Abstract
Background
Perioperative blood transfusions have been associated with worse oncological outcome in several types of cancer. The objective of this study was to assess the effect of perioperative blood transfusions on time to recurrence and overall survival (OS) in patients who underwent curative-intent resection of perihilar cholangiocarcinoma (PHC).
Methods
This retrospective cohort study included consecutive patients with resected PHC between 1992 and 2013 in a specialized center. Patients with 90-day mortality after surgery were excluded. Patients who did and did not receive perioperative blood transfusions were compared using univariable Kaplan–Meier analysis and multivariable Cox regression.
Results
Of 145 included patients, 80 (55.2%) received perioperative blood transfusions. The median OS was 49 months for patients without and 41 months for patients with blood transfusions (P = 0.46). In risk-adjusted multivariable Cox regression analysis, blood transfusion was not associated with OS (HR 1.00, 95% CI 0.59–1.68, P = 0.99) or time to recurrence (HR 1.00, 95% CI 0.57–1.78, P = 0.99). In addition, no differences in effect were found between different types of blood products transfused.
Conclusion
Blood transfusion was not associated with survival or time to recurrence after curative resection of PHC in this series. The alleged association is presumably related to the circumstances necessitating blood transfusions.
Introduction
Perihilar cholangiocarcinoma (PHC) is the most common type of cholangiocarcinoma,1 and originates at or near the biliary confluence. Surgical resection is the only potentially curative treatment for PHC, yielding a median overall survival of 19 to 39 months.2, 3
Complete excision of PHC requires extended liver resection, which may cause significant blood loss. Perioperative blood transfusions with packed red blood cells (pRBC) are used to compensate for critical blood loss, but have been associated with increased risk of tumor recurrence and decreased long-term outcome in several tumor types, including colorectal, prostate, lung, and head and neck cancer.4, 5, 6, 7, 8, 9, 10, 11 The effect of blood transfusions on prognosis is attributed to a distinct pathology of immunosuppression, known as transfusion-related immune modulation (TRIM). Blood transfusion seems to provoke an immune deviation12 and changes in the anti-inflammatory/pro-inflammatory environment.13, 14, 15 These substantial alterations form a complex and dynamic interplay creating a pro-tumor environment, which has been suggested to facilitate growth of residual cancer cells at the resection margin, transformation of micro-metastases into clinical metastases, or both.
Despite multiple studies showing an association between perioperative blood transfusion and prognosis, it is unclear whether this effect is caused by clinical circumstances requiring transfusions or is due to the blood transfusion itself.16, 17 Conflicting results have been reported after resection of cholangiocarcinoma.18, 19, 20, 21, 22, 23 Some studies included both proximal and distal cholangiocarcinoma, but these should be regarded as distinct tumor entities with different prognosis, as the latter involves the pancreatic head.24, 25 The aim of this study was to assess the effect of perioperative blood transfusions on overall survival (OS) and time to recurrence in patients who recovered after resection of PHC, thus excluding patients who died from post-operative complications. As a secondary analysis, we also assessed the individual effects of different transfusion products.5
Methods
A retrospective database was used, identifying 167 consecutive patients who underwent a curative-intent resection of PHC at a single center (Academic Medical Center, Amsterdam, The Netherlands) between 1992 and 2013. All patients who died within 90 days after resection (n = 22; 13.2%) were excluded. These patients most likely died from perioperative complications, which is a potential confounder when assessing the effect of transfusion on long-term outcome.26 PHC was defined as a pathologically confirmed biliary malignancy originating at the biliary confluence, right or left hepatic duct, or common hepatic duct.24 Patient selection and perioperative management have been described previously.27 Briefly, patients underwent routine preoperative biliary drainage, and preoperative low-dose radiotherapy (3 × 3.5 Gy) to prevent seeding metastases. An extrahepatic bile duct resection without liver resection was performed in patients with Bismuth type I tumors. For Bismuth type 2, 3 and 4 tumors, resection encompassed excision of the liver hilum en bloc with (extended) hemihepatectomy, excision of the portal vein bifurcation when involved, and complete lymphadenectomy of the hepatoduodenal ligament. Caudate lobectomy was performed in most patients since the late 90s.
Data collection and definitions
Perioperative transfusion was defined as administration of one or more blood products within seven days before or after surgery. Blood transfusions were further classified into the different blood products administered, consisting of pRBC, fresh-frozen plasma (FFP) or platelets. Overall survival was measured from the date of surgery to the date of death. Patients were censored when alive at January 1st, 2014. Since survival status was synchronized with the Dutch municipal register, no patients were lost to follow-up of overall survival. Time to recurrence was measured from the time of surgery to the time of the first recurrence on imaging. No adjuvant chemotherapy was administered after initial curative resection. Clinical follow-up was performed routinely every three months in the first year after surgery and every six months in the following five years. Laboratory tests and follow-up CT scans were performed in the first six months to detect early recurrence, and in later course when indicated. Patients who had no observed recurrence were censored at the time of the last follow-up (not necessarily with imaging) prior to January 2014. Major complications were graded according to the Clavien–Dindo classification28; severe morbidity included grade III and IV complications (grade V, post-operative death, was excluded from the analysis).
