Abstract
Background
Long-term incidence of endocrine and exocrine insufficiency after pancreatectomy is poorly described. We analyze the long-term risks of pancreatic insufficiency after pancreatectomy.
Methods
Subjects who underwent pancreatectomy from 2002 to 2012 were identified from a prospective database (n = 227). Subjects who underwent total pancreatectomy or pancreatitis surgery were excluded. New post-operative endocrine and exocrine insufficiency was defined as the need for new pharmacologic intervention within 1000 days from resection.
Results
28 (16%) of 178 subjects without pre-existing endocrine insufficiency developed post-operative endocrine insufficiency: 7 (25%) did so within 30 days, 8 (29%) between 30 and 90 days, and 13 (46%) after 90 days. 94 (43%) of 214 subjects without pre-operative exocrine insufficiency developed exocrine insufficiency: 20 (21%) did so within 30 days, 29 (31%) between 30 and 90 days, and 45 (48%) after 90 days. Adjuvant radiation was associated with new endocrine insufficiency. On multivariate regression, pancreaticoduodenectomy and chemotherapy were associated with a greater risk of exocrine insufficiency.
Conclusion
Reporting 30-day functional outcomes for pancreatic resection is insufficient, as nearly 45% of subjects who develop disease do so after 90 days. Reporting of at least 90-day outcomes may more reliably assess risk for post-operative endocrine and exocrine insufficiency.
Introduction
Pancreatectomy has been increasingly utilized in recent decades for both benign and malignant entities. Owing to the refinement of surgical technique as well as improvement in patient selection and perioperative care, pancreatic resection can be performed safely with a morbidity rate of 4.6–46%.1, 2 While the use of parenchymal-preserving resections such as enucleation and central pancreatectomy aim to reduce the risk of post-operative endocrine and exocrine insufficiency, these complications still result in a detrimental impact on quality of life.
While many studies have reported these complications, few have excluded pancreatitis patients, who are more prone to these specific complications and biasing the outcomes. Even fewer have reported long-term outcomes. The presence of such data may allow for a more accurate means of educating patient about long-term risks following resection.
A study of the Society of Thoracic Surgery (STS) mortality database revealed significantly greater mortality after lung resection at 90 days compared to 30 days,3 suggesting that standard 30-day outcome reporting is inadequate. Work in hepatic resection demonstrated that 30-day reporting of mortality may be misleading. The authors proposed that perioperative outcomes for hepatic resection should be reported with the 90-day benchmark.4 However, such a study has not been reported following pancreatic surgery, which may limit physicians from appropriately educating their patients preoperatively about these potential risks.
In light of the limited data, we aim to report the short- and long-term incidence of new endocrine and exocrine insufficiency after pancreatic resection for non-pancreatitis etiologies. We also seek to determine if the standard reporting of 30-day outcomes for these complications is sufficient and to identify predictive factors for new post-operative endocrine and exocrine insufficiency after pancreatectomy.
Methods
Subjects who underwent pancreatic resection from January 2002 to December 2012 at a tertiary care center were identified from a prospectively maintained pancreatic surgery database. Subjects who underwent total pancreatectomy or surgery for pancreatitis were excluded. Demographic, histopathologic, operative, perioperative, and follow-up data were collected. The study was conducted according to institutional human research committee procedures, and was reviewed and approved by the University of Massachusetts Medical School Institutional Review Board.
Development of pancreatic insufficiency was defined by the need for new pharmacological intervention, such as pancreatic enzymes, insulin or oral hypoglycemic medications that persisted beyond discharge after initial surgery. Subjects who received insulin in the immediate perioperative period that was not continued at discharge were excluded. Initiation of pancreatic enzymes by members of the pancreatic team involved in the subject's care was based on symptom development or on serologic data (such as new hyperglycemia). Data on subjects with pre-resection endocrine and exocrine insufficiency (such as need for escalation or continuation of medication) were collected. However, these data are not included in the current analysis and are not considered “new onset.” Subjects who developed pancreatic insufficiency within 1000 days were included in analysis. The median time to development of pancreatic insufficiency was calculated from the first post-operative day to the first date of newly documented pharmacological initiation.
