Abstract
AIM: To investigate the effect of Euphorbia esula (E. esula) extract in inhibiting proliferation and inducing apoptosis in SGC-7901 cells.
METHODS: E. esula extract at different concentrations was used to inhibit proliferation and induce apoptosis of human gastric carcinoma SGC-7901 cells. Inhibition of proliferation was detected with thiazolyl blue assay, and apoptosis was detected with fluorescence microscopy, transmission electron microscopy, and flow cytometry. The mechanisms were studied by measurement of caspase-3 and caspase-8 activities and Bax and Bcl2 mRNA expression.
RESULTS: The thiazolyl blue assay showed that SGC-7901 cell viability and proliferation were inhibited significantly by E. esula extract in a time- and concentration-dependent manner. Fluorescence microscopy revealed that the cell nuclei showed the characteristic changes of apoptosis, such as uneven staining and chromatin marginalization. Some key features of apoptosis were also observed under transmission electron microscopy, which included cellular shrinkage and the foaming or bubbling phenomenon. When the cells were analyzed by flow cytometry, a sub-G1 peak could be seen clearly. Spectrophotometric assay of caspase-3 and caspase-8 activities in the treated cells showed an approximately two-fold increase. Reverse transcription polymerase chain reaction showed that Bax mRNA expression was upregulated, while Bcl2 mRNA expression was downregulated.
CONCLUSION: E. esula extract inhibited proliferation and induced apoptosis in SGC-7901 cells, in a caspase-dependent manner, involving upregulation of Bax and downregulation of Bcl2.
Keywords: Euphorbia esula Linn, Apoptosis, Gastric carcinoma, Caspase, Bax, Bcl2
Core tip: Latex of Euphorbia esula (E. esula) is used to treat benign growths in traditional Chinese medicine. This led us to design an experiment to establish whether latex of E. esula can cause apoptosis. We found that E. esula extract inhibited proliferation and induced apoptosis in SGC-7901 cells, and that the action was caspase dependent and involved upregulation of Bax gene and downregulation of Bcl2 gene.
INTRODUCTION
Euphorbia esula (E. esula) Linn is a herbaceous perennial plant belonging to the family Euphorbiaceae, which is native to many countries in the world, including China[1-4]. One of the characteristic features of the plant is its abundance of latex; a kind of white milk-like fluid that readily flows out when the stem or leaf of the plant is broken. For that reason, the plant is also known as milky juice herb in some areas of China. One major use of this plant in traditional Chinese medicine is to treat skin warts (a kind of non-cancerous growth, or papilloma). The fresh latex of the plant is applied several times to the surface of the growth, which results in gradual shrinkage and final disappearance of the lump.
This prompted us to speculate whether certain ingredients in the latex cause apoptosis in the wart cells. In the present study, we collected the fresh wild plant from the mountainous area of Yanan, extracted the latex from the herb with water at room temperature or 4 °C, and used the extract to induce apoptosis in the SGC-7901 cell line, a continuous cell line originally isolated from human gastric carcinoma. Gastric carcinoma is the leading cause of cancer mortality in rural China and the second most common cause worldwide, including Chinese cities[5].
Our study demonstrated that E. esula extract inhibited proliferation and induced apoptosis in SGC-7901 cells. The action was caspase dependent and involved upregulation of Bax gene transcription and downregulation of Bcl2 gene transcription.
MATERIALS AND METHODS
Preparation of E. esula extract
The aboveground part of wild E. esula was collected from the area around Yanan City in early June (Figure 1). The fresh herb was washed clean, first with running water and then with ultrapure water, and cut into small pieces. Latex of the plant was gathered by squeezing the plant pieces in ultrapure water with a machine, and the fluid was filtered with analytical filter paper (slow type ) at 4 °C. The resulting liquid, which was clear and brown in color, was air-dried at 50 °C. The dry substance was weighed and then dissolved and diluted with ultrapure water to obtain a concentration of 20 g/L. The final solution was made aseptic by passing through a 220-nm filter membrane (Minisart; Sartorius, Goettigen, Germany) and stored at -20 °C.
Figure 1.

Euphorbia esula Linn collected in Yan'an, China in June.
Cell line maintenance
Human gastric carcinoma cell line SGC-7901 was obtained from the Cell Resource Center of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). The cells were maintained in modified RPMI 1640 medium (Hyclone; Thermo Fisher Scientific, Beijing, China) supplemented with 2.05 mmol/L L-glutamine, 100 mL/L fetal bovine serum (FBS; Hangzhou Sijiqing Biological Engineering Materials, Hangzhou, China), 100 U/mL penicillin, and 100 mg/L streptomycin, and incubated at 37 °C with 50 mL/L CO2, and 95% humidity.