Statistical analysis
Continuous data are expressed as mean (±standard deviation) or median (±interquartile range (IQR)) as applicable. For comparing continuous variables a t-test or Mann–Whitney U test was used; for comparing proportions, Fisher's exact test or Chi square test was used. Firstly, characteristics between patients with and without a blood transfusion were compared, including patient demographics, comorbidities, preoperative status and treatment, and perioperative details. Survival was analyzed using a Kaplan–Meier survival plot, and compared with a log rank test. All models in Cox multivariable survival analysis were adjusted for age, sex and known prognostic factors including resection margin, lymph node stage, tumor differentiation,29 and major complications.30 The same prognostic factors were used in the analysis assessing the impact of different blood products of OS.
Statistical analysis was performed using SPSS (version 20, SPSS Inc., Chicago, IL). Two sided P-values < 0.05 were considered significant.
Results
Patient characteristics
Of 145 patients included, 80 (55.2%) received a perioperative blood transfusion: 26 patients (17.9%) received one or two blood units, and 54 patients (37.2%) received more than two blood units (total range, 1–47). Evaluation of different time spans shows a decrease in administered blood transfusions; 1992–1999 20 (62.5%), 2000–2006 25 (67.6%), 2007–2014 35 (46.1%). Table 1 details baseline and intra-operative characteristics of patients with and without perioperative transfusion. Patients receiving blood products were younger, more often jaundiced at presentation, and more often suffered from preoperative cholangitis. Also, the disease was more extensive in patients who had received perioperative blood transfusion, as evidenced by a higher Bismuth classification and Blumgart stage on preoperative imaging, and use of more extended resections and portal vein reconstruction. Patients with a perioperative blood transfusion more often had a major post-operative complication.
Table 1.
Baseline and intra-operative characteristics of patients with and without perioperative transfusion for perihilar cholangiocarcinoma. Variables are expressed as numbers (count) and percentages, unless stated otherwise
| Variable | Total (N = 145) | No transfusion N = 65 | Transfusion N = 80 | P-valuea |
|---|---|---|---|---|
| Age (years), mean (sd) | 63 ± 11.1 | 66 ± 10.7 | 59 ± 10.7 | 0.001 |
| Sex | 0.108 | |||
| Female | 56 | 21 (37.5) | 35 (62.5) | |
| Male | 89 | 44 (49.4) | 45 (50.6) | |
| ASA classification | 0.389 | |||
| 1–2 | 126 | 54 (42.9) | 72 (57.1) | |
| 3 | 16 | 8 (50.0) | 8 (50.0) | |
| Jaundice at presentation | 0.050 | |||
| No | 30 | 18 (60.0) | 12 (40.0) | |
| Yes | 112 | 46 (41.1) | 66 (58.9) | |
| Preoperative biliary drainage | 0.074 | |||
| No | 19 | 11 (57.9) | 8 (42.1) | |
| Percutaneous | 5 | 1 (20.0) | 4 (80.0) | |
| Endoscopic | 89 | 44 (49.4) | 45 (50.6) | |
| Both | 32 | 9 (28.1) | 23 (71.9) | |
| Preoperative cholangitis | 0.012 | |||
| No | 105 | 55 (52.4) | 50 (47.6) | |
| Yes | 27 | 7 (25.9) | 20 (74.1) | |
| Preoperative total bilirubin (μmol/L), median (IQR) | 14 ± 8 | 11 ± 10 | 19 ± 28 | 0.488 |
| Preoperative hemoglobin, mean (sd) | 7.9 ± 1.1 | 8.0 ± 1.0 | 7.8 ± 1.1 | 0.197 |
| Blumgart classification | 0.002 | |||
| 1 | 70 | 40 (57.1) | 30 (42.9) | |
| 2 | 29 | 7 (24.1) | 22 (75.9) | |
| 3 | 22 | 6 (27.3) | 16 (72.7) | |
| Bismuth classification | <0.001 | |||
| Left or right duct only | 16 | 13 (81.2) | 3 (18.8) | |
| 1/2 | 41 | 29 (70.7) | 12 (29.3) | |
| 3A/B | 69 | 17 (24.6) | 52 (75.4) | |
| 4 | 19 | 6 (31.6) | 13 (68.4) | |
| Type of resection | 0.001 | |||
| No major hepatectomy | 43 | 29 (67.4) | 14 (32.6) | |
| (Ext.) left hepatectomy | 54 | 23 (42.6) | 31 (57.4) | |
| (Ext.) right hepatectomy | 48 | 13 (27.1) | 35 (72.9) | |
| Caudate lobe resection | 0.129 | |||
| No | 65 | 33 (50.8) | 32 (49.2) | |
| Yes | 80 | 32 (40.0) | 48 (60.0) | |
| PV reconstruction | 0.023 | |||
| No | 118 | 58 (49.2) | 60 (50.8) | |
| Yes | 27 | 7 (25.9) | 20 (74.1) | |
| Blood loss (100 ml), mean (sd) | 22.8 ± 15.4 | 12.5 ± 6.9 | 31.8 ± 15.1 | <0.001 |
| Major post-operative complications (Clavien grade 3 or 4) | 0.002 | |||
| No | 94 | 51 (54.3) | 43 (45.7) | |
| Yes | 50 | 14 (28.0) | 36 (72.0) |
N, number of patients.
