Abstract
New strategies are needed for prevention of biofilm formation. We have previously shown that 24 hr of 2,000 µA of direct current (DC) reduces Staphylococcus epidermidis biofilm formation in vitro. Herein, we examined the effect of a lower amount of DC exposure on S. epidermidis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Propionibacterium acnes, and Candida albicans biofilm formation. 12 hr of 500 µA DC decreased S. epidermidis, S. aureus, E. coli, and P. aeruginosa biofilm formation on Teflon discs by 2, 1, 1, and 2 log10 cfu/cm2, respectively (p < 0.05). Reductions in S. epidermidis, S. aureus, and E. coli biofilm formation were observed with as few as 12 hr of 200 µA DC (2, 2 and 0.4 log10 cfu/cm2, resp.); a 1 log10 cfu/cm2 reduction in P. aeruginosa biofilm formation was observed at 36 hr. 24 hr of 500 µA DC decreased C. albicans biofilm formation on Teflon discs by 2 log10 cfu/cm2. No reduction in P. acnes biofilm formation was observed. 1 and 2 log10 cfu/cm2 reductions in E. coli and S. epidermidis biofilm formation on titanium discs, respectively, were observed with 12 hr of exposure to 500 µA. Electrical current is a potential strategy to reduce biofilm formation on medical biomaterials.
1. Introduction
Biofilms are associated with a variety of persistent infections as a result of their propensity to form and grow on foreign bodies. Compared with planktonic forms, organisms in biofilms exhibit increased resistance to the host immune system and antimicrobial therapy [1]; for this reason, the management of biofilm-associated infections is challenging. Today, many of these infections are definitively managed using medical device removal, an intervention that is both costly and inconvenient [2].
Given that biofilm-associated infections are difficult to manage, prevention strategies are ideal [3]. Most preventive approaches utilize antimicrobials or antiseptics [4–8]; however, considering that biofilms can survive in the presence of high concentrations of antimicrobial agents, new prophylactic strategies are needed. Chemical and mechanical strategies such as silver or gallium ions, cationic molecules, and other disinfectants have been studied as coatings of indwelling devices [9–12]. Substances with antibiofilm activity, such as lactoferrin or synthesized chalcones [13–15], as well as low acoustic energy [16, 17] have shown some ability to prevent biofilm formation. None of these strategies has, however, solved the clinical challenge of biofilm-associated infections.
The initial step of biofilm formation on medical devices involves adhesion of organisms to medical implant surfaces by electrostatic forces which are largely repulsive, as both are negatively charged [18]. Direct current (DC) may augment repulsive electrostatic forces between organisms and medical implants [19–22]. In addition, DC may impact biofilm formation by changing physical conditions (e.g., temperature, pH) at the implant surface and through the accumulation of products of oxidative stress [20, 23–27].
Previous studies have demonstrated that DC exhibits bactericidal activity against established biofilms [20, 22, 23, 25, 28]. The bactericidal effect of DC against sessile cells suggests that this strategy may be useful to reduce biofilm formation [19]. In a previous study, we showed that 24 hours of 2,000 µA DC reduced S. epidermidis biofilm formation [29]. Whether lower amperage of DC would also reduce biofilm formation and whether our findings with S. epidermidis generalize to other microorganisms are unknown.
The use of DC to reduce biofilm formation may provide a new strategy to prevent biofilm formation in clinical practice. It has the potential benefit of eliminating the use of traditional antimicrobials and therefore decreasing the risk of selecting resistance to these agents. Herein, we examined the effect of different amperages and delivery durations of DC in reducing formation of biofilms of five bacterial and one fungal species.
2. Materials and Methods
2.1. Microorganisms
S. epidermidis Xen 43 [30], Staphylococcus aureus Xen 30 [31], Escherichia coli (IDRL-7029, prosthetic hip infection clinical isolate), Pseudomonas aeruginosa Xen 5 [32], Candida albicans (GDH2346, mouth infection clinical isolate), and Propionibacterium acnes (IDRL-7676, prosthetic shoulder infection clinical isolate) were studied. The Xen strains were generous gifts of PerkinElmer Caliper Life Sciences (formerly Xenogen Corp., Waltham, MA); GDH2346 was from Drs. Jyotsna Chandra and Mahmoud Ghannoum (University Hospitals of Cleveland and Case Western Reserve University, Cleveland, OH).
