Abstract
Evidence from neuropathological, genetic, animal model, and biochemical studies has indicated that the accumulation of amyloid-beta (Aβ) is associated with, and probably induces, profound neuronal changes in brain regions critical for memory and cognition in the development of Alzheimer's disease (AD). There is considerable evidence that synapses are particularly vulnerable to AD, establishing synaptic dysfunction as one of the earliest events in pathogenesis, prior to neuronal loss. It is clear that excessive Aβ levels can disrupt excitatory synaptic transmission and plasticity, mainly due to dysregulation of the AMPA and NMDA glutamate receptors in the brain. Importantly, AMPA receptors are the principal glutamate receptors that mediate fast excitatory neurotransmission. This is essential for synaptic plasticity, a cellular correlate of learning and memory, which are the cognitive functions that are most disrupted in AD. Here we review recent advances in the field and provide insights into the molecular mechanisms that underlie Aβ-induced dysfunction of AMPA receptor trafficking. This review focuses primarily on NMDA receptor- and metabotropic glutamate receptor-mediated signaling. In particular, we highlight several mechanisms that underlie synaptic long-term depression as common signaling pathways that are hijacked by the neurotoxic effects of Aβ.
1. Introduction
Alzheimer's disease (AD) is the most common cause of dementia among the aging population. Early memory deficits and progressive loss of higher cognitive functions are common clinical features of AD patients. Pathologically, AD is characterized by insoluble aggregates of extracellular amyloid-beta (Aβ) peptides (senile plaques) and intracellular filaments composed of hyperphosphorylated tau (neurofibrillary tangles) in the brain. Strong evidence from human genetics and transgenic mouse models has implicated Aβ in the etiology and pathogenesis of AD [1]. Aβ peptides are derived from β-secretase- and γ-secretase-mediated sequential proteolytic cleavage of the amyloid-precursor protein (APP), with Aβ 1–40 and Aβ 1–42 being the most abundant species [2]. Many human mutations associated with familial AD, such as those that are found in genes encoding APP and the catalytic subunit of γ-secretase, presenilin (PS1 and PS2), promote amyloidogenic processing of APP, leading to enhanced Aβ production [3]. Recent studies have shown that soluble oligomeric forms of Aβ (ranging from dimers and trimers to dodecamers) exert potent and acute neurotoxic effects on the structure and function of synapses, including reduced excitatory synaptic transmission, loss of dendritic spines, and aberrant neuronal network activity [4, 5]. These deleterious effects could contribute to the cognitive deficit and memory loss associated with AD, indicating that “synaptic failure” is likely to be one of the earliest events that occurs in the pathogenesis of AD prior to neuronal loss [6–8].
The majority of fast excitatory synaptic transmission in the mammalian central nervous system is mediated by the release of glutamate from the presynaptic terminal and its binding to glutamate receptors on the postsynaptic membrane. The ionotropic glutamate receptors consist of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), NMDA (N-methyl-D-aspartate), and kainate receptors. Among these, AMPA receptors (AMPARs) are the principal receptors that mediate fast excitatory synaptic transmission in the mammalian brain. They are tetrameric assemblies of two dimers of four potential subunits (GluA1–GluA4) encoded by distinct genes, GRIA1–GRIA4. The predominant AMPARs expressed in the hippocampal and cortical pyramidal neurons are composed of GluA1/GluA2 and GluA2/GluA3 subunits [9]. Brief periods of high neuronal activity open NMDA receptors (NMDARs) and induce Ca2+ influx, leading to a long-lasting increase in synaptic efficacy, known as long-term potentiation (LTP), which is characterized by an increase in the number of AMPARs on the postsynaptic membrane and spine growth. In contrast, repetitive low frequency stimulation leads to the removal of synaptic AMPARs to produce long-term depression (LTD), that is, a decrease in synaptic strength. It has long been postulated that these forms of synaptic plasticity represent a cellular correlate of learning and memory [10].
One of the key mechanisms underlying synaptic plasticity is the tight control of AMPAR number at synapses. This requires a balance between the biosynthesis (number of receptors being produced), membrane trafficking (the movement of receptors to and from the plasma membrane via exocytosis and endocytosis), and degradation of receptors (receptor turnover), all of which are dynamically regulated by AMPAR interacting proteins as well as by various posttranslational modifications that occur on their cytoplasmic carboxyl terminal domains [11, 12]. Aberrant trafficking of AMPARs usually leads to impaired synaptic plasticity and deficits in learning and memory [11]. Importantly, several studies have demonstrated a role for Aβ in promoting AMPAR endocytosis and hence synaptic depression [13–16]. This review focuses primarily on NMDAR and metabotropic glutamate receptor- (mGluR-) mediated signaling. In particular, we highlight several mechanisms that underlie synaptic LTD as common signaling pathways that are hijacked by the neurotoxic effects of Aβ. Several pharmacological agents that target these pathways and are efficacious in inhibiting or reversing the neurotoxic effects of Aβ on glutamatergic neurotransmission and synaptic plasticity are also discussed.
2. Aβ Alters Synaptic Plasticity In Vitro and In Vivo
The ability of neurons to modulate their synaptic strength is widely believed to be a cellular correlate of learning and memory. NMDAR-dependent LTP and LTD are two major forms of synaptic plasticity that are best studied in the hippocampus, a region of the brain that is both critical for memory formation and highly vulnerable to Aβ toxicity. It is well established that synthetic soluble Aβ oligomers [17, 18] or those secreted from cell lines overexpressing APP [19] acutely and potently block hippocampal LTP at high concentration. More recent studies have further shown that soluble Aβ dimers, but not Aβ monomers, either prepared by chemical cross-linking or extracted directly from postmortem AD brains, are extremely potent in inhibiting hippocampal LTP both in vitro and in vivo [4, 20]. Congruent with the LTP hypothesis of long-term memory, injection of these soluble Aβ oligomers into the rat hippocampus disrupts cognitive function and learned behavior [4, 21]. Most transgenic AD mouse models overexpressing different familial AD mutations, such as Tg2576 (APPSwe; K670N/M671L), PDAPP (APPInd; V717F), 3xTg (APPswe, Tau P301L, and PS1 M146V), and 5xFAD (APPswe, APPFlorida; I716V, APPLondon; V717I, PS1 M146L, and PS1 L286V), generally display impairments in LTP and cognition [22–26]. Notably, some AD transgenic mice show abnormal LTP and learning deficits well in advance of plaque formation [22, 27, 28]. Collectively, these results lend support to the idea that soluble oligomeric Aβ plays a key role in disrupting synaptic plasticity. More importantly, studies performed in human subjects have also revealed deficits in LTP-like cortical plasticity in mild-to-moderate AD patients [29–31].
Consistent with the fact that Aβ induces an impairment in LTP, soluble Aβ oligomers have been demonstrated to facilitate the expression of LTD in the hippocampus [4, 17, 32]. Although the exact mechanisms underlying Aβ-induced LTD remain equivocal, they have been shown to involve internalization of NMDA- and AMPA-type glutamate receptors, dendritic spine shrinkage, and eventual synaptic loss [14, 16, 33, 34].
