Abstract
AIM: To determine serum vitamin D levels and colonic vitamin D receptor (VDR) expression in inflammatory bowel disease (IBD) and non-IBD patients and correlate these with histopathology.
METHODS: Puerto Rican IBD (n = 10) and non-IBD (n = 10) patients ≥ 21 years old scheduled for colonoscopy were recruited. Each patient completed a questionnaire and provided a serum sample and a colonic biopsy of normal-appearing mucosa. For IBD patients, an additional biopsy was collected from visually diseased mucosa. Serum vitamin D levels were measured by ultra-performance liquid chromatography and mass spectrometry. Hematoxylin and eosin stained tissue sections from colonic biopsies were classified histologically as normal or colitis (active/inactive), and scored for the degree of inflammation present (0-3, inactive/absent to severe). Tissue sections from colonic biopsies were also stained by immunohistochemistry for VDR, for which representative diagnostic areas were photographed and scored for staining intensity using a 4-point scale.
RESULTS: The IBD cohort was significantly younger (40.40 ± 5.27, P < 0.05) than the non-IBD cohort (56.70 ± 1.64) with a higher prevalence of vitamin D deficiency (40% vs 20%, respectively) and insufficiency (70% vs 50%, respectively). Histologic inflammation was significantly higher in visually diseased mucosa from IBD patients (1.95 ± 0.25) than in normal-appearing mucosa from control patients (0.25 ± 0.08, P < 0.01) and from IBD patients (0.65 ± 0.36, P < 0.05) and correlated inversely with VDR expression in visually diseased colonic tissue from IBD patients (r = -0.44, P < 0.05) and from IBD patients with Crohn’s disease (r = -0.69, P < 0.05), but not in normal-appearing colonic tissue from control patients or IBD patients. Control and IBD patient serum vitamin D levels correlated positively with VDR expression in normal colon from control and IBD patients (r = 0.38, P < 0.05) and with patient age (r = 0.54, P < 0.01).
CONCLUSION: Levels of serum vitamin D correlate positively with colonic VDR expression in visually normal mucosa whereas inflammation correlates negatively with colonic VDR expression in visually diseased mucosa in Puerto Rican patients.
Keywords: Colitis, Inflammation, Vitamin D, Vitamin D receptor, Inflammatory bowel disease
Core tip: Our study examines for the first time the relationship between serum vitamin D levels, colonic vitamin D receptor (VDR) expression, and histologic disease activity. We show in Puerto Rican patients that colonic VDR expression and inflammation are negatively correlated in endoscopically and histologically diseased colon and that serum vitamin D levels positively correlate with VDR expression in endoscopically and histologically normal colon. These findings contribute to our understanding of the role of vitamin D and VDR in patients with inflammatory bowel disease and could affect the current care of these patients.
INTRODUCTION
Although the incidence and prevalence of inflammatory bowel disease (IBD) is stable in first world countries, IBD is becoming more frequent in other geographic areas and in minority populations such as blacks and Hispanics, where they were rarely seen[1]. Puerto Rico is no exception, and the rate at which ulcerative colitis and Crohn’s disease are diagnosed is increasing[2,3]. Vendrell et al[4] found that the prevalence of IBD among insured Puerto Ricans in 2005 is among the highest described for Hispanic populations. Vitamin D deficiency is common among patients with IBD, yet evaluation of vitamin D status is not currently considered standard of care in these patients, and guidelines for treating vitamin D deficiency in these patients are nonexistent[5]. Increasing evidence suggests that vitamin D is an environmental factor linked to the pathogenesis and severity of IBD, including Crohn’s disease and ulcerative colitis[6,7].
Vitamin D is a hormone precursor present in two forms: ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). Cholecalciferol is synthesized in the skin upon sunlight exposure and is subsequently converted to 25-hydroxyvitamin D, the major inactive circulating vitamin D metabolite. 25-hydroxyvitamin D has a long half-life of about 3 wk, making it the best biomarker to evaluate vitamin D status[8,9]. The active form of vitamin D, 1,25(OH)2D3 (calcitriol), exerts its biological functions via the vitamin D receptor (VDR), a member of a superfamily of nuclear hormone receptors. One of the major roles of vitamin D and VDR is to regulate intestinal absorption and serum levels of calcium and phosphate. Many tissues and cells in the body, including immune cells and colonocytes, express VDR and possess the enzymes necessary to produce local 1,25(OH)2D3[6]. The main source of local 1,25(OH)2D3 is the intestinal autocrine/paracrine vitamin D system, which plays a critical role in maintaining both mucosal immunity and normal growth of epithelial cells[9]. Vitamin D-VDR signaling results in anti-inflammatory, immune-modulating, anti-mitotic, pro-differentiating, and pro-apoptotic effects, which are attributable to the hundreds of genes that contain vitamin D responsive elements (VDRE)[9-11]. These properties are critical in the context of immune diseases, such as IBD, and in cancer prevention; however, the mechanisms by which vitamin D exerts these properties are not well understood.
Importantly, the mechanisms by which circulating vitamin D levels regulate colonic VDR expression in homeostasis and disease states are also incompletely understood. Studies in bone cells have revealed that the VDR gene contains VDRE, suggesting that vitamin D can increase colonic VDR expression[12]. However, treatment of Caco-2 colon cancer cells with calcitriol resulted in increased transcript levels of CYP24A1, the major 1,25(OH)2D3 inactivating enzyme[13], and xenografts of HT29 colon cancer cells overexpressing CYP24A1 are more proliferative and invasive[14]. Additionally, SNAIL1 has been shown to repress VDR expression in colon cancer cells[15-17]. Any situation that impairs the 1,25(OH)2D3/VDR system at the intestinal mucosal level, such as vitamin D deficiency or increased CYP24A1 or SNAIL expression, may increase risk for development or progression of IBD and colorectal cancer (CRC)[9], one of the most severe complications for patients with long-standing IBD. In fact, IBD colitis patients are six times more likely to develop CRC than the general population[9], and this risk increases with disease severity, duration, and extent[18]. Interestingly, we recently found differential expression of VDR in Puerto Rican patients with colitis-associated and sporadic colorectal neoplasia, with a significant decrease in VDR expression in sporadic dysplasia and CRC compared with normal and colitis-associated CRC tissue[19].