Overall survival
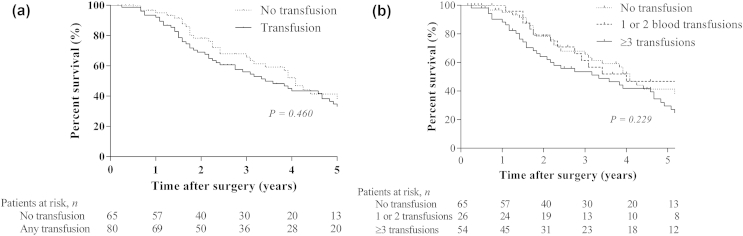
The median OS in the study cohort was 47 months (95% confidence interval [CI] 39–55). The median follow-up among survivors was 47 months (range 4–244). At last follow-up, 83 patients (57.2%) had died. Median OS of patients receiving a blood transfusion was 41 months compared to 49 months in patients without blood transfusions, which was not significant in univariable analysis (P = 0.46; Fig. 1a). To further explore a potential dose-dependent association, patients were stratified for the number of blood products transfused (none, 1 or 2, or ≥3). A trend towards worse OS was observed, but the effect remained non-significant (P = 0.23; Fig. 1b). Patients with three or more transfusions had a median OS of 39 months compared to a median OS of 41 months in patients who received one or two blood products. Multivariable analysis was performed to assess the association between one or more units transfused versus no units transfused. Risk-adjustment revealed significant associations between OS and known prognostic factors, including lymph node involvement, R1 resection, and tumor differentiation, but no association with blood transfusion (Table 2).
Figure 1.
Kaplan-Meier curves for overall survival
Table 2.
Prognostic factors for overall survival
| Variable | Est. median survival (months) | Cox regression |
||||
|---|---|---|---|---|---|---|
| Univariable HR (95% CI) | P-value | Multivariable HR (95% CI) | P-value | |||
| Blood transfusion | No | 49 | Reference | 0.461 | Reference | 0.993 |
| Yes | 41 | 1.18 (0.76–1.83) | 1.00 (0.59–1.68) | |||
| Age | Per year | NA | 1.00 (0.98–1.02) | 0.651 | 0.99 (0.96–1.01) | 0.150 |
| Sex | Female | 47 | Reference | 0.474 | Reference | 0.854 |
| Male | 47 | 1.18 (0.75–1.84) | 1.05 (0.63–1.74) | |||
| Type of resection | No major hepatectomy | 41 | Reference | 0.516 | Reference | 0.890 |
| Major hepatectomy | 48 | 0.86 (0.55–1.35) | 1.04 (0.61–1.76) | |||
| Portal vein reconstruction | No | 46 | Reference | 0.057 | Reference | 0.028 |
| Yes | NAa | 0.51 (0.25–1.02) | 0.43 (0.20–0.91) | |||
| Margin | R0 | 49 | Reference | 0.099 | Reference | 0.015 |
| R1 | 38 | 1.46 (0.93–2.28) | 1.93 (1.14–3.28) | |||
| Lymph node metastases | N0 | 49 | Reference | 0.001 | Reference | 0.001 |
| N1 | 27 | 2.27 (1.38–3.73) | 2.49 (1.17–4.28) | |||
| Tumor differentiation | Well | 68 | Reference | 0.009 | Reference | 0.014 |
| Moderate/poor | 38 | 2.21 (1.22–4.02) | 2.15 (1.17–3.96) | |||
| Major complications | No | 49 | Reference | 0.185 | Reference | 0.218 |
| Yes | 29 | 1.36 (0.86–2.15) | 1.37 (0.83–2.28) | |||
HR, hazard ratio; CI, confidence interval; NA, not available.
The median survival in patients with PV reconstruction is not available, this is because more than half of the patients is alive. The median has not been reached.
Time to recurrence
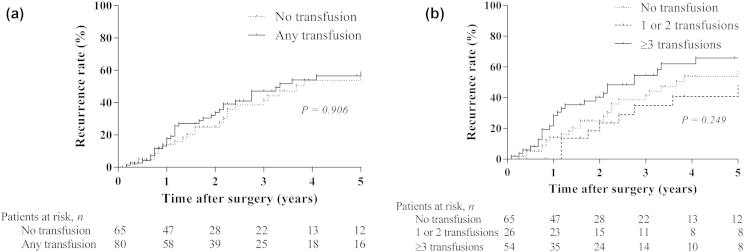
Recurrence status was unknown in 12 patients who were lost to follow-up of recurrences (8.3%). Among the other 133 patients, tumor recurrence was detected in 72 patients (54.1%) during follow up. Initial recurrences were classified according to the 7th edition of the AJCC staging system24 as local recurrence in 20 patients (15.0%), distant recurrence in 39 patients (29.3%), or both in 13 patients (9.8%). The median time to recurrence in all patients was 39 months; the median time to recurrence was 39 months among patients receiving a blood transfusion, and 44 months among patients without blood transfusions (P = 0.91; Fig. 2a). Similar to OS, stratification for the number of blood products (none, 1–2, or ≥3) revealed a trend in time to recurrence, but the effect was non-significant (P = 0.25; Fig. 2b). Interestingly, patients with one or two units transfused had a non-significant longer time to recurrence compared to patients with no transfusions (median 61 versus 44 months, respectively). Time to recurrence was shortest in patients with three or more units transfused (median 33 months). Finally, the association between one or more transfused units (versus no units transfused) was assessed in multivariable analysis. Again, risk-adjustment revealed significant associations of time to recurrence with known prognostic factors, including lymph node involvement, tumor differentiation and major complications but no association with one or more units transfused. (Table 3)
Figure 2.