Cell viability assay with thiazolyl blue reagent
SGC-7901 cells cultivated to logarithmic phase were digested with 2.5 g/L trypsin and a cell suspension of 6 × 104 cells/mL was made with RPMI 1640 containing 100 mL/L FBS. The cells were seeded in two 96-well culture plates (NUNC microwell plates, Thermo Scientific, Roskilde, Denmark), 200 μL per well and incubated at 37 °C with 50 mL/L CO2 and 95% humidity. Twenty-four hours later, when the cells had grown to approximately 70% confluence, the culture medium was replaced with the same volume of fresh 100 mL/L FBS-RPMI 1640 medium containing different concentrations of E. esula extract at 5, 10, 20, 40, 80 and 160 mg/L; there were eight wells for each dilution. The culture medium in an additional eight wells was replaced with the same culture medium without E. esula extract, 200 μL per well also, to be used as a control. The cells were further incubated for 24 h for one plate and 48 h for the other. At the end of the incubation, thiazolyl blue (Sigma-Aldrich, St Louis, MO, United States) solution at 5 mg/mL was added to all of the wells at 20 μL per well. After further incubating at 37 °C, 50 mL/L CO2, for 4 h, the supernatant containing thiazolyl blue was discarded and dimethyl sulfoxide was added to all wells at 150 μL per well. The plate was oscillated slowly with a horizontal oscillator for 10 min to dissolve the Formazan crystals fully. After that, the optical density (OD) of the testing wells and the control wells was obtained at 490 nm wavelength with a Microplate reader (Model 680; Bio-Rad, United States). The arithmetic means of the eight wells for each dilution and the control wells were taken to draw a dose-response relationship polygon curve. Rates of cell proliferation inhibition were calculated using the following formula: cell viability inhibition rate = (average OD value of control wells - average OD value of testing wells)/average OD value of control wells.
Fluorescence microscopic detection of SGC-7901 cell apoptosis after E. esula extract treatment
SGC-7901 cells were cultured until the monolayer reached approximately 80% confluence. The cells were digested with 2.5 g/L trypsin and made into a suspension of 6 × 104 cells/mL with RPMI 1640 containing 100 mL/L FBS. A sterile coverslip was placed on the bottom of each well of a six-well culture plate, and 500 μL of the cell suspension was applied to the center of each coverslip. After 2 h incubation at 37 °C with 50 mL/L CO2 and 95% humidity, 1.5 mL of cell culture medium (RPMI 1640 containing 100 mL/L FBS) was added to each well of the six-well culture plate, which was then put into the incubator for further cultivation. Twenty hours later, after removing the used culture medium, E. esula extract at concentrations of 40 and 80 mg/L, diluted with RPMI 1640 containing 100 mL/L FBS, was added to the testing wells; 2.0 mL per well. For control wells, 2.0 mL RPMI 1640 containing 100 mL/L FBS only was added. After incubating for 24 h under the same conditions, the used culture medium was discarded and 500 μL 5 mg/L Hoechst 33258 (Sigma-Aldrich) was added to the coverslip of each well and allowed to stain in the dark at room temperature for 5 min. The coverslips were removed from the wells and each was mounted on a glass slide with the cell side downward. Nuclear morphological changes were observed using fluorescence microscopy excited by a UV light source (excitation 352 nm, emission 461 nm).
Examination of SGC-7901 cell apoptosis using transmission electron microscopy
SGC-7901 cells in logarithmic phase, prepared at a density of 3 × 104 cells, were seeded in a six-well culture plate (2 mL per well) and cultured at 37 °C and 50 mL/L CO2 for 24 h. The cell culture medium was replaced with 100 mL/L FBS-RPMI 1640 containing 40 mg/L of E. esula extract. For control wells, only 100 mL/L FBS-RPMI 1640 culture medium was added. After cultivation at 37 °C and 50 mL/L CO2 for 24 h, cells were harvested with 2.5 g/L trypsin digestion, rinsed with PBS, and pelleted at 290 × g for 6 min. The cell pellets were fixed with 1.5 g/L glutaraldehyde for 90 min, rinsed three times with 0.18 mol/L sucrose, and, after lying overnight, post-fixed in 1 g/L osmium tetroxide for 1 h, and rinsed. The cells were dehydrated in a graded series of ethanol, soaked in a mixture of an equal volume of ethanol and embedding medium at 37 °C for 3 h, and embedded in epoxy resin. The blocks were cut using a Leica ultramicrotome. The slices were stained with uranyl acetate and lead citrate and examined using a JEM-1011 transmission electron microscope (JEOL, Japan); photographs of representative fields were taken.