3. Mechanisms Underlying Aβ-Induced Deficits in AMPAR Function
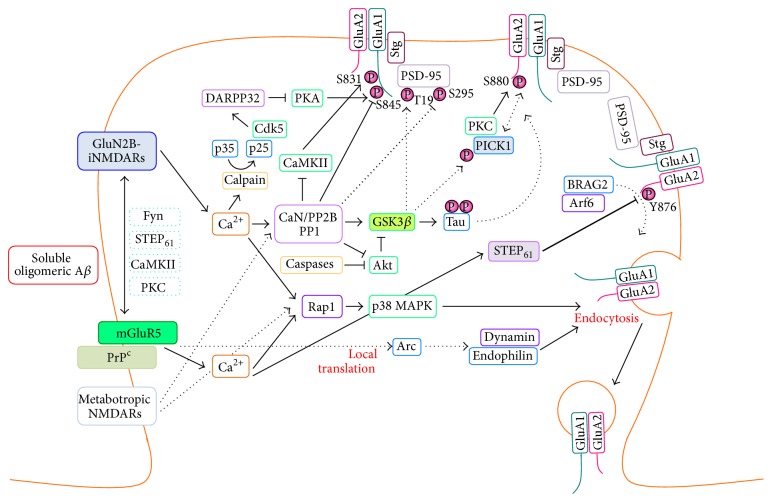
Dynamic trafficking of AMPARs to and from synapses is a critical mechanism underlying the induction of synaptic plasticity. Defects in the endocytosis and lysosomal trafficking pathways are known to contribute significantly to AD pathogenesis [35]. Consistent with this notion, overexpression of APP and a high concentration of soluble oligomeric Aβ are able to induce the removal of surface AMPARs at synapses, leading to synaptic depression and inhibition of LTP [14, 19, 36, 37]. Mechanistically, these neurotoxic effects of Aβ are mediated by high levels of glutamate at synapses as a result of a disrupted glutamate reuptake process [32] that subsequently leads to aberrant activation of NMDARs, mGluRs, and the cellular prion protein (PrPC), as well as elevated levels of AMPAR ubiquitination. Activation of these signaling pathways in turn promotes synaptic depression, via common pathways shared with LTD as summarized in Figure 1, which are discussed in detail in the following sections.
Figure 1.
Signaling mechanisms involved in Aβ-induced AMPAR internalization. Soluble Aβ oligomers activate ionotropic NMDA (iNMDA) and metabotropic glutamate (mGlu) receptors, leading to an increase in intracellular Ca2+. Ca2+ subsequently activates a number of signal transduction cascades involving protein phosphatases (calcineurin, PP1, and STEP61) and protein kinases (Cdk5, PKC, and GSK3β) to modulate the phosphorylation of AMPAR subunits, as well as intracellular signaling and scaffolding molecules. Activation of these pathways, which are commonly shared with LTD, promotes AMPAR internalization and synaptic depression. The cross talk between NMDAR and mGluR5 signaling can be modulated by factors such as Fyn, CaMKII, PKC, and STEP61. The involvement of metabotropic NMDARs in mediating the neurotoxic effects of Aβ, which do not involve the flux of Ca2+, has recently been proposed, albeit this remains controversial. Dotted arrows indicate events that are inferred from the study of LTD and have not been shown to be directly involved in Aβ-mediated signaling. Thicker lines indicate common pathways, while colored boxes indicate potential therapeutics targets for AD.
3.1. NMDARs
NMDAR-dependent LTD induced by low frequency stimulation or by direct application of NMDA (chemically induced LTD) triggers Ca2+ entry into the postsynaptic compartment and activates protein phosphatase 2B (PP2B, also known as calcineurin), which in turn leads to the activation of protein phosphatase 1 (PP1) [38, 39]. PP1 and PP2B are known to mediate NMDAR-induced AMPAR internalization by dephosphorylating the GluA1 subunit of AMPARs at Ser-845 [40, 41], a protein kinase A (PKA) site that is crucial for maintaining the stability of AMPARs at perisynaptic sites and LTP [42–44]. NMDAR-dependent LTD also induces the p38 mitogen activated protein kinase (p38 MAPK) signaling pathway via the activation of Rap small GTPases, leading to the removal of AMPARs [45, 46].
Emerging evidence demonstrates that toxic levels of Aβ aberrantly enhance the activity of NMDARs in favor of LTD induction, thereby preventing LTP [32, 37, 47]. In cultured neurons and acute brain slices, soluble oligomeric Aβ induces excessive influx of Ca2+ through the GluN2B-containing extrasynaptic NMDARs, which subsequently activates the Rap-p38 MAPK signaling pathway, as well as the protein phosphatases, PP1 and calcineurin [13, 14, 16, 32, 37, 48–50]. One of the consequences of Aβ-induced activation of calcineurin is reduced phosphorylation of Ser-845, which induces AMPAR endocytosis and impairs the synaptic incorporation of these receptors [16]. Consistent with this finding, APPSwe,Ind transgenic mice display lower levels of Ser-845 phosphorylation, a phenomenon that correlates well with the loss of AMPARs on the cell surface and deficits in initial learning and memory [16].
Another key substrate of PP1 the activity of which is required for the expression of NMDAR-dependent LTD is glycogen synthase kinase-3β (GSK3β) [51]. PP1 can activate GSK3β by a direct dephosphorylation mechanism, as well as via the modulation of the upstream caspase–Akt signaling pathways, which are also crucial for AMPAR internalization and LTD [51, 52]. Interestingly, both GSK3β and caspases are enzymes that have been widely implicated in AD. Indeed, it has been demonstrated that inhibition of LTP by Aβ is mediated by the caspase 3, Akt1, and GSK3β signaling pathway [32, 53]. Paradoxically, however, GSK3β activity has also been reported to play a role in maintaining AMPAR synaptic expression under basal conditions as its inhibition leads to the loss of surface AMPAR expression by controlling the rate of AMPAR internalization [54]. However, during NMDAR-dependent LTD, GSK3β may preferentially phosphorylate other substrates including the key scaffolding protein in excitatory synapses, postsynaptic density-95 (PSD-95). PSD-95 stabilizes AMPARs at synapses through its interaction with transmembrane AMPAR regulatory proteins (TARPs), auxiliary subunits of AMPARs [55]. Overexpression of PSD-95 promotes synaptic maturation and enhances synaptic strength, whereas PSD-95 knockdown results in the opposite effects [56–60]. It appears that GSK3β phosphorylation of PSD-95 at Thr-19, following its dephosphorylation at Ser-295 by PP1, destabilizes and mobilizes PSD-95 away from the PSD, resulting in increased AMPAR internalization [61, 62]. Whether or not the phosphorylation status of PSD-95 is modulated by oligomeric Aβ via the GSK3β and PP1 signaling pathways remains to be determined.
GSK3β is also a major kinase that phosphorylates the microtubule-associated protein tau [63, 64]. Aβ causes tau hyperphosphorylation and mislocalization from axons to somatodendritic compartments, where it accumulates and mediates Aβ-induced downregulation of surface AMPARs [65–68]. Recent studies have shown that NMDAR-induced GSK3β phosphorylation of tau at Ser-396 is required for hippocampal LTD by enhancing the interaction between the GluA2 subunits of AMPARs with the protein interacting with C-kinase 1 (PICK1) [69, 70], a process that is fundamental for AMPAR internalization and/or intracellular retention during LTD [71–76]. Furthermore, phosphorylation of PICK1 by GSK3β at Ser-416 has also been reported to augment this interaction [77].