Statistical analyses were performed using Intercooled Stata software, version 12.0 (StataCorp, College Station, TX). Categorical variables were analyzed utilizing Fisher's Exact test and Pearson's chi-squared test. Continuous variables were analyzed using the student t-test for variables with a normal distribution, and the Mann–Whitney rank sum test for variables without normal distribution. Univariate variables with statistical significance were included in the multivariate model. Statistical significance was accepted at a p-value of less than 0.05.
Results
Patient and tumor characteristics
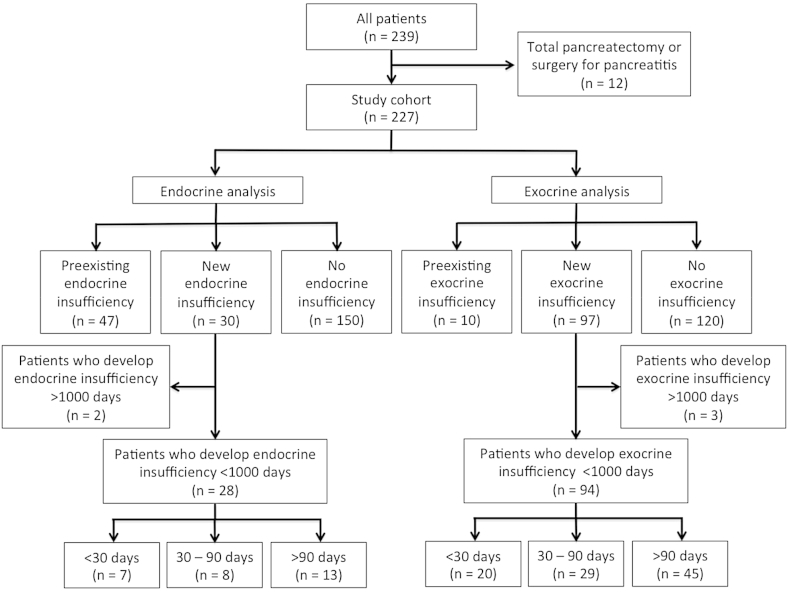
Of the 239 subjects who underwent pancreatic resection from January 2002 to December 2012, 12 subjects were excluded due to the presence of total pancreatectomy (n = 5) or surgery for pancreatitis (n = 7). A total of 227 subjects were analyzed (Fig. 1). The mean age was 62 years old (range: 22–88 years). Median follow-up was 21 months (range: 0–114 months). The majority were females (n = 120, 53%). Tumors were most commonly located at the pancreatic head (n = 150, 66%), followed by body (15%) and tail (12%). One hundred and fifty nine (70%) subjects underwent pancreaticoduodenectomy (PD), while 63 (28%) subjects had distal pancreatectomy (DP), and 5 (2%) underwent enucleation (Table 1).
Figure 1.
Study scheme
Table 1.