2.2. Treatment Device
Experiments were performed using polycarbonate channeled chambers designed and fabricated by the Mayo Division of Engineering (Figure 1). Each chamber contained a groove into which a 12.5 × 1 mm Teflon or titanium disc was inserted, positioned vertically. Cylindrical platinum electrodes, 1.5 × 55 mm, were placed in each chamber, 3 mm from the disc, with 1 cm of electrode extended above the chamber for the purpose of connecting the electrode to a current generator.
Figure 1.
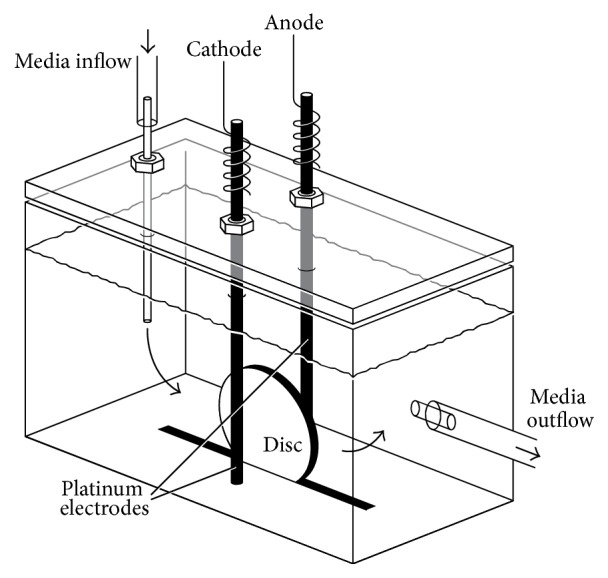
Setup of the treatment device. Electrodes are 3 mm from the disc.
2.3. Electricity Generator
A power source (Keithley 2400 SourceMeter) or an 8-channel computer controlled current generator (designed by Mayo Division of Engineering) was used to deliver direct current (200 or 500 µA).
2.4. S. epidermidis, S. aureus, P. aeruginosa, and E. coli Studies
Microorganisms were subcultured from frozen aliquots onto BBL™ Trypticase™ Soy Agar with 5% sheep blood plates (TSA II, Becton Dickinson Franklin Lakes, NJ) and incubated overnight at 37°C in 5% CO2. One colony was added to 3 mL of Trypticase soy broth (TSB) and grown for 1-2 hours at 37°C on an orbital shaker. The broth was adjusted to a 0.5 McFarland standard and added to a previously described semisynthetic medium [25] supplemented with 64 mL of 1% glucose and TSB (10%) to a final bacterial concentration of 103 colony forming units (cfu)/mL.
A continuous flow (3 mL/hour) of the semisynthetic medium containing 103 cfu/mL test organism was delivered to the polycarbonate treatment chambers containing Teflon or titanium discs. After 2 hours (for Gram-negative bacilli) or 4 hours (for Gram-positive cocci), the semisynthetic medium containing the test organism was changed to a phosphate buffer (12.78 mg Na2HPO4, 6.15 mg KH2PO4, and 19.2 mg glucose in 1000 mL sterile water) without bacteria, also flowing at 3 mL/hour.
DC (0, 200, or 500 µA) was delivered (starting at the same time that semisynthetic medium with bacteria flow started) for either 4, 8, 12, 16, 20, or 24 hours of a 24-hour period when testing 500 µA DC, or 12, 24, 36, or 48 hours of a 48-hour period when testing 200 µA DC, with 0 µA controls tested at each time point. Testing was performed at 37°C for Gram-positive cocci and at room temperature for Gram-negative bacilli.
After 24 hours when using 500 µA or 48 hours when using 200 µA, discs were aseptically removed from the test chambers, planktonic organisms rinsed off by gently dipping the discs into sterile saline, and the discs placed into sterile tubes containing 1 mL of sterile saline. Biofilm organisms were removed by vortexing and sonication in an ultrasound bath (40 KHz, 320 mW/cm2) for 5 minutes [33]. Suspensions of disaggregated biofilms were quantitatively cultured. The medium that remained in the chamber (planktonic organisms) after 24 or 48 hours was also quantitatively cultured. Each test was done in triplicate for each microorganism. Biofilm results were expressed as log10 cfu/cm2; planktonic results were expressed as log10 cfu/mL.
2.5. C. albicans Studies
The method described above was performed with the following modifications. C. albicans was subcultured from frozen aliquots and incubated for 48 hours at 30°C in room air, only 0 and 500 µA DC for 24 and 48 hours were tested, semisynthetic medium was changed to phosphate buffer after 4 hours, and experiments were conducted at room temperature.