Flow cytometry analysis of SGC-7901 cell apoptosis
SGC-7901 cells were seeded in two six-well plates at 6 × 104/mL in RPMI 1640 containing 100 mL/L FBS (2 mL per well) and cultivated at 37 °C with 50 mL/L CO2 and 95% humidity. Twenty-four hours later, when the cell monolayer reached approximately 80% confluence, the old culture medium of one of the two plates was discarded, and the cells were treated with E. esula extract at concentrations of 40 and 80 mg/L, and diluted with RPMI 1640 containing 100 mL/L FBS. Both plates were cultivated continually under the same conditions. Twenty-four hours later, cells of the other plate were treated with E. esula extract in the same way. The culture medium of the control wells was replaced with the same volume of RPMI 1640 containing 100 mL/L FBS. After culturing for a further 24 h, the cells were collected with 2.5 g/L trypsin digestion, passed through a 400-mesh nylon screen (38-μm sieve opening) to eliminate any cell clumps, and washed twice with ice-cold PBS. After the second wash, the cell pellets were resuspended with ice-cold 70% ethanol and placed at 4 °C for 30 min (ethanol fixation). The cells were washed again with ice-cold PBS and resuspended with ice-cold RNase solution (BioDev-Tech, Beijing, China, kept at -20 °C, diluted with ice-cold PBS at 50 mg/L), and kept at room temperature for 20 min. Finally, the cells were centrifuged at 129 × g for 5 min, resuspended with 1 mL ice-cold PBS, and kept at -20 °C until use. When we were ready for flow cytometry analysis, 100 μL propidium iodide (Sigma-Aldrich) solution (100 mg/L) was added to 1 mL cell suspension and mixed thoroughly. After keeping at room temperature in the dark for 30 min, the apoptotic peak (sub-G1 peak) was detected using a flow cytometer (Beckman Coulter, United States).
Spectrophotometry of caspase-3 and caspase-8 activity in cells treated with E. esula extract
SGC-7901 cells incubated in 25-cm2 culture flasks at 37 °C with 50 mL/L CO2 and 95% humidity were allowed to grow to approximately 80% confluence, and then E. esula extract was added at a final concentration of 40 mg/L and the cells were incubated again under the same conditions. Twenty-four hours later, the cells were harvested with 2.5 g/L trypsin digestion and washed with ice-cold PBS. After the supernatant was removed, cell lysis buffer was added (50 μL lysis solution for 2 × 106 cells) and the cells were resuspended and kept in an ice bath for 30 min. During that time, vortex oscillation of the centrifugal tube was carried out four times for 10 s each. The cell lysate was centrifuged at 4 °C, 12879 × g for 15 min. The supernatant was transferred carefully to a new centrifuge tube and placed on ice. Caspase-3 and caspase-8 activities were measured immediately according to the instructions of Caspase Activity Colorimetric Assay Kit (Nanjing Biobox Biotech. Co. Ltd, Nanjing, China). OD values were read at 405 nm using a Microplate reader (Model 680; Bio-Rad).
Reverse transcription polymerase chain reaction detection of dose-effect dependence of Bax and Bcl2 mRNA expression after E. esula extract treatment
SGC-7901 cells were seeded in four 25-cm2 culture flasks and allowed to grow to approximately 80% confluence. The cells were treated with E. esula extract at concentrations of 20, 40 and 80 mg/L. One of the flasks was used as a control. After 24 h incubation, the cells were harvested with 2.5 g/L trypsin, washed with ice-cold PBS and pelleted at 290 × g, for 6 min. Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, United States). Diethylpyrocarbonate water was used to dissolve the RNA. A small amount was taken from all extracted total RNA samples for quality and quantity analysis using UV spectrophotometry and agarose gel electrophoresis. The extracted RNA samples were stored at -80 °C until further use. Reverse transcription for cDNA was performed using 1 μg total RNA in a reaction volume of 20 μL. Two microliters of cDNA product was taken to perform PCR amplification in a 25-μL reaction system. β-Actin gene was used as an internal reference to normalize the expression of the target genes. Primer sequences of the Bax, Bcl2 and β-actin genes are shown in Table 1. The PCR mixture was subjected to 30 cycles with the following conditions: activation at 95 °C for 5 min; denaturation at 95 °C for 5 s; annealing at 62 °C for 10 s; and extension at 60 °C for 10 s. Finally, the PCR products were subjected to 1.5% agarose gel electrophoresis separation.