Demographics and perioperative variables of pancreatectomy subjects
| Variable | Subjects (N = 227) |
|---|---|
| Age, years (range) | 62 (22–88) |
| BMI, kg/m2 (range) | 27 (16–56) |
| Gender, N (%) | |
| Male | 107 (47) |
| Female | 120 (53) |
| Resection Type, N (%) | |
| Pancreaticoduodenectomy | 159 (70) |
| Distal pancreatectomy | 63 (28) |
| Enucleation | 5 (2) |
| Pathology, N (%) | |
| Benign | |
| IPMN | 35 (15) |
| Serous cystadenoma | 10 (4) |
| Mucinous cystadenoma | 7 (3) |
| GIST | 2 (1) |
| Others | 18 (8) |
| Malignant | |
| PDAC | 100 (44) |
| Ampullary adenocarcinoma | 28 (12) |
| Neuroendocrine carcinoma | 13 (6) |
| Cholangiocarcinoma | 9 (4) |
| Duodenal adenocarcinoma | 4 (2) |
| Metastasis | 1 (0.4) |
| Tumor location, N (%) | |
| Head | 150 (66) |
| Body | 35 (15) |
| Tail | 27 (12) |
| Unknown | 15 (7) |
| Tumor size, cm (range) | 3.0 (0.2–17.0) |
| Pancreatitis on pathology, N (%) | |
| Yes | 57 (25) |
| No | 153 (67) |
| Unknown | 17 (7) |
| Chemotherapy, N (%) | |
| Neoadjuvant | 18 (8) |
| Adjuvant | 109 (48) |
| Radiation, N (%) | |
| Neoadjuvant | 17 (8) |
| Adjuvant | 80 (35) |
BMI, body mass index; GIST, gastrointestinal stromal tumor; IPMN, intraductal papillary mucinous neoplasm; PDAC, pancreatic ductal adenocarcinoma.
Seventy-two (32%) were found to have non-invasive disease, including intraductal papillary mucinous neoplasm (n = 35, 15%), serous cystadenoma (4%), mucinous cystadenoma (3%) and gastrointestinal stromal tumor (1%). The majority (n = 155, 68%) had malignant disease, most commonly pancreatic ductal adenocarcinoma (PDAC; 44%), ampullary adenocarcinoma (12%), neuroendocrine carcinoma (6%) and cholangiocarcinoma (4%; Table 1).
Mean tumor size was 3.0 cm (range: 0.2 cm–17.0 cm). Fifty-seven (25%) subjects were incidentally found to have pancreatitis on pathologic analysis, but surgery was not performed for this indication. Eighteen (8%) subjects underwent neoadjuvant chemotherapy, and 109 (48%) underwent adjuvant chemotherapy. Seventeen (8%) underwent neoadjuvant radiation therapy while 80 (35%) underwent adjuvant radiation therapy (Table 1).
Incidence of post-operative pancreatic endocrine and exocrine insufficiency
Forty-seven (21%) subjects had preoperative endocrine insufficiency and need for pharmacologic intervention. Of the remaining previously unaffected (n = 180), two were excluded due to development beyond 1000 days. Twenty-eight (16%) developed post-operative endocrine insufficiency with a median time to development of 72 days (range: 0–906 days) and were included in the analysis. Of these, 7 (25%) subjects did so within 30 days, 8 (29%) between 30 and 90 days and 13 (46%) after 90 days (Fig. 1).
Ten subjects had pre-existing exocrine insufficiency. Of the previously unaffected subjects (n = 217), three were excluded from analysis due to time to disease. Ninety-four (43%) subjects developed exocrine insufficiency over a median of 75 days (range: 0–881 days). Twenty (21%) subjects developed the deficiency within 30 days of resection, whereas 31% (n = 29) between 30 and 90 days of resection, and 48% (n = 45) after 90 days (Fig. 1).
Of the 122 new events of pancreatic endocrine and exocrine insufficiency, 78% of these events (n = 95) developed after the 30-day time point and 48% (n = 58) after the 90-day time point.
Predictive factors of post-operative endocrine and exocrine insufficiency
On the univariate analysis, subjects who received adjuvant radiation therapy were more likely to develop post-operative endocrine insufficiency (54% vs 34%, p = 0.048). Relative risk of developing post-operative endocrine insufficiency is 1.9 in patients undergoing adjuvant radiation therapy vs those that did not. Additional perioperative factors were not associated with post-operative endocrine insufficiency (Table 2). Multivariate analysis was not performed for endocrine insufficiency as only one significant variable was identified on univariate analysis.
Table 2.