Kaplan-Meier curves for time to recurrence
Table 3.
Prognostic factors for time to recurrence
| Variable | Est. median time to recurrence (months) | Cox regression |
||||
|---|---|---|---|---|---|---|
| Univariable HR (95% CI) | P-value | Multivariable HR (95% CI) | P-value | |||
| Blood transfusion | No | 44 | Reference | 0.906 | Reference | 0.989 |
| Yes | 39 | 0.97 (0.59–1.59) | 1.00 (0.57–1.78) | |||
| Age | Per year | NA | 0.99 (0.97–1.02) | 0.528 | 1.00 (0.97–1.02) | 0.636 |
| Sex | Female | 61 | Reference | 0.313 | Reference | 0.710 |
| Male | 39 | 1.30 (0.78–2.16) | 1.12 (0.62–2.02) | |||
| Type of resection | No major hepatectomy | 36 | Reference | 0.263 | Reference | 0.656 |
| Major hepatectomy | 44 | 0.75 (0.46–1.24) | 0.88 (0.49–1.57) | |||
| Portal vein reconstruction | No | 40 | Reference | 0.610 | Reference | 0.354 |
| Yes | NAa | 0.61 (0.30–1.24) | 0.69 (0.31–1.52) | |||
| Margin | R0 | 44 | Reference | 0.703 | Reference | 0.392 |
| R1 | 33 | 1.11 (0.65–1.90) | 1.31 (0.71–2.41) | |||
| Lymph node metastasis | N0 | 68 | Reference | <0.001 | Reference | <0.001 |
| N1 | 16 | 3.63 (2.13–6.20) | 3.85 (2.13–6.96) | |||
| Tumor differentiation | Well | Reference | 0.012 | Reference | 0.011 | |
| Moderate/poor | 33 | 2.49 (1.22–5.06) | 2.56 (1.24–5.31) | |||
| Major complications | No | 46 | Reference | 0.118 | Reference | 0.071 |
| Yes | 27 | 1.51 (0.90–2.51) | 1.67 (0.96–2.91) | |||
HR, hazard ratio; CI, confidence interval; NA, not available.
The median survival in patients with PV reconstruction is not available, this is because more than half of the patients is alive. The median has not been reached.
Red blood cells, platelets concentrates and fresh-frozen plasma
Seventy-seven patients received pRBC (53.1%), 47 patients received FFP (32.4%) and 9 patients received platelet concentrates in (6.2%). Univariable analysis showed that FFP had a non-significant adverse effect on OS (HR 1.36, 95% CI 0.87–2.12). After risk-adjustment in multivariable analysis, none of the three had a significant effect on OS. (Table 4)
Table 4.
Blood product-specific effect on overall survival
| No. (%) | Univariable HR (95% CI) | P-value | Multivariable HR (95% CI) | P-value | |
|---|---|---|---|---|---|
| Packed red blood cells | 77 (53.1%) | 1.18 (0.76–1.82) | 0.470 | 0.88 (0.54–1.44) | 0.601 |
| Fresh-frozen plasma | 47 (32.4%) | 1.36 (0.87–2.12) | 0.172 | 1.05 (0.66–1.68) | 0.874 |
| Platelet concentrates | 9 (6.2%) | 0.64 (0.23–1.75) | 0.383 | 0.69 (0.25–1.95) | 0.694 |
HR, hazard ratio; CI, confidence interval.
Discussion
We set out to assess the effect of perioperative blood transfusions on long-term outcomes after resection of perihilar cholangiocarcinoma in a single center, specialized in biliary tract surgery. After appropriate risk-adjustment, no effect of blood transfusion was found on tumor recurrence or overall survival.
Blood transfusions have been associated with worse oncological outcomes in several types of cancer,9, 16, 17, 31, 32 including multiple studies in cholangiocarcinoma.21, 22, 33 These studies attributed this association to transfusion-related immune modulation (TRIM), which is characterized by an increase in T helper type 2 (Th2) and regulatory T-cells. Th2 cytokines promote matrix metalloproteinase expression, invasiveness and metastasis of tumor cells.13 This in turn leads to down-regulation of the secretion of T helper type 1 cytokines, which normally suppress tumor growth and initiate changes to the inflammatory environment.12 Recent experimental evidence supports these findings.34, 35, 36, 37 Taking this into account, in combination with other side effects such as transfusion-related acute lung injury (TRALI), current guidelines recommend a cautious policy to use perioperative blood transfusions only when it is highly required.38
The alleged association between blood transfusion and overall survival were not corroborated in more recent studies. Several studies found no effect of blood transfusion on overall survival,39, 40 including several studies in cholangiocarcinoma (Table 5). Amongst others,18, 19, 23, 41 Muller et al. found no effect of blood transfusions in a propensity-matched analysis of patients undergoing resection of distal or proximal extrahepatic cholangiocarcinoma.41 The present study adds weight to that observation by analyzing a large homogenous cohort restricted to patients undergoing resection of perihilar cholangiocarcinoma, which is associated with a higher risk of blood loss because of the extended liver resections required to achieve complete removal of the tumor. Moreover, we excluded all patients who died within 90 days after surgery, thereby eliminating a potential confounding bias of perioperative complications and truly focusing the analysis on long-term outcome. We found no association with time to tumor recurrence and no association with overall survival, neither in unadjusted nor in risk-adjusted analysis.