GluA2 can be phosphorylated by protein kinase C (PKC) at Ser-880 and by the protein tyrosine kinase of the sarcoma (Src) family at Tyr-876, both of which are required for AMPAR internalization and LTD [78–80]. GluA2 phosphorylation at these sites differentially regulates the interaction of the subunit with PICK1 and glutamate receptor interacting proteins (GRIP) 1 and 2 [80, 81]. GRIP1 plays an important role in stabilizing AMPARs at synapses and is essential for LTD [72, 79]. Given that phosphorylation of GluA2 weakens the interaction of the subunit with GRIP1, but not PICK1, it has been postulated that LTD involves destabilization and detachment of GluA2 from synapses, allowing AMPARs to be internalized. In accord with the role of Aβ in inducing aberrant AMPAR endocytosis, one study has observed that oligomeric Aβ increases PKC-mediated phosphorylation of GluA2 at Ser-880 and subsequently reduces surface expression of AMPARs in cultured hippocampal neurons [15]. More importantly, several molecular and pharmacological manipulations that inhibit GluA2 internalization potently prevent Aβ-induced synaptic depression and rescue memory impairment in AD mice. These include the GluA2-R845A mutant [14], GluA2-3Y peptides [82], and a small molecule PICK1 inhibitor [83].
A new mechanism underlying the pathological action of Aβ that involves the cyclin-dependent kinase 5- (Cdk5-) activating peptide, p25, has recently been described by Seo et al. [26]. Elevated levels of p25 have been implicated in many neurodegenerative diseases, including AD [84]. In their study, Seo et al. found that Aβ induces calpain-mediated cleavage of p35 into p25 in the hippocampus, a process that requires the activity of GluN2B-containing NMDARs and Ca2+/calmodulin-dependent protein kinase II (CaMKII). The Aβ-induced elevation in p25/Cdk5 activity subsequently enhances the phosphorylation of dopamine- and cyclic adenosine monophosphate-regulated neuronal phosphoprotein (DARPP-32) at Thr-75, thereby inhibiting the activity of PKA [85]. In a synergistic manner, Aβ also triggers dephosphorylation of DARPP-32 at Thr-34, presumably by calcineurin, thereby releasing its inhibition on PP1 [86, 87]. These converging mechanisms eventually lead to the loss of GluA1 phosphorylation at Ser-845 and induce AMPAR internalization and synaptic depression. Remarkably, genetic inhibition of p25 generation rescues LTP and memory deficits in 5xFAD transgenic mice [26].
In addition to promoting the internalization of AMPARs, oligomeric Aβ can also act through mechanisms that prevent the forward trafficking of AMPARs towards the plasma membrane. Aβ has been shown to cause aberrant redistribution of CaMKII from the synaptic to the cytosolic fraction both in cultured neurons and in the brain of APPswe transgenic mice [88]. CaMKII can potentiate AMPAR-mediated transmission via (a) phosphorylation of GluA1 at Ser-831 to enhance AMPAR channel conductance, (b) phosphorylation of the TARP, stargazin, to facilitate synaptic recruitment of AMPARs, and (c) potentiation of the Ras-ERK (extracellular signal-regulated kinase) pathway to promote AMPAR insertion into the plasma membrane [45, 89–91]. Consistent with the role of CaMKII in synaptic potentiation, exposure of soluble Aβ oligomers reduces surface GluA1 clusters in cultured neurons, concomitant with decreased AMPAR synaptic responses in cortical pyramidal neurons recorded from acute brain slices of APPswe transgenic mice [88].
Aβ has been shown to interact with NMDARs [92, 93] and to reduce their surface expression through endocytosis [33]. Aβ-induced internalization of NMDARs involves dephosphorylation of the GluN2B subunit at Tyr-1472 by STEP61 (striatal-enriched protein tyrosine phosphatase 61), the expression of which is upregulated in several AD mouse models, as well as in the postmortem prefrontal cortex of AD patients [33, 94–96]. The fact that Aβ enhances the internalization of NMDARs seems counterintuitive given the role of NMDARs in mediating AMPAR endocytosis, spine loss, and ultimately excitotoxicity in neurons. Recent studies on the putative oligomeric Aβ receptor, PrPC, have provided insights into two potential mechanisms that regulate NMDAR function [97, 98]. Firstly, soluble oligomeric Aβ binding to PrPC activates the tyrosine kinase Fyn, which initially phosphorylates GluN2B and transiently enhances NMDAR function, before the STEP61 level increases and dephosphorylates GluN2B [99]. Secondly, Aβ disrupts the ability of PrPC to limit excessive NMDAR activity in a copper-dependent manner, potentially by chelating copper ions and preventing them from binding to PrPC, thereby producing large nondesensitizing steady-state NMDAR currents [100]. Albeit controversial, loss of PrPC function has been reported to prevent Aβ-induced LTP and memory impairment in mice [98, 101–105]. Despite this, the role of PrPC in regulating AMPAR trafficking has not been directly examined.
Recent studies by Kessels and colleagues have challenged the central role of NMDAR-mediated Ca2+ influx in Aβ-induced synaptic depression [106]. It is well established that the neurotoxic effects of oligomeric Aβ on synapses can be blocked by the NMDAR antagonist, D-APV (D-2-amino-5-phosphonopentanoic acid), which prevents glutamate binding and blocks the activation of NMDARs. However, noncompetitive NMDAR antagonists that block ion flow through the receptor, such as MK-801, ketamine, and 7-chlorokynurenic acid, are not able to rescue Aβ-mediated synaptic depression [106, 107]. A similar finding was recently reported for oligomeric Aβ-induced dendritic spine loss [108]. Consistent with the idea that Aβ operates through shared pathways with LTD, metabotropic, but not ionotropic, NMDAR function has been shown to be required for NMDAR-dependent LTD in the hippocampus by activating the p38 MAPK signaling pathway [109]. In fact, ligand binding to the extracellular domain of NMDARs induces conformational change and movement of their cytoplasmic tails, allowing PP1 to dephosphorylate CaMKII together with other signaling molecules that contribute to synaptic depression [110, 111]. Although the role of metabotropic NMDARs remains controversial [46], it does offer an explanation for the fact that the FDA-approved NMDAR antagonist, memantine, has poor efficacy in treating early-stage AD [112]. Further research is warranted, as delineating the metabotropic NMDAR signaling pathway may shed light on new strategies for the development of future AD drugs.
3.2. mGluRs
mGluRs belong to the G-protein-coupled receptor superfamily that modulates neuronal excitability, synaptic transmission, and plasticity in the central nervous system [113]. Group I mGluRs, which consist of two members, mGluR1 and mGluR5, predominantly localize to the postsynaptic membrane and are canonically coupled to Gα q/11 to activate phospholipase Cβ (PLCβ) that catalyzes the hydrolysis of phosphoinositides into inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). Subsequently, these second messengers trigger the release of Ca2+ from intracellular stores and activate PKC, respectively. Group I mGluRs, and more specifically mGluR5, are the predominant receptors that mediate mGluR-dependent LTD in the hippocampus and have been widely implicated in AD [114].