Univariate analysis of predictive factors of post-operative endocrine insufficiency
| Variables | Presence of new post-operative endocrine insufficiency (n = 28)a | Absence of new post-operative endocrine insufficiency (n = 150)a | p-value |
|---|---|---|---|
| Gender, N (%) | |||
| Female | 15 (54) | 79 (53) | 0.927 |
| Male | 13 (46) | 71 (47) | |
| BMI, kg/m2 (range) | 25.4 ± 3.5 | 27.7 ± 7.1 | 0.282 |
| Pancreaticoduodenectomy, N (%) | 19 (68) | 104 (70) | 0.799 |
| Malignant pathology, N (%) | 19 (73) | 91 (72) | 0.929 |
| Partial gastrectomy, N (%) | 12 (46) | 53 (38) | 0.410 |
| Location, tail N (%) | 4 (15) | 19 (13) | 0.795 |
| Pathologic presence of pancreatitis, N (%) | 10 (38) | 39 (28) | 0.287 |
| Neoadjuvant chemotherapy, N (%) | 1 (4) | 12 (8) | 0.408 |
| Adjuvant chemotherapy, N (%) | 18 (64) | 69 (49) | 0.138 |
| Neoadjuvant radiation therapy, N (%) | 1 (4) | 12 (8) | 0.408 |
| Adjuvant radiation therapy, N (%) | 15 (54) | 48 (34) | 0.048 |
Due to missing data points, percentages are based upon n of available data points.
Male gender (p = 0.014), PD (p < 0.001), malignant pathology (p = 0.002), presence of a partial gastrectomy (p = 0.001), tail location of lesion (p = 0.036), neoadjuvant chemotherapy (p = 0.050), adjuvant chemotherapy (p < 0.001) and adjuvant radiation therapy (p < 0.001) were associated with the development of post-operative exocrine insufficiency (Table 3). Body mass index (BMI), pathologic presence of pancreatitis and neoadjuvant radiation therapy were not associated with development of post-operative exocrine insufficiency. All risk factors were adjusted with multivariate regression.
Table 3.
Univariate analysis of predictive factors of post-operative exocrine insufficiency
| Variables | Presence of new post-operative exocrine insufficiency (n = 94)a | Absence of new post-operative exocrine insufficiency (n = 120)a | p-value |
|---|---|---|---|
| Gender, N (%) | |||
| Female | 40 (43) | 71 (59) | 0.014 |
| Male | 54 (57) | 49 (41) | |
| Body mass index (kg/m2) | 27.9 ± 7.0 | 28.3 ± 6.9 | 0.745 |
| Pancreaticoduodenectomy, N (%) | 80 (85) | 71 (60) | <0.001 |
| Malignant pathology, N (%) | 72 (83) | 61 (62) | 0.002 |
| Partial gastrectomy, N (%) | 50 (56) | 38 (33) | 0.001 |
| Location, tail, N (%) | 7 (8) | 20 (18) | 0.036 |
| Pathologic presence of pancreatitis, N (%) | 24 (27) | 28 (25) | 0.724 |
| Neoadjuvant chemotherapy, N (%) | 9 (10) | 4 (3) | 0.050 |
| Adjuvant chemotherapy, N (%) | 62 (69) | 40 (35) | <0.001 |
| Neoadjuvant radiation therapy, N (%) | 8 (9) | 5 (4) | 0.175 |
| Adjuvant radiation therapy, N (%) | 48 (53) | 29 (26) | <0.001 |
Due to missing data points, percentages are based upon n of available data points.
On multivariate analysis, the presence of adjuvant chemotherapy (OR 3.10, p < 0.001) and the presence of a PD (OR 2.57, p = 0.010) were associated with the development of post-operative exocrine insufficiency. Relative risk of developing post-operative exocrine insufficiency is 2.3 in patients undergoing PD (compared to DP and enucleation) and 2.2 in patients undergoing adjuvant chemotherapy. Gender, malignant pathology, presence of partial gastrectomy, neoadjuvant chemotherapy and adjuvant radiation therapy did not maintain statistical significance in the final model (Table 4).
Table 4.
