Abstract
Background
Colorectal cancer (CRC) accounts for 9.7% of all cancers with 1.4 million new cases diagnosed each year. 19–31% of CRC patients develop colorectal liver metastases (CRLM), and 23–38% develop extra-hepatic disease (EHD). The aim of this systematic review was to determine overall survival (OS) in patients resected for CRLM and known EHD.
Methods
A systematic review was undertaken to identify studies reporting OS after resection for CRLM in the presence of EHD. Proportional meta-analyses and relative risk of death before five years were assessed between patient groups.
Results
A total of 15,144 patients with CRLM (2308 with EHD) from 52 studies were included. Three and 5-year OS were 58% and 26% for lung, 37% and 17% for peritoneum, and 35% and 15% for lymph nodes, respectively. The combined relative risk of death by five years was 1.49 (95% CI = 1.34–1.66) for lung, 1.59 (95% CI = 1.16–2.17) for peritoneal and 1.70 (95% CI = 1.57–1.84) for lymph node EHD, in favour of resection in the absence of EHD.
Conclusion
This review supports attempts at R0 resection in selected patients and rejects the notion that EHD is an absolute contraindication to resection.
Introduction
Colorectal cancer (CRC) is a major health burden with a world-wide estimate of 1.4 million new cases annually resulting in approximately 694,000 deaths.1 Approximately 19–31% of all patients with CRC present with, or subsequently develop, liver metastases (CRLM). These are defined as either synchronous if found at the time of presentation of the primary tumour or metachronous if identified at a later date. At diagnosis, a further 23–38% of patients already have, or will develop extra-hepatic disease (EHD).2, 3, 4 EHD is defined as either synchronous or metachronous to the primary CRC and/or the CRLM.
Over the past 10 years widespread use of modern chemotherapeutic and biological agents, combined with careful case selection and improved surgical techniques, have markedly improved outcomes in patients with metastatic CRC.5, 6, 7, 8 The presence of limited EHD is no longer considered an absolute contra-indication to liver resection as long as the future remnant liver is of sufficient volume, the patient is fit for a major operation, and there is potential for an R0 resection at both sites.9, 10, 11, 12, 13, 14, 15, 16, 17, 18
The current literature is difficult to interpret in relation to the benefit of removing EHD due to selection variability, multi-modal treatment regimens and the inherent subjectivity of the term ‘resectable’. Compounding this difficulty are the numerous permutations of possible presentations regarding the timing of both the CRLM and EHD. This ambiguity is reflected in numerous inconsistencies in consensus statements and guidelines regarding the value of resection of CRLM in the presence of EHD.19, 20
The aim of this systematic review was to determine overall survival (OS) in patients who underwent resection of CRLM and known EHD (synchronous or prior to the CRLM). Patients were stratified by site of EHD and then comparisons made between outcomes in this group and those who underwent resection of CRLM in the absence of EHD.
Materials and methods
The study protocol for this systematic review followed the PRISMA checklist (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and consulted the MOOSE checklist (Meta-analysis of Observational Studies in Epidemiology) for relevant additions.21, 22
Eligibility criteria
Full-text English language studies of adult human patients published between December 2004 and December 2014 were considered for this review. Case reports, systematic reviews, meta-analyses and studies of recurrence were excluded.
Search
A systematic search was applied to PubMed, Embase, Cinahl and Medline databases up to December 2014 to identify studies reporting resection of CRLM in the presence of known EHD with the terms referenced in Fig. 1. All articles were vetted by title then abstract, with the full text of the remaining articles examined for inclusion. Reference lists of all included articles were searched for further studies also meeting inclusion criteria.
Figure 1.
Search strategy applied to PubMed database
Study selection
Selection criteria were predefined and applied to results of the search strategy. Original studies reporting OS in patients undergoing first-time curative liver resection for CRLM with known EHD were included in the systematic review. Studies were excluded if follow-up was less than three years, resection was undertaken for palliative purposes or if the study population was exclusive. All included patients' had undergone prior curative resection of the primary tumour. Only patients undergoing resection of both CRLM and synchronous or previous EHD were included in these analyses; outcomes in patients whose EHD was detected after resection for CRLM were excluded.
Data collection
Reported survival, mortality, morbidity, demographic, peri-operative and chemotherapy (no stratification) data specific to patients resected for CRLM with EHD were extracted.
Level of evidence/risk of bias
Level of evidence for each study was assessed using the Oxford Centre for Evidence-Based Medicine (CEBM) Levels of Evidence.23 The methodological tool described by Downs and Black was modified for non-randomized studies by excluding the power calculation and applied to all included studies.24
Outcomes
Primary outcome measures were proportionally-weighted OS by EHD site (lung, peritoneum and lymph nodes) for those patients undergoing both CRLM and EHD resection and relative risk (RR) of death before five years comparing those resected for CRLM and EHD to those resected for CRLM without EHD.