Table 1.
Primer sequences of Bax, Bcl-2 and β-actin genes
| Gene | Forward primer | Backward primer |
| Bax | TTTGCTTCAGGGTTTCATCC | GGAGGAAGTCCAATGTCCAG |
| Bcl-2 | GGATGCCTTTGTGGAACTGT | ATTCGACGTTTTGCCTGAAG |
| β-actin | GAAAATCTGGCCACCCACCT | GGGGTGTTGAAGGTCTCAAA |
Statistical analysis
Experimental data were presented as the mean ± SD. Differences between treatments and controls, and among different treatment groups, were assessed by analysis of variance or a χ2 test. P < 0.05 was considered statistically significant.
RESULTS
Viability of SGC-7901 cells after treatment with E. esula extract detected with thiazolyl blue assay
The thiazolyl blue assay showed that the viability or proliferative activity of human gastric carcinoma SGC-7901 cells was significantly inhibited by E. esula extract, in an obvious time- and concentration-dependent manner. SGC-7901 cells treated with different concentrations of E. esula extract all showed a significant inhibitory effect compared with the controls (P < 0.01). With the increasing drug concentration, there was a significant trend of intensification of cell growth inhibition (P < 0.01). The inhibitory effect was more notable with treatment for 48 h than 24 h (P < 0.05) (Table 2).
Table 2.
Inhibition of SGC-7901 cells by Euphorbia esula extract
| E. esula (mg/L) |
OD490 (mean ± SD) |
Inhibition rate (%) |
||
| 24 h | 48 h | 24 h | 48 h | |
| 0 (control) | 0.827 ± 0.020 | 0.818 ± 0.021 | 0 | 0 |
| 5 | 0.728 ± 0.018b | 0.712 ± 0.020b | 11.97b | 12.96b |
| 10 | 0.645 ± 0.020bd | 0.620 ± 0.019bd | 22.00bd | 24.21bd |
| 20 | 0.574 ± 0.019bd | 0.501 ± 0.018bcd | 30.59bd | 38.75bcd |
| 40 | 0.447 ± 0.017bd | 0.397 ± 0.015bcd | 45.95bd | 51.47bcd |
| 80 | 0.338 ± 0.021bd | 0.274 ± 0.017bcd | 59.13bd | 66.50bcd |
| 160 | 0.269 ± 0.019bd | 0.214 ± 0.016bcd | 67.47bd | 73.84bcd |
P < 0.01 vs control;
P < 0.01 vs previous concentration;
P < 0.05 vs 24 h treatment. n = 8. E. esula: Euphorbia esula.
Apoptotic nuclear morphology observed using fluorescence microscopy after treating SGC-7901 cells with E. esula extract
SGC-7901 cells were treated with E. esula extract, stained by fluorescent dye Hoechst 33258, and examined under a UV-excited fluorescence microscopy. The nuclei of all cells were blue in color. The cells that were treated with E. esula extract showed the characteristic nuclear morphological changes of apoptosis, such as nuclear condensation and uneven staining, chromatin marginalization, and nuclear debris. In addition, enhancement of blue fluorescence was observed in the nuclei of these cells. The apoptotic nuclear morphological changes were more prominent in the cells treated with 80 mg/L E. esula extract than those treated with 40 mg/L extract. In contrast, the nuclei of the control cells showed a shallower and evenly distributed blue color (Figure 2).
Figure 2.
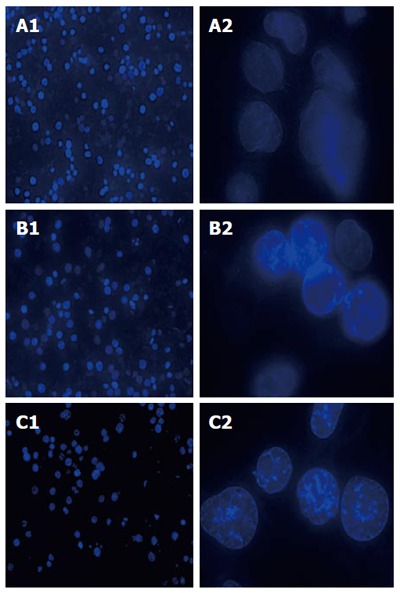
Nuclear morphological changes observed using a fluorescence microscopy in SGC-7901 cells treated with Euphorbia esula extract at different concentrations and for 24 h, stained by Hoechst 33258. A1: Untreated, magnification × 200; A2: Untreated, magnification × 1000; B1: 40 mg/L, magnification × 200; B2: 40 mg/L, magnification × 1000; C1: 80 mg/L, magnification × 200; C2: 80 mg/L, magnification × 1000.