Table 5.
Overview of recent studies assessing the effect blood transfusion on overall survival after resection of cholangiocarcinoma
| Author, reference, publication year | N | Type of cholangio-carcinoma | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|---|---|
| HR or RR (95% CI) | P-value | HR or RR (95% CI) | P-value | |||
| Studies with significant association | ||||||
| Young et al. 201121 | 83 | Perihilar | NA | 0.01 | 2.00 (1.09–3.69) | 0.03 |
| Nagino et al. 201322 | 574 | Perihilar | NA | 0.002 | 1.35 (1.06–1.72) | 0.02 |
| Studies with non-significant association | ||||||
| Muller et al. 201441 | 128 | Any extrahepatic (hilar or distal) | 2.05 (1.19–3.51) | 0.01 | 1.14 (0.52–2.48) | 0.75 |
| Chauhan et al. 201118 | 51 | Hilar | NA | 0.37 | – | |
| Furusuwa et al. 201419 | 144 | Hilar | NA | 0.02 | 1.49 (0.90–2.47) | 0.12 |
| Li et al. 201423 | 58a | Intrahepatic | NA | 0.05 | 1.98 (1.05–3.72) | 0.44 |
| Present study | 145 | Perihilar | 1.18 (0.76–1.83) | 0.46 | 1.00 (0.59–1.68) | 0.99 |
N, number of patients in the study; HR, hazard ratio; RR, relative risk; NA, not available.
Perioperative blood transfusion cut off was 600 mL.
In the abovementioned studies, the presumed effect of blood transfusion on long-term outcomes disappeared when the analysis was adjusted for the circumstances that require the blood transfusion. Additionally, one study showed no effect of blood transfusion in a large cohort of veterans undergoing surgery when the analysis was adjusted for perioperative complications.26 Other studies have suggested that anemia-induced hypoxia is the actual contributor to tumor recurrence instead of the blood transfusion.42, 43, 44 This theory was supported by the long-term results of a clinical trial comparing allogeneic versus autologous blood transfusion in colorectal cancer surgery.45 Counter-intuitively, this study showed worse overall and disease free survival after autologous transfusion, which was attributed to the induction of iatrogenic anemia before surgery. Furthermore, recent evidence demonstrates that a higher hemoglobin level mediates the response to radiation through delivery of oxygen to the tumor.46 These studies fit into a generally changing attitude towards perioperative blood transfusion: the assumption that perioperative blood transfusion is safe in terms of long-term outcome, and can be helpful when it is clinically required. A meta-analysis could provide a higher level of evidence to shed more light on this discussion. In that perspective, publication of negative studies is equally important as publishing positive studies, in order to prevent publication bias.
In our study, a relatively large amount of patients (55%) received blood transfusion, reflecting a liberal transfusion policy during the study period. Some studies reported less frequent use of blood transfusions during resection of PHC,41, 47 whereas other studies reported transfusion rates similar to the present study.18, 19, 39 The applied transfusion policy is apparently justified since no association of blood transfusion with survival has been found.
Up to now, little is reported about the association between survival and fresh-frozen plasma or platelet concentrates,5, 48 as literature mainly focuses on the effect of packed red blood cells.49, 50 An interesting finding of our study is that the distinction between blood products has no influence on the outcome. Comparable results were reported for example by Tomimaru et al., who found that FFP transfusion did not affect cancer prognosis following hepatic resection for HCC.51 McGrath et al. concluded that platelet transfusions in cardiac surgery have no effect on morbidity after cardiac surgery.52
Several studies have shown a dose-dependent relationship, with three or more units of transfused blood almost doubling the risk observed with one or two units.9, 17, 53, 54 Although, the principal analysis found no effect of blood transfusions on long-term outcomes, we cannot exclude a potential effect of massive blood transfusion, which could still cause immune modulation and alterations to the inflammatory response. Moreover, blood transfusion remains a costly therapy and unnecessary blood transfusions should be avoided. Transfusions can still cause alloimmunization, transmission of viral diseases, graft-versus-host disease, and an increased post-operative infection rate.4, 6, 7, 55 It is important to make an individualized decision on the use of blood transfusion in every patient undergoing cancer surgery, in conjunction with other precautionary measures.5
This retrospective study has several limitations. The cohort stretched over a long time period (1992–2013), which may have introduced heterogeneity related to changes in perioperative care. On the other hand, this approach enabled us to analyze the effect of blood transfusion in the largest cohort of resected PHC to date. Nevertheless, it remains a relatively small sample size with which a type II error cannot be ruled out. Furthermore, no information was available on the storage time of pRBC's, which may also affect outcome. Limiting the study to perihilar cholangiocarcinoma improved homogeneity, as distal and intrahepatic cholangiocarcinoma are regarded as different tumor entities with their own specific treatment and prognosis. Although, we performed risk-adjustment for a variety of observed confounding factors, potential bias due to unknown or unobserved confounders cannot be excluded.