Statistical analysis
Freeman–Tukey transformations were used to obtain proportional OS, while the X2 test with k−1 degrees of freedom was used to assess RR of death by five years.25 Survival data were expressed as pooled OS or RR and because significant heterogeneity (I2) was found, more conservative random-effects methods were used.26 P values were calculated with the X2 test or Freeman–Tukey transformation as appropriate; P < 0.05 was considered statistically significant. Data analysis was performed using Review Manager 5.0 software (Cochrane Collaboration, Oxford, UK) and MedCalc for Windows, version 12.5 (Ostend, Belgium).25, 26, 27
Results
Selection
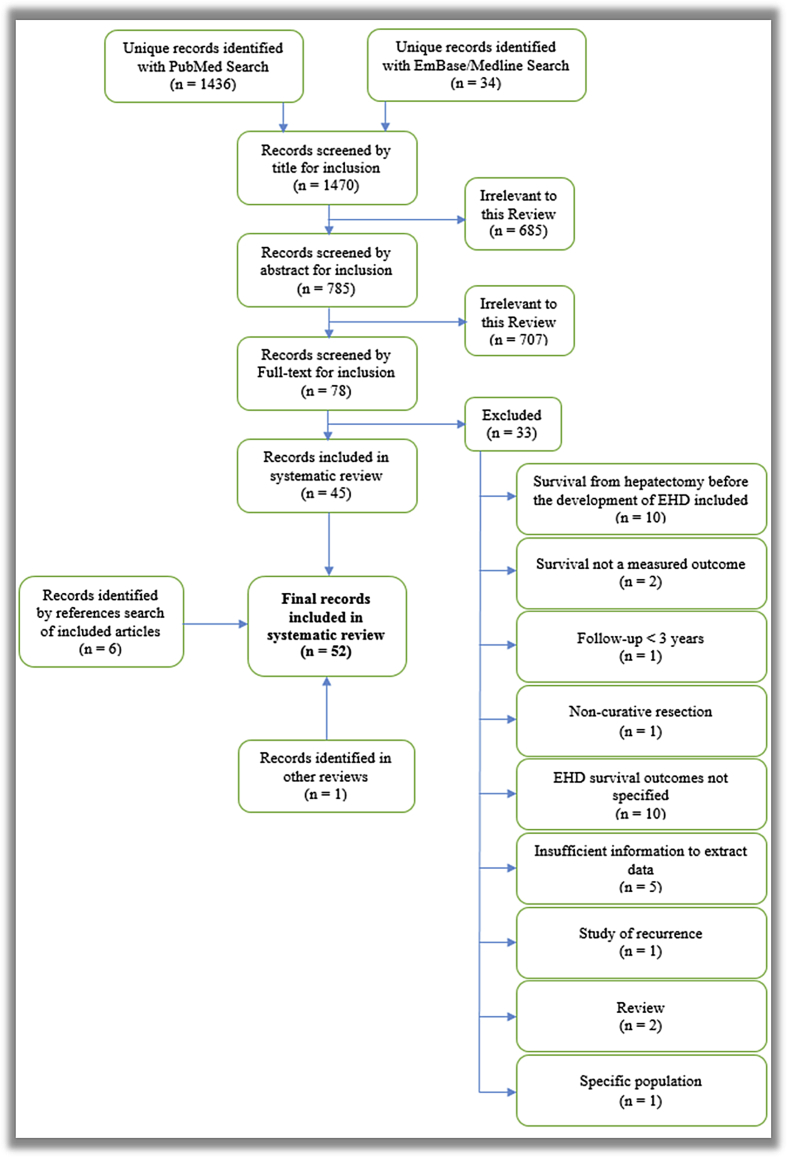
Constrained by year, language, study type and population this search returned 1470 unique articles. Vetting as per Appendix 1 yielded 45 studies whose reference lists were searched manually, identifying six further studies whose references were also manually examined. One study was identified while background-researching other reviews in this field. This process yielded 52 articles for inclusion in the systematic review, from which data were extracted.
Study characteristics
The 52 studies included in this review examined a total of 15,144 patients who had hepatic resection for CRLM. Of these 15,144 patients, 2308 presented with EHD known at hepatic resection. Three-hundred seventy-two of these patients with EHD did not progress to resection of both CRLM and EHD and were therefore excluded from further analysis. The remaining 1936 patients underwent hepatic resection plus resection of the EHD and comprise the population in the following analyses (Table 1).
Table 1.
Characteristics of included studies reporting overall survival after resection in patients with CLM and EHD16, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89
| First author | Year of publication | Study period start | Study period end | CLM patients (n) | CLM patients with EHD (n) | CLM resected, EHD not resected (n) | CLM and EHD resected (n) |
|---|---|---|---|---|---|---|---|
| Elias | 2005 | 1987 | 2000 | 308 | 84 | 84 | |
| Elias | 2006 | 1993 | 2003 | 24 | 24 | 24 | |
| Minagawa | 2006 | 1980 | 2002 | 187 | 39 | 39 | |
| Shah | 2006 | 1992 | 2002 | 39 | 12 | 12 | |
| Tanaka | 2006 | 1992 | 2004 | 53 | 14 | 10 | |
| Favero | 2007 | 1989 | 2005 | 5 | 5 | 5 | |
| Figueras | 2007 | 1990 | 2004 | 501 | 73 | 7 | 66 |
| Kianmanesh | 2007 | 1996 | 2005 | 43 | 16 | 16 | |
| Kornprat | 2007 | 1998 | 2002 | 98 | 18 | 18 | |
| Miller | 2007 | 1981 | 2000 | 131 | 46 | 46 | |
| Niu | 2007 | 1990 | 2006 | 402 | 63 | 63 | |
| Takahashi | 2007 | 1992 | 2005 | 30 | 12 | 11 | |
| Tamandl | 2007 | 2001 | 2004 | 200 | 18 | 18 | |
| Tanaka | 2007 | 1985 | 1999 | 156 | 20 | 20 | |
| Tsukioka | 2007 | 1990 | 2006 | 46 | 4 | 4 | |
| Zakaria | 2007 | 1960 | 1995 | 662 | 35 | 35 | |
| Adam | 2008 | 1992 | 2006 | 757 | 47 | 47 | |
| Aoki | 2008 | 1988 | 2005 | 187 | 37 | 37 | |
| Bennett | 2008 | 2002 | 2004 | 59 | 22 | 22 | |
| Rees | 2008 | 1987 | 2005 | 929 | 164 | 136 | |
| Tanaka | 2008 | 1987 | 2006 | 85 | 14 | 14 | |
| Wicherts | 2008 | 1992 | 2007 | 817 | 7 | 7 | |
| Byam | 2009 | 1995 | 2008 | 383 | 39 | 39 | |
| Carpizo | 2009 | 1992 | 2007 | 1369 | 127 | 10 | 117 |
| Chua | 2009 | 1997 | 2008 | 55 | 16 | 16 | |
| Karanjia | 2009 | 1996 | 2006 | 283 | 12 | 12 | |
| Lordan | 2009 | 1996 | 2006 | 285 | 4 | 4 | |
| Marudanayagam | 2009 | 2000 | 2007 | 43 | 10 | 10 | |
| Neeff | 2009 | 1987 | 2006 | 44 | 13 | 13 | |
| Oussoultzoglou | 2009 | 2000 | 2006 | 45 | 45 | 45 | |
| Reissfelder | 2009 | 2002 | 2008 | 281 | 40 | 40 | |
| Varban | 2009 | 1991 | 2007 | 142 | 14 | 14 | |
| Elias | 2010 | 1990 | 2007 | 543 | 77 | 77 | |
| Hemming | 2010 | 1996 | 2009 | 40 | 13 | 13 | |
| House | 2010 | 1985 | 2004 | 1600 | 229 | 229 | |
| Maithel | 2010 | 2004 | 2006 | 160 | 68 | 68 | |
| van der Pool | 2010 | 2000 | 2008 | 272 | 21 | 21 | |
| Adam | 2011 | 1990 | 2006 | 186 | 186 | 59 | 127 |
| Pulitano | 2011 | 1996 | 2007 | 1629 | 171 | 171 | |
| Beppu | 2012 | 2000 | 2004 | 727 | 82 | 82 | |
| Gomez | 2012 | 2006 | 2010 | 184 | 30 | 30 | |
| Kawano | 2012 | 1997 | 2008 | 35 | 8 | 8 | |
| Pulitano | 2012 | 1996 | 2007 | 61 | 61 | 61 | |
| Allard | 2013 | 1985 | 2010 | 42 | 42 | 30 | |
| Edwards | 2013 | 2002 | 2012 | 4 | 4 | 4 | |
| Hattori | 2013 | 1999 | 2009 | 96 | 29 | 29 | |
| Ishibashi | 2013 | 2000 | 2008 | 61 | 13 | 3 | |
| Maggioria | 2013 | 1993 | 2009 | 98 | 37 | 37 | |
| Marin | 2013 | 1996 | 2010 | 44 | 21 | 21 | |
| Mavros | 2013 | 1982 | 2011 | 97 | 97 | 97 | |
| Meimarakisa | 2013 | 1981 | 2009 | 543 | 13 | 13 | |
| Liu | 2014 | 2000 | 2012 | 73 | 12 | 12 | |
| Total | 15,144 | 2308 | 317 | 1936 | |||
| Median | 136.5 | 23 | 30 | 21 | |||
| Range | 4–1629 | 4–229 | 7–68 | 3–229 |
Case control study, all others are observational.