2.6. Anaerobic Studies
For P. acnes, experiments were performed as stated above with the following modifications. The organism was subcultured from frozen aliquots and incubated for 72 hours at 37°C under anaerobic conditions, only 0 and 500 µA DC for 24 and 48 hours were tested, semisynthetic medium was changed to phosphate buffer after 4 hours, and experiments were conducted at 37°C in an anaerobic chamber (Coy Laboratory Products, Grass Lake, MI). In addition to performing experiments under aerobic conditions, S. epidermidis experiments were run under anaerobic conditions using 0 and 500 µA DC for 24 hours.
2.7. Titanium Disc Studies
We compared the difference between S. epidermidis and E. coli biofilm formation on titanium discs using 0, 200, and 500 µA of DC for 12 and 24 hours and the treatment device and methods described above.
2.8. Statistical Methods
Reductions in biofilm or planktonic cells were calculated comparing quantitative cultures of discs or surrounding fluid in chambers exposed and not exposed to electrical current. Statistical analyses were performed using SAS software (SAS Institute, Inc., Cary, NC). A one-way analysis of variance was performed with each current delivery strategy and no current delivery using the Wilcoxon rank sum test to determine if electricity reduced biofilm formation. All tests were two-sided; p values < 0.05 were considered statistically significant.
3. Results
3.1. S. epidermidis, S. aureus, P. aeruginosa, and E. coli Studies
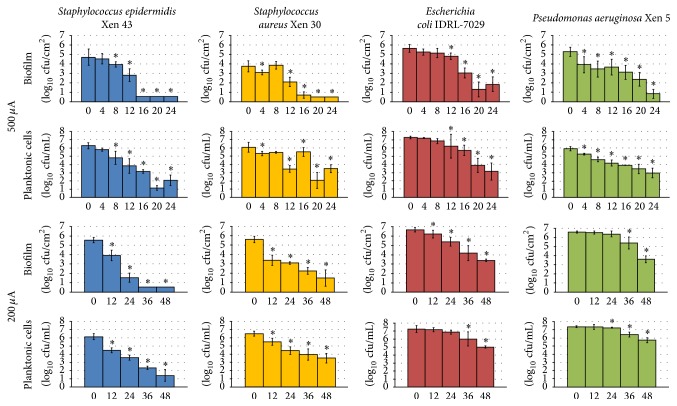
Time- and dose-dependent reductions in biofilm formation on Teflon discs were observed for S. epidermidis, S. aureus, E. coli, and P. aeruginosa, using 500 and 200 µA (Figure 2). For S. epidermidis, a 1 log10 cfu/cm2 reduction in biofilm formation was observed starting at 8 hours of exposure to 500 µA, with a 4 log10 cfu/cm2 reduction observed after 16 hours of exposure to 500 µA or 24 hours of exposure to 200 µA. For S. aureus, there were 2 log10 cfu/cm2 reductions in biofilm formation with 12 or more hours of exposure to 200 and 500 µA. For E. coli, there were 1 and 4 log10 cfu/cm2 reductions in biofilm formation with 12 and 24 hours of exposure to 500 µA, respectively; a similar but smaller effect was observed with 200 µA, with a 4 log10 cfu/cm2 reduction observed with 48 hours of exposure. For P. aeruginosa, a 1 log10 cfu/cm2 reduction in biofilm formation was observed with 4 hours of exposure to 500 µA or 36 hours of exposure to 200 µA, with a 4 log10 cfu/cm2 reduction being observed after 24 hours of exposure to 500 µA. Overall, significant reductions in biofilm formation were observed using 500 µA for at least 12 hours (p = 0.0495) and 200 µA for at least 36 hours (p < 0.05) for all four bacteria studied. Significant differences in amounts of planktonic cells were observed using 500 µA for at least 12 hours (p = 0.0495) and 200 µA for at least 36 hours (p = 0.0495) for all four bacteria studied.
Figure 2.
Results of quantitative cultures of Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa biofilms on Teflon discs and associated planktonic cells with 200 and 500 µA DC started at the time of bacterial seeding of the discs. The x-axis shows hours of DC exposure. The y-axis shows results of quantitative cultures in log10/cm2 for biofilm and log10/mL for planktonic cultures. The 0 µA controls that were tested at each time point were combined for graphical purposes. ∗Statistical significance compared to exposure to no current (p < 0.05).