Morphological changes of SGC-7901 cells after treatment with E. esula extract observed with transmission electron microscope
After treatment with E. esula extract at 40 mg/L for 24 h, SGC-7901 cells showed some characteristic features of apoptosis under the transmission electron microscope. These included cellular shrinkage, foaming or bubbling of cytoplasm, as well as chromatin condensation and marginalization. The untreated control cells did not show these phenomena (Figure 3).
Figure 3.
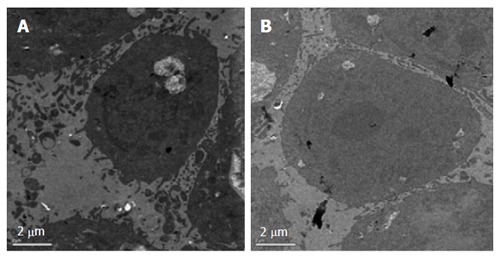
Morphology of SGC-7901 cells observed using a transmission electron microscope. A: After treatment with 40 mg/L Euphorbia esula extract for 24 h; B: Untreated cells. Bar = 2 μm.
Flow cytometry showed the apoptotic peak in SGC-7901 cells treated with E. esula extract
SGC-7901 cells were treated with 40 and 80 mg/L E. esula extract for 24 and 48 h and were detected by flow cytometry. Sub-G1 or the apoptotic peak, which is a characteristic feature of cells undergoing apoptosis, resulting from cellular DNA loss, could be seen clearly, and was more obvious in cells treated with the higher concentration and for a longer period. The untreated SGC-7901 cells did not show this feature (Figure 4).
Figure 4.
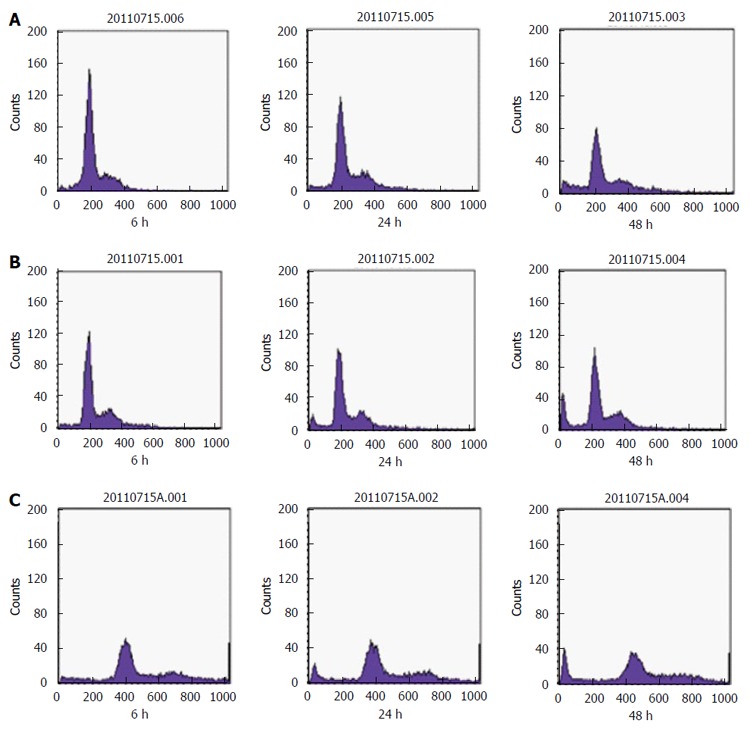
Flow cytometry detection of the apoptotic peak. SGC-7901 cells were treated with different concentrations of Euphorbia esula (E. esula) extract and flow cytometry was performed at three time points. A: Untreated SGC-7901 cells used as a control; B: Treated with 40 mg/L E. esula extract; C: Treated with 80 mg/L E. esula extract.
