [DOI] [PubMed] [Google Scholar]
- 17.Rice J., Sauerbrei E.E., Semogas P., Cooperberg P.L., Burhenne H.J. Sonographic appearance of adenomyomatosis of the gallbladder. J Clin Ultrasound. 1981;9:336–337. doi: 10.1002/jcu.1870090615. [DOI] [PubMed] [Google Scholar]
- 18.Pellino G., Sciaudone G., Candilio G., Perna G., Santoriello A., Canonico S. Stepwise approach and surgery for gallbladder adenomyomatosis: a mini-review. Hepatobiliary Pancreat Dis Int. 2013;12:136–142. doi: 10.1016/s1499-3872(13)60022-3. [DOI] [PubMed] [Google Scholar]
- 19.Xie X.H., Xu H.X., Xie X.Y., Lu M.D., Kuang M., Xu Z.F. Differential diagnosis between benign and malignant gallbladder diseases with real-time contrast-enhanced ultrasound. Eur Radiol. 2010;20:239–248. doi: 10.1007/s00330-009-1538-8. [DOI] [PubMed] [Google Scholar]
- 20.Stunell H., Buckley O., Geoghegan T., O'Brien J., Ward E., Torreggiani W. Imaging of adenomyomatosis of the gall bladder. J Med Imaging Radiat Oncol. 2008;52:109–117. doi: 10.1111/j.1440-1673.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- 21.Ash-Miles J., Roach H., Virjee J., Callaway M. More than just stones: a pictorial review of common and less common gallbladder pathologies. Curr Probl Diagn Radiol. 2008;37:189–202. doi: 10.1067/j.cpradiol.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Yoon J.H., Cha S.S., Han S.S., Lee S.J., Kang M.S. Gallbladder adenomyomatosis: imaging findings. Abdom Imaging. 2006;31:555–563. doi: 10.1007/s00261-005-0230-y. [DOI] [PubMed] [Google Scholar]
- 23.Rahmouni A., Bargoin R., Herment A., Bargoin N., Vasile N. Color Doppler twinkling artifact in hyperechoic regions. Radiology. 1996;199:269–271. doi: 10.1148/radiology.199.1.8633158. [DOI] [PubMed] [Google Scholar]
- 24.Liu L.N., Xu H.X., Lu M.D., Xie X.Y., Wang W.P., Hu B. Contrast-enhanced ultrasound in the diagnosis of gallbladder diseases: a multi-center experience. PLoS One. 2012;7:e48371. doi: 10.1371/journal.pone.0048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadamoto Y., Oda S., Tanaka M., Harada N., Kubo H., Eguchi T. A useful approach to the differential diagnosis of small polypoid lesions of the gallbladder, utilizing an endoscopic ultrasound scoring system. Endoscopy. 2002;34:959–965. doi: 10.1055/s-2002-35859. [DOI] [PubMed] [Google Scholar]
- 26.Azuma T., Yoshikawa T., Araida T., Takasaki K. Differential diagnosis of polypoid lesions of the gallbladder by endoscopic ultrasonography. Am J Surg. 2001;181:65–70. doi: 10.1016/s0002-9610(00)00526-2. [DOI] [PubMed] [Google Scholar]
- 27.Akatsu T., Aiura K., Shimazu M., Ueda M., Wakabayashi G., Tanabe M. Can endoscopic ultrasonography differentiate nonneoplastic from neoplastic gallbladder polyps? Dig Dis Sci. 2006;51:416–421. doi: 10.1007/s10620-006-3146-7. [DOI] [PubMed] [Google Scholar]