Since DC reduced S. epidermidis, S. aureus, E. coli, and P. aeruginosa biofilm formation on Teflon discs, we next tested whether this effect would be observed with yeast and an anaerobic bacterium on Teflon discs, as well as with S. epidermidis and E. coli on titanium discs.
3.2. C. albicans Studies
A 3 log10 cfu/cm2 reduction in C. albicans biofilm formation on Teflon discs was detected after 24 hours of exposure to 500 µA DC (Figure 3).
Figure 3.
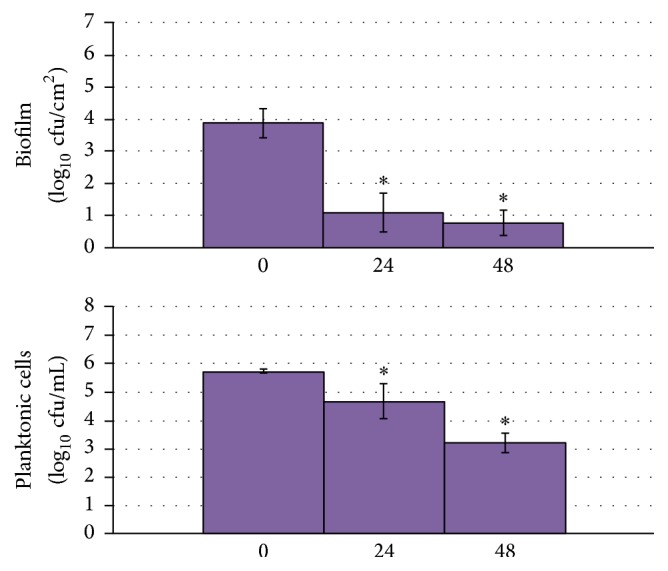
Results of quantitative cultures of Candida albicans biofilms on Teflon discs and associated planktonic cells with 500 µA DC exposure started at the time of candidal seeding of the discs. The x-axis shows hours of DC exposure. The y-axis shows results of quantitative cultures in log10/cm2 for biofilm and log10/mL for planktonic cultures. The 0 µA controls that were tested at each time point were combined for graphical purposes. ∗Statistical significance compared to exposure to no current (p < 0.05).
3.3. Anaerobic Studies
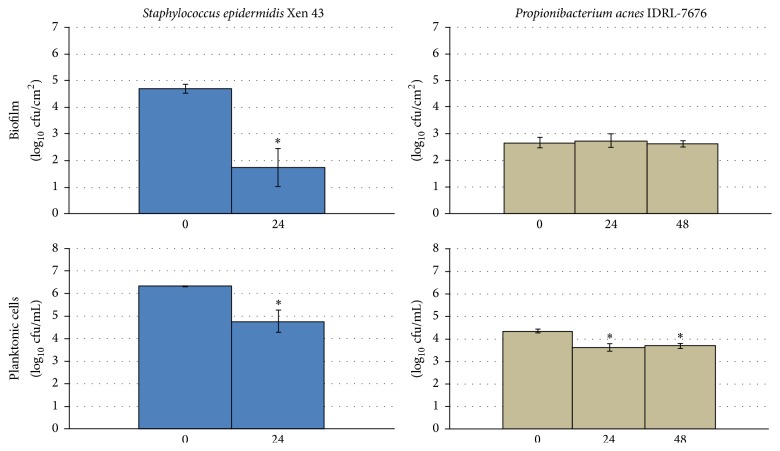
There was no reduction in P. acnes biofilm formation with 48 hours of exposure to 500 µA DC (Figure 4), although there was a 1 log10 cfu/mL reduction in planktonic P. acnes with exposure to 500 µA DC for 24 hours. A 3 log10 cfu/cm2 reduction in S. epidermidis biofilm was observed with 24 hours of exposure to 500 µA (Figure 4).
Figure 4.
Results of quantitative cultures of Staphylococcus epidermidis and Propionibacterium acnes biofilms on Teflon discs and associated planktonic cells with 500 µA DC exposure started at the time of bacterial seeding of the discs for experiments performed under anaerobic conditions. The x-axis shows hours of DC exposure. The y-axis shows results of quantitative cultures in log10/cm2 for biofilm and log10/mL for planktonic cultures. For P. acnes, the 0 µA controls that were tested at each time point were combined for graphical purposes. ∗Statistical significance compared to exposure to no current (p < 0.05).