Recent animal studies have demonstrated that vitamin D supplementation ameliorates, whereas vitamin D or VDR deficiency worsens, inflammation in murine models of IBD[20,21], providing strong evidence for vitamin D as an anti-inflammatory immunomodulator in IBD. Furthermore, in vivo and in vitro studies have shown that combining vitamin D with steroids, immunomodulators, or biologics produces synergistic effects[22-24]. In vitro studies have suggested that VDR plays a critical role in preserving intestinal mucosal barrier integrity[10,25]. It has also been demonstrated that 1,25(OH)2D3 signaling through VDR is a direct and important inducer of NOD2 expression[26]. A key downstream signaling consequence of NOD2 activation by the agonist muramyl dipeptide is stimulation of NF-κB transcription factor function, which induces expression of the gene encoding defensin β2 (DEFβ2/HBD2), a gut antimicrobial peptide that plays an important role in the mucosal immune barrier and whose deficiency has been linked to colonic Crohn’s disease[27].
There is a paucity of data on the role of vitamin D in IBD disease activity preventing firm conclusions regarding its use as a predictor of disease severity. Nevertheless, Joseph et al found that 79% of patients with Crohn’s disease in India were vitamin D deficient and that the clinical disease activity correlated negatively with serum 25-hydroxyvitamin D levels[28]. Ulitsky et al. reported a high rate (49.8%) of vitamin D deficiency in a cohort of 504 IBD patients in the north-central United States and an independent association of this deficiency with lower quality of life and greater disease activity of Crohn’s disease[29]. There is also some evidence that vitamin D supplementation may help in maintaining remission in patients with Crohn’s disease[30].
In light of the protective and regulatory effects that vitamin D has on the colonic epithelial barrier and immune system, respectively, we postulate that patients with IBD who have low levels of vitamin D are susceptible to progression to severe disease and perpetuation of chronic mucosal inflammation, proven risk factors for CRC in these patients. Elucidating the role of vitamin D and VDR in patients with IBD could therefore affect the current care of these patients. In the present study, we examine the relationship between serum vitamin D levels, colonic VDR expression, and histologic disease activity. To our knowledge, this relationship has not previously been studied in either a Puerto Rican or any other IBD population.
MATERIALS AND METHODS
Use of human subjects and internal review board approval
This study was carried out in compliance with all NIH regulations concerning the Protection of Human Subjects. The study was approved by the University of Puerto Rico Medical Sciences Campus Institutional Review Board under protocol number 1250313.
Patients and collection of samples
Puerto Rican patients (both sexes) over 21 years old with a documented diagnosis of ulcerative colitis or Crohn’s disease by standard endoscopic, histologic, radiologic, and/or clinical criteria who were seen at the University of Puerto Rico Center for IBD and were undergoing colonoscopy and biopsy for clinical indications were offered participation in this study. Patients were informed about the purpose of the study, benefits and risks involved, and their rights and confidentiality. Patients over 21 years old undergoing a colonoscopy for other reasons (but not due to suspected IBD, chronic diarrhea, malabsorption syndrome, or malignancy) were recruited as controls. Patients or controls who had used vitamin D, vitamin D plus calcium, or multivitamin supplementation during the previous 3 mo were excluded from study participation. Patients who agreed to participate signed the IRB-approved informed consent form and completed a questionnaire to gather demographic, lifestyle, and disease-related information. Our study included 10 controls and 10 patients with IBD (Table 1, also see Table 2 for additional disease and demographic information on individual participants). Two colon biopsies were collected from IBD patients, one from visually diseased mucosa and one from normal appearing mucosa. Controls provided one colonic biopsy, which was subsequently confirmed as histologically normal. A serum blood sample was collected from all patients and stored at -80 °C until time of analysis.
Table 1.
Demographic information for control and inflammatory bowel disease patients
| Control (n = 10) | IBD (n = 10) | |
| Sex | ||
| Male | 4 | 6 |
| Female | 6 | 4 |
| Mean age (yr) | 56.70 ± 1.64 | 40.40 ± 5.27a |
| IBD diagnosis | ||
| CD | 7 | |
| UC | 3 |
P < 0.05. CD: Crohn’s disease; IBD: Inflammatory bowel disease; UC: Ulcerative colitis.
Table 2.