It is well established that mGluR-dependent LTD requires the internalization of GluA2-containing AMPARs, leading to a long-term reduction in the number of surface AMPARs [115, 116]. One of the mechanisms that regulates mGluR-induced AMPAR endocytosis involves the phosphorylation of GluA2 at Ser-880 by PKC, a process that is facilitated by PICK1 [117–119]. However, in the CA1 region of the hippocampus, internalization of AMPARs does not require PKC but instead relies on the dephosphorylation of GluA2 at Tyr-876 by STEP61 [120–122]. Dephosphorylation of GluA2 stimulates the binding of BRAG2 (brefeldin resistant Arf GEF 2), which in turn activates the small GTPase Arf6 through augmentation of its GEF (guanine-nucleotide exchange factor) activity and promotes AMPAR endocytosis [122]. In accordance with this model, it has been reported that Aβ-induced internalization of AMPARs requires STEP61 activity [95]. Genetic deletion of STEP61 restores the number of AMPARs on the postsynaptic membrane, enhances LTP, and improves cognitive function in AD mice [95, 123]. A new small molecule inhibitor of STEP61, TC-2153, has recently been shown to reverse cognitive deficits in 3xTg AD mice [124]. Like NMDAR-dependent LTD, mGluR-mediated LTD also involves the Rap1-p38 MAPK signal transduction pathway to facilitate AMPAR internalization via the formation of the GDI-Rab5 complex [125–127]. In addition, a role for ERK in mGluR-dependent LTD has also been reported [128].
One unique feature of mGluR-dependent LTD is its requirement for rapid translation of preexisting mRNAs (local protein translation) in dendrites [129]. mGluR-dependent de novo protein synthesis can be regulated through multiple pathways, including the PI3K-Akt-mTOR (mammalian target of rapamycin) and ERK signaling pathways that converge on the initiation and elongation factors of protein translation [130]. Several mRNA encoding proteins that regulate AMPAR trafficking are locally translated during mGluR-dependent LTD, including the activity-regulated cytoskeleton-associated protein (Arc), microtubule-associated protein 1B (MAP1B), and STEP [121, 131–133]. All of these proteins are known to facilitate the internalization of AMPARs. MAP1B is a known GRIP1 binding protein [134]. Given that GRIP1 stabilizes AMPARs at synapses, the newly synthesized MAP1B may sequester GRIP, hence loosening its interaction with GluA2. On the other hand, Arc interacts with the endocytic proteins, endophilin and dynamin, and is able to enhance dynamin polymerization and GTPase activity, thereby promoting AMPAR endocytosis [135, 136]. Interestingly, soluble oligomeric Aβ rapidly induces Arc expression in neurons, which may contribute to the loss of AMPARs from the plasma membrane [137]. Moreover, Arc also regulates the endosomal trafficking of APP and BACE1, as well as PS1, a mechanism that is essential for the activity-dependent production of Aβ in the brain, and genetic deletion of Arc reduces the Aβ load in APPswe;PS1ΔE9 transgenic AD mice [138]. This may serve as a positive feedback mechanism underlying the overproduction of Aβ in the pathophysiology of AD.
Studies from several laboratories have implicated the mGluR-dependent signaling pathway in the neurotoxic effects of Aβ on synaptic function [4, 14, 139–143]. Notably, genetic and pharmacological inhibition of mGluR5 prevents oligomeric Aβ-induced impairment in LTP, spine loss, and cognitive deficits in AD mouse models [139, 142–145]. More recently, a seminal study by Strittmatter and colleagues identified an interaction between mGluR5 and PrPC, which together act as a coreceptor for oligomeric Aβ [144]. They also revealed an essential role for mGluR5 and PrPC coupling in the pathology of AD [146]. Mechanistically, mGluR5 links PrPC to key intracellular signaling molecules, such as Homer1b/c, Pyk2, Fyn, and CaMKII, all of which play major roles in synaptic plasticity [144, 146, 147]. When neurons are exposed to oligomeric Aβ, the PrPC-mGluR5 complex mediates the aberrant activation of Pyk2, Fyn, and CaMKII, causing altered neuronal states that lead to impaired LTP [99, 144, 146]. It is interesting to note that Aβ also induces a biphasic alteration in CaMKII activity, resembling that of Fyn, in a PrPC-mGluR5-dependent manner, and that this is accompanied by the increased association of mGlu5 with CaMKII [146]. Given that mGluR5 activation enhances NMDAR forward trafficking through CaMKII-mediated phosphorylation of GluN2B at Ser-1303 [148], it is hypothesized that Aβ-induced enhancement of the association between mGluR5 and CaMKII may prevent synaptic potentiation. Furthermore, pharmacological activation of mGluR5 in the presence of PrPC causes a redistribution of CaMKII into the cytoplasm [147], which may have an impact on AMPAR trafficking.
Interestingly, the cross talk between mGluR5 and NMDAR signaling is bidirectional. Not only can mGluR5 potentiate NMDAR currents through CaMKII and PKC signaling pathways [148, 149], but also activation of NMDARs can potentiate mGluR5 responses under physiological conditions [150, 151]. This involves the NMDAR-dependent activation of calcineurin that dephosphorylates mGluR5 and reduces receptor desensitization. However, a high concentration of NMDA can induce PKC-dependent mGluR5 phosphorylation and inhibit mGluR5 responses [152]. Although the interaction of mGluR5 and NMDARs has been implicated in synaptic plasticity and various animal behaviors [153–156], their alteration in the presence of Aβ binding to PrPC and how this impacts on AMPAR trafficking remain unclear.
3.3. Protein Ubiquitination
Posttranslational ubiquitination, a regulatory signal that controls protein trafficking and turnover, has recently emerged as an important mechanism that regulates AMPAR function [157, 158]. All AMPAR subunits undergo activity-dependent ubiquitination in cultured neurons, a process that is Ca2+-dependent and requires the activity of L-type voltage-gated Ca2+ channels [159–161]. The primary E3 ligases that catalyze the ubiquitination of GluA1 and GluA2 subunits are Nedd4-1 and RNF167, respectively [160, 162]. While the role of protein ubiquitination on the GluA1 and GluA2 subunits in ligand-induced AMPAR endocytosis remains controversial, it is well accepted that ubiquitination of AMPARs regulates the intracellular sorting of receptors into late endosomes for degradation [159–161, 163]. Under normal conditions, the degradation of AMPARs is required for protein homeostasis to ensure turning over of old or used receptors in order to maintain healthy levels of AMPARs in neurons. However, when the ubiquitin pathway is hijacked (e.g., by elevated levels of Aβ), there is an excessive downregulation of AMPARs and synaptic depression. Indeed, a new finding has demonstrated a role for naturally secreted and synthetic Aβ in promoting the ubiquitination of AMPARs by Nedd4-1 [164]. Interestingly, knocking down Nedd4-1 rescued Aβ-induced synaptic deficits, including reduced glutamatergic synaptic transmission, decreased levels of surface AMPARs, and the loss of dendritic spines. These findings have important implications in targeting ubiquitin E3 ligases as potential drug targets for the treatment of AD.