Level of evidence/risk of bias
The studies in this review are comprised of level 2b (observational cohort) and 3b (case–control) as per the Oxford CEBM guideline.23 Median modified Downs and Black methodology score for included studies is 15/26 (IQR = 14–17) (Supplementary Fig. 1).
Patient, disease, operative and post-operative characteristics
Twenty-one studies report patient characteristics specific to those resected for CRLM in the presence of EHD (Supplementary Table 1a). The median reported values for the number of liver lesions was two, and the median size of the largest lesion was 38 mm. Fifty-four percent of all patients in these studies were male. Supplementary Table 1b shows that in the 17 studies reporting characteristics of the primary tumour and liver metastases 75% of patients had a colon primary, 74% had primary tumour lymph node involvement, 48% had CRLM's present synchronous to the primary tumour, and 42% had unilateral liver disease at the time of resection (all values median). Finally, in 13 studies reporting specific R0 resection rates for our cohort of interest, a median 85% of patients had an R0 resection (Supplementary Table 1c).
Morbidity and mortality
In patients who underwent resection of CRLM in the presence of EHD, 10 studies report a median mortality of 1.8% (range 0–8%), while 16 studies report a median morbidity of 30.5% (range 12.5–80%) (Supplementary Table 2).
Resection of liver & lung metastases
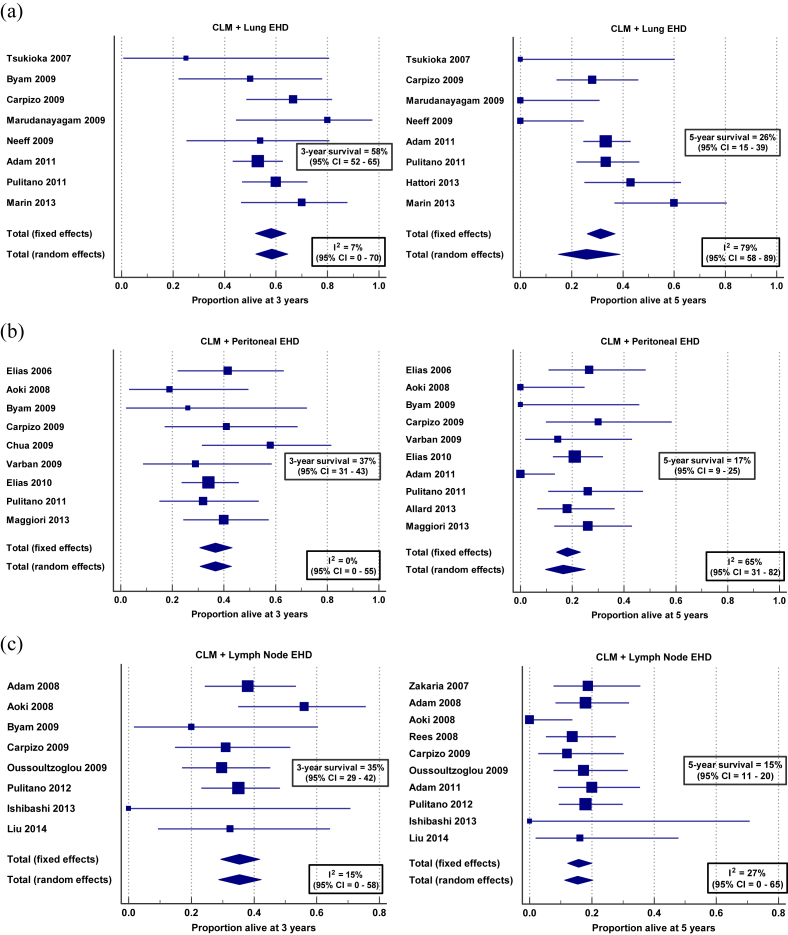
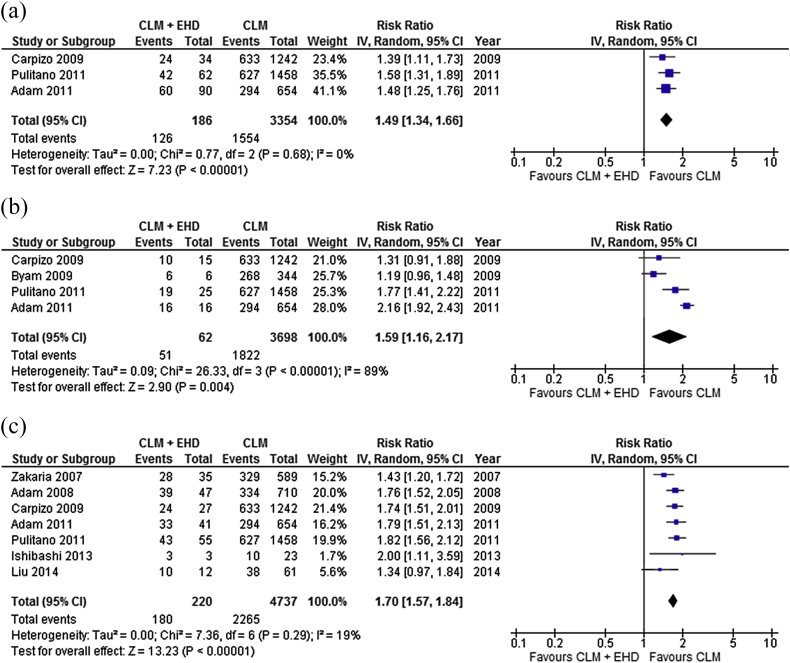
Twenty-three studies15, 18, 19, 20, 21, 31, 36, 37, 42, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 report survival after resection of CRLM in a total of 574 patients with lung metastases. This patient group accounts for 25% of all patients resected for CRLM and EHD, and 4% of all patients resected for CRLM (Supplementary Table 3). Proportional meta-analysis of survival data for those in whom both CRLM and known EHD was resected reveal three and 5-year OS of 58% (95% CI = 52–65%, I2 = 7%) and 26% (95% CI = 15–39%, I2 = 79%) (Fig. 2a), while brief quantitative analysis shows that this group has a relative risk (RR) of mortality by five years after resection of 1.49 (95% CI = 1.34–1.66) when compared to patients resected for CRLM without EHD (Fig. 3a). Reported median OS of this cohort is 42 months (Supplementary Table 4a).