In conclusion, perioperative blood transfusion was not associated with overall survival or time to recurrence in this large single-center cohort of patients who recovered from resection of perihilar cholangiocarcinoma. Therefore, the use of blood transfusion during or after surgery for perihilar cholangiocarcinoma seems safe in terms of long-term outcome. The alleged association of perioperative blood transfusions with worse outcomes after curative resection of PHC is presumably due to the circumstances necessitating blood transfusions instead of the blood transfusion per se.
Funding sources
None.
Conflicts of interest
None declared.
References
- 1.Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014. CA – Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Popescu I., Dumitrascu T. Curative-intent surgery for hilar cholangiocarcinoma: prognostic factors for clinical decision making. Langenbecks Arch Surg. 2014;399:693–705. doi: 10.1007/s00423-014-1210-x. [DOI] [PubMed] [Google Scholar]
- 3.D'Angelica M.I., Jarnagin W.R., Blumgart L.H. Resectable hilar cholangiocarcinoma: surgical treatment and long-term outcome. Surg Today. 2004;34:885–890. doi: 10.1007/s00595-004-2832-3. [DOI] [PubMed] [Google Scholar]
- 4.Carson J.L., Carless P.A., Hebert P.C. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4:CD002042. doi: 10.1002/14651858.CD002042.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cata J.P., Wang H., Gottumukkala V., Reuben J., Sessler D.I. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salpeter S.R., Buckley J.S., Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med. 2014;127:124–131. doi: 10.1016/j.amjmed.2013.09.017. e3. [DOI] [PubMed] [Google Scholar]
- 7.Theodoraki K., Markatou M., Rizos D., Fassoulaki A. The impact of two different transfusion strategies on patient immune response during major abdominal surgery: a preliminary report. J Immunol Res. 2014;2014:945829. doi: 10.1155/2014/945829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Churchhouse A.M., Mathews T.J., McBride O.M., Dunning J. Does blood transfusion increase the chance of recurrence in patients undergoing surgery for lung cancer? Interact Cardiovasc Thorac Surg. 2012;14:85–90. doi: 10.1093/icvts/ivr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunka I., Dostalik J., Martinek L., Gunkova P., Mazur M. Impact of blood transfusions on survival and recurrence in colorectal cancer surgery. Indian J Surg. 2013;75:94–101. doi: 10.1007/s12262-012-0427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miki C., Hiro J., Ojima E., Inoue Y., Mohri Y., Kusunoki M. Perioperative allogeneic blood transfusion, the related cytokine response and long-term survival after potentially curative resection of colorectal cancer. Clin Oncol. 2006;18:60–66. doi: 10.1016/j.clon.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Luan H., Ye F., Wu L., Zhou Y., Jiang J. Perioperative blood transfusion adversely affects prognosis after resection of lung cancer: a systematic review and a meta-analysis. BMC Surg. 2014;14:34. doi: 10.1186/1471-2482-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vamvakas E.C. Possible mechanisms of allogeneic blood transfusion-associated postoperative infection. Transfus Med Rev. 2002;16:144–160. doi: 10.1053/tmrv.2002.31463. [DOI] [PubMed] [Google Scholar]
- 13.Vamvakas E.C., Blajchman M.A. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–348. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Lacy A.M., Garcia-Valdecasas J.C., Delgado S., Castells A., Taura P., Pique J.M. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 15.Bouvy N.D., Marquet R.L., Jeekel H., Bonjer H.J. Impact of gas(less) laparoscopy and laparotomy on peritoneal tumor growth and abdominal wall metastases. Ann Surg. 1996;224:694–700. doi: 10.1097/00000658-199612000-00005. discussion-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busch O.R., Hop W.C., Hoynck van Papendrecht M.A., Marquet R.L., Jeekel J. Blood transfusions and prognosis in colorectal cancer. N Engl J Med. 1993;328:1372–1376. doi: 10.1056/NEJM199305133281902. [DOI] [PubMed] [Google Scholar]
- 17.Amato A., Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006:CD005033. doi: 10.1002/14651858.CD005033.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chauhan A., House M.G., Pitt H.A., Nakeeb A., Howard T.J., Zyromski N.J. Post-operative morbidity results in decreased long-term survival after resection for hilar cholangiocarcinoma. HPB. 2011;13:139–147. doi: 10.1111/j.1477-2574.2010.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furusawa N., Kobayashi A., Yokoyama T., Shimizu A., Motoyama H., Miyagawa S. Surgical treatment of 144 cases of hilar cholangiocarcinoma without liver-related mortality. World J Surg. 2014;38:1164–1176. doi: 10.1007/s00268-013-2394-x. [DOI] [PubMed] [Google Scholar]