Multivariate analysis of predictive factors of post-operative exocrine insufficiency
| Variables | Odds ratio | 95% Confidence intervals | p-value |
|---|---|---|---|
| Pancreaticoduodenectomy | 3.10 | 1.248–5.293 | 0.010 |
| Adjuvant chemotherapy | 2.57 | 1.682–5.739 | <0.001 |
Discussion
The development of pancreatic insufficiency after pancreatic surgery is an understudied topic even though it has been reported as a common clinical manifestation after pancreatic surgery.5, 6 Early recognition of pancreatic insufficiency after surgery and early initiation of treatment was initially emphasized.7 Over the past two decades, there has been a movement towards prevention of pancreatic insufficiency by promoting pancreatic parenchymal-preserving techniques for benign and pre-malignant pancreatic disease.8, 9, 10
Long-term incidence of post-operative endocrine insufficiency is estimated to be between 8 and 49%11, 12 and up to 53–73% for exocrine insufficiency.13 These numbers are heavily biased towards those with pancreatitis, who have an increased likelihood of developing this complication due to poor functional reserve. Few studies evaluate the risk of post-operative pancreatic insufficiency for patients undergoing resection for neoplasia, who theoretically have a more normal remnant pancreas. In a study of 162 subjects undergoing pancreatic resection for benign tumors, 14–18% developed endocrine insufficiency and 18–33% developed exocrine insufficiency after DP and PD, respectively with a follow-up of 5 years.5 Our current study reports a similar incidence of endocrine insufficiency (13%) but an increased risk of exocrine insufficiency (43%). This could be because we included malignant disease in our study, which may increase the risk of exocrine insufficiency due to tumor obstruction and the receipt of chemoradiotherapy, increasing the risk of pancreatic fibrosis and reduced function.
The current standard of publicly reported outcomes as set forth by the Center of Medicare and Medicaid (CMS) is often a 30-day measure. However, the 30-day measure may not adequately capture the incidence of a variable to accurately portray its true impact. In a retrospective review of lung resections, 30-day mortality rates underestimated 90-day mortality rates by six fold.14 Even in the gastrointestinal literature, Damhuis et al. also reported increased post-operative mortality rates at 90 days as compared to 30 days for eight different cancer types, including gastric resection (13.7% vs 9.3%).15 Other complications of gastrointestinal surgery, such as anastomotic leak, were also found to demonstrate a similar trend.16 Similarly, within our analysis, 30-day outcome reporting of endocrine insufficiency and exocrine insufficiency significantly underestimates the true incidence: 30-day reporting did not capture 78% of cases of endocrine and exocrine insufficiency that ultimately developed in the post-operative period. Endocrine and exocrine insufficiency are functional outcomes. Particularly in light of our data that 48% of subjects develop exocrine insufficiency even after 90 days, it is possible that with a larger follow-up study that allows for analysis of longer-term follow-up, one might better determine if 90 days, or a longer time point, should serve as the gold standard for post-pancreatectomy functional assessment. We believe that the reporting of post-operative pancreatic insufficiency should not be limited to the 30-day reporting measure as it underestimates its true incidence of these complications. These data suggest that the risk is not limited to the first 30-day window and with increased awareness of the long-term complications after resection, one can better design preoperative risk discussions and discussion and post-operative management strategies.