4. Concluding Remarks
Research over the past decade has provided strong evidence that the cognitive deficit associated with AD is caused by the neurotoxic effects of soluble Aβ oligomers on synaptic function. Increasing evidence indicates that the trafficking of AMPARs, which is essential to multiple forms of synaptic and structural plasticity in the brain, is aberrantly dysregulated by oligomeric Aβ and manifests as impairments in LTP, learning, and memory. It is particularly encouraging to learn that pharmacological and genetic manipulations that block endocytosis or enhance the forward trafficking of AMPARs can rescue LTP and reverse cognitive deficits in AD mice. Given that Aβ-induced AMPAR internalization requires the same adaptor proteins as the conventional trafficking pathway, it will be challenging to minimize unwanted side effects. Hence, further research is needed to identify specific targets for improving the memory deficits associated with AD. Rapid progress has been made in delineating the molecular mechanisms and signaling pathways underlying the loss of AMPARs from the plasma membrane induced by oligomeric Aβ (Figure 1). The discovery of PrPC as a receptor for soluble Aβ oligomers that signals through NMDA and mGluR5 receptor has underscored the importance of glutamatergic signaling in the etiology of AD. It is likely that these receptors act cooperatively to mediate the synaptotoxic effects of Aβ, highlighting the need for further investigation of the associated signaling mechanisms with a view to developing more effective therapeutic strategies for the treatment of AD.
Acknowledgments
The authors thank Rowan Tweedale for editing of this paper. This work is supported by grants from the John T. Reid Charitable Trusts, the Alzheimer's Australia Dementia Research Foundation (DGP14-57), and the Australian National Health and Medical Research Council (APP1083209 and APP1099114) to Victor Anggono. Sumasri Guntupalli is a recipient of a University of Queensland International Scholarship.
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Sumasri Guntupalli and Jocelyn Widagdo contributed equally to this work.
References
- 1.Selkoe D. J. Alzheimer's disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 2.Walsh D. M., Minogue A. M., Sala Frigerio C., Fadeeva J. V., Wasco W., Selkoe D. J. The APP family of proteins: similarities and differences. Biochemical Society Transactions. 2007;35(2):416–420. doi: 10.1042/bst0350416. [DOI] [PubMed] [Google Scholar]
- 3.Scheuner D., Eckman C., Jensen M., et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nature Medicine. 1996;2(8):864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 4.Shankar G. M., Li S., Mehta T. H., et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nature Medicine. 2008;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palop J. J., Chin J., Roberson E. D., et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of alzheimer's disease. Neuron. 2007;55(5):697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mucke L., Selkoe D. J. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harbor Perspectives in Medicine. 2012;2(7) doi: 10.1101/cshperspect.a006338.a006338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haass C., Selkoe D. J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nature Reviews Molecular Cell Biology. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 8.Sheng M., Sabatini B. L., Südhof T. C. Synapses and Alzheimer's disease. Cold Spring Harbor Perspectives in Biology. 2012;4(5) doi: 10.1101/cshperspect.a005777.a005777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu W., Shi Y., Jackson A. C., et al. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62(2):254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessels H. W., Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61(3):340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anggono V., Huganir R. L. Regulation of AMPA receptor trafficking and synaptic plasticity. Current Opinion in Neurobiology. 2012;22(3):461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu W., Roche K. W. Posttranslational regulation of AMPA receptor trafficking and function. Current Opinion in Neurobiology. 2012;22(3):470–479. doi: 10.1016/j.conb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao W.-Q., Santini F., Breese R., et al. Inhibition of calcineurin-mediated endocytosis and α-amino-3-hydroxy- 5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid β oligomer-induced synaptic disruption. The Journal of Biological Chemistry. 2010;285(10):7619–7632. doi: 10.1074/jbc.m109.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh H., Boehm J., Sato C., et al. AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron. 2006;52(5):831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S.-J., Gasperini R., Foa L., Small D. H. Amyloid-β decreases cell-surface AMPA receptors by increasing intracellular calcium and phosphorylation of GluR2. Journal of Alzheimer's Disease. 2010;21(2):655–666. doi: 10.3233/jad-2010-091654. [DOI] [PubMed] [Google Scholar]
- 16.Miñano-Molina A. J., España J., Martín E., et al. Soluble oligomers of amyloid-β peptide disrupt membrane trafficking of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor contributing to early synapse dysfunction. Journal of Biological Chemistry. 2011;286(31):27311–27321. doi: 10.1074/jbc.M111.227504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J. H., Anwyl R., Suh Y. H., Djamgoz M. B., Rowan M. J. Use-dependent effects of amyloidogenic fragments of (beta)-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. The Journal of Neuroscience. 2001;21(4):1327–1333. doi: 10.1523/JNEUROSCI.21-04-01327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert M. P., Barlow A. K., Chromy B. A., et al. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(11):6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh D. M., Klyubin I., Fadeeva J. V., et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 20.Hu N.-W., Smith I. M., Walsh D. M., Rowan M. J. Soluble amyloid-β peptides potently disrupt hippocampal synaptic plasticity in the absence of cerebrovascular dysfunction in vivo. Brain. 2008;131(9):2414–2424. doi: 10.1093/brain/awn174. [DOI] [PubMed] [Google Scholar]
- 21.Cleary J. P., Walsh D. M., Hofmeister J. J., et al. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nature Neuroscience. 2005;8(1):79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 22.Chapman P. F., White G. L., Jones M. W., et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nature Neuroscience. 1999;2(3):271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 23.Larson J., Lynch G., Games D., Seubert P. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Research. 1999;840(1-2):23–35. doi: 10.1016/S0006-8993(99)01698-4. [DOI] [PubMed] [Google Scholar]
- 24.Oddo S., Caccamo A., Shepherd J. D., et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 25.Cantanelli P., Sperduti S., Ciavardelli D., Stuppia L., Gatta V., Sensi S. L. Age-dependent modifications of AMPA receptor subunit expression levels and related cognitive effects in 3xTg-AD mice. Frontiers in Aging Neuroscience. 2014;6, article 200 doi: 10.3389/fnagi.2014.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo J., Giusti-Rodríguez P., Zhou Y., et al. Activity-dependent p25 generation regulates synaptic plasticity and aβ-induced cognitive impairment. Cell. 2014;157(2):486–498. doi: 10.1016/j.cell.2014.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsia A. Y., Masliah E., Mcconlogue L., et al. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(6):3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberson E. D., Halabisky B., Yoo J. W., et al. Amyloid-β/fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of alzheimer's disease. The Journal of Neuroscience. 2011;31(2):700–711. doi: 10.1523/jneurosci.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inghilleri M., Conte A., Frasca V., et al. Altered response to rTMS in patients with Alzheimer's disease. Clinical Neurophysiology. 2006;117(1):103–109. doi: 10.1016/j.clinph.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Battaglia F., Wang H.-Y., Ghilardi M. F., et al. Cortical plasticity in Alzheimer's disease in humans and rodents. Biological Psychiatry. 2007;62(12):1405–1412. doi: 10.1016/j.biopsych.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 31.Koch G., Di Lorenzo F., Bonnì S., Ponzo V., Caltagirone C., Martorana A. Impaired LTP-but not LTD-like cortical plasticity in Alzheimer's disease patients. Journal of Alzheimer's Disease. 2012;31(3):593–599. doi: 10.3233/JAD-2012-120532. [DOI] [PubMed] [Google Scholar]