Demographic and disease information for control and inflammatory bowel disease patients
| Patient ID | Age (yr) | Sex | Diagnosis | IBD duration (yr) | Medications | Colonoscopy findings |
| UC1 | 63 | F | Ulcerative colitis | 15 | Mesalamine, Azathioprine | Proctosigmoiditis |
| CD1 | 29 | F | Crohn’s disease | 2 | Adalimumab | Active mild colitis |
| CD2 | 41 | M | Crohn’s disease1 | 16 | Enalapril | Active Crohn’s disease at ileocolonic anastomosis |
| UC2 | 25 | M | Ulcerative colitis | 2 | Sulfazalazine | Mild proctosigmoiditis |
| CD3 | 23 | M | Crohn’s disease | 3 | Adalimumab, Azathioprine, folic acid | Active Crohn’s disease with ileocecal valve involvement |
| CD4 | 55 | M | Crohn’s disease1 | 10 | Mesalamine | Crohn’s ileocolitis with small external hemorrhoids |
| CD5 | 38 | M | Crohn’s disease1 | 8 | Infliximab | Active disease at ileocolonic anastomosis |
| CD6 | 32 | F | Crohn’s disease1 | 8 | Infliximab | Mild sigmoiditis with internal/external hemorrhoids |
| CD7 | 28 | M | Crohn’s disease1 | 2 | Adalimumab | Friable ileocecal valve anastomosis with small aphtous ulcer; mild distal proctitis |
| UC3 | 70 | F | Ulcerative colitis | 8 | Mesalamine | Mild proctitis |
| C1 | 50 | F | HTN, dyslipidemia, DM-2 | - | Irbesartan, Simvastatine, Glipizide, Insulin, Clonazepam | Normal study |
| C2 | 55 | F | HTN | - | Losartan | Single diminutive sessile polyp |
| C3 | 67 | M | DM-2, post-liver transplant secondary to ETOH | - | Folic acid, Glipizide, Tacrolimus | Hemorrhoids internal/external |
| C4 | 54 | M | HIV | - | Efavirnez, Tenofovir, Lamivudine | External hemorrhoids |
| C5 | 63 | M | HTN, hypothyroidism, CKD, post-liver transplant secondary to ETOH | - | Tacrolimus, Propranolol, Pepcid, Simvastatin | Normal study |
| C6 | 57 | F | Hypothyroidism, GERD | - | Ranitidine, Omeprazole, Levothyroxine | |
| C7 | 55 | M | HTN, dyslipidemia, anxiety, depression | - | Losartan, Clopidrogel, Amlodipine, Simvastatin, Clonazepam, Trazodone, Venlafaxine | Two polyps < 1 cm, both tubular adenomas |
| C8 | 59 | F | Hypothyroidism, depression, anxiety | - | Levothyroxine, Gabapentin, Sertraline, Clonazepam, Estazolam | Moderate pandiverticulosis |
| C9 | 56 | F | DM-2, dyslipidemia, NASH | - | Simvastatin, Aspirin, Gliburide | Normal sigmoidoscopy/normal virtual colonoscopy |
| C10 | 51 | F | None reported | - | none | Two small sessile polyps-hyperplastic polyps; left side diverticulosis |
IBD-related surgery. F: Female; M: Male; HTN: Hypertension; DM-2: Type 2 diabetes mellitus; HIV: Human immunodeficiency virus; CKD: Chronic kidney disease; GERD: Gastroesophageal reflux disease; NASH: Nonalcoholic steatohepatitis; IBD: Inflammatory bowel disease.
Histological analysis
Tissue sections (2-4 μm) were stained with hematoxylin and eosin and analyzed independently by two pathologists (CIG and AAI). All biopsies were classified histologically as normal, inactive colitis, or active colitis, based on Geboes et al[31]. The degree of inflammation for each biopsy site was recorded as follows: 0 = inactive/absent, 1 = mild, 2 = moderate, or 3 = severe.
Vitamin D levels
Serum samples were analyzed for 25-hydroxyvitamin D levels by ultra-performance liquid chromatography and mass spectrometry at the De Diego Research Foundation Bioanalytical Laboratory (Agilent; detection limit 7.5 ng/mL). Samples were classified as deficient (< 20 ng/mL) or not deficient (≥ 20 ng/mL) and as sufficient (≥ 30 ng/mL) or insufficient (< 30 ng/mL).
Vitamin D receptor immunohistochemical staining
Tissue sections were stained for VDR by immunohistochemistry, and VDR expression was scored as previously described[19]. Briefly, formalin-fixed, paraffin-embedded tissue sections mounted on charged glass slides were deparaffinized with Hemo-De xylene-substitute and rehydrated with graded ethanol dilutions and distilled water. Endogenous peroxidase activity was quenched with hydrogen peroxide (3%, aqueous). Heat-induced antigen retrieval was performed using citrate-EDTA buffer (10 mmol/L; 2 mmol/L EDTA, 0.05% Tween 20, pH 6.2) at 95 °C to 99 °C for 40 min and at room temperature for 20 min. After samples were blocked with normal goat serum (Biogenex, San Ramon, CA), tissues were incubated overnight with the anti-VDR antibody (Ab3508; Abcam Inc, dilution 1:2000). Secondary detection of the primary antibody was achieved using the peroxidase-based Super Sensitive Link-Label IHC Detection System (Biogenex) and 3,3-diaminobenzidine chromogen solution (Biogenex). Nuclei were counterstained with hematoxylin before dehydration, clearing, and coverslipping. Representative areas containing the diagnosis of interest (normal, inactive colitis, active colitis) were photographed and scored in triplicate by observers blinded to the patients’ diagnoses. Staining intensity was scored using a four-point scale (0-3), where 0 indicates the weakest staining and 3 indicates the strongest staining[19].
Statistical analysis
The statistical significance of differences in inflammation scores and VDR expression between control mucosa, normal-appearing mucosa from IBD patients, and visually diseased mucosa from IBD patients was determined using the Kruskal-Wallis test followed by the Dunn multiple comparisons test. Differences in age and serum 25-hydroxyvitamin D levels between control and IBD patients were assessed using the Mann-Whitney test. Spearman correlation coefficient was used for analyzing correlations between VDR expression, inflammation scores, serum 25-hydroxyvitamin D levels, and age. All statistical analyses were performed with GraphPad Prism v6.0a (GraphPad Software, San Diego, CA, United States). Data are presented as mean ± SE, and statistical significance was established at P < 0.05.
RESULTS
The control and IBD groups had similar numbers of patients from both sexes; however, as might be expected, IBD patients were significantly younger than controls (P < 0.05, Table 1). Most IBD patients (7/10) had a diagnosis of Crohn’s disease.
More IBD patients have vitamin D insufficiency and deficiency than control patients
Mean serum 25-hydroxyvitamin D levels were not significantly or substantially different between control (27.61 ± 3.36 ng/mL) and IBD patients (25.18 ± 2.36 ng/mL; Figure 1A). Nevertheless, twice as many IBD patients (4/10) had vitamin D deficiency (< 20 ng/mL) compared to controls (2/10; Figure 1B). Furthermore, a higher percentage of IBD patients (7/10) had insufficient levels of vitamin D (< 30 ng/mL) compared to controls (5/10; Figure 1C), half of which had vitamin D insufficiency. Serum vitamin D levels did not significantly correlate with colonic inflammation scores.