Figure 2.
Proportional meta-analyses of three and 5-year overall survival in patients resected for CRLM and EHD in the (a) lung,16, 34, 39, 40, 72, 77, 78, 81, 87 (b) peritoneum16, 29, 33, 34, 35, 37, 38, 39, 40, 41, 42 and (c) lymph nodes16, 33, 34, 36, 39, 47, 48, 50, 52, 53, 55
Figure 3.
Forest plot comparing 5-year overall survival in patients resected for CRLM without EHD versus patients resected for both CRLM and EHD in the (a) lung,16, 39, 40 (b) peritoneum16, 34, 39, 40 and (c) lymph nodes16, 39, 40, 47, 48, 53, 55
Resection of liver & peritoneal metastases
Sixteen studies16, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 report survival after resection of CRLM in a total of 378 patients with peritoneal metastases, accounting for 17% of all patients resected for CRLM and EHD, and 3% of all patients resected for CRLM (Supplementary Table 3). Proportional meta-analysis of survival data for those in whom both CRLM and known EHD was resected show three and 5-year OS of 37% (95% CI = 31–43%, I2 = 0%) and 17% (95% CI = 9–25%, I2 = 65%) (Fig. 2b), while brief quantitative analysis shows a RR of mortality by five years for this group of 1.59 (95% CI = 1.16–2.17) compared to those resected for CRLM alone (Fig. 3b). Reported median OS of this cohort is 29 months (Supplementary Table 4b).
Resection of liver & nodal metastases
Twenty-one studies16, 28, 31, 33, 34, 36, 39, 40, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 report survival after resection of CRLM in a total of 559 patients with nodal disease, which accounts for 24% of all patients resected for CRLM and EHD, and 4% of all patients resected for CRLM (Supplementary Table 3). Proportional meta-analysis of survival data for those in whom both CRLM and known EHD was resected reveal three and 5-year OS of 35% (95% CI = 29–41%, I2 = 15%) and 15% (95% CI = 11–20%, I2 = 27%) (Fig. 2c), while brief quantitative analysis shows a RR of mortality by five years after resection for this group of 1.70 (95% CI = 1.57–1.84), compared to patients resected for CRLM without EHD (Fig. 3c). Reported median OS of this cohort is 25 months (Supplementary Table 4c).
Resection at other sites
In nine studies, a total of 175 patients were reported to have a locally invasive metastasis, accounting for 8% of all patient with EHD and 1% of all patients with CRLM in this review. Four studies explicitly state 3-year, 5-year and median survivals of 40%, 23% and 27 months in patients with metastatic spread to local structures – including the diaphragm, the inferior vena cava (IVC) or biliary tree. Eight studies totalling 98 patients (4% of those with EHD in this review) had EHD at rare sites (bone, brain, ovary, spleen, etc.), while three studies reported a median 5-year survival of 51% in patients grouped together by presentation at these rare metastatic locations (Supplementary Tables 3 & 4d–e).
Finally, 16 studies report 3-year, 5-year and median survivals of 43%, 26% and 32 months in patients where all EHD is lumped into one category and six studies report median 3-year, 5-year and median survivals of 26%, 14% and 17 months in patients with multiple sites of EHD (Supplementary Table 4f–g). Two studies report only that EHD of an unknown site has been resected.56, 57
R0 versus R1/2
Quantitative analysis of R0 versus R1/2 resection showed a relative risk of death by five years of 0.73 (95% CI = 0.60–0.90) in those for whom both CRLM and EHD achieved R0 resection (Supplementary Fig. 5).
Discussion
Without treatment the median survival and 5-year overall survival of patients with CRLM is eight months and 0%, respectively.58, 59 The prognosis worsens when there are both liver and extra-hepatic metastases. Complete R0 resection of isolated CRLM offers 5-year survivals of up to 50%. However, if EHD is left in situ survival decreases dramatically to less than 20%.60, 61 An R0 resection is an essential component for long-term survival and this was confirmed in the current systematic review. Although best available chemotherapy can prolong survival in patients with CRLM and EHD to as much as 12–13 months, and even provide a 1% 5-year survival, evidence from available cohorts shows that no treatment modality approaches outcomes offered by curative resection.34, 62, 63 This is the first review to use a proportional meta-analysis of overall survival in patients presenting for CRLM and EHD resection. Although it is difficult to pool heterogeneous data from a group of observational studies, this method was chosen because it gives statistical weight to data based on cohort size, and therefore more accurately approximates true survival outcomes when compared to a median of values from multiple studies.
This study demonstrates that resection of CRLM and lung EHD, most often performed as staged procedures, is associated with a 42-month median survival and a 26% 5-year survival. When CRLM and EHD confined to the peritoneum (all volumes of disease were treated together in this review) are resected patients can expect a median 29-month survival and a 17% 5-year survival. Finally, when CRLM and lymph node metastases are resected, patients achieve a 25-month median survival and a 15% 5-year survival. It is worth noting that these studies include highly selected patients, most of whom are also treated with combination chemotherapy and/or biological agents. Nevertheless, these survival data exceed the best outcomes in patients receiving systemic chemotherapy alone.60, 62, 63 In contrast, patients who undergo resection of EHD at multiple sites have a median survival of only 17 months which is similar to what can be expected with chemotherapy alone.63 The worse outcomes with EHD at multiple sites may reflect the lower R0 resection rates achieved in these patients.
Mortality rates after resection of isolated CRLM in specialist centres are usually less than three percent.59 Therefore, the median 1.8% mortality rate demonstrated in this review confirms that resection of CRLM + EHD can be done safely.
Reported median overall survivals for resection of CRLM and EHD in the lung, peritoneum and lymph nodes of 42, 29 and 25 months in this study are comparable to previous reviews. Hwang et al. reported 45-month, 29-month and 26-month median overall survivals while Chua et al. reported similar figures of 41, 25 and 25 months for these same disease sites.64, 65 In contrast to these previous reviews, the present study extracted data specific to patients presenting with CRLM and known EHD from within cohorts that are often only investigating EHD as a prognostic factor in a larger cohort. Importantly, this review separated survival data from those patients whose EHD was either left in situ, of ambiguous fate, or was discovered after a previous liver resection. Furthermore, this review is the first to summarize OS after resection of CRLM and EHD as a proportional meta-analysis. This is a more representative measure of true outcome compared with using median overall survival figures alone. Unfortunately, it was not possible with the available data to present overall 5-year DFS figures which would give the best indication of possible cure after resection. We believe that future studies would benefit from a more concerted focus on 5-year DFS.