- 28.Sugiyama M., Atomi Y., Yamato T. Endoscopic ultrasonography for differential diagnosis of polypoid gall bladder lesions: analysis in surgical and follow up series. Gut. 2000;46:250–254. doi: 10.1136/gut.46.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park C.H., Chung M.J., Oh T.G., Park J.Y., Bang S., Park S.W. Differential diagnosis between gallbladder adenomas and cholesterol polyps on contrast-enhanced harmonic endoscopic ultrasonography. Surg Endosc. 2013;27:1414–1421. doi: 10.1007/s00464-012-2620-x. [DOI] [PubMed] [Google Scholar]
- 30.Kim H.J., Park J.H., Park D.I., Cho Y.K., Sohn C.I., Jeon W.K. Clinical usefulness of endoscopic ultrasonography in the differential diagnosis of gallbladder wall thickening. Dig Dis Sci. 2012;57:508–515. doi: 10.1007/s10620-011-1870-0. [DOI] [PubMed] [Google Scholar]
- 31.Ching B.H., Yeh B.M., Westphalen A.C., Joe B.N., Qayyum A., Coakley F.V. CT differentiation of adenomyomatosis and gallbladder cancer. AJR Am J Roentgenol. 2007;189:62–66. doi: 10.2214/AJR.06.0866. [DOI] [PubMed] [Google Scholar]
- 32.Kim S.J., Lee J.M., Lee J.Y., Kim S.H., Han J.K., Choi B.I. Analysis of enhancement pattern of flat gallbladder wall thickening on MDCT to differentiate gallbladder cancer from cholecystitis. AJR Am J Roentgenol. 2008;191:765–771. doi: 10.2214/AJR.07.3331. [DOI] [PubMed] [Google Scholar]
- 33.Catalano O.A., Sahani D.V., Kalva S.P., Cushing M.S., Hahn P.F., Brown J.J. MR imaging of the gallbladder: a pictorial essay. Radiographics. 2008;28:135–155. doi: 10.1148/rg.281065183. quiz 324. [DOI] [PubMed] [Google Scholar]
- 34.Jung S.E., Lee J.M., Lee K., Rha S.E., Choi B.G., Kim E.K. Gallbladder wall thickening: MR imaging and pathologic correlation with emphasis on layered pattern. Eur Radiol. 2005;15:694–701. doi: 10.1007/s00330-004-2539-2. [DOI] [PubMed] [Google Scholar]
- 35.Haradome H., Ichikawa T., Sou H., Yoshikawa T., Nakamura A., Araki T. The pearl necklace sign: an imaging sign of adenomyomatosis of the gallbladder at MR cholangiopancreatography. Radiology. 2003;227:80–88. doi: 10.1148/radiol.2271011378. [DOI] [PubMed] [Google Scholar]
- 36.Elsayes K.M., Oliveira E.P., Narra V.R., El-Merhi F.M., Brown J.J. Magnetic resonance imaging of the gallbladder: spectrum of abnormalities. Acta Radiol. 2007;48:476–482. doi: 10.1080/02841850701324102. [DOI] [PubMed] [Google Scholar]
- 37.Adusumilli S., Siegelman E.S. MR imaging of the gallbladder. Magn Reson Imaging Clin N Am. 2002;10:165–184. doi: 10.1016/s1064-9689(03)00055-2. [DOI] [PubMed] [Google Scholar]
- 38.Koh T., Taniguchi H., Kunishima S., Yamagishi H. Possibility of differential diagnosis of small polypoid lesions in the gallbladder using FDG-PET. Clin Positron Imaging. 2000;3:213–218. doi: 10.1016/s1095-0397(00)00100-x. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki K., Watada S., Yoko M., Nakahara T., Kumamoto Y. Successful diagnosis of gallbladder carcinoma coexisting with adenomyomatosis by 18F-FDG-PET–report of a case. J Gastrointest Cancer. 2011;42:252–256. doi: 10.1007/s12029-010-9221-5. [DOI] [PubMed] [Google Scholar]
- 40.Maldjian P.D., Ghesani N., Ahmed S., Liu Y. Adenomyomatosis of the gallbladder: another cause for a “hot” gallbladder on 18F-FDG PET. AJR Am J Roentgenol. 2007;189:W36–W38. doi: 10.2214/AJR.05.1284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.