Figure 1.
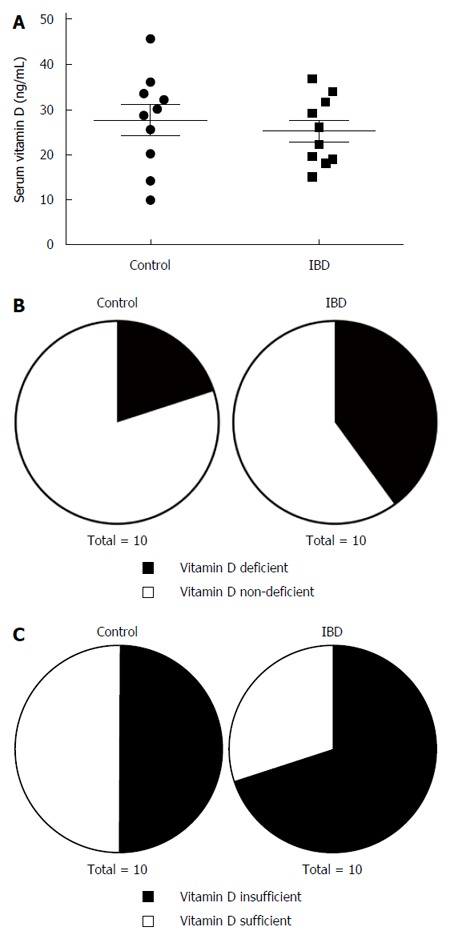
Vitamin D status in control patients and patients with inflammatory bowel disease. A: Serum levels of 25-hydroxyvitamin D3 in control and inflammatory bowel disease (IBD) patients (n = 10 patients/group). B: Proportion of control and IBD patients with vitamin D deficiency (< 20 ng/mL). C: Proportion of control and IBD patients with vitamin D insufficiency (< 30 ng/mL).
Inflammation scores, but not VDR expression levels, are increased in diseased colon from IBD patients
As expected, visually diseased mucosa from IBD patients had significantly higher inflammation scores (1.95 ± 0.25) than normal-appearing mucosa from IBD (0.65 ± 0.36, P < 0.05) or controls (0.25 ± 0.08, P < 0.01; Figure 2A). Inflammation scores were consistently higher in visually diseased than in normal appearing mucosa for all but two IBD patients. Statistically significant differences in VDR immunohistochemistry scores were not observed in comparisons between control mucosa (2.03 ± 0.20), normal appearing mucosa from IBD patients (1.93 ± 0.24), and visually diseased mucosa from IBD patients (2.30 ± 0.21; Figure 2B). VDR immunostaining was mainly found within glandular epithelial cells and lamina propria cells and was observed in both the nuclear and the cytoplasmic compartments.
Figure 2.
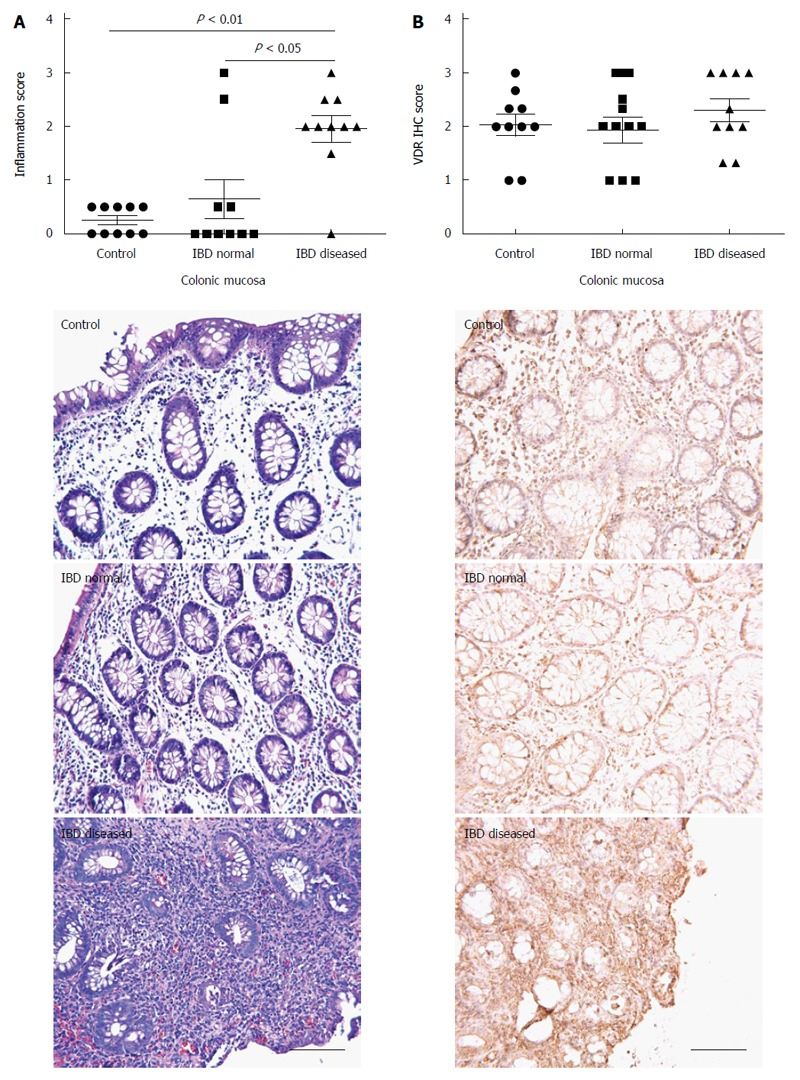
Colonic inflammatory and vitamin D receptor status in control and inflammatory bowel disease patients. Inflammation score (A) and VDR expression (B) for colonic mucosa from control patients, normal appearing mucosa from IBD patients, and visually diseased mucosa from IBD patients (n = 10 per group). Each IBD patient contributed a biopsy from normal appearing mucosa and another biopsy from visually diseased mucosa. VDR: Vitamin D receptor; IBD: Inflammatory bowel disease.