This review was limited by the available literature, specifically by the heterogeneity between observational studies and the lack of high-level evidence comparing similar patient groups. Expectedly, the data analysed show significant relative risks of death by 5-years after resection of CRLM and EHD in the lungs (RR = 1.49), peritoneum (RR = 1.59) and lymph nodes (RR = 1.70) compared with patients resected for CRLM in the absence of EHD. More interesting comparisons would involve patients with similar CRLM and EHD treated with resection of all metastases versus either resection of CRLM only or best medical therapy. However, there is a paucity of data available and therefore it was not possible to compare these cohorts.66, 67 Level 2b and 3b evidence, coupled with a median methodological score of only 15/26 (Supplementary Fig. 1), indicates a strong selection bias in many of the studies, and poor overall quality of available evidence for analysis.
A further limitation of the data reported here was the fact that all lymph node metastases were combined together for the sake of simplifying the analysis. This is problematic as there may be marked differences in survival depending on which lymph nodes are involved. Metastases in nodes at the hepatic pedicle confer a more favourable prognosis than metastases at more distant sites. This was demonstrated by Adam et al. who found a 25% 5-year survival in patients with involvement of the portal pedicle compared with a 0% 5-year survival when there were metastases in the para-aortic region.48 These findings have been confirmed in other studies.36, 52
Another limitation of this review was the length of time over which the pooled patients were treated, and consequently the variation in the types and duration of chemotherapy and biological agents used. It was not possible in the analysis to tease out potential contributions of the evolving medical treatments to overall survival. This is an important area of future study to determine the true impact of resection of EHD on long-term outcomes.68
The wide gap in survival outcomes reported for patients resected for CRLM and EHD compared with other treatment modalities supports attempts at R0 resection where appropriate, and in carefully selected patients. Specifically, CRLM resection should be considered even if EHD is present, as long as it is possible to achieve an R0 resection at both sites. This is especially the case for EHD confined to the lungs or when there is limited spread to the peritoneum. However, the exact number or extent of metastases that are appropriate for resection in the presence of resectable CRLM's is still unclear. With regard to lymph node metastases, there are insufficient data to make firm conclusions. Certainly, individual studies36, 48, 52 demonstrate reasonable outcomes after resection of nodes at the hepatic pedicle, albeit involving small numbers of patients. However, less favourable outcomes after resection of lymph nodes at distant sites must be weighed against the peri-operative risks.
Considering that the evidence generated in this review is limited by the non-randomized nature of the studies, the heterogeneity between those observational studies and the highly selected cohorts they present, it is impossible to make specific selection recommendations based on these findings. Instead, this review supports increased consideration of resection for patients presenting with CRLM and known EHD, rejects the notion that EHD is an absolute contraindication to liver resection and encourages further study of both OS and DFS to better elucidate the objective benefit conferred by resection of EHD.
Funding sources
None.
Conflicts of interest
None to declare.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.hpb.2015.12.004.
Appendix 1. Flow diagram depicting acquisition of reviewed articles.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Kune G.A., Kune S., Field B., White R., Brough W., Schellenberger R. Survival in patients with large-bowel cancer. A population-based investigation from the Melbourne Colorectal Cancer Study. Dis Colon Rectum. 1990;33:938–946. doi: 10.1007/BF02139103. [DOI] [PubMed] [Google Scholar]
- 3.Manfredi S., Lepage C., Hatem C., Coatmeur O., Faivre J., Bouvier A.M. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Geest L.G., Lam-Boer J., Koopman M., Verhoef C., Elferink M.A., de Wilt J.H. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 2015;32:457–465. doi: 10.1007/s10585-015-9719-0. [DOI] [PubMed] [Google Scholar]
- 5.Kohne C.H., van Cutsem E., Wils J., Bokemeyer C., El-Serafi M., Lutz M.P. Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European Organisation for Research and Treatment of Cancer Gastrointestinal Group Study 40986. J Clin Oncol. 2005;23:4856–4865. doi: 10.1200/JCO.2005.05.546. [DOI] [PubMed] [Google Scholar]
- 6.Giantonio B.J., Catalano P.J., Meropol N.J., O'Dwyer P.J., Mitchell E.P., Alberts S.R. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E., Kohne C.H., Hitre E., Zaluski J., Chang Chien C.R., Makhson A. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher D.J., Kemeny N. Metastatic colorectal cancer: from improved survival to potential cure. Oncology. 2010;78:237–248. doi: 10.1159/000315730. [DOI] [PubMed] [Google Scholar]
- 9.Adam R., Vinet E. Regional treatment of metastasis: surgery of colorectal liver metastases. Ann Oncol. 2004;15(Suppl. 4):iv103–iv106. doi: 10.1093/annonc/mdh912. [DOI] [PubMed] [Google Scholar]
- 10.Elias D., Ouellet J.F., Bellon N., Pignon J.P., Pocard M., Lasser P. Extrahepatic disease does not contraindicate hepatectomy for colorectal liver metastases. Br J Surg. 2003;90:567–574. doi: 10.1002/bjs.4071. [DOI] [PubMed] [Google Scholar]
- 11.Hugh T.J., Kinsella A.R., Poston G.J. Management strategies for colorectal liver metastases–Part II. Surg Oncol. 1997;6:31–48. doi: 10.1016/s0960-7404(97)00003-0. [DOI] [PubMed] [Google Scholar]
- 12.Pawlik T.M., Schulick R.D., Choti M.A. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51–64. doi: 10.1634/theoncologist.2007-0142. [DOI] [PubMed] [Google Scholar]
- 13.Poston G.J., Adam R., Alberts S., Curley S., Figueras J., Haller D. OncoSurge: a strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol. 2005;23:7125–7134. doi: 10.1200/JCO.2005.08.722. [DOI] [PubMed] [Google Scholar]