3.4. Titanium Disc Studies
1 and 2 log10 cfu/cm2 reductions in E. coli and S. epidermidis biofilm formation on titanium discs, respectively, were observed with 12 hours of exposure to 500 µA (p = 0.0495). A 1 log10 cfu/cm2 reduction in biofilm formation was observed for both E. coli and S. epidermidis on titanium discs with 24 hours of exposure to 200 µA (p = 0.0495). The overall magnitude of the effect observed with titanium and Teflon discs was similar for both bacteria, although E. coli (means of 5 versus 6 log10 cfu/cm2, p = 0.0009) but not S. epidermidis (p = 0.0765) formed slightly less biofilm on untreated titanium than Teflon discs.
4. Discussion
Results of these studies demonstrate that DC reduces Staphylococcus species, E. coli, P. aeruginosa, and C. albicans biofilm formation. Since these microorganisms are frequently involved in biofilm-associated infections, these findings are of potential clinical interest.
Although our results are consistent with previous data showing a bactericidal effect of DC against sessile and planktonic cells [22, 25, 26, 34], previous studies have focused on treatment of established biofilms. Our results provide evidence that DC can reduce biofilm formation by staphylococci, Gram-negative bacilli, and Candida species. We observed both dose- and time-dependent responses using the strategy studied. Overall, a reduction in biofilm formation was measureable within 12 hours of application of 500 µA DC; when applying 200 µA of DC, an effect was observed after 36 hours of current application. The same effect was observed for planktonic bacteria and yeast.
DC may reduce the formation of biofilms by preventing adherence of bacterial cells to surfaces [20], through augmentation of the noncovalent forces between organisms, and in our study Teflon and titanium discs. However, the decrease in the observed planktonic cell population suggests that there may be additional active mechanisms. Direct damage from DC to bacteria or yeast by electroporation and/or production of reactive oxygen species, as well as generation of other toxic substances, has been proposed. Chlorine has been identified as a toxic substance that plays a role in the bactericidal effect of electrical current against established biofilms [27]. The absence of an effect against P. acnes biofilm formation may be explained by the involvement of reactive oxygen species in the mechanism underlying the antibiofilm activity of electrical current. The contribution of reactive oxygen species to this process is also supported by the decreased effect observed under anaerobic conditions with S. epidermidis.
Electrode composition may impact the activity observed. We used platinum electrodes to avoid corrosion associated with stainless steel electrodes [28]. Differences in bactericidal effect have been described when using different electrode materials; we observed less antibiofilm effect when using stainless steel compared with platinum electrodes (data not shown). It is possible that platinum complexes contributed to the effect observed [35].
Although most of our experiments were performed using Teflon discs, we demonstrated a similar effect using titanium discs, which is of clinical relevance since titanium is used in the construction of orthopedic implants.
Further investigation is needed to determine the appropriate dose and time of administration of DC for reduction of biofilm formation. Future work could explore the capacity of cells to adhere to a surface that has been previously exposed to electrical current and intermittent DC administration. Ultimately, in vivo studies will be required to address efficacy and safety.
Overall, our results demonstrate that biofilm formation can be reduced using low dose DC. Potentially, this strategy could be used during surgery to prevent early infection and contamination of newly implanted foreign bodies.
Acknowledgments
The authors thank the Mayo Division of Engineering for designing and fabricating the generator and the chambers and Jorge Parra-Ruiz for making the stay at Mayo Clinic possible for Maria Ruiz-Ruigomez and Jon Badiola. Research reported in this paper was supported by the National Institute of Allergy and Infectious Diseases under Award Number R01 AI091594.
Disclosure
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Anwar H., Dasgupta M. K., Costerton J. W. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrobial Agents and Chemotherapy. 1990;34(11):2043–2046. doi: 10.1128/aac.34.11.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmen J. C., Roeder B. L., Nelson J. L., et al. Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics. American Journal of Infection Control. 2005;33(2):78–82. doi: 10.1016/j.ajic.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslam S., Darouiche R. O. Role of antibiofilm-antimicrobial agents in controlling device-related infections. International Journal of Artificial Organs. 2011;34(9):752–758. doi: 10.5301/ijao.5000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Z., Liang S. Y., Marschall J. Current strategies for the prevention and management of central line-associated bloodstream infections. Infection and Drug Resistance. 2010;3:147–163. doi: 10.2147/idr.s10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendriks J. G. E., van Horn J. R., van der Mei H. C., Busscher H. J. Backgrounds of antibiotic-loaded bone cement and prosthesis-related infection. Biomaterials. 2004;25(3):545–556. doi: 10.1016/s0142-9612(03)00554-4. [DOI] [PubMed] [Google Scholar]
- 6.Jiranek W. A., Hanssen A. D., Greenwald A. S. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. The Journal of Bone & Joint Surgery—American Volume. 2006;88(11):2487–2500. doi: 10.2106/jbjs.e.01126. [DOI] [PubMed] [Google Scholar]
- 7.Percival S. L., Kite P. Intravascular catheters and biofilm control. Journal of Vascular Access. 2007;8(2):69–80. [PubMed] [Google Scholar]
- 8.Raad I., Mohamed J. A., Reitzel R. A., et al. Improved antibiotic-impregnated catheters with extended-spectrum activity against resistant bacteria and fungi. Antimicrobial Agents and Chemotherapy. 2012;56(2):935–941. doi: 10.1128/AAC.05836-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cochis A., Azzimonti B., Della Valle C., Chiesa R., Arciola C. R., Rimondini L. Biofilm formation on titanium implants counteracted by grafting gallium and silver ions. Journal of Biomedical Materials Research Part A. 2015;103(3):1176–1187. doi: 10.1002/jbm.a.35270. [DOI] [PubMed] [Google Scholar]
- 10.Kesel S., Mader A., Seeberger P. H., Lieleg O., Opitz M. Carbohydrate coating reduces adhesion of biofilm-forming Bacillus subtilis to gold surfaces. Applied and Environmental Microbiology. 2014;80(19):5911–5917. doi: 10.1128/aem.01600-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loo C.-Y., Young P. M., Lee W.-H., Cavaliere R., Whitchurch C. B., Rohanizadeh R. Non-cytotoxic silver nanoparticle-polyvinyl alcohol hydrogels with anti-biofilm activity: designed as coatings for endotracheal tube materials. Biofouling. 2014;30(7):773–788. doi: 10.1080/08927014.2014.926475. [DOI] [PubMed] [Google Scholar]
- 12.Sathyanarayanan M. B., Balachandranath R., Genji Srinivasulu Y., Kannaiyan S. K., Subbiahdoss G. The effect of gold and iron-oxide nanoparticles on biofilm-forming pathogens. ISRN Microbiology. 2013;2013:5. doi: 10.1155/2013/272086.272086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ammons M. C., Copié V. Mini-review: lactoferrin: a bioinspired, anti-biofilm therapeutic. Biofouling. 2013;29(4):443–455. doi: 10.1080/08927014.2013.773317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozic D. D., Milenkovic M., Ivkovic B., Cirkovic I. Newly-synthesized chalcones-inhibition of adherence and biofilm formation of methicillin-resistant Staphylococcus aureus . Brazilian Journal of Microbiology. 2014;45(1):263–270. doi: 10.1590/s1517-83822014000100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lesman-Movshovich E., Lerrer B., Gilboa-Garber N. Blocking of Pseudomonas aeruginosa lectins by human milk glycans. Canadian Journal of Microbiology. 2003;49(3):230–235. doi: 10.1139/w03-027. [DOI] [PubMed] [Google Scholar]
- 16.Hazan Z., Zumeris J., Jacob H., et al. Effective prevention of microbial biofilm formation on medical devices by low-energy surface acoustic waves. Antimicrobial Agents and Chemotherapy. 2006;50(12):4144–4152. doi: 10.1128/aac.00418-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebeaux D., Ghigo J.-M., Lucet J.-C. Device-related infections: pathophysiology and prevention. Revue du Praticien. 2014;64(5):620–625. [PubMed] [Google Scholar]
- 18.Jucker B. A., Harms H., Zehnder A. J. B. Adhesion of the positively charged bacterium Stenotrophomonas (Xanthomonas)maltophilia 70401 to glass and teflon. Journal of Bacteriology. 1996;178(18):5472–5479. doi: 10.1128/jb.178.18.5472-5479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W.-K., Brown M. R. W., Elliott T. S. J. Mechanisms of the bactericidal activity of low amperage electric current (DC) Journal of Antimicrobial Chemotherapy. 1997;39(6):687–695. doi: 10.1093/jac/39.6.687. [DOI] [PubMed] [Google Scholar]
- 20.Pareilleux A., Sicard N. Lethal effects of electric current on Escherichia coli . Applied Microbiology. 1970;19(3):421–424. doi: 10.1128/am.19.3.421-424.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueshima M., Tanaka S., Nakamura S., Yamashita K. Manipulation of bacterial adhesion and proliferation by surface charges of electrically polarized hydroxyapatite. Journal of Biomedical Materials Research. 2002;60(4):578–584. doi: 10.1002/jbm.10113. [DOI] [PubMed] [Google Scholar]
- 22.van der Borden A. J., van der Mei H. C., Busscher H. J. Electric block current induced detachment from surgical stainless steel and decreased viability of Staphylococcus epidermidis . Biomaterials. 2005;26(33):6731–6735. doi: 10.1016/j.biomaterials.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 23.Del Pozo J. L., Rouse M. S., Euba G., et al. The electricidal effect is active in an experimental model of Staphylococcus epidermidis chronic foreign body osteomyelitis. Antimicrobial Agents and Chemotherapy. 2009;53(10):4064–4068. doi: 10.1128/aac.00432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Pozo J. L., Rouse M. S., Mandrekar J. N., Sampedro M. F., Steckelberg J. M., Patel R. Effect of electrical current on the activities of antimicrobial agents against Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis biofilms. Antimicrobial Agents and Chemotherapy. 2009;53(1):35–40. doi: 10.1128/aac.00237-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.del Pozo J. L., Rouse M. S., Mandrekar J. N., Steckelberg J. M., Patel R. The electricidal effect: reduction of Staphylococcus and Pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrobial Agents and Chemotherapy. 2009;53(1):41–45. doi: 10.1128/aac.00680-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.del Pozo J. L., Rouse M. S., Patel R. Bioelectric effect and bacterial biofilms. A systematic review. International Journal of Artificial Organs. 2008;31(9):786–795. doi: 10.1177/039139880803100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandvik E. L., McLeod B. R., Parker A. E., Stewart P. S. Direct electric current treatment under physiologic saline conditions kills Staphylococcus epidermidis biofilms via electrolytic generation of hypochlorous acid. PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0055118.e55118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt-Malan S. M., Karau M. J., Cede J., et al. Antibiofilm activity of low-amperage continuous and intermittent direct electrical current. Antimicrobial Agents and Chemotherapy. 2015;59(8):4610–4615. doi: 10.1128/AAC.00483-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Pozo J. L., Rouse M. S., Euba G., et al. Prevention of Staphylococcus epidermidis biofilm formation using electrical current. Journal of Applied Biomaterials & Functional Materials. 2014;12(2):e81–e83. doi: 10.5301/jabfm.5000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vuong C., Kocianova S., Yu J., Kadurugamuwa J. L., Otto M. Development of real-time in vivo imaging of device-related Staphylococcus epidermidis infection in mice and influence of animal immune status on susceptibility to infection. Journal of Infectious Diseases. 2008;198(2):258–261. doi: 10.1086/589307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caliper Life Sciences. Bioware™ Microorganism—Staphylococcus aureus Xen30. Hopkinton, Mass, USA: Caliper Life Sciences; 2008. http://www.perkinelmer.com/Content/TDLotSheet/119241-%20Xen30.pdf. [Google Scholar]
- 32.Caliper Life Sciences. Bioware™ Microorganism—Pseudomonas aeruginosa Xen5. Hopkinton, Mass, USA: Caliper Life Sciences; 2008. http://www.perkinelmer.com/Content/TDLotSheet/119228-%20Xen05.pdf. [Google Scholar]
- 33.Trampuz A., Piper K. E., Jacobson M. J., et al. Sonication of removed hip and knee prostheses for diagnosis of infection. The New England Journal of Medicine. 2007;357(7):654–663. doi: 10.1056/nejmoa061588. [DOI] [PubMed] [Google Scholar]
- 34.Freebairn D., Linton D., Harkin-Jones E., Jones D. S., Gilmore B. F., Gorman S. P. Electrical methods of controlling bacterial adhesion and biofilm on device surfaces. Expert Review of Medical Devices. 2013;10(1):85–103. doi: 10.1586/erd.12.70. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg B., Van Camp L., Grimley E. B., Thomson A. J. The inhibition of growth or cell division in Escherichia coli by different ionic species of platinum(IV) complexes. The Journal of Biological Chemistry. 1967;242(6):1347–1352. [PubMed] [Google Scholar]