VDR expression negatively correlates with inflammatory status in the colon of IBD patients
VDR immunohistochemistry and inflammation scores did not correlate significantly in colonic tissue from controls (r = -0.36, P > 0.05; Figure 3A), but correlated inversely in visually diseased tissue from IBD patients (r = -0.44, P < 0.05, Figure 3B). This inverse correlation was stronger in IBD patients who had Crohn’s disease (r = -0.69, P < 0.05; Figure 3C). Statistically significant correlations between VDR immunohistochemistry and inflammation scores were not observed for normal-appearing colonic tissue from IBD patients.
Figure 3.
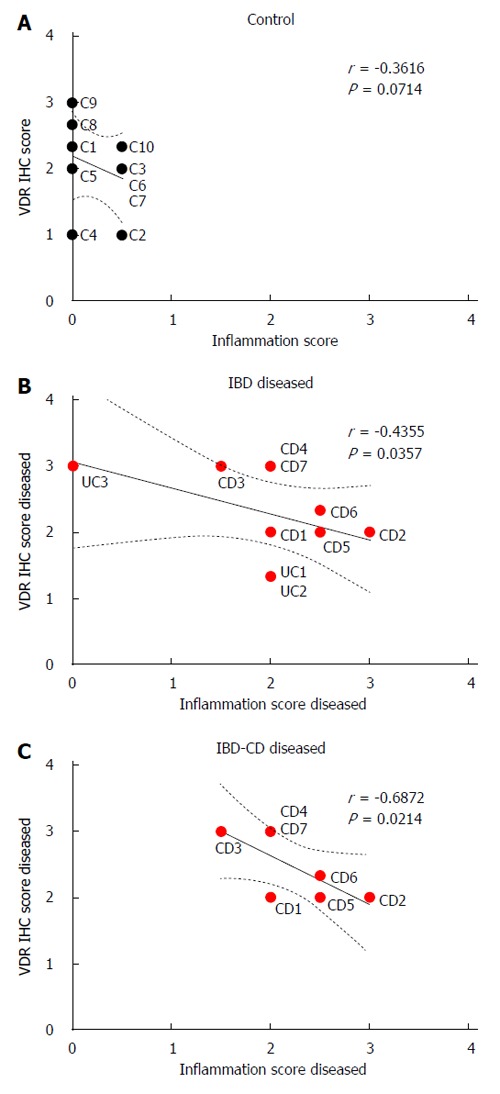
Correlation between inflammation and vitamin D receptor. Immunohistochemistry (IHC) scores for vitamin D receptor and inflammation scores in colonic mucosa from control patients (A), inflammatory bowel disease (IBD) patients (B), and patients with Crohn’s disease (CD) (C). In B and C, inflammation and VDR scores from visually diseased mucosa were used for IBD and CD patients (red circles).
VDR expression in normal appearing mucosa from control and IBD patients positively correlates with serum vitamin D levels
Colonic VDR immunohistochemistry scores did not correlate with serum vitamin D levels in control and IBD patients when VDR immunohistochemistry scores from visually diseased mucosa from IBD patients were used (r = 0.13, P > 0.05; Figure 4A). When VDR immunohistochemistry scores from normal-appearing mucosa from IBD patients were used, colonic VDR immunohistochemistry scores positively correlated with serum vitamin D levels in controls and IBD patients (r = 0.38, P < 0.05; Figure 4B).
Figure 4.
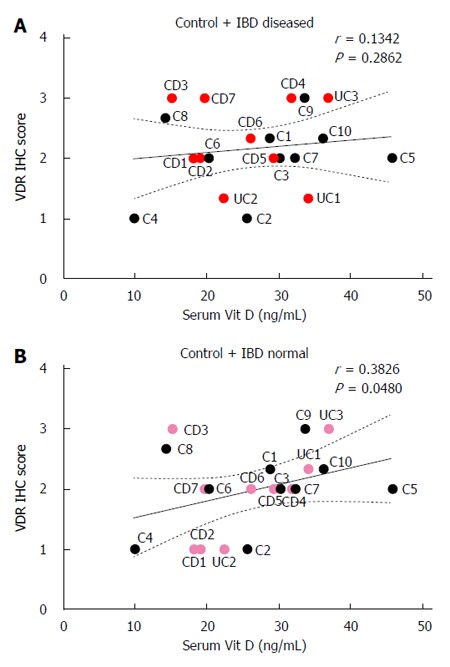
Correlation between serum 25-hydroxyvitamin D3 levels and vitamin D receptor immunohistochemistry scores in control and inflammatory bowel disease patients. A: VDR scores from visually diseased mucosa were used for IBD patients (red circles); B: VDR scores from normal appearing mucosa were used for IBD patients (pink circles). VDR: Vitamin D receptor; CD: Crohn’s disease; UC: Ulcerative colitis; IBD: Inflammatory bowel disease; IHC: Immunohistochemistry.
Colonic inflammatory status negatively correlates with patient age
Colonic inflammation scores were inversely correlated with patient age in both control and IBD patients when inflammation scores from visually diseased mucosa were used for patients with IBD (r = -0.57, P < 0.01; Figure 5).
Figure 5.