- 14.Scheele J., Stangl R., Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 15.Narita M., Oussoultzoglou E., Bachellier P., Jaeck D., Uemoto S. Post-hepatectomy liver failure in patients with colorectal liver metastases. Surg Today. 2015;45:1218–1226. doi: 10.1007/s00595-015-1113-7. [DOI] [PubMed] [Google Scholar]
- 16.Carpizo D.R., Are C., Jarnagin W., Dematteo R., Fong Y., Gonen M. Liver resection for metastatic colorectal cancer in patients with concurrent extrahepatic disease: results in 127 patients treated at a single center. Ann Surg Oncol. 2009;16:2138–2146. doi: 10.1245/s10434-009-0521-6. [DOI] [PubMed] [Google Scholar]
- 17.Hughes K.S., Simon R., Songhorabodi S., Adson M.A., Ilstrup D.M., Fortner J.G. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278–284. [PubMed] [Google Scholar]
- 18.van Ooijen B., Wiggers T., Meijer S., van der Heijde M.N., Slooff M.J., van de Velde C.J. Hepatic resections for colorectal metastases in The Netherlands. A multiinstitutional 10-year study. Cancer. 1992;70:28–34. doi: 10.1002/1097-0142(19920701)70:1<28::aid-cncr2820700105>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Adams R.B., Aloia T.A., Loyer E., Pawlik T.M., Taouli B., Vauthey J.N. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB. 2013;15:91–103. doi: 10.1111/j.1477-2574.2012.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siriwardena A.K., Mason J.M., Mullamitha S., Hancock H.C., Jegatheeswaran S. Management of colorectal cancer presenting with synchronous liver metastases. Nat Rev Clin Oncol. 2014;11:446–459. doi: 10.1038/nrclinonc.2014.90. [DOI] [PubMed] [Google Scholar]
- 21.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Phillips B., Ball C., Sackett D., Badenoch D., Straus S., Haynes B. CEBM; 2009. Oxford Centre for evidence-based medicine – levels of evidence (March 2009) [Google Scholar]
- 24.Downs S.H., Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman M.F., Tukey J.W. Transformations related to the angular and the square root. Ann Math Stat. 1950:607–611. [Google Scholar]
- 26.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Mantel N., Haenszel W. Statistical aspects of the analysis of data from retrospective studies. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 28.Elias D., Liberale G., Vernerey D., Pocard M., Ducreux M., Boige V. Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol. 2005;12:900–909. doi: 10.1245/ASO.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Elias D., Benizri E., Pocard M., Ducreux M., Boige V., Lasser P. Treatment of synchronous peritoneal carcinomatosis and liver metastases from colorectal cancer. Eur J Surg Oncol. 2006;32:632–636. doi: 10.1016/j.ejso.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Minagawa M., Yamamoto J., Miwa S., Sakamoto Y., Kokudo N., Kosuge T. Selection criteria for simultaneous resection in patients with synchronous liver metastasis. Arch Surg. 2006;141:1006–1012. doi: 10.1001/archsurg.141.10.1006. discussion 1013. [DOI] [PubMed] [Google Scholar]
- 31.Figueras J., Torras J., Valls C., Llado L., Ramos E., Marti-Rague J. Surgical resection of colorectal liver metastases in patients with expanded indications: a single-center experience with 501 patients. Dis Colon Rectum. 2007;50:478–488. doi: 10.1007/s10350-006-0817-6. [DOI] [PubMed] [Google Scholar]
- 32.Kianmanesh R., Scaringi S., Sabate J.M., Castel B., Pons-Kerjean N., Coffin B. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg. 2007;245:597–603. doi: 10.1097/01.sla.0000255561.87771.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoki T., Umekita N., Tanaka S., Noda K., Warabi M., Kitamura M. Prognostic value of concomitant resection of extrahepatic disease in patients with liver metastases of colorectal origin. Surgery. 2008;143:706–714. doi: 10.1016/j.surg.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Byam J., Reuter N.P., Woodall C.E., Scoggins C.R., McMasters K.M., Martin R.C. Should hepatic metastatic colorectal cancer patients with extrahepatic disease undergo liver resection/ablation? Ann Surg Oncol. 2009;16:3064–3069. doi: 10.1245/s10434-009-0693-0. [DOI] [PubMed] [Google Scholar]
- 35.Chua T.C., Yan T.D., Zhao J., Morris D.L. Peritoneal carcinomatosis and liver metastases from colorectal cancer treated with cytoreductive surgery perioperative intraperitoneal chemotherapy and liver resection. Eur J Surg Oncol. 2009;35:1299–1305. doi: 10.1016/j.ejso.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Oussoultzoglou E., Romain B., Panaro F., Rosso E., Pessaux P., Bachellier P. Long-term survival after liver resection for colorectal liver metastases in patients with hepatic pedicle lymph nodes involvement in the era of new chemotherapy regimens. Ann Surg. 2009;249:879–886. doi: 10.1097/SLA.0b013e3181a334d9. [DOI] [PubMed] [Google Scholar]
- 37.Varban O., Levine E.A., Stewart J.H., McCoy T.P., Shen P. Outcomes associated with cytoreductive surgery and intraperitoneal hyperthermic chemotherapy in colorectal cancer patients with peritoneal surface disease and hepatic metastases. Cancer. 2009;115:3427–3436. doi: 10.1002/cncr.24385. [DOI] [PubMed] [Google Scholar]
- 38.Elias D., Gilly F., Boutitie F., Quenet F., Bereder J.M., Mansvelt B. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 39.Adam R., de Haas R.J., Wicherts D.A., Vibert E., Salloum C., Azoulay D. Concomitant extrahepatic disease in patients with colorectal liver metastases: when is there a place for surgery? Ann Surg. 2011;253:349–359. doi: 10.1097/SLA.0b013e318207bf2c. [DOI] [PubMed] [Google Scholar]
- 40.Pulitano C., Bodingbauer M., Aldrighetti L., de Jong M.C., Castillo F., Schulick R.D. Liver resection for colorectal metastases in presence of extrahepatic disease: results from an international multi-institutional analysis. Ann Surg Oncol. 2011;18:1380–1388. doi: 10.1245/s10434-010-1459-4. [DOI] [PubMed] [Google Scholar]
- 41.Allard M.A., Adam R., Ruiz A., Vibert E., Paule B., Levi F. Is unexpected peritoneal carcinomatosis still a contraindication for resection of colorectal liver metastases? Combined resection of colorectal liver metastases with peritoneal deposits discovered intra-operatively. Eur J Surg Oncol. 2013;39:981–987. doi: 10.1016/j.ejso.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Maggiori L., Goere D., Viana B., Tzanis D., Dumont F., Honore C. Should patients with peritoneal carcinomatosis of colorectal origin with synchronous liver metastases be treated with a curative intent? A case-control study. Ann Surg. 2013;258:116–121. doi: 10.1097/SLA.0b013e3182778089. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka K., Shimada H., Nagano Y., Endo I., Sekido H., Togo S. Outcome after hepatic resection versus combined resection and microwave ablation for multiple bilobar colorectal metastases to the liver. Surgery. 2006;139:263–273. doi: 10.1016/j.surg.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 44.Favero A., Benzoni E., Zompicchiatti A., Rossit L., Bresadola F., De Anna D. Surgery in hepatic and extrahepatic colorectal metastases. G Chir. 2007;28:307–311. [PubMed] [Google Scholar]
- 45.Kornprat P., Jarnagin W.R., Gonen M., DeMatteo R.P., Fong Y., Blumgart L.H. Outcome after hepatectomy for multiple (four or more) colorectal metastases in the era of effective chemotherapy. Ann Surg Oncol. 2007;14:1151–1160. doi: 10.1245/s10434-006-9068-y. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi S., Nagai K., Saito N., Konishi M., Nakagohri T., Gotohda N. Multiple resections for hepatic and pulmonary metastases of colorectal carcinoma. Jpn J Clin Oncol. 2007;37:186–192. doi: 10.1093/jjco/hym006. [DOI] [PubMed] [Google Scholar]
- 47.Zakaria S., Donohue J.H., Que F.G., Farnell M.B., Schleck C.D., Ilstrup D.M. Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg. 2007;246:183–191. doi: 10.1097/SLA.0b013e3180603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adam R., de Haas R.J., Wicherts D.A., Aloia T.A., Delvart V., Azoulay D. Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? J Clin Oncol. 2008;26:3672–3680. doi: 10.1200/JCO.2007.15.7297. [DOI] [PubMed] [Google Scholar]
- 49.Bennett J.J., Schmidt C.R., Klimstra D.S., Grobmyer S.R., Ishill N.M., D'Angelica M. Perihepatic lymph node micrometastases impact outcome after partial hepatectomy for colorectal metastases. Ann Surg Oncol. 2008;15:1130–1136. doi: 10.1245/s10434-007-9802-0. [DOI] [PubMed] [Google Scholar]
- 50.Rees M., Tekkis P.P., Welsh F.K., O'Rourke T., John T.G. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 51.Maithel S.K., Ginsberg M.S., D'Amico F., DeMatteo R.P., Allen P.J., Fong Y. Natural history of patients with subcentimeter pulmonary nodules undergoing hepatic resection for metastatic colorectal cancer. J Am Coll Surg. 2010;210:31–38. doi: 10.1016/j.jamcollsurg.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pulitano C., Bodingbauer M., Aldrighetti L., Choti M.A., Castillo F., Schulick R.D. Colorectal liver metastasis in the setting of lymph node metastasis: defining the benefit of surgical resection. Ann Surg Oncol. 2012;19:435–442. doi: 10.1245/s10434-011-1902-1. [DOI] [PubMed] [Google Scholar]
- 53.Ishibashi K., Ishida H., Ohsawa T., Okada N., Kumamoto K., Haga N. Impact of hepatic lymph node metastasis on survival of patients with synchronous resectable or unresectable liver metastases of colorectal cancer. Tech Coloproctol. 2013;17:51–57. doi: 10.1007/s10151-012-0881-y. [DOI] [PubMed] [Google Scholar]
- 54.Mavros M.N., Hyder O., Pulitano C., Aldrighetti L., Pawlik T.M. Survival of patients operated for colorectal liver metastases and concomitant extra-hepatic disease: external validation of a prognostic model. J Surg Oncol. 2013;107:481–485. doi: 10.1002/jso.23260. [DOI] [PubMed] [Google Scholar]
- 55.Liu W., Yan X.L., Wang K., Bao Q., Sun Y., Xing B.C. The outcome of liver resection and lymphadenectomy for hilar lymph node involvement in colorectal cancer liver metastases. Int J Colorectal Dis. 2014;29:737–745. doi: 10.1007/s00384-014-1863-5. [DOI] [PubMed] [Google Scholar]
- 56.House M.G., Ito H., Gonen M., Fong Y., Allen P.J., DeMatteo R.P. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–752. doi: 10.1016/j.jamcollsurg.2009.12.040. 752–745. [DOI] [PubMed] [Google Scholar]
- 57.Tamandl D., Gruenberger B., Herberger B., Schoppmann S., Bodingbauer M., Schindl M. Selective resection of colorectal liver metastases. Eur J Surg Oncol. 2007;33:174–182. doi: 10.1016/j.ejso.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Adson M.A. Resection of liver metastases–when is it worthwhile? World J Surg. 1987;11:511–520. doi: 10.1007/BF01655817. [DOI] [PubMed] [Google Scholar]
- 59.Simmonds P.C., Primrose J.N., Colquitt J.L., Garden O.J., Poston G.J., Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Jong M.C., Pulitano C., Ribero D., Strub J., Mentha G., Schulick R.D. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 61.Fong Y., Fortner J., Sun R.L., Brennan M.F., Blumgart L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferrarotto R., Pathak P., Maru D., Agarwal A., Overman M., Hoff P.M. Durable complete responses in metastatic colorectal cancer treated with chemotherapy alone. Clin Colorectal Cancer. 2011;10:178–182. doi: 10.1016/j.clcc.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 63.Simmonds P.C. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ. 2000;321:531–535. doi: 10.1136/bmj.321.7260.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chua T.C., Saxena A., Liauw W., Chu F., Morris D.L. Hepatectomy and resection of concomitant extrahepatic disease for colorectal liver metastases–a systematic review. Eur J Cancer. 2012;48:1757–1765. doi: 10.1016/j.ejca.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 65.Hwang M., Jayakrishnan T.T., Green D.E., George B., Thomas J.P., Groeschl R.T. Systematic review of outcomes of patients undergoing resection for colorectal liver metastases in the setting of extra hepatic disease. Eur J Cancer. 2014;50:1747–1757. doi: 10.1016/j.ejca.2014.03.277. [DOI] [PubMed] [Google Scholar]
- 66.Mise Y., Kopetz S., Mehran R.J., Aloia T.A., Conrad C., Brudvik K.W. Is complete liver resection without resection of synchronous lung metastases justified? Ann Surg Oncol. 2014;22:1218–1226. doi: 10.1245/s10434-014-4207-3. [DOI] [PubMed] [Google Scholar]
- 67.Robinson B.J., Rice T.W., Strong S.A., Rybicki L.A., Blackstone E.H. Is resection of pulmonary and hepatic metastases warranted in patients with colorectal cancer? J Thorac Cardiovasc Surg. 1999;117:66–75. doi: 10.1016/s0022-5223(99)70470-8. discussion 75–66. [DOI] [PubMed] [Google Scholar]
- 68.Center VUUM. Chemotherapy and Maximal Tumor Debulking of Multi-organ Colorectal Cancer Metastases – Full Text View – ClinicalTrials.gov 2015. Available from: https://clinicaltrials.gov/ct2/show/NCT01792934.