- 20.de Boer M.T., Molenaar I.Q., Porte R.J. Impact of blood loss on outcome after liver resection. Dig Surg. 2007;24:259–264. doi: 10.1159/000103656. [DOI] [PubMed] [Google Scholar]
- 21.Young A.L., Igami T., Senda Y., Adair R., Farid S., Toogood G.J. Evolution of the surgical management of perihilar cholangiocarcinoma in a Western centre demonstrates improved survival with endoscopic biliary drainage and reduced use of blood transfusion. HPB. 2011;13:483–493. doi: 10.1111/j.1477-2574.2011.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagino M., Ebata T., Yokoyama Y., Igami T., Sugawara G., Takahashi Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258:129–140. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]
- 23.Li H., Wu J.S., Wang X.T., Lv P., Gong L.S., Liu G. Factors predicting surgical resection in patients with intrahepatic cholangiocarcinoma and cirrhosis. J Investig Surg – Off J Acad Surg Res. 2014;27:219–225. doi: 10.3109/08941939.2014.880138. [DOI] [PubMed] [Google Scholar]
- 24.Edge S.B., Compton C.C. The American Joint Committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 25.van der Gaag N.A., Kloek J.J., de Bakker J.K., Musters B., Geskus R.B., Busch O.R. Survival analysis and prognostic nomogram for patients undergoing resection of extrahepatic cholangiocarcinoma. Ann Oncol – Off J Eur Soc Med Oncol/ESMO. 2012;23:2642–2649. doi: 10.1093/annonc/mds077. [DOI] [PubMed] [Google Scholar]
- 26.Lee J., Radulescue V., Porhomayon J., Pourafkari L., Arora P., Dosluoglu H.H. The role of perioperative transfusion on long-term survival of veterans undergoing surgery. Ann Surg. 2015 Jan;261:104–110. doi: 10.1097/SLA.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 27.van Gulik T.M., Kloek J.J., Ruys A.T., Busch O.R., van Tienhoven G.J., Lameris J.S. Multidisciplinary management of hilar cholangiocarcinoma (Klatskin tumor): extended resection is associated with improved survival. Eur J Surg Oncol – J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2011;37:65–71. doi: 10.1016/j.ejso.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groot Koerkamp B., Wiggers J.K., Gonen M., Doussot A., Allen P.J., Besselink M.G. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann Oncol – Off J Eur Soc Med Oncol/ESMO. 2015 Sep;26:1930–1935. doi: 10.1093/annonc/mdv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pucher P.H., Aggarwal R., Qurashi M., Darzi A. Meta-analysis of the effect of postoperative in-hospital morbidity on long-term patient survival. Br J Surg. 2014;101:1499–1508. doi: 10.1002/bjs.9615. [DOI] [PubMed] [Google Scholar]
- 31.Cata J.P., Chukka V., Wang H., Feng L., Gottumukkala V., Martinez F. Perioperative blood transfusions and survival in patients with non-small cell lung cancer: a retrospective study. BMC Anesthesiol. 2013;13:42. doi: 10.1186/1471-2253-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han D.H., Choi G.H., Park J.Y., Ahn S.H., Kim K.S., Choi J.S. Lesson from 610 liver resections of hepatocellular carcinoma in a single center over 10 years. World J Surg Oncol. 2014;12:192. doi: 10.1186/1477-7819-12-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura N., Toyoki Y., Ishido K., Kudo D., Yakoshi Y., Tsutsumi S. Perioperative blood transfusion as a Poor prognostic factor after Aggressive surgical resection for hilar cholangiocarcinoma. J Gastrointest Surg – Off J Soc Surg Aliment Tract. 2015 Jun;19:1194–1195. doi: 10.1007/s11605-015-2823-2. [DOI] [PubMed] [Google Scholar]
- 34.Ghio M., Contini P., Negrini S., Mazzei C., Zocchi M.R., Poggi A. Down regulation of human natural killer cell-mediated cytolysis induced by blood transfusion: role of transforming growth factor-beta(1), soluble Fas ligand, and soluble Class I human leukocyte antigen. Transfusion. 2011;51:1567–1573. doi: 10.1111/j.1537-2995.2010.03000.x. [DOI] [PubMed] [Google Scholar]
- 35.Baumgartner J.M., Nydam T.L., Clarke J.H., Banerjee A., Silliman C.C., McCarter M.D. Red blood cell supernatant potentiates LPS-induced proinflammatory cytokine response from peripheral blood mononuclear cells. J Interferon Cytokine Res – Off J Int Soc Interferon Cytokine Res. 2009;29:333–338. doi: 10.1089/jir.2008.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Upile T., Jerjes W., Mahil J., Upile N., Sudhoff H., Wright A. An explanation for the worsened prognosis in some cancer patients of perioperative transfusion: the time-dependent release of biologically active growth factors from stored blood products. Eur Arch Oto-Rhino-Laryngol – Off J Eur Fed Oto-Rhino-Laryngol Soc. 2011;268:1789–1794. doi: 10.1007/s00405-011-1525-y. [DOI] [PubMed] [Google Scholar]