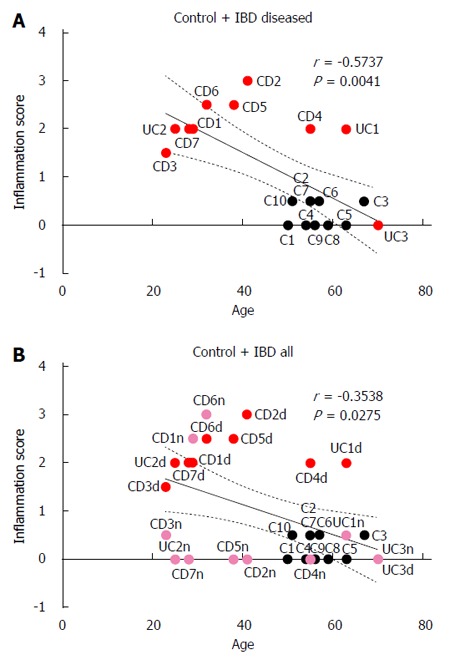
Correlation between age and inflammation scores for control and inflammatory bowel disease patients. A: inflammation scores from visually diseased mucosa were used for IBD patients (red circles); B: inflammation scores from both normal appearing mucosa (pink circles) and visually diseased mucosa (red circles) were used for IBD patients. CD: Crohn’s disease; UC: Ulcerative colitis; IBD: Inflammatory bowel disease.
Serum vitamin D levels positively correlate with patient age
Serum vitamin D levels correlated positively with patient age in IBD patients alone (r = 0.81, P < 0.01; Figure 6A) and in control and IBD patients (r = 0.53, P < 0.01; Figure 6B).
Figure 6.
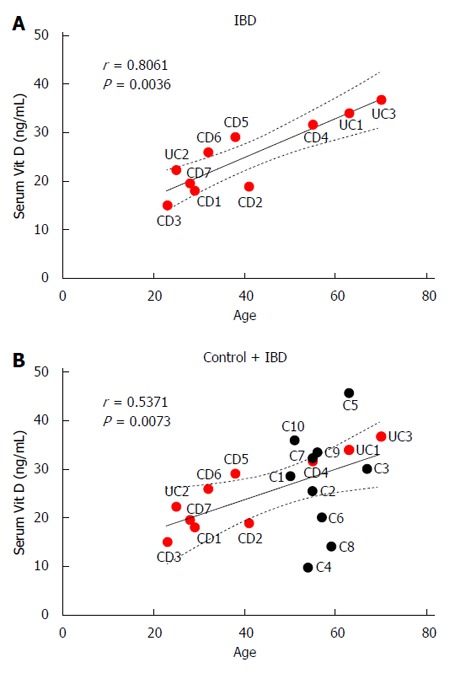
Correlation between age and serum 25-hydroxyvitamin D3 levels for inflammatory bowel disease patients (A) and all patients (B).
DISCUSSION
In the present study, we describe vitamin D status in IBD and non-IBD patients (controls) from Puerto Rico and examine how this relates to colonic inflammation and VDR expression. The prevalence of vitamin D deficiency determined for our Puerto Rican IBD cohort was 40% compared with a prevalence of 20% for the control group. Interestingly, although IBD patients have lower vitamin D levels, we found that both Hispanic cohorts have vitamin D insufficiency, despite living on a sunny tropical island. Vitamin D deficiency has been shown to be fairly common in the United States (41.6%), especially among blacks (82.1%) and Hispanics (69.2%)[32]. In a genome-wide association study examining 25-hydroxyvitamin D concentrations in 33996 individuals of European descent, Wang et al. found that variants near genes involved in cholesterol synthesis, hydroxylation, and vitamin D transport affect vitamin D status[33]. They concluded that these genetic variations identify individuals who have a substantially raised risk of vitamin D insufficiency. It is therefore possible that environmental factors as well as genetic polymorphisms might be affecting vitamin D metabolism in our study population.
Although the expression of VDR has been reported to be decreased in patients with ulcerative colitis, dysplasia, and colitis-associated CRC[34], we have previously shown that in Puerto Rican patients there is a significant decrease in colonic VDR expression in sporadic dysplasia and CRC but not in colitis-associated cancer or in IBD, when compared to normal tissue[19]. In the present study, no significant differences were found in VDR expression between cohorts, which is consistent with our previous results, or between sources of colonic samples in IBD patients, despite finding significantly higher inflammation scores in visually diseased mucosa when compared to normal-appearing mucosa from IBD patients. Although 1,25(OH)2D3 is thought to regulate VDR expression in target tissues[35], the mechanism for regulation of colonic VDR expression in relation to vitamin D is currently unknown (i.e., positive or negative feedback).
In our study, decreased colonic VDR expression was found to be associated with higher histologic inflammation scores, which reflect disease activity, in diseased mucosa. Our study also found a positive correlation between VDR scores from normal appearing mucosa (controls and IBD patients) and serum vitamin D levels but not with VDR scores from mucosa that appeared diseased, suggesting the following points. First, colonic VDR expression is regulated via a positive-feedback mechanism. Second, the disease process disrupts the vitamin D-VDR system in diseased areas of the colon. These points concur with those reported by Ham et al[36], who found lower serum vitamin D levels in patients with active IBD than in patients in remission. Low serum vitamin D levels may truly reflect insufficiency/deficiency or could result from 25-hydroxyvitamin D being converted to the active form of vitamin D3. Moreover, differences in IBD presentation between Hispanics and non-Hispanic whites have been reported[37]; however, there is a paucity of data on the disease course in these populations. Similarly, the metabolism of vitamin D may differ among ethnic groups, where other environmental factors, such as geographical location, and comorbidities, such as obesity, could influence the prevalence of vitamin D deficiency[38]. In addition to the intricacies of vitamin D pathways, the amount of active vitamin D necessary at a molecular level to exert its diverse properties in the intestines, especially in regard to patients with IBD, is unknown.