Caspase-3 and caspase-8 activation in SGC-7901 cells treated with E. esula extract
After treating SGC-7901 cells with 40 mg/L E. esula extract for 24 h, caspase-3 and caspase-8 activities were measured by a colorimetric assay, and mean OD405 values were obtained from blank wells, control wells and E. esula extract-treated wells, which were 0.038, 0.195 and 0.349 for caspase-3 (Figure 5A), and 0.038, 0.162 and 0.252 for caspase-8 (Figure 5B), respectively. The fold-increases of caspase-3 and caspase-8 activity in E. esula extract-treated SGC-7901 cells were 1.98 and 1.73, respectively, as determined by the following formula: Fold increase = mean OD of testing wells - mean OD of blank wells)/mean OD of control wells - mean OD of blank wells.
Figure 5.
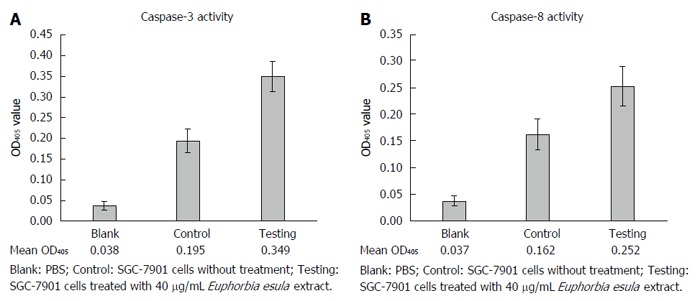
Activation of caspase-3 and caspase-8 in SGC-7901 cells after treatment with Euphorbia esula extract, measured using spectrophotometry. Concentration of Euphorbia esula extract: 40 mg/L; duration of treatment: 24 h; n = 3.
Dose-dependent alteration of Bax and Bcl2 mRNA expression in SGC-7901 cells treated with E. esula extract
RT-PCR and agarose gel electrophoresis showed a dose-dependent increase in the Bax mRNA expression level and a dose-dependent decrease in the Bcl2 mRNA expression level in SGC-7901 cells treated with E. esula extract. Bax mRNA expression increased with increasing concentration of E. esula extract, whereas Bcl2 mRNA expression decreased with increasing concentration of E. esula extract (Figure 6).
Figure 6.
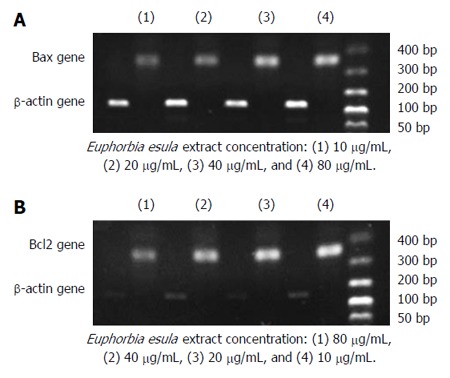
Agarose gel electrophoresis of RT-PCR products showing dose-dependent alterations of Bax and Bcl2 gene expression in SGC-7901 cells treated with Euphorbia esula extract. A: Bax mRNA level increased with the concentration of Euphorbia esula (E. esula) extract; B: Bcl2 mRNA level increased with decreased concentration of E. esula extract.
DISCUSSION
Malignant tumors are a serious threat to human health, and their morbidity and mortality are among the highest of all disease categories worldwide[5]. Looking for effective anti-tumor drugs with fewer side effects is a great challenge and an urgent task for the entire medical and pharmacological community. Currently, extracting ingredients from plants or other natural resources to be used as anti-tumor drugs is a noteworthy direction in research[6-8].
Chemically synthesized drugs reached their peak phase in the western world during the 1950s and 1960s, but since the 1970s, people have become more conscious of the undesired aspects of chemical synthetic drugs, that is, their side effects and toxicity. In the meantime, natural drugs, because of their fewer side effects and lower toxicity, have gradually been taken seriously. In addition, natural medicines have lower development costs than chemical synthetic drugs[9-12]. In entering the 21st century, under the influence of the transformation of medicine model and the new concept of “returning to nature”, the exploration and research of natural resources for medications is becoming even more prevalent. At the present time, the phrase natural medicine has become an attractive and frequently used term in the medical profession, as well as among lay people, and searching for active ingredients from natural resources for health care and for the prevention and treatment of diseases has become a major trend worldwide. Many researchers believe that in the near future, natural medicines will become the mainstream medical market[13].