- 32.Li S., Hong S., Shepardson N. E., Walsh D. M., Shankar G. M., Selkoe D. Soluble oligomers of amyloid β protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62(6):788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder E. M., Nong Y., Almeida C. G., et al. Regulation of NMDA receptor trafficking by amyloid-β . Nature Neuroscience. 2005;8(8):1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 34.Almeida C. G., Tampellini D., Takahashi R. H., et al. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiology of Disease. 2005;20(2):187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Pimplikar S. W., Nixon R. A., Robakis N. K., Shen J., Tsai L.-H. Amyloid-independent mechanisms in Alzheimer's disease pathogenesis. The Journal of Neuroscience. 2010;30(45):14946–14954. doi: 10.1523/jneurosci.4305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klyubin I., Walsh D. M., Lemere C. A., et al. Amyloid β protein immunotherapy neutralizes Aβ oligomers that disrupt synaptic plasticity in vivo. Nature Medicine. 2005;11(5):556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- 37.Li S., Jin M., Koeglsperger T., Shepardson N. E., Shankar G. M., Selkoe D. J. Soluble aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. The Journal of Neuroscience. 2011;31(18):6627–6638. doi: 10.1523/jneurosci.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulkey R. M., Herron C. E., Malenka R. C. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261(5124):1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- 39.Mulkey R. M., Endo S., Shenolikar S., Malenka R. C. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369(6480):486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- 40.Beattie E. C., Carroll R. C., Yu X., et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nature Neuroscience. 2000;3(12):1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 41.Ehlers M. D. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28(2):511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 42.He K., Song L., Cummings L. W., Goldman J., Huganir R. L., Lee H.-K. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(47):20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh M. C., Derkach V. A., Guire E. S., Soderling T. R. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. Journal of Biological Chemistry. 2006;281(2):752–758. doi: 10.1074/jbc.m509677200. [DOI] [PubMed] [Google Scholar]
- 44.Man H.-Y., Sekine-Aizawa Y., Huganir R. L. Regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(9):3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu J. J., Qin Y., Zhao M., Van Aelst L., Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110(4):443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- 46.Babiec W. E., Guglietta R., Jami S. A., Morishita W., Malenka R. C., O'Dell T. J. Ionotropic NMDA receptor signaling is required for the induction of long-term depression in the mouse hippocampal CA1 region. Journal of Neuroscience. 2014;34(15):5285–5290. doi: 10.1523/JNEUROSCI.5419-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferreira S. T., Klein W. L. The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer's disease. Neurobiology of Learning and Memory. 2011;96(4):529–543. doi: 10.1016/j.nlm.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly B. L., Ferreira A. β-Amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. Journal of Biological Chemistry. 2006;281(38):28079–28089. doi: 10.1074/jbc.m605081200. [DOI] [PubMed] [Google Scholar]
- 49.Knobloch M., Farinelli M., Konietzko U., Nitsch R. M., Mansuy I. M. Aβ oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. Journal of Neuroscience. 2007;27(29):7648–7653. doi: 10.1523/jneurosci.0395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shankar G. M., Bloodgood B. L., Townsend M., Walsh D. M., Selkoe D. J., Sabatini B. L. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. The Journal of Neuroscience. 2007;27(11):2866–2875. doi: 10.1523/jneurosci.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peineau S., Taghibiglou C., Bradley C., et al. LTP inhibits LTD in the hippocampus via regulation of GSK3β . Neuron. 2007;53(5):703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 52.Li Z., Jo J., Jia J.-M., et al. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141(5):859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jo J., Whitcomb D. J., Olsen K. M., et al. Aβ 1−42 &inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3β . Nature Neuroscience. 2011;14(5):545–547. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- 54.Wei J., Liu W., Yan Z. Regulation of AMPA receptor trafficking and function by glycogen synthase kinase 3. The Journal of Biological Chemistry. 2010;285(34):26369–26376. doi: 10.1074/jbc.m110.121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen L., Chetkovich D. M., Petralia R. S., et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408(6815):936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 56.Elias G. M., Funke L., Stein V., Grant S. G., Bredt D. S., Nicoll R. A. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52(2):307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 57.El-Husseini A. E.-D., Schnell E., Chetkovich D. M., Nicoll R. A., Bredt D. S. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290(5495):1364–1368. [PubMed] [Google Scholar]
- 58.Schnell E., Sizemore M., Karimzadegan S., Chen L., Bredt D. S., Nicoll R. A. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ehrlich I., Klein M., Rumpel S., Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(10):4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Béïque J.-C., Lin D.-T., Kang M.-G., Aizawa H., Takamiya K., Huganir R. L. Synapse-specific regulation of AMPA receptor function by PSD-95. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(51):19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim M. J., Futai K., Jo J., Hayashi Y., Cho K., Sheng M. Synaptic accumulation of PSD-95 and synaptic function regulated by phosphorylation of serine-295 of PSD-95. Neuron. 2007;56(3):488–502. doi: 10.1016/j.neuron.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Nelson C. D., Kim M. J., Hsin H., Chen Y., Sheng M. Phosphorylation of threonine-19 of PSD-95 by GSK-3β is required for PSD-95 mobilization and long-term depression. The Journal of Neuroscience. 2013;33(29):12122–12135. doi: 10.1523/jneurosci.0131-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lovestone S., Reynolds C. H., Latimer D., et al. Alzheimer's disease-like phosphorylation of the microtubule-associated protein tau by glycogen synthase kinase-3 in transfected mammalian cells. Current Biology. 1994;4(12):1077–1086. doi: 10.1016/S0960-9822(00)00246-3. [DOI] [PubMed] [Google Scholar]
- 64.Takashima A., Honda T., Yasutake K., et al. Activation of tau protein kinase I/glycogen synthase kinase-3β by amyloid β peptide (25–35) enhances phosphorylation of tau in hippocampal neurons. Neuroscience Research. 1998;31(4):317–323. doi: 10.1016/s0168-0102(98)00061-3. [DOI] [PubMed] [Google Scholar]
- 65.Hoover B. R., Reed M. N., Su J., et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68(6):1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ittner L. M., Ke Y. D., Delerue F., et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer's disease mouse models. Cell. 2010;142(3):387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 67.Zempel H., Thies E., Mandelkow E., Mandelkow E.-M. Aβ oligomers cause localized Ca2+ elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. Journal of Neuroscience. 2010;30(36):11938–11950. doi: 10.1523/jneurosci.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller E. C., Teravskis P. J., Dummer B. W., Zhao X., Huganir R. L., Liao D. Tau phosphorylation and tau mislocalization mediate soluble Aβ oligomer-induced AMPA glutamate receptor signaling deficits. European Journal of Neuroscience. 2014;39(7):1214–1224. doi: 10.1111/ejn.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kimura T., Whitcomb D. J., Jo J., et al. Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1633) doi: 10.1098/rstb.2013.0144.20130144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Regan P., Piers T., Yi J. H., et al. Tau phosphorylation at serine 396 residue is required for hippocampal LTD. Journal of Neuroscience. 2015;35(12):4804–4812. doi: 10.1523/jneurosci.2842-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Citri A., Bhattacharyya S., Ma C., et al. Calcium binding to PICK1 is essential for the intracellular retention of AMPA receptors underlying long-term depression. Journal of Neuroscience. 2010;30(49):16437–16452. doi: 10.1523/jneurosci.4478-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim C.-H., Hee Jung Chung, Lee H.-K., Huganir R. L. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(20):11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin D.-T., Huganir R. L. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. Journal of Neuroscience. 