Potential limitations of this study include the small sample size and the fact that patients in the control group were older than IBD patients, which prevented age matching. This age difference between the groups is likely responsible for the correlation between age and colonic inflammation that we observed in this study. It is unknown how the age of the patient, comorbidities, and current medication use alter the mechanisms of vitamin D in the body. Interestingly, our findings suggest that age directly correlates with higher serum levels of vitamin D in IBD patients, but other studies suggest a decline in vitamin D levels with aging due to decreased absorption. Our control group was found to have vitamin D insufficiency and not sufficiency as expected; therefore, it was not a suitable “control cohort” for the purposes of this study.
In conclusion, the natural history of IBD in Puerto Ricans and Hispanics in general warrants further investigation. We previously found that the incidence and prevalence of this disease were increased in our population, with a particular aggressive disease behavior among younger patients[3,4]. Our current investigation showed a positive correlation between serum vitamin D levels and VDR expression in normal appearing colonic mucosa and a negative correlation between VDR expression and inflammation in diseased mucosa, particularly among patients with Crohn’s disease from the IBD cohort. Future studies should examine whether these relationships also exist in other populations, Hispanic or otherwise. The identification of potentially correctible risk factors, such as vitamin D deficiency, may result in disease modification strategies that reduce the severity of these chronic conditions, decrease the risk of developing CRC, and improve quality of life.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Gil Diaz for aiding with the recruitment process and Rasa Hamilton (Moffitt Cancer Center) for editorial assistance. Preliminary data from this study were presented at the Digestive Diseases of the Caribbean (San Juan, Puerto Rico, April 2015) and at the American College of Gastroenterology Annual Scientific Meeting (Honolulu, Hawaii, October 2015).
COMMENTS
Background
Increasing evidence suggests that vitamin D is an environmental factor linked to the pathogenesis and severity of inflammatory bowel disease (IBD). Of concern, patients with IBD are more likely to develop colorectal cancer than the general population, and low levels of vitamin D are associated with an elevated risk of this malignancy.
Research frontiers
The relationship between serum vitamin D levels and colonic vitamin D receptor (VDR) expression remains unclear. Furthermore, the effect of inflammation or disease activity on this relationship is incompletely understood.
Innovations and breakthroughs
To the authors knowledge, the relationship between serum vitamin D levels, colonic VDR expression, and histologic disease activity has not previously been studied in either a Puerto Rican or any other IBD population. The authors show, for the first time, that in normal mucosa serum vitamin D levels correlate with colonic VDR expression and that in diseased IBD mucosa VDR expression negatively correlates with inflammation. Curiously, they also show that serum vitamin D levels correlate with age in IBD patients from Puerto Rico.
Applications
This study suggests that vitamin D insufficiency and deficiency could be a potentially correctible risk factor in the Puerto Rican population. Targeting the vitamin D-VDR axis together with disease modification strategies could reduce the severity of these chronic conditions, decrease the risk of developing CRC, and improve quality of life. Additionally, colonic VDR expression in conjunction with histological analysis could serve the dual role of indicating relative vitamin D status in normal mucosa and disease activity in mucosa affected by IBD.
Terminology
IBD is a chronic and debilitating condition of the intestines that manifests as Crohn’s disease or ulcerative colitis. Crohn’s disease can affect any part of the gastrointestinal tract, including the colon or large intestine, in a discontinuous manner, whereas ulcerative colitis usually affects the colon in a continuous manner, starting at the rectum and spreading proximally. Cholecalciferol, or vitamin D, is a lipid-soluble vitamin that is involved in regulating calcium and phosphate balance and in many other processes. The major inactive, circulating metabolite of vitamin D is 25-hydroxyvitamin D, and the active form of vitamin D is known as calcitriol, or 1,25-dihydroxyvitamin D. The effects of calcitriol are mediated by the vitamin D receptor (VDR), a nuclear hormone receptor.
Peer-review
This work focused on a small group of subjects with and without IBD, and elucidated relationships between gut inflammation, vitamin D and VDR staining, and the study raises some interesting findings that are worthy of publication/circulation.
Footnotes
Supported by National Institutes of Health Grants, No. R25GM082406 (to Isidro RA); and U54CA163071 to Appleyard CB; the Office of Research from the Ponce Research Institute at Ponce Health Sciences University; William Townsend Porter Predoctoral Fellowship from the American Physiological Society (to Isidro RA); the PHSU Molecular and Genomics Core Laboratory, RCMI Grant No. RR003050/MD007579; the Puerto Rico Clinical and Translational Research Consortium, National Institutes of Health Grant U54MD007587 (to Abreu Y, Medero P and Torres EA).
Institutional review board statement: The study was reviewed and approved by the University of Puerto Rico Medical Sciences Campus Institutional Review Board (protocol number 1250313).
Informed consent statement: All subjects gave written informed consent prior to study inclusion.