In the present study, we used a fresh aqueous extract from E. esula Linn to induce apoptosis in human gastric carcinoma SGC-7901 cells and found that it reduced cellular viability and induced apoptosis. We also investigated the underlying mechanisms and found that the activity of caspase-8 was 1.73-fold increased, that of caspase-3 was 1.98-fold increased, the expression of Bax mRNA was upregulated and the expression of Bcl2 mRNA was downregulated. These results indicated that E. esula extract/latex must contain some ingredients that inhibit SGC-7901 cell proliferation and induce apoptosis; the latter being dependent on activation of caspases and regulated by Bax and Bcl2 gene products. The results suggested that E. esula extract/latex induces SGC-7901 cell apoptosis through extrinsic signaling or a membrane-receptor-dependent pathway. In this pathway, the extracellular inducer acts on membrane receptors that transduce the triggering signal intracellularly through several steps and leads to caspase-8 activation[14,15]. The process is usually regulated by a balance between the proapoptotic proteins, such as Bax, Bid, Bak and Bad, and the antiapoptotic proteins, such as Bcl2 and Bcl-XL, and might involve alteration of mitochondrial membrane permeability and release of cytochrome C[16-18]. Finally, the apoptotic effector molecule caspase-3 is activated and the cell death program is executed[19]. It is not clear whether caspase-9 is also involved in the process of E. esula extract-induced SGC-7901 cell apoptosis.
E. esula latex is used to treat skin warts in traditional Chinese medicine. Both skin warts and cervical cancers are caused by human papillomaviruses (HPVs); therefore, when we initially designed the experiments to test whether E. esula induces apoptosis, we naturally thought of using a cell line that originated from cervical cancer and the HeLa cell line was the choice. A preliminary comparative study using E. esula extract to induce apoptosis in the HeLa cell line and several other cancerous cell lines including human gastric carcinoma SGC-7901 cells, human lung cancer A549 cells, human liver cancer HepG2 Cells, and human breast cancer MCF-7 cells showed that E. esula induced apoptosis in all these cell lines, but that there were no significant differences between HeLa cells and the other cell lines in their response to E. esula treatment (unpublished work). The results suggested that E. esula might not exert its action interfering with HPV carcinogenesis or HPV oncogene/oncoprotein actions. Thus, we used human gastric carcinoma SGC-7901 cells in our research because gastric cancer is the second leading cause of cancer-related death in the world (and is the number one in most Chinese rural areas, including the countryside around the city in which the authors work). The fact that E. esula did not show significant differences in inducing apoptosis among different cancerous cell lines could be an advantage, because if we could isolate the effective ingredient(s), it might be used to treat cancers of other tissue origins. It is not clear yet whether E. esula extract induces apoptosis more effectively in cancer cells than non-cancerous cells. If the answer is yes then the drug made from E. esula effective ingredient(s) would be very promising; if not, a technique should be established to deliver the drug to/into cancer cells.
To study further the mechanisms of E. esula extract-induced SGC-7901 cell apoptosis, we could detect the subcellular distribution of cytochrome C and measure the enzymatic activity of caspase-9 in E. esula extract-treated SGC-7901 cells. Future experiments will also include a comparison between primary cultures of normal tissues and continuous cell lines to see if there are any differences in their responses to E. esula-induced apoptosis. In particular, future work should include the isolation and identification of the effective ingredient(s) in E. esula extract that inhibit cancer cell proliferation and trigger cancer cells to undergo apoptosis.
COMMENTS
Background
Euphorbia esula (E. esula) is used to treat skin warts in Chinese folk medicine. The latex of the plant is applied several times to the surface of the warts and the lump shrinks gradually and finally disappears. This prompted the authors to design the present study to determine whether the latex can cause apoptosis in tumor cells.
Research frontiers
The side effects and toxicity of chemically synthetic drugs cannot be ignored; therefore, natural medicines have gradually been taken seriously. In addition to their advantages of fewer side effects and lower toxicity, natural medicines have lower development costs than chemical drugs. As a result, the exploration and research of natural resources for medications is becoming more prevalent.
Innovations and breakthroughs
This study showed clearly that E. esula extract inhibited proliferation and induced apoptosis effectively in SGC-7901 cells, a cancerous cell line originally isolated from human gastric carcinoma, and that the action was caspase dependent.
Applications
After the effective ingredient in E. esula that inhibits cancer cell proliferation and triggers cancer cells to undergo apoptosis is isolated or extracted, it can be used to develop medicines for clinical use.
Terminology
E. esula Linn is an herbaceous perennial plant belonging to the family Euphorbiaceae. The plant is rich in a type of milky juice or latex.
Peer-review
This is a well written and set up study. The authors give a sufficient overview of the study background and clearly stated the hypothesis of the study. The aim of the study is fulfilled. The results are presented sufficiently well and have been discussed well; the 6 figures and the table give a good overview of the results and are presented correctly.
Footnotes
Supported by Shaanxi Provincial High-Level University Construction Project in Basic Medical Sciences, No. 2013SXTS02; and Yan’an Science and Technology Department, No. 2014HM-05.
Institutional review board statement: This study was reviewed and approved by the Academic Committee of Medical College of Yan’an University, Yan’an, China.
Conflict-of-interest statement: The authors declare that there is no conflict of interest related to this study.
Data sharing statement: No additional unpublished data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 25, 2015
First decision: December 11, 2015
Article in press: January 11, 2016
P- Reviewer: Vorobjova T S- Editor: Ma YJ L- Editor: Stewart G E- Editor: Wang CH
References
- 1.Chinese academy of sciences editorial board for Flora of China. Euphorbia esula Linn. In: Flora of China 44(3), editor. Beijing: Science Press; 2006. pp. 125–126. [Google Scholar]
- 2.Hou SL. Detailed annotation of eight hundred kinds of Chinese medicines. 2nd ed. Zhengzhou: Henan Science and Technology Press; 2009. p. 638. [Google Scholar]
- 3.Xu GJ, Wang Q. Color spectrum of Chinese herbal medicines. 3rd ed. Fuzhou: Fujian Science and Technology Press; 2009. pp. 808–809; 146-147. [Google Scholar]
- 4.Liu YF, Fu ZY. Confusion resulted from improper use of traditional Chinese medicine names and suggested ways of clarification. Zhongguo Yiyao Daobao. 2011;13:1593–1594. [Google Scholar]
- 5.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 6.Tian HY, Wang AH, Fu ZY. Study on the induction of apoptosis in human gastric carcinoma SGC7901 cells by Euphorbia lunulata extracts. Huaxi Yaoxue Zazhi. 2014;29:527–530. [Google Scholar]
- 7.Li XJ, Mi ZK, Fu ZY. Mechanisms of ginsenoside and oridonin inducing cancer cell apoptosis. Guoji Zhongliuxue Zazhi. 2011;38:263–266. [Google Scholar]
- 8.Siu D. Natural products and their role in cancer therapy. Med Oncol. 2011;28:888–900. doi: 10.1007/s12032-010-9528-x. [DOI] [PubMed] [Google Scholar]
- 9.Bell IR, Sarter B, Koithan M, Banerji P, Banerji P, Jain S, Ives J. Integrative nanomedicine: treating cancer with nanoscale natural products. Glob Adv Health Med. 2014;3:36–53. doi: 10.7453/gahmj.2013.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fulda S. Modulation of apoptosis by natural products for cancer therapy. Planta Med. 2010;76:1075–1079. doi: 10.1055/s-0030-1249961. [DOI] [PubMed] [Google Scholar]
- 11.Basmadjian C, Zhao Q, Bentouhami E, Djehal A, Nebigil CG, Johnson RA, Serova M, de Gramont A, Faivre S, Raymond E, et al. Cancer wars: natural products strike back. Front Chem. 2014;2:20. doi: 10.3389/fchem.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattern DJ, Valiante V, Unkles SE, Brakhage AA. Synthetic biology of fungal natural products. Front Microbiol. 2015;6:775. doi: 10.3389/fmicb.2015.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta RG, Murillo G, Naithani R, Peng X. Cancer chemoprevention by natural products: how far have we come? Pharm Res. 2010;27:950–961. doi: 10.1007/s11095-010-0085-y. [DOI] [PubMed] [Google Scholar]
- 14.Fulda S. Targeting extrinsic apoptosis in cancer: Challenges and opportunities. Semin Cell Dev Biol. 2015;39:20–25. doi: 10.1016/j.semcdb.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Fiandalo MV, Kyprianou N. Caspase control: protagonists of cancer cell apoptosis. Exp Oncol. 2012;34:165–175. [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez J, Tait SW. Mitochondrial apoptosis: killing cancer using the enemy within. Br J Cancer. 2015;112:957–962. doi: 10.1038/bjc.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flusberg DA, Sorger PK. Surviving apoptosis: life-death signaling in single cells. Trends Cell Biol. 2015;25:446–458. doi: 10.1016/j.tcb.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao F, Fu Z, Tian H, He Z. The Euphorbia lunulata Bge extract inhibits proliferation of human hepatoma HepG2 cells and induces apoptosis. J BUON. 2013;18:491–495. [PubMed] [Google Scholar]
- 19.Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev. 2015;16:2129–2144. doi: 10.7314/apjcp.2015.16.6.2129. [DOI] [PubMed] [Google Scholar]