Several studies have evaluated risk factors leading to development of post-operative endocrine and exocrine insufficiency. In previous reports, there was a higher incidence of post-operative endocrine insufficiency in subjects undergoing PD5, 17 and total pancreatectomy.18 We have excluded subjects who underwent total pancreatectomies from our analysis because pancreatic insufficiency is a known outcome of this procedure after patients are rendered apancreatic.18, 19 Other reported risk factors for post-operative endocrine insufficiency include previous acute pancreatitis episodes,20 pre-existing chronic pancreatitis,21 preoperative glucose intolerance,22 pancreatic specimen size,22, 23 body mass index (BMI) and pancreatic texture.24 While many of some of these factors could not be analyzed in the current study, the data fail to show that risk factors, like PD, increase the risk of post-operative diabetes. However, our study demonstrated that subjects who underwent radiation therapy (54% vs 34%, p = 0.05) have a greater likelihood of developing post-operative endocrine insufficiency, possibly related to radiation-induced pancreatic fibrosis, as previous authors demonstrated in long-term survivors of childhood cancer.25
Reported risk factors for exocrine insufficiency include PD,5 preoperative endocrine insufficiency, hard pancreatic texture13 and malignancy.26 Sikkens et al. demonstrated that the prevalence of preoperative exocrine insufficiency in subjects with ampullary tumors was 66%, which quickly escalated to 92% after a median follow-up of 2 months.27 In univariate analysis, PD, malignant pathology, presence of partial gastrectomy (associated with standard PD), tail location of lesion, neoadjuvant chemotherapy, adjuvant chemotherapy and adjuvant radiation therapy were found to be significant risk factors for development of disease. On multivariate analysis, only PD and adjuvant chemotherapy maintained their statistical significance. The majority of subjects undergoing PD have tumors at the head of the pancreas predisposing them to pancreatic atrophy or fibrosis due to pancreatic ductal obstruction.28 While there is no data to support our finding that chemotherapy may be associated with post-operative exocrine insufficiency, one can hypothesize that this finding may be related to the underlying pathology resulting in pancreatic burnout or that chemotherapy induces a fibrotic reaction that further diminishes from the function of the pancreatic remnant.
We hope that our data can help provide a platform for future studies on functional outcomes after pancreatic surgery. Our study results should be interpreted within its context. It is retrospective in nature and therefore several clinical variables are not available. We cannot determine the specific factors (such as poor remnant reserve, anastomotic stricture [including those related to pancreatic leak], tumor obstruction, chemotherapy or radiation-induced fibrosis, duct size) that ultimately drive the long-term functional deficiency. However, a larger, prospective study should incorporate these variables, which may contribute to long-term outcomes.
In conclusion, the incidence of pancreatic endocrine and exocrine insufficiency after pancreatectomy is underestimated with the 30-day outcomes reporting as standardized by CMS. This study serves as the largest to date evaluating these complications after pancreatectomy for neoplasia and demonstrates an incidence of 16% and 43% for new-onset endocrine and exocrine insufficiency, respectively. Moreover, this study demonstrates that these complications largely occur after 30 days: more than 75% of these new cases occur after the 30-day benchmark. Therefore, 30-day outcome reporting is insufficient after pancreatic resection. In order to educate subjects undergoing pancreatic resection, at least 90-day functional outcomes should be reported.
Conflicts of interest
None declared.
Acknowledgments
We would like to thank the Pancreatic Cancer Alliance and the Linda J. Verville Cancer Research Foundation for internal support of this study.
Footnotes
Presented at the American Pancreas Association 2013 Annual Meeting, Miami, FL, Oct 19–Nov 2, 2013.