- 69.Beppu T., Sakamoto Y., Hasegawa K., Honda G., Tanaka K., Kotera Y. A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a Project Study for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2012;19:72–84. doi: 10.1007/s00534-011-0460-z. [DOI] [PubMed] [Google Scholar]
- 70.Edwards J., Scoggins C., McMasters K., Martin R. Combined pancreas and liver therapies: resection and ablation in hepato-pancreatico-biliary malignancies. J Surg Oncol. 2013;107:709–712. doi: 10.1002/jso.23318. [DOI] [PubMed] [Google Scholar]
- 71.Gomez D., Kamali D., Dunn W.K., Beckingham I.J., Brooks A., Cameron I.C. Outcomes in patients with indeterminate pulmonary nodules undergoing resection for colorectal liver metastases. HPB. 2012;14:448–454. doi: 10.1111/j.1477-2574.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hattori N., Kanemitsu Y., Komori K., Shimizu Y., Sano T., Senda Y. Outcomes after hepatic and pulmonary metastasectomies compared with pulmonary metastasectomy alone in patients with colorectal cancer metastasis to liver and lungs. World J Surg. 2013;37:1315–1321. doi: 10.1007/s00268-013-1954-4. [DOI] [PubMed] [Google Scholar]
- 73.Hemming A.W., Magliocca J.F., Fujita S., Kayler L.K., Hochwald S., Zendejas I. Combined resection of the liver and pancreas for malignancy. J Am Coll Surg. 2010;210:808–814. doi: 10.1016/j.jamcollsurg.2009.12.007. 814–806. [DOI] [PubMed] [Google Scholar]
- 74.Karanjia N.D., Lordan J.T., Fawcett W.J., Quiney N., Worthington T.R. Survival and recurrence after neo-adjuvant chemotherapy and liver resection for colorectal metastases: a ten year study. Eur J Surg Oncol. 2009;35:838–843. doi: 10.1016/j.ejso.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 75.Kawano D., Takeo S., Tsukamoto S., Katsura M., Masuyama E., Nakaji Y. Prediction of the prognosis and surgical indications for pulmonary metastectomy from colorectal carcinoma in patients with combined hepatic metastases. Lung Cancer. 2012;75:209–212. doi: 10.1016/j.lungcan.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 76.Lordan J.T., Riga A., Worthington T.R., Karanjia N.D. Early and long-term outcomes of patients undergoing liver resection and diaphragm excision for advanced colorectal liver metastases. Ann R Coll Surg Engl. 2009;91:483–488. doi: 10.1308/003588409X432176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marin C., Robles R., Lopez Conesa A., Torres J., Flores D.P., Parrilla P. Outcome of strict patient selection for surgical treatment of hepatic and pulmonary metastases from colorectal cancer. Dis Colon Rectum. 2013;56:43–50. doi: 10.1097/DCR.0b013e3182739f5e. [DOI] [PubMed] [Google Scholar]
- 78.Marudanayagam R., Ramkumar K., Shanmugam V., Langman G., Rajesh P., Coldham C. Long-term outcome after sequential resections of liver and lung metastases from colorectal carcinoma. HPB. 2009;11:671–676. doi: 10.1111/j.1477-2574.2009.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meimarakis G., Angele M., Conrad C., Schauer R., Weidenhagen R., Crispin A. Combined resection of colorectal hepatic-pulmonary metastases shows improved outcome over chemotherapy alone. Langenbecks Arch Surg. 2013;398:265–276. doi: 10.1007/s00423-012-1046-1. [DOI] [PubMed] [Google Scholar]
- 80.Miller G., Biernacki P., Kemeny N.E., Gonen M., Downey R., Jarnagin W.R. Outcomes after resection of synchronous or metachronous hepatic and pulmonary colorectal metastases. J Am Coll Surg. 2007;205:231–238. doi: 10.1016/j.jamcollsurg.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 81.Neeff H., Horth W., Makowiec F., Fischer E., Imdahl A., Hopt U.T. Outcome after resection of hepatic and pulmonary metastases of colorectal cancer. J Gastrointest Surg. 2009;13:1813–1820. doi: 10.1007/s11605-009-0960-1. [DOI] [PubMed] [Google Scholar]
- 82.Niu R., Yan T.D., Zhu J.C., Black D., Chu F., Morris D.L. Recurrence and survival outcomes after hepatic resection with or without cryotherapy for liver metastases from colorectal carcinoma. Ann Surg Oncol. 2007;14:2078–2087. doi: 10.1245/s10434-007-9400-1. [DOI] [PubMed] [Google Scholar]
- 83.Reissfelder C., Rahbari N.N., Koch M., Ulrich A., Pfeilschifter I., Waltert A. Validation of prognostic scoring systems for patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16:3279–3288. doi: 10.1245/s10434-009-0654-7. [DOI] [PubMed] [Google Scholar]
- 84.Shah S.A., Haddad R., Al-Sukhni W., Kim R.D., Greig P.D., Grant D.R. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg. 2006;202:468–475. doi: 10.1016/j.jamcollsurg.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 85.Tanaka K., Shimada H., Matsumoto C., Matsuo K., Takeda K., Nagano Y. Impact of the degree of liver resection on survival for patients with multiple liver metastases from colorectal cancer. World J Surg. 2008;32:2057–2069. doi: 10.1007/s00268-008-9610-0. [DOI] [PubMed] [Google Scholar]
- 86.Tanaka K., Shimada H., Ueda M., Matsuo K., Endo I., Togo S. Long-term characteristics of 5-year survivors after liver resection for colorectal metastases. Ann Surg Oncol. 2007;14:1336–1346. doi: 10.1245/s10434-006-9071-3. [DOI] [PubMed] [Google Scholar]
- 87.Tsukioka T., Nishiyama N., Iwata T., Nagano K., Izumi N., Mizuguchi S. Pulmonary metastasis from colorectal carcinoma with hepatic metastasis. Gen Thorac Cardiovasc Surg. 2007;55:455–460. doi: 10.1007/s11748-007-0165-z. [DOI] [PubMed] [Google Scholar]
- 88.van der Pool A.E., Lalmahomed Z.S., Ozbay Y., de Wilt J.H., Eggermont A.M., Jzermans J.N. ‘Staged’ liver resection in synchronous and metachronous colorectal hepatic metastases: differences in clinicopathological features and outcome. Colorectal Dis. 2010;12:e229–235. doi: 10.1111/j.1463-1318.2009.02135.x. [DOI] [PubMed] [Google Scholar]
- 89.Wicherts D.A., Miller R., de Haas R.J., Bitsakou G., Vibert E., Veilhan L.A. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248:994–1005. doi: 10.1097/SLA.0b013e3181907fd9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.