- 37.Long K., Meier C., Ward M., Williams D., Woodward J., Bernard A. Immunologic profiles of red blood cells using in vitro models of transfusion. J Surg Res. 2013;184:567–571. doi: 10.1016/j.jss.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramsey G., Wagar E.A., Grimm E.E., Friedberg R.C., Souers R.J., Lehman C.M. Red blood cell transfusion practices: a college of American pathologists q-probes study of compliance with audit criteria in 128 hospitals. Arch Pathol Lab Med. 2015;139:351–355. doi: 10.5858/arpa.2013-0756-CP. [DOI] [PubMed] [Google Scholar]
- 39.Warschkow R., Guller U., Koberle D., Muller S.A., Steffen T., Thurnheer M. Perioperative blood transfusions do not impact overall and disease-free survival after curative rectal cancer resection: a propensity score analysis. Ann Surg. 2014;259:131–138. doi: 10.1097/SLA.0b013e318287ab4d. [DOI] [PubMed] [Google Scholar]
- 40.Pang T.C., Spiro C., Ramacciotti T., Choi J., Drummond M., Sweeney E. Complications following liver resection for colorectal metastases do not impact on longterm outcome. HPB. 2015 Feb;17:185–193. doi: 10.1111/hpb.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller S.A., Mehrabi A., Rahbari N.N., Warschkow R., Elbers H., Leowardi C. Allogeneic blood transfusion does not affect outcome after curative resection for advanced cholangiocarcinoma. Ann Surg Oncol. 2014;21:155–164. doi: 10.1245/s10434-013-3226-9. [DOI] [PubMed] [Google Scholar]
- 42.Vaupel P., Mayer A. Hypoxia and anemia: effects on tumor biology and treatment resistance. Transfus Clin Biol – J de la Soc Francaise de Transfus Sang. 2005;12:5–10. doi: 10.1016/j.tracli.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Leo C., Giaccia A.J., Denko N.C. The hypoxic tumor microenvironment and gene expression. Semin Radiat Oncol. 2004;14:207–214. doi: 10.1016/j.semradonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Bindra R.S., Crosby M.E., Glazer P.M. Regulation of DNA repair in hypoxic cancer cells. Cancer Metastasis Rev. 2007;26:249–260. doi: 10.1007/s10555-007-9061-3. [DOI] [PubMed] [Google Scholar]
- 45.Harlaar J.J., Gosselink M.P., Hop W.C., Lange J.F., Busch O.R., Jeekel H. Blood transfusions and prognosis in colorectal cancer: long-term results of a randomized controlled trial. Ann Surg. 2012;256:681–686. doi: 10.1097/SLA.0b013e318271cedf. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 46.Katahira-Suzuki R., Hata M., Tateishi U., Taguchi T., Takano S., Omura-Minamisawa M. Definitive chemo-radiotherapy for squamous cell carcinoma of the pharynx: impact of baseline low hemoglobin level (<12 g/dL) and post-radiation therapy F-18 FDG-PET/CT. Ann Nucl Med. 2015;29:37–45. doi: 10.1007/s12149-014-0907-9. [DOI] [PubMed] [Google Scholar]
- 47.Liu C.L., Fan S.T., Lo C.M., Tso W.K., Lam C.M., Wong J. Improved operative and survival outcomes of surgical treatment for hilar cholangiocarcinoma. Br J Surg. 2006;93:1488–1494. doi: 10.1002/bjs.5482. [DOI] [PubMed] [Google Scholar]
- 48.Spiess B.D., Royston D., Levy J.H., Fitch J., Dietrich W., Body S. Platelet transfusions during coronary artery bypass graft surgery are associated with serious adverse outcomes. Transfusion. 2004;44:1143–1148. doi: 10.1111/j.1537-2995.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- 49.Cohen B., Matot I. Aged erythrocytes: a fine wine or sour grapes? Br J Anaesth. 2013;111(Suppl. 1):i62–70. doi: 10.1093/bja/aet405. [DOI] [PubMed] [Google Scholar]
- 50.Bernard A.C., Davenport D.L., Chang P.K., Vaughan T.B., Zwischenberger J.B. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg. 2009;208 doi: 10.1016/j.jamcollsurg.2008.11.019. 931–7, 7 e1–2; discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 51.Tomimaru Y., Wada H., Marubashi S., Kobayashi S., Eguchi H., Takeda Y. Fresh frozen plasma transfusion does not affect outcomes following hepatic resection for hepatocellular carcinoma. World J Gastroenterol – WJG. 2010;16:5603–5610. doi: 10.3748/wjg.v16.i44.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGrath T., Koch C.G., Xu M., Li L., Mihaljevic T., Figueroa P. Platelet transfusion in cardiac surgery does not confer increased risk for adverse morbid outcomes. Ann Thorac Surg. 2008;86:543–553. doi: 10.1016/j.athoracsur.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 53.Yao H.S., Wang Q., Wang W.J., Hu Z.Q. Intraoperative allogeneic red blood cell transfusion in ampullary cancer outcome after curative pancreatoduodenectomy: a clinical study and meta-analysis. World J Surg. 2008;32:2038–2046. doi: 10.1007/s00268-008-9675-9. [DOI] [PubMed] [Google Scholar]
- 54.Philips P., Farmer R.W., Scoggins C.R., McMasters K.M., Martin R.C., 2nd Caudate lobe resections: a single-center experience and evaluation of factors predictive of outcomes. World J Surg Oncol. 2013;11:220. doi: 10.1186/1477-7819-11-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rohde J.M., Dimcheff D.E., Blumberg N., Saint S., Langa K.M., Kuhn L. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311:1317–1326. doi: 10.1001/jama.2014.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]