References
- 1.Palanivelu C., Shetty R., Jani K., Sendhilkumar K., Rajan P.S., Maheshkumar G.S. Laparoscopic distal pancreatectomy: results of a prospective non-randomized study from a tertiary center. Surg Endosc. 2007;21:373–377. doi: 10.1007/s00464-006-9020-z. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-del Castillo C., Morales-Oyarvide V., McGrath D., Wargo J.A., Ferrone C.R., Thayer S.P. Evolution of the Whipple procedure at the Massachusetts General Hospital. Surgery. 2012;152(3 Suppl. 1):S56–S63. doi: 10.1016/j.surg.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryant A.S., Rudemiller K., Cerfolio R.J. The 30- versus 90-day operative mortality after pulmonary resection. Ann Thorac Surg. 2010;89:1717–1722. doi: 10.1016/j.athoracsur.2010.01.069. discussion 22–3. [DOI] [PubMed] [Google Scholar]
- 4.Mayo S.C., Shore A.D., Nathan H., Edil B.H., Hirose K., Anders R.A. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB – Off J Int Hepato Pancreato Biliary Assoc. 2011;13:473–482. doi: 10.1111/j.1477-2574.2011.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falconi M., Mantovani W., Crippa S., Mascetta G., Salvia R., Pederzoli P. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. 2008;95:85–91. doi: 10.1002/bjs.5652. [DOI] [PubMed] [Google Scholar]
- 6.Kahl S., Malfertheiner P. Exocrine and endocrine pancreatic insufficiency after pancreatic surgery. Best Pract Res Clin Gastroenterol. 2004;18:947–955. doi: 10.1016/j.bpg.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Sikkens E.C., Cahen D.L., van Eijck C., Kuipers E.J., Bruno M.J. The daily practice of pancreatic enzyme replacement therapy after pancreatic surgery: a northern European survey: enzyme replacement after surgery. J Gastrointest Surg – Off J Soc Surg Alimentary Tract. 2012;16:1487–1492. doi: 10.1007/s11605-012-1927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiNorcia J., Lee M.K., Reavey P.L., Genkinger J.M., Lee J.A., Schrope B.A. One hundred thirty resections for pancreatic neuroendocrine tumor: evaluating the impact of minimally invasive and parenchyma-sparing techniques. J Gastrointest Surg – Off J Soc Surg Alimentary Tract. 2010;14:1536–1546. doi: 10.1007/s11605-010-1319-3. [DOI] [PubMed] [Google Scholar]
- 9.Cauley C.E., Pitt H.A., Ziegler K.M., Nakeeb A., Schmidt C.M., Zyromski N.J. Pancreatic enucleation: improved outcomes compared to resection. J Gastrointest Surg – Off J Soc Surg Alimentary Tract. 2012;16:1347–1353. doi: 10.1007/s11605-012-1893-7. [DOI] [PubMed] [Google Scholar]
- 10.Falconi M., Zerbi A., Crippa S., Balzano G., Boninsegna L., Capitanio V. Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol. 2010;17:1621–1627. doi: 10.1245/s10434-010-0949-8. [DOI] [PubMed] [Google Scholar]
- 11.Huang J.J., Yeo C.J., Sohn T.A., Lillemoe K.D., Sauter P.K., Coleman J. Quality of life and outcomes after pancreaticoduodenectomy. Ann Surg. 2000;231:890–898. doi: 10.1097/00000658-200006000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemaire E., O'Toole D., Sauvanet A., Hammel P., Belghiti J., Ruszniewski P. Functional and morphological changes in the pancreatic remnant following pancreaticoduodenectomy with pancreaticogastric anastomosis. Br J Surg. 2000;87:434–438. doi: 10.1046/j.1365-2168.2000.01388.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura H., Murakami Y., Uemura K., Hayashidani Y., Sudo T., Ohge H. Predictive factors for exocrine pancreatic insufficiency after pancreatoduodenectomy with pancreaticogastrostomy. J Gastrointest Surg – Off J Soc Surg Alimentary Tract. 2009;13:1321–1327. doi: 10.1007/s11605-009-0896-5. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y., McMurry T.L., Isbell J.M., Stukenborg G.J., Kozower B.D. Readmission after lung cancer resection is associated with a 6-fold increase in 90-day postoperative mortality. J Thorac Cardiovasc Surg. 2014;148 doi: 10.1016/j.jtcvs.2014.04.026. 2261–2267 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damhuis R.A., Wijnhoven B.P., Plaisier P.W., Kirkels W.J., Kranse R., van Lanschot J.J. Comparison of 30-day, 90-day and in-hospital postoperative mortality for eight different cancer types. Br J Surg. 2012;99:1149–1154. doi: 10.1002/bjs.8813. [DOI] [PubMed] [Google Scholar]