Conflict-of-interest statement: The authors have no conflicts of interest to disclose.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 26, 2015
First decision: November 13, 2015
Article in press: January 18, 2016
P- Reviewer: Day AS, de Silva AP, Garg M, Kuo SM, Laverny G S- Editor: Gong ZM L- Editor: A E- Editor: Ma S
References
- 1.Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006;12:6102–6108. doi: 10.3748/wjg.v12.i38.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appleyard CB, Hernández G, Rios-Bedoya CF. Basic epidemiology of inflammatory bowel disease in Puerto Rico. Inflamm Bowel Dis. 2004;10:106–111. doi: 10.1097/00054725-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Torres EA, De Jesús R, Pérez CM, Iñesta M, Torres D, Morell C, Just E. Prevalence of inflammatory bowel disease in an insured population in Puerto Rico during 1996. P R Health Sci J. 2003;22:253–258. [PubMed] [Google Scholar]
- 4.Vendrell R, Venegas HL, Pérez CM, Morell C, Roman RV, Torres EA. Differences in prevalence of inflammatory bowel disease in Puerto Rico between commercial and government-sponsored managed health care insured individuals. Bol Asoc Med P R. 2013;105:15–19. [PubMed] [Google Scholar]
- 5.Pappa HM, Grand RJ, Gordon CM. Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm Bowel Dis. 2006;12:1162–1174. doi: 10.1097/01.mib.0000236929.74040.b0. [DOI] [PubMed] [Google Scholar]
- 6.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229:1136–1142. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 7.Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc Soc Exp Biol Med. 2000;223:230–233. doi: 10.1046/j.1525-1373.2000.22333.x. [DOI] [PubMed] [Google Scholar]
- 8.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross HS, Nittke T, Kallay E. Colonic vitamin D metabolism: implications for the pathogenesis of inflammatory bowel disease and colorectal cancer. Mol Cell Endocrinol. 2011;347:70–79. doi: 10.1016/j.mce.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Raman M, Milestone AN, Walters JR, Hart AL, Ghosh S. Vitamin D and gastrointestinal diseases: inflammatory bowel disease and colorectal cancer. Therap Adv Gastroenterol. 2011;4:49–62. doi: 10.1177/1756283X10377820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pike JW, Lee SM, Meyer MB. Regulation of gene expression by 1,25-dihydroxyvitamin D3 in bone cells: exploiting new approaches and defining new mechanisms. Bonekey Rep. 2014;3:482. doi: 10.1038/bonekey.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kósa JP, Horváth P, Wölfling J, Kovács D, Balla B, Mátyus P, Horváth E, Speer G, Takács I, Nagy Z, et al. CYP24A1 inhibition facilitates the anti-tumor effect of vitamin D3 on colorectal cancer cells. World J Gastroenterol. 2013;19:2621–2628. doi: 10.3748/wjg.v19.i17.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Höbaus J, Tennakoon S, Heffeter P, Groeschel C, Aggarwal A, Hummel DM, Thiem U, Marculescu R, Berger W, Kállay E. Impact of CYP24A1 overexpression on growth of colorectal tumour xenografts in mice fed with vitamin D and soy. Int J Cancer. 2016;138:440–450. doi: 10.1002/ijc.29717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia V, Falzon M. Restoration of the anti-proliferative and anti-migratory effects of 1,25-dihydroxyvitamin D by silibinin in vitamin D-resistant colon cancer cells. Cancer Lett. 2015;362:199–207. doi: 10.1016/j.canlet.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larriba MJ, Bonilla F, Muñoz A. The transcription factors Snail1 and Snail2 repress vitamin D receptor during colon cancer progression. J Steroid Biochem Mol Biol. 2010;121:106–109. doi: 10.1016/j.jsbmb.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Pálmer HG, Larriba MJ, García JM, Ordóñez-Morán P, Peña C, Peiró S, Puig I, Rodríguez R, de la Fuente R, Bernad A, et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med. 2004;10:917–919. doi: 10.1038/nm1095. [DOI] [PubMed] [Google Scholar]
- 18.Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746–74, 774.e1-4; quiz e12-3. doi: 10.1053/j.gastro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 19.Isidro RA, Cruz ML, Isidro AA, Baez A, Arroyo A, González-Marqués WA, González-Keelan C, Torres EA, Appleyard CB. Immunohistochemical expression of SP-NK-1R-EGFR pathway and VDR in colonic inflammation and neoplasia. World J Gastroenterol. 2015;21:1749–1758. doi: 10.3748/wjg.v21.i6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 21.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 23.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stio M, Treves C, Martinesi M, Bonanomi AG. Biochemical effects of KH 1060 and anti-TNF monoclonal antibody on human peripheral blood mononuclear cells. Int Immunopharmacol. 2005;5:649–659. doi: 10.1016/j.intimp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–G216. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 26.Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, Dionne S, Servant MJ, Bitton A, Seidman EG, et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285:2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wehkamp J, Koslowski M, Wang G, Stange EF. Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn‘s disease. Mucosal Immunol. 2008;1 Suppl 1:S67–S74. doi: 10.1038/mi.2008.48. [DOI] [PubMed] [Google Scholar]
- 28.Joseph AJ, George B, Pulimood AB, Seshadri MS, Chacko A. 25 (OH) vitamin D level in Crohn’s disease: association with sun exposure & amp; disease activity. Indian J Med Res. 2009;130:133–137. [PubMed] [Google Scholar]
- 29.Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, Issa M. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35:308–316. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
- 30.Nicholson I, Dalzell AM, El-Matary W. Vitamin D as a therapy for colitis: a systematic review. J Crohns Colitis. 2012;6:405–411. doi: 10.1016/j.crohns.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404–409. doi: 10.1136/gut.47.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wada K, Tanaka H, Maeda K, Inoue T, Noda E, Amano R, Kubo N, Muguruma K, Yamada N, Yashiro M, et al. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol Rep. 2009;22:1021–1025. doi: 10.3892/or_00000530. [DOI] [PubMed] [Google Scholar]
- 35.Dusso A, Slatopolsky E. Vitamin D and Renal Disease. In: Feldman D, Pike J, Adams J, editors. Vitamin D. 3th ed. USA: Academic Press; 2011. pp. 1325–1358. [Google Scholar]
- 36.Ham M, Longhi MS, Lahiff C, Cheifetz A, Robson S, Moss AC. Vitamin D levels in adults with Crohn’s disease are responsive to disease activity and treatment. Inflamm Bowel Dis. 2014;20:856–860. doi: 10.1097/MIB.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Damas OM, Jahann DA, Reznik R, McCauley JL, Tamariz L, Deshpande AR, Abreu MT, Sussman DA. Phenotypic manifestations of inflammatory bowel disease differ between Hispanics and non-Hispanic whites: results of a large cohort study. Am J Gastroenterol. 2013;108:231–239. doi: 10.1038/ajg.2012.393. [DOI] [PubMed] [Google Scholar]
- 38.Young KA, Engelman CD, Langefeld CD, Hairston KG, Haffner SM, Bryer-Ash M, Norris JM. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab. 2009;94:3306–3313. doi: 10.1210/jc.2009-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]