2007;27(50):13903–13908. doi: 10.1523/jneurosci.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thorsen T. S., Madsen K. L., Rebola N., et al. Identification of a small-molecule inhibitor of the PICK1 PDZ domain that inhibits hippocampal LTP and LTD. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(1):413–418. doi: 10.1073/pnas.0902225107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rocca D. L., Martin S., Jenkins E. L., Hanley J. G. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nature Cell Biology. 2008;10(3):259–271. doi: 10.1038/ncb1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anggono V., Koç-Schmitz Y., Widagdo J., et al. PICK1 interacts with PACSIN to regulate AMPA receptor internalization and cerebellar long-term depression. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(34):13976–13981. doi: 10.1073/pnas.1312467110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yagishita S., Murayama M., Ebihara T., Maruyama K., Takashima A. Glycogen synthase kinase 3β-mediated phosphorylation in the most C-terminal Region of Protein Interacting with C Kinase 1 (PICK1) regulates the binding of PICK1 to glutamate receptor subunit GluA2. The Journal of Biological Chemistry. 2015;290(49):29438–29448. doi: 10.1074/jbc.m114.619668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahmadian G., Ju W., Liu L., et al. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. The EMBO Journal. 2004;23(5):1040–1050. doi: 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seidenman K. J., Steinberg J. P., Huganir R., Malinow R. Glutamate receptor subunit 2 serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. Journal of Neuroscience. 2003;23(27):9220–9228. doi: 10.1523/JNEUROSCI.23-27-09220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hayashi T., Huganir R. L. Tyrosine phosphorylation and regulation of the AMPA receptor by Src family tyrosine kinases. Journal of Neuroscience. 2004;24(27):6152–6160. doi: 10.1523/JNEUROSCI.0799-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hee Jung Chung, Xia J., Scannevin R. H., Zhang X., Huganir R. L. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. Journal of Neuroscience. 2000;20(19):7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dong Z., Han H., Li H., et al. Long-term potentiation decay and memory loss are mediated by AMPAR endocytosis. The Journal of Clinical Investigation. 2015;125(1):234–247. doi: 10.1172/jci77888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alfonso S., Kessels H. W., Banos C. C., et al. Synapto-depressive effects of amyloid beta require PICK1. European Journal of Neuroscience. 2014;39(7):1225–1233. doi: 10.1111/ejn.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Su S. C., Tsai L.-H. Cyclin-dependent kinases in brain development and disease. Annual Review of Cell and Developmental Biology. 2011;27:465–491. doi: 10.1146/annurev-cellbio-092910-154023. [DOI] [PubMed] [Google Scholar]
- 85.Bibb J. A., Snyder G. L., Nishi A., et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402(6762):669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 86.Hemmings H. C., Jr., Greengard P., Lim Tung H. Y., Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310(5977):503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- 87.Halpain S., Girault J.-A., Greengard P. Activation of NMDA receptors induces dephosphorylation of DARPP-32 in rat striatal slices. Nature. 1990;343(6256):369–372. doi: 10.1038/343369a0. [DOI] [PubMed] [Google Scholar]
- 88.Gu Z., Liu W., Yan Z. β-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. Journal of Biological Chemistry. 2009;284(16):10639–10649. doi: 10.1074/jbc.m806508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kristensen A. S., Jenkins M. A., Banke T. G., et al. Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nature Neuroscience. 2011;14(6):727–735. doi: 10.1038/nn.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lisman J., Yasuda R., Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nature Reviews Neuroscience. 2012;13(3):169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tomita S., Stein V., Stocker T. J., Nicoll R. A., Bredt D. S. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45(2):269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 92.De Felice F. G., Velasco P. T., Lambert M. P., et al. Aβ oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. The Journal of Biological Chemistry. 2007;282(15):11590–11601. doi: 10.1074/jbc.m607483200. [DOI] [PubMed] [Google Scholar]
- 93.Decker H., Jürgensen S., Adrover M. F., et al. N-Methyl-D-aspartate receptors are required for synaptic targeting of Alzheimer's toxic amyloid-β peptide oligomers. Journal of Neurochemistry. 2010;115(6):1520–1529. doi: 10.1111/j.1471-4159.2010.07058.x. [DOI] [PubMed] [Google Scholar]
- 94.Kurup P., Zhang Y., Xu J., et al. Aβ-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61 . The Journal of Neuroscience. 2010;30(17):5948–5957. doi: 10.1523/jneurosci.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y., Kurup P., Xu J., et al. Reduced levels of the tyrosine phosphatase STEP block beta amyloid-mediated GluA1/GluA2 receptor internalization. Journal of Neurochemistry. 2011;119(3):664–672. doi: 10.1111/j.1471-4159.2011.07450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chin J., Palop J. J., Puoliväli J., et al. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer's disease. The Journal of Neuroscience. 2005;25(42):9694–9703. doi: 10.1523/jneurosci.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen S., Yadav S. P., Surewicz W. K. Interaction between human prion protein and amyloid-β (Aβ) oligomers: role of N-terminal residues. Journal of Biological Chemistry. 2010;285(34):26377–26383. doi: 10.1074/jbc.m110.145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laurén J., Gimbel D. A., Nygaard H. B., Gilbert J. W., Strittmatter S. M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-Β oligomers. Nature. 2009;457(7233):1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Um J. W., Nygaard H. B., Heiss J. K., et al. Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nature Neuroscience. 2012;15(9):1227–1235. doi: 10.1038/nn.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.You H., Tsutsui S., Hameed S., et al. Aβ neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-D-aspartate receptors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(5):1737–1742. doi: 10.1073/pnas.1110789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gimbel D. A., Nygaard H. B., Coffey E. E., et al. Memory impairment in transgenic alzheimer mice requires cellular prion protein. Journal of Neuroscience. 2010;30(18):6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kessels H. W., Nguyen L. N., Nabavi S., Malinow R. The prion protein as a receptor for amyloid-γ 2. Nature. 2010;466(7308):E3–E5. doi: 10.1038/nature09217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barry A. E., Klyubin I., Mc Donald J. M., et al. Alzheimer's disease brain-derived amyloid-β-mediated inhibition of LTP In Vivo is prevented by immunotargeting cellular prion protein. Journal of Neuroscience. 2011;31(20):7259–7263. doi: 10.1523/jneurosci.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Freir D. B., Nicoll A. J., Klyubin I., et al. Interaction between prion protein and toxic amyloid β assemblies can be therapeutically targeted at multiple sites. Nature Communications. 2011;2, article 336 doi: 10.1038/ncomms1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Calella A. M., Farinelli M., Nuvolone M., et al. Prion protein and Aβ-related synaptic toxicity impairment. EMBO Molecular Medicine. 2010;2(8):306–314. doi: 10.1002/emmm.201000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kessels H. W., Nabavi S., Malinow R. Metabotropic NMDA receptor function is required for β-amyloid-induced synaptic depression. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(10):4033–4038. doi: 10.1073/pnas.1219605110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tamburri A., Dudilot A., Licea S., Bourgeois C., Boehm J. NMDA-receptor activation but not ion flux is required for amyloid-beta induced synaptic depression. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0065350.e65350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Birnbaum J. H., Bali J., Rajendran L., Nitsch R. M., Tackenberg C. Calcium flux-independent NMDA receptor activity is required for Aβ oligomer-induced synaptic loss. Cell Death & Disease. 2015;6, article e1791 doi: 10.1038/cddis.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nabavi S., Kessels H. W., Alfonso S., Aow J., Fox R., Malinow R. Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(10):4027–4032. doi: 10.1073/pnas.1219454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aow J., Dore K., Malinow R. Conformational signaling required for synaptic plasticity by the NMDA receptor complex. Proceedings of the National Academy of Sciences. 2015;112(47):14711–14716. doi: 10.1073/pnas.1520029112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dore K., Aow J., Malinow R. Agonist binding to the NMDA receptor drives movement of its cytoplasmic domain without ion flow. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(47):14705–14710. doi: 10.1073/pnas.1520023112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schneider L. S., Dagerman K. S., Higgins J. P. T., McShane R. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Archives of Neurology. 2011;68(8):991–998. doi: 10.1001/archneurol.2011.69. [DOI] [PubMed] [Google Scholar]