- 16.Hyman N., Manchester T.L., Osler T., Burns B., Cataldo P.A. Anastomotic leaks after intestinal anastomosis: it's later than you think. Ann Surg. 2007;245:254–258. doi: 10.1097/01.sla.0000225083.27182.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugito K., Furuya T., Kaneda H., Masuko T., Ohashi K., Inoue M. Long-term follow-up of nutritional status, pancreatic function, and morphological changes of the pancreatic remnant after pancreatic tumor resection in children. Pancreas. 2012;41:554–559. doi: 10.1097/MPA.0b013e318232a6e2. [DOI] [PubMed] [Google Scholar]
- 18.Barbier L., Jamal W., Dokmak S., Aussilhou B., Corcos O., Ruszniewski P. Impact of total pancreatectomy: short- and long-term assessment. HPB – Off J Int Hepato Pancreato Biliary Assoc. 2013:882–892. doi: 10.1111/hpb.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epelboym I., Winner M., DiNorcia J., Lee M.K., Lee J.A., Schrope B. Quality of life in patients after total pancreatectomy is comparable with quality of life in patients who undergo a partial pancreatic resection. J Surg Res. 2014;187:189–196. doi: 10.1016/j.jss.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Das S.L., Singh P.P., Phillips A.R., Murphy R., Windsor J.A., Petrov M.S. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut. 2014;63:818–831. doi: 10.1136/gutjnl-2013-305062. [DOI] [PubMed] [Google Scholar]
- 21.Ewald N., Kaufmann C., Raspe A., Kloer H.U., Bretzel R.G., Hardt P.D. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c) Diabetes Metab Res Rev. 2012;28:338–342. doi: 10.1002/dmrr.2260. [DOI] [PubMed] [Google Scholar]
- 22.Ferrara M.J., Lohse C., Kudva Y.C., Farnell M.B., Que F.G., Reid-Lombardo K.M. Immediate post-resection diabetes mellitus after pancreaticoduodenectomy: incidence and risk factors. HPB – Off J Int Hepato Pancreato Biliary Assoc. 2013;15:170–174. doi: 10.1111/j.1477-2574.2012.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slezak L.A., Andersen D.K. Pancreatic resection: effects on glucose metabolism. World J Surg. 2001;25:452–460. doi: 10.1007/s002680020337. [DOI] [PubMed] [Google Scholar]
- 24.Hirata K., Nakata B., Amano R., Yamazoe S., Kimura K., Hirakawa K. Predictive factors for change of diabetes mellitus status after pancreatectomy in preoperative diabetic and nondiabetic patients. J Gastrointest Surg – Off J Soc Surg Alimentary Tract. 2014;18:1597–1603. doi: 10.1007/s11605-014-2521-5. [DOI] [PubMed] [Google Scholar]
- 25.Meacham L.R., Sklar C.A., Li S., Liu Q., Gimpel N., Yasui Y. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med. 2009;169:1381–1388. doi: 10.1001/archinternmed.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto J., Traverso L.W. Exocrine function following the whipple operation as assessed by stool elastase. J Gastrointest Surg – Off J Soc Surg Alimentary Tract. 2006;10:1225–1229. doi: 10.1016/j.gassur.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Sikkens E.C., Cahen D.L., de Wit J., Looman C.W., van Eijck C., Bruno M.J. A prospective assessment of the natural course of the exocrine pancreatic function in patients with a pancreatic head tumor. J Clin Gastroenterol. 2014;48:e43–e46. doi: 10.1097/MCG.0b013e31829f56e7. [DOI] [PubMed] [Google Scholar]
- 28.Fong Z.V., Tan W.P., Lavu H., Kennedy E.P., Mitchell D.G., Koniaris L.G. Preoperative imaging for resectable periampullary cancer: clinicopathologic implications of reported radiographic findings. J Gastrointest Surg – Off J Soc Surg Alimentary Tract. 2013;17:1098–1106. doi: 10.1007/s11605-013-2181-x. [DOI] [PubMed] [Google Scholar]