Abstract
Perivascular epithelioid cell tumor (PEComa) of the pancreas is an unusual tumor deriving from mesenchyma. This paper described a case of pancreatic PEComa, which was initially suspected as neuroendocrine carcinoma by biopsy, and therefore surgical treatment was recommended due to undetermined diagnosis. Examination of the surgical specimen under a microscope showed that the tumor cell’s morphology was epithelioid or spindle-shaped, and ranged in a nested pattern. Additionally, these cells had a large extent of acidophilic cytoplasm, no mitotic figures, and expressed HMB-45, melan-p, and smooth muscle actin immunohistochemically. Pathological examination indicated that PEComa originated from the pancreas, but symptoms related to tuberous sclerosis were absent. Since PEComa is extremely rare in the pancreas, it is likely to be ignored in differential diagnosis. In conclusion, our article highlighted the clinicopathological features of PEComa, and we conducted a literature review focusing on PEComa so as to deepen the understanding of this tumor type.
Keywords: Pancreas, Perivascular epithelioid cell tumor, HMB-45, Immunohistochemistry, Clinicopathological feature
Core tip: Perivascular epithelioid cell tumor (PEComa) of the pancreas is an unusual tumor deriving from mesenchyma. Via describing a rare case of pancreatic PEComa, we highlight the clinicopathological features of PEComa and conduct a literature review focusing on this tumor type so as to deepen the understanding of the subject. We also reviewed the biological behavior, prognosis, and therapeutic strategy of PEComa.
INTRODUCTION
Perivascular epithelioid cell tumor (PEComa) is an extremely rare tumor derived from mesenchymal tissue, with characteristics of perivascular epithelioid cells (PEC) in histology and immunohistochemistry[1]. Microscopically, the morphology of PEC is of epithelial origin, containing a bright cytoplasm or fine grained eosinophilic shapes with positive PAS staining. Moreover, the nuclei of PEC are relatively small, and are round or oval shaped with small nucleolus; intranuclear inclusion bodies can be observed occasionally. PEC, distributing in the perivascular region in a radial pattern, is amylase intolerant, shows epithelioid features in nearby vessels, and becomes spindle-shaped when distant from vessels. The proportion of epithelioid cells and spindle cells is different depending on the patient. In immunohistochemistry staining, PEC usually expresses HMB45 and melan-A. PEC is featured with melanosome in ultrastructure, and is rich in glycogen and cytoplasmic filaments[2].
PEComas can be classified as angiomyolipomas, clear cell “sugar” tumors of the lung, lymphangioleiomyomatoses (LAM), and other PEComas characterized by similar histological and immunohistochemical presentations[3]. Although PEComas are benign in most cases, Bonetti et al[4] reported four abdominopelvic sarcomas of PEC in young women, raising concern about malignant PEComas that result in regional tissue infiltration, multiple metastases, and even patient death[5-7]. As for PEComas of the pancreas, only two malignant cases with liver metastasis have been reported in the literature[8,9].
Herein, we report a 50-year-old female with benign PEComas of the pancreas that could not be definitely diagnosed preoperatively. A literature review on pancreatic PEComa, with special consideration of pathological diagnosis, was also performed.
CASE REPORT
Clinical characteristics
A 50-year-old female patient was admitted to our hospital in November 2013 because of abdominal ultrasound (US) findings of a space-occupying lesion in the head of pancreas, which could not be clearly diagnosed. The patient was a lifelong nonsmoker who consumed no alcohol and had no history of family-inherited diseases. The patient denied any history of surgery or trauma. CT examination demonstrated no significant abnormality in the morphology or density of the pancreas, nor was there any pancreatic duct dilatation (Figure 1A). Moreover, the peripancreatic fat space was clearly demarcated and no retroperitoneal lymph nodes were enlarged. On the arterial phase, there was a relatively low density of nodules of approximately 1 cm × 1.4 cm in the uncus of the pancreas. On the portal venous and delayed phases, the nodules enhanced gradually and slightly, with a significantly lower density than the surrounding pancreatic tissue level, whereas the pathologically changed border was well-defined on the delayed phase. Magnetic resonance (MR) imaging (Figure 1B) showed that a round abnormal signal 1.7 cm × 1.4 cm in size was found in the head and uncus of the pancreas; T1WI showed a low signal clearly distinctive of normal pancreatic tissue surrounding a relatively high signal. In T2WI, the mass was difficult to distinguish from the surrounding pancreatic tissue due to the equal signal. Endoscopic ultrasound (EUS) showed a hypoechoic region 1.6 cm × 1.4 cm in size in the uncus of the pancreas. This region had clear boundaries and echo was not equal inside the low echo region. Pancreatic tail shape remained regular and the pancreatic duct was not dilated. There was no dilatation of the intrahepatic bile duct or enlargement of lymph nodes.
Figure 1.
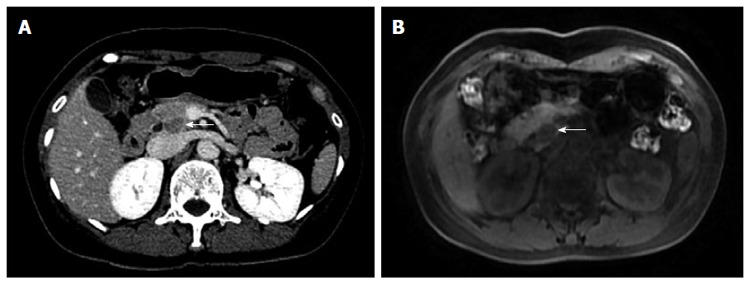
Abdomen computed tomography-scan. A: CT delayed phase: a relatively low density of nodules (arrow) of approximately 1 cm × 1.4 cm in the uncus of the pancreas; B: Round abnormal signal (arrow) of 1.7 cm × 1.4 cm was found in the head and uncus of the pancreas, T1WI showed low signal, clearly contrasting with normal pancreatic tissue surrounding a relatively high signal.
Methods and materials
The patient underwent endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) (ECHO-25G, Cook Company). The subsequent biopsy specimen was fixed in formalin, dehydrated by gradient ethanol, dewaxed by xylol, imbedded in paraffin, cut into sections for microscopic examination, and then stained with hematoxylin-eosin.
All biopsy and surgical specimens underwent immunohistochemical staining by the EliVisionTM Plus method. Immunochemical staining was performed for all specimens by using the following markers: CD56, Cam5.2 (cytokeratin), neuron specific enolase (NSE), chromogranin A (CgA), alpha-1-antitrypsin (α-AT), CD34, CD56, S-100, TFE3, estrogen receptor (ER), progesterone receptor (PR), calponin, synaptophysin (Syn), Pax-8, β-catenin, CD117, melan-A, HMB-45, smooth muscle actin (SMA), epithelial membrane antigen (EMA), vimentin, p53, and Ki-67. All specimens were heated at 37 °C for 1 h and kept at 4 °C overnight. Staining intensity was recorded as positive (> 10% of plasma and nucleus stained) or negative. Ki-67 was evaluated by percentage. All information on antibodies used is summarized in Table 1.
Table 1.
Characteristics of antibodies used in immunohistochemistry and staining conditions
| Antibody | Clone | Source | Dilution |
| CD56 | 56C04 | Maxim | 1:100 |
| CAM5.2 | CAM5.2 | Maxim | 1:50 |
| NSE | E27 | Maxim | 1:100 |
| CgA | SP12 | Maxim | 1:100 |
| α-AT | Polyclonal | DAKO | 1:100 |
| CD34 | QBEnd/10 | Maxim | 1:100 |
| P53 | D0-7 | Maxim | 1:200 |
| S-100 | SP11 | Thermo | 1:100 |
| TFE3 | MRQ-37 | Maxim | 1:50 |
| ER | 1D5 | Maxim | 1:100 |
| PR | 1A6 | Maxim | 1:100 |
| Calponin | CALP | Maxim | 1:100 |
| Syn | Polyclonal | Maxim | 1:100 |
| Pax-8 | ZR-1 | Gene Tech | 1:100 |
| β-catenin | CAT-5H10 | Maxim | 1:100 |
| CD117 | YR145, 2E4 | Maxim | 1:100 |
| Melan-A | A103 | Maxim | 1:100 |
| HMB-45 | HMB45 | Maxim | 1:50 |
| SMA | 1A4 | Gene Tech | 1:100 |
| EMA | E29 | Maxim | 1:100 |
| Vimentin | V9 | Maxim | 1:200 |
| Ki-67 | MX006 | Maxim | 1:200 |
Results
Biopsy specimen: Spindle-shaped nucleus can be found in some cell lumps with sporadically distributed cells in the background, suggesting that they were malignant cells derived from unidentified sources. In DNA ploidy analysis, a few DNA ploidy-abnormal cells were found.
Cytological results: Cytology smear showed that cells were distributed in irregular lumps, with nuclear sizes varying slightly and arranged mussily. Enriched plasma and vaguely defined boundary were identified in the cells (Figure 2).
Figure 2.
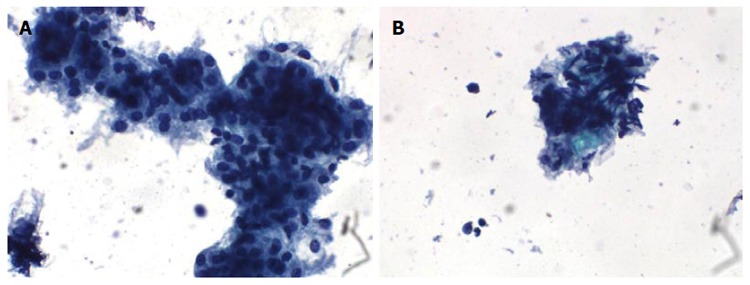
Cytology results. A: Cells were irregular lumps distributed with medium nuclei sizes, arranged in a disorderly manner, and with crowded overlap; B: Tumor cell with abundant cytoplasm and unclear boundary. Messily arranged spindle cell nuclei can be seen in some cell clumps. Scattered single cells were found in the background.
Tumor cells were epithelioid or spindle-shaped with large size and nested distribution (Figure 3). Tumor cells contained a bright cytoplasm or fine grained eosinophilic shapes with suspicion of clear cell carcinoma. Therefore, gastrointestinal stromal tumor, neuro-endocrine tumor, acinic cell carcinoma, solid pseudo-papillary tumor, and metastatic clear-cell carcinoma should be excluded. The immunohistochemistry results were as follows: CD56 (+), CAM5.2 (-), P53 (-), NSE (-), vimentin (-), Ki67 (2%), CgA (-), α1-ACT (-), Syn (-), Pax-8 (-), β-catenin (positive in plasma), CD117 (-). Since PEComa of the pancreas had not been diagnosed in our hospital, this tumor was not considered to be PEComa preoperatively. Based on cytological and immunohistochemical results, neuro-endocrine tumor was suspected, but acinic cell carcinoma and solid pseudo-papillary tumor were not excluded, leading to operation being suggested.
Figure 3.
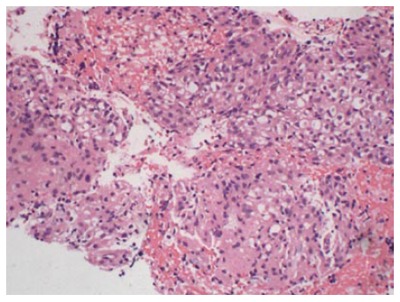
Biopsy specimen. Hematoxylin-eosin staining shows the epithelial tumor cells with bright or slightly eosinophilic granules with nested distribution.
Surgical specimen: A mass 2 cm × 2 cm in size in the head of the pancreas was discovered during operation and was of medium texture with a complete capsule. The border was clear between the mass and adjacent tissues, and metastatic lesions were not found.
Gross examination (Figure 4): the pancreas, duodenum, and gall bladder were resected; the size of the pancreas head was 8 cm × 4 cm × 3 cm, with a mass 2 cm × 1 cm × 1 cm in size that was 4 cm away from the surgical margin. The transection of the mass was grayish with a solid soft texture.
Figure 4.
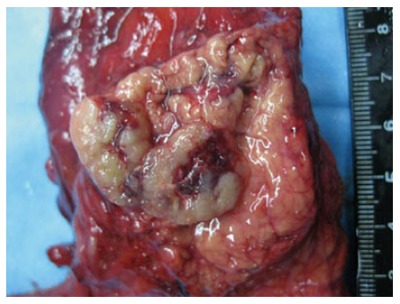
Gross examination. Tumor 2 cm × 2 cm in size in pancreatic head with clear boundaries. Cross-section of gray and solid soft texture. Metastatic lesions were not found.
Microscopic examination: the boundary between tumor and adjacent tissue was clear. Tumor cells were arranged in nests, with some tumor cells growing around arteries. Mounts of vessel were in mesenchyma with hyaline degeneration (Figure 5B). The morphology of tumor cells was epithelioid or spindle-shaped (Figure 5C and D) and ranging in a nested pattern. These cells had plenty of acidophil cytoplasm and no mitotic figures. There was no fat tissue found in the tumor. Lymphatic invasion was not observed in this patient.
Figure 5.
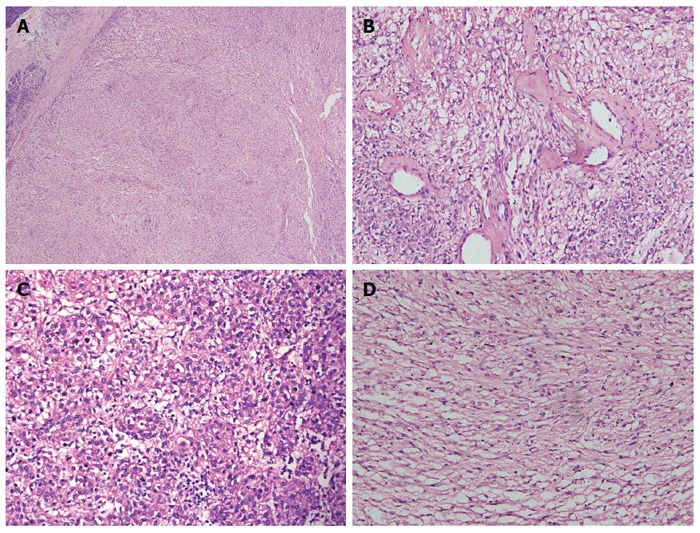
Microscopic examination. A: Microscopy showing clear boundaries between the tumor and adjacent pancreatic tissue; B: Mounts of vessel were in mesenchyma, with hyaline degeneration present; C: Epithelioid tumor cell; D: Spindle-shaped tumor cell with bright or slightly eosinophilic granule. A, B, C and D: Hematoxylin-eosin staining.
Immunohistochemistry (Figure 6): melan-A (+), HMB45 (+), SMA (+) CD56 (+), Ki67 (1%), CAM5.2 (-), P53 (-), NSE (-), vimentin (-), CgA (-), α-AT (-), Syn (-), Pax-8 (-), β-catenin (positive in plasma), CD117 (-), CD34 (positive in vessel), S-100 (-), TFE3 (-), ER (-), PR (-), calponin (-), and MSA (-).
Figure 6.
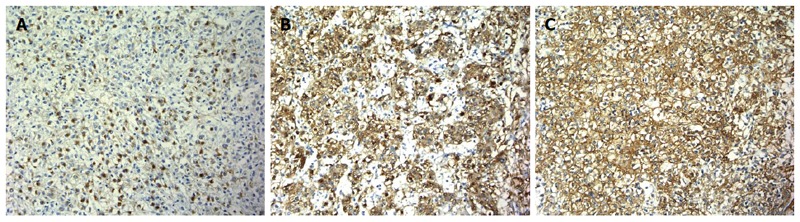
Immunohistochemistry. A: Tumor cells expressed HMB-45; B: Tumor cells are immunophenotypically positive for the melanocytic marker melan-A; C: Tumor cells are immunophenotypically positive for the smooth muscle marker SMA. Original magnification × 200. A, B and C: Immunostaining.
Diagnosis: PEComa in the head of the pancreas.
Follow-up
No recurrence or distant metastases were observed during follow-up of 14 mo. However, Nagata et al[8] reported that benign PEComa could recur after surgery, meaning that it is necessary to follow-up and re-examine patients.
DISCUSSION
PEComa of the pancreas is extremely rare, with only twelve previous cases[2-5,8-17] being reported during the last decade. Combining these previous cases with ours, we found that the patients had a mean age of 52 years (range: 31 to 62 years) and were mostly female (11 females and 2 males). The morbidity of PEComa in females was significantly higher than that in males, suggesting that one risk factor of PEComa is related to sex hormones. Additionally, some studies revealed that progesterone receptors (PR) were expressed in PEComas immunohistochemically, especially in LAM and renal AKL[18]. However, in these thirteen cases, PR was negatively expressed in five PEComas cases and only one PEComa partially expressed ER[15]. Tuberous sclerosis (TSC) containing TSC1 or TSC2 gene deletion can be seen in some PEComas, especially in renal AKL. Located on chromatosome 9q and 16p respectively, TSC1 and TSC2 play a pivotal role in the Rheb-mTOR-P7OS6K pathway[1]. Nevertheless, all the PEComas of the pancreas presented without TSC.
Abdominal pain is the main initial symptom in pancreatic PEComa, although a few asymptomatic cases have been found during health examinations or follow-up examinations for other disease. In PEComa sites, the head of pancreas accounted for six cases, the body for five cases, and the uncus for two. Tumor size varied from 15 mm to 100 mm, with an average of 23 mm. The tumor ruptured in one case.
In pathological morphology, akin to previous cases, the PEComa reported in our case was a solid homogeneous nodule with a mainly clear boundary and that only partially infiltrated to adjacent tissue. Tumor cells that manifested during histological results were mainly made up of clear cells or eosinophilic cells with nested, fascial, or laminar distributions. Tumor cells were epithelioid or spindle-shaped with plenty of glycogen. Nucleolus atypia and small nucleolus can be found in the tumor cells. Mounts of vessel were in mesenchyma, with some tumor cells growing around arteries. Adipose tissue, which would make the diagnosis easier, is rarely presented in PEComa. In the 12 previously reported cases of pancreatic PEComa, adipose tissue was found in 2 cases[8,11]. In one of the two cases, liver metastasis occurred 27 mo after surgery[8]. Therefore, it seems that the biological behavior of the tumor is not related to the existence of fat tissue. Immunostaining shows that PEComa generally has both smooth muscle cells and evidence of melanoma. Folpe’s[19] study indicated that HMB-45 is the most sensitive marker for melanoma cells (96%), followed by melan-A (72%) and microphthalmia-associated transcription factor (MiTF) (50%). In their study, all cases expressed at least one marker of melanoma cells and 80% of cases expressed smooth muscle actin, especially in epithelioid cells. In the reported cases of PEComa of the pancreas, only one of the 13 cases did not express α-SMA[9], which was also the only case of malignant PEComa in the pancreas with epithelioid tumor cells. Therefore, we proposed that the lack of smooth muscle expression may serve as a malignant indicator of PEComa. All three indicators previously reported in pancreatic PEComa expression are as follows: HMB-45 (13/13), melan-A (8/9), and α-SMA (12/13). Meanwhile, MiTF and CD63 were expressed in tumor cells. CD117 was positive in a few cases. PEComa also expressed TFE3 and cyclin D1, expressed desmin to a lesser extent, and did not generally express S-100 and CK.
To date, the biological behavior and histologic origin of pancreatic PEComas are unknown. Although the pervasive concept is that PEComas are usually benign, increasing reports indicate that PEComas may have malignant potential, despite there being no consensus in how to evaluate PEComa. In 2005, Folpe et al[19] suggested that a tumor which meets at least two points of the following criteria should be considered a malignant PEComa based on previous reports about PEComa: (1) tumor diameter >50 mm; (2) tumors with an invasive growth pattern; (3) tumors that possess advanced nucleus and cell richness; (4) mitotic count higher than 1/50 HPF; and (5) necrosis and vascular infiltration. However, it is difficult to confirm the accuracy of Folpe’s criteria in distinguishing malignant PEComa from those that are benign, owing to the rare cases of PEComa[5]. In the 13 cases, only one[9] can be regarded as malignant PEComa according to Folpe’s criteria, in which liver metastasis occurred 6 mo after surgery. However, this patient had a family history of breast cancer and BRCA2 gene mutation. The patient undertook radiotherapy and chemotherapy because of breast cancer 10 years prior. Therefore, whether malignant PEComa resulted from BRCA2 gene mutation or chemotherapy and radiotherapy was unknown. Additionally, invasive PEComa cannot be diagnosed as malignant, whereas multiple liver metastases were found 27 mo after surgery in this patient. More cases need to be analyzed in order to evaluate the recurrent risk of PEComa and develop an effective therapy. If histology results show some malignant features, such as mitosis index, tumor cell pleomorphism, and invasive growth, close patient follow-up is then required.
In diagnosis and differential diagnosis, PEComas, especially the type that mainly present with epithelioid cells or spindle-shaped cells, should be distinguished from pancreatic clear cell neuroendocrine tumor, solid pseudopapillary tumor, metastatic renal clear carcinoma, metastatic gastrointestinal stromal, metastatic melanoma, or soft tissue clear cell sarcoma[13]. (1) Clear cellular neuroendocrine carcinoma: PEComa and clear cellular neuroendocrine carcinoma are similar in morphology and feature plenty of plasma and lipid droplets[20]. They can be distinguished by immunohistochemistry. Chromogranin and synaptophysin are positive in clear cellular neuroendocrine carcinoma, but negative in PEComa; (2) solid pseudopapillary tumor: tumor cells are eosinophilic and neutrophilic, and papillary structure can be seen under a microscope. Sometimes hemorrhagic necroses are found inside the tumor. Immunohistochemically, CK, CD56, synaptophysin, and β-catenin are positive, while HMB-45 is negative[21]; (3) clear cell carcinoma: cytokeratin is widely expressed in clear cell carcinoma, while melanin is expressed in PEComa; (4) gastrointestinal stromal tumor (GIST): epithelioid cells mixed with spindle-shaped cells in both PEComa and gastrointestinal stromal tumor, but a great deal of vasoganglion and clear and eosinophilic tumor cells exist in PEComa, which lacks fibroblast-like cells. PEComas mostly do not express CD117, but in some cases do express CD117 during immunostaining. Molecular detection shows no c-kit gene mutation. CD34 is positive in GIST, while PEComa does not express CD34[21]; (5) metastatic melanoma: owing to positive expression of melanin in PEComa, differential diagnosis is necessary to distinguish PEComa from metastatic melanoma. In most cases, it is possible to differentiate PEComa with myogenic markers from metastatic melanoma with s-100; and (6) alveolar soft part sarcoma: because of its organ-like clear cell structure, it is also important to differentiate PEComa from alveolar soft part sarcoma. Alveolar soft part sarcoma is a malignant tumor which often contains high levels of nuclear atypia and clear nucleoli. Vascular invasion is common in alveolar soft part sarcoma, which is only found in malignant PEComa. Immunohistochemically, melanoma-derived markers and myogenic markers are not expressed in alveolar soft part sarcoma.
Currently, EUS-FNA serves as one of the most important preoperative diagnosis methods, and is widely used in clinical diagnosis. In the aforementioned 13 pancreatic PEComa cases, 9 performed EUS-FNA examination prior to operation. However, 5 cases could not be diagnosed clearly. Therefore, when a cell arrangement and shape resembling that of neuroendocrine tumor is visible under microscope, we must take into account the possibility of PEComa.
In conclusion, as an unusual tumor deriving from mesenchyma, PEComa of the pancreas is always benign. Cases concomitant with TSC or other syndromes have not yet been reported. Complete surgical resection is the main treatment for PEComa, although the necessity of resection and the timing of surgical treatment are relatively limited. Furthermore, for cases with huge inoperable tumors and multiple metastases, effective treatment is lacking, as the effect of traditional radiotherapy and chemotherapy is poor. Wagner et al[22] reported three cases of malignant PEComa that reacted to mTOR inhibitor sirolimus radiological examination, indicating that mTOR inhibitor may serve as a candidate for future targeted chemotherapy drugs, although further study is needed. For benign PEComa, it is recommended that regular patient follow-up be performed, while aggressive therapy is not recommended.
COMMENTS
Case characteristics
A 50-year-old female patient admitted to hospital because of abdominal ultrasound findings of a space-occupying lesion in the head of pancreas, which could not be clearly diagnosed.
Clinical diagnosis
Upon physical examination, the patient had no clinical abnormalities and was diagnosed with a pancreas head mass according to the previous diagnosis.
Differential diagnosis
Pancreatic clear cell neuroendocrine tumor, solid pseudopapillary tumor, metastatic renal clear carcinoma, metastatic gastrointestinal stromal, metastatic melanoma, or soft tissue clear cell sarcoma.
Laboratory diagnosis
Laboratory tests showed no abnormal values.
Imaging diagnosis
Contrast-enhanced computed tomography scan, magnetic resonance imaging, and endoscopic ultrasound all revealed a space-occupying lesion in the uncus of the pancreas.
Pathological diagnosis
By cytological, histological, and immunohistochemical examination, the pathological diagnosis was of perivascular epithelioid cell tumor (PEComa), 2 cm × 2 cm × 1 cm, in the head of the pancreas.
Experiences and lessons
PEComa originating from the pancreas is very rare and we reveal the clinicopathological features of it. Caution is needed in order to differentiate this entity from other pancreatic tumors, especially pancreatic clear cell neuroendocrine tumor, solid pseudopapillary tumor, metastatic renal clear carcinoma, metastatic gastrointestinal stromal, metastatic melanoma, and soft tissue clear cell sarcoma.
Peer-review
This article highlights the clinical characteristics of PEComa of the pancreas and offered an excellent methodology to diagnose the disease. The information in this article is worthwhile to the reader.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Shanghai Changhai Hospital Ethnic Committee.
Informed consent statement: The study was performed after obtaining the patient’s informed consent. The patient was treated according to the provisions of the Helsinki criteria.
Conflict-of-interest statement: We declare that we have no commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 12, 2015
First decision: December 11, 2015
Article in press: January 11, 2016
P- Reviewer: Efthymiou A, Huerta-Franco MR S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Wang CH
References
- 1.Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48:75–82. doi: 10.1111/j.1365-2559.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 2.Hirabayashi K, Nakamura N, Kajiwara H, Hori S, Kawaguchi Y, Yamashita T, Dowaki S, Imaizumi T, Osamura RY. Perivascular epithelioid cell tumor (PEComa) of the pancreas: immunoelectron microscopy and review of the literature. Pathol Int. 2009;59:650–655. doi: 10.1111/j.1440-1827.2009.02421.x. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher CD, Organization WH, Cancer IAfRo. WHO classification of tumours of soft tissue and bone. IARC press; 2013. [Google Scholar]
- 4.Bonetti F, Martignoni G, Colato C, Manfrin E, Gambacorta M, Faleri M, Bacchi C, Sin VC, Wong NL, Coady M, et al. Abdominopelvic sarcoma of perivascular epithelioid cells. Report of four cases in young women, one with tuberous sclerosis. Mod Pathol. 2001;14:563–568. doi: 10.1038/modpathol.3880351. [DOI] [PubMed] [Google Scholar]
- 5.Zemet R, Mazeh H, Neuman T, Freund HR, Eid A. Asymptomatic pancreatic perivascular epithelial cell tumor (PEComa) in a male patient: report and literature review. JOP. 2011;12:55–58. [PubMed] [Google Scholar]
- 6.Ribalta T, Lloreta J, Munné A, Serrano S, Cardesa A. Malignant pigmented clear cell epithelioid tumor of the kidney: clear cell (“sugar”) tumor versus malignant melanoma. Hum Pathol. 2000;31:516–519. doi: 10.1053/hp.2000.6717. [DOI] [PubMed] [Google Scholar]
- 7.Eble JN, Amin MB, Young RH. Epithelioid angiomyolipoma of the kidney: a report of five cases with a prominent and diagnostically confusing epithelioid smooth muscle component. Am J Surg Pathol. 1997;21:1123–1130. doi: 10.1097/00000478-199710000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Nagata S, Yuki M, Tomoeda M, Kubo C, Yoshizawa H, Kitamura M, Uehara H, Katayama K, Nakayama H, Okamoto Y, et al. Perivascular epithelioid cell neoplasm (PEComa) originating from the pancreas and metastasizing to the liver. Pancreas. 2011;40:1155–1157. doi: 10.1097/MPA.0b013e318221fc0e. [DOI] [PubMed] [Google Scholar]
- 9.Mourra N, Lazure T, Colas C, Arrive L, de Gramont A. Perivascular epithelioid cell tumor: the first malignant case report in the pancreas. Appl Immunohistochem Mol Morphol. 2013;21:e1–e4. doi: 10.1097/PAI.0b013e3182392bb6. [DOI] [PubMed] [Google Scholar]
- 10.Zamboni G, Pea M, Martignoni G, Zancanaro C, Faccioli G, Gilioli E, Pederzoli P, Bonetti F. Clear cell “sugar” tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol. 1996;20:722–730. doi: 10.1097/00000478-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Heywood G, Smyrk TC, Donohue JH. Primary angiomyolipoma of the pancreas. Pancreas. 2004;28:443–445. doi: 10.1097/00006676-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Ramuz O, Lelong B, Giovannini M, Delpero JR, Rochaix P, Xerri L, Hassoun J, Flejou JF, Monges G. “Sugar” tumor of the pancreas: a rare entity that is diagnosable on preoperative fine-needle biopsies. Virchows Arch. 2005;446:555–559. doi: 10.1007/s00428-005-1216-4. [DOI] [PubMed] [Google Scholar]
- 13.Périgny M, Larochelle O, Hammel P, Sauvanet A, Dokmak S, Belghiti J, Ruszniewski P, Vilgrain V, Bedossa P, Couvelard A. [Pancreatic perivascular epithelioid cell tumor (PEComa)] Ann Pathol. 2008;28:138–142. doi: 10.1016/j.annpat.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Baez JC, Landry JM, Saltzman JR, Qian X, Zinner MJ, Mortelé KJ. Pancreatic PEComa (sugar tumor): MDCT and EUS features. JOP. 2009;10:679–682. [PubMed] [Google Scholar]
- 15.Finzi G, Micello D, Wizemann G, Sessa F, Capella C. Pancreatic PEComa: a case report with ultrastructural localization of HMB-45 within melanosomes. Ultrastruct Pathol. 2012;36:124–129. doi: 10.3109/01913123.2011.642463. [DOI] [PubMed] [Google Scholar]
- 16.Al-Haddad M, Cramer HM, Muram T, Wang X, Pitt HA. Perivascular epithelioid cell tumor: an unusual pancreatic mass diagnosed by EUS-FNA. Gastrointest Endosc. 2013;78:165; discussion 165–167. doi: 10.1016/j.gie.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Okuwaki K, Kida M, Masutani H, Yamauchi H, Katagiri H, Mikami T, Miyazawa S, Iwai T, Takezawa M, Imaizumi H, et al. A resected perivascular epithelioid cell tumor (PEComa) of the pancreas diagnosed using endoscopic ultrasound-guided fine-needle aspiration. Intern Med. 2013;52:2061–2066. doi: 10.2169/internalmedicine.52.0746. [DOI] [PubMed] [Google Scholar]
- 18.Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch. 2008;452:119–132. doi: 10.1007/s00428-007-0509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558–1575. doi: 10.1097/01.pas.0000173232.22117.37. [DOI] [PubMed] [Google Scholar]
- 20.Singh R, Basturk O, Klimstra DS, Zamboni G, Chetty R, Hussain S, La Rosa S, Yilmaz A, Capelli P, Capella C, et al. Lipid-rich variant of pancreatic endocrine neoplasms. Am J Surg Pathol. 2006;30:194–200. doi: 10.1097/01.pas.0000184819.71752.ad. [DOI] [PubMed] [Google Scholar]
- 21.Abraham SC, Klimstra DS, Wilentz RE, Yeo CJ, Conlon K, Brennan M, Cameron JL, Wu TT, Hruban RH. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160:1361–1369. doi: 10.1016/s0002-9440(10)62563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner AJ, Malinowska-Kolodziej I, Morgan JA, Qin W, Fletcher CD, Vena N, Ligon AH, Antonescu CR, Ramaiya NH, Demetri GD, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28:835–840. doi: 10.1200/JCO.2009.25.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]