- 113.Niswender C. M., Conn P. J. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annual Review of Pharmacology and Toxicology. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lüscher C., Huber K. M. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65(4):445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Snyder E. M., Philpot B. D., Huber K. M., Dong X., Fallon J. R., Bear M. F. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nature Neuroscience. 2001;4(11):1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 116.Wang Y. T., Linden D. J. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25(3):635–647. doi: 10.1016/S0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- 117.Xia J., Chung H. J., Wihler C., Huganir R. L., Linden D. J. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28(2):499–510. doi: 10.1016/S0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 118.Chung H. J., Steinberg J. P., Huganir R. L., Linden D. J. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300(5626):1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- 119.Steinberg J. P., Takamiya K., Shen Y., et al. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49(6):845–860. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 120.Moult P. R., Gladding C. M., Sanderson T. M., et al. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. The Journal of Neuroscience. 2006;26(9):2544–2554. doi: 10.1523/jneurosci.4322-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y., Venkitaramani D. V., Gladding C. M., et al. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. Journal of Neuroscience. 2008;28(42):10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scholz R., Berberich S., Rathgeber L., Kolleker A., Köhr G., Kornau H.-C. AMPA receptor signaling through BRAG2 and Arf6 critical for long-term synaptic depression. Neuron. 2010;66(5):768–780. doi: 10.1016/j.neuron.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y., Kurup P., Xu J., et al. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive andcellular deficits in an Alzheimer's disease mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(44):19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu J., Chatterjee M., Baguley T. D., et al. Inhibitor of the tyrosine phosphatase STEP reverses cognitive deficits in a mouse model of Alzheimer's disease. PLoS Biology. 2014;12(8) doi: 10.1371/journal.pbio.1001923.e1001923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rush A. M., Wu J., Rowan M. J., Anwyl R. Group I metabotropic glutamate receptor (mGluR)-dependent long-term depression mediated via p38 mitogen-activated protein kinase is inhibited by previous high-frequency stimulation and activation of mGluRs and protein kinase C in the rat dentate gyrus in vitro. Journal of Neuroscience. 2002;22(14):6121–6128. doi: 10.1523/JNEUROSCI.22-14-06121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moult P. R., Corrêa S. A. L., Collingridge G. L., Fitzjohn S. M., Bashir Z. I. Co-activation of p38 mitogen-activated protein kinase and protein tyrosine phosphatase underlies metabotropic glutamate receptor-dependent long-term depression. Journal of Physiology. 2008;586(10):2499–2510. doi: 10.1113/jphysiol.2008.153122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huang C.-C., You J.-L., Wu M.-Y., Hsu K.-S. Rap1-induced p38 mitogen-activated protein kinase activation facilitates AMPA receptor trafficking via the GDI·Rab5 complex: potential role in (S)-3,5-dihydroxyphenylglycine-induced long term depression. Journal of Biological Chemistry. 2004;279(13):12286–12292. doi: 10.1074/jbc.m312868200. [DOI] [PubMed] [Google Scholar]
- 128.Gallagher S. M., Daly C. A., Bear M. F., Huber K. M. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. Journal of Neuroscience. 2004;24(20):4859–4864. doi: 10.1523/jneurosci.5407-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huber K. M., Kayser M. S., Bear M. F. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288(5469):1254–1256. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 130.Waung M. W., Huber K. M. Protein translation in synaptic plasticity: mGluR-LTD, Fragile X. Current Opinion in Neurobiology. 2009;19(3):319–326. doi: 10.1016/j.conb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Waung M. W., Pfeiffer B. E., Nosyreva E. D., Ronesi J. A., Huber K. M. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59(1):84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Park S., Park J. M., Kim S., et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59(1):70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Davidkova G., Carroll R. C. Characterization of the role of microtubule-associated protein 1B in metabotropic glutamate receptor-mediated endocytosis of AMPA receptors in hippocampus. The Journal of Neuroscience. 2007;27(48):13273–13278. doi: 10.1523/jneurosci.3334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Seog D.-H. Glutamate receptor-interacting protein 1 protein binds to the microtubule-associated protein. Bioscience, Biotechnology and Biochemistry. 2004;68(8):1808–1810. doi: 10.1271/bbb.68.1808. [DOI] [PubMed] [Google Scholar]
- 135.Byers C. E., Barylko B., Ross J. A., et al. Enhancement of dynamin polymerization and GTPase activity by Arc/Arg3.1. Biochimica et Biophysica Acta (BBA): General Subjects. 2015;1850(6):1310–1318. doi: 10.1016/j.bbagen.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chowdhury S., Shepherd J. D., Okuno H., et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52(3):445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lacor P. N., Buniel M. C., Chang L., et al. Synaptic targeting by Alzheimer's-related amyloid β oligomers. Journal of Neuroscience. 2004;24(45):10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu J., Petralia R. S., Kurushima H., et al. Arc/Arg3.1 regulates an endosomal pathway essential for activity-dependent β-amyloid generation. Cell. 2011;147(3):615–628. doi: 10.1016/j.cell.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Renner M., Lacor P. N., Velasco P. T., et al. Deleterious effects of amyloid β oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66(5):739–754. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Q., Walsh D. M., Rowan M. J., Selkoe D. J., Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid β-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. The Journal of Neuroscience. 2004;24(13):3370–3378. doi: 10.1523/jneurosci.1633-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen X., Lin R., Chang L., et al. Enhancement of long-term depression by soluble amyloid β protein in rat hippocampus is mediated by metabotropic glutamate receptor and involves activation of p38MAPK, STEP and caspase-3. Neuroscience. 2013;253:435–443. doi: 10.1016/j.neuroscience.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 142.Rammes G., Hasenjäger A., Sroka-Saidi K., Deussing J. M., Parsons C. G. Therapeutic significance of NR2B-containing NMDA receptors and mGluR5 metabotropic glutamate receptors in mediating the synaptotoxic effects of β-amyloid oligomers on long-term potentiation (LTP) in murine hippocampal slices. Neuropharmacology. 2011;60(6):982–990. doi: 10.1016/j.neuropharm.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 143.Hu N. W., Nicoll A. J., Zhang D., et al. mGlu5 receptors and cellular prion protein mediate amyloid-β-facilitated synaptic long-term depression in vivo. Nature Communications. 2014;5, article 3374 doi: 10.1038/ncomms4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Um J. W., Kaufman A. C., Kostylev M., et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer aβ oligomer bound to cellular prion protein. Neuron. 2013;79(5):887–902. doi: 10.1016/j.neuron.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hamilton A., Esseltine J. L., Devries R. A., Cregan S. P., Ferguson S. S. G. Metabotropic glutamate receptor 5 knockout reduces cognitive impairment and pathogenesis in a mouse model of Alzheimer's disease. Molecular Brain. 2014;7, article 40 doi: 10.1186/1756-6606-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haas L. T., Salazar S. V., Kostylev M. A., Um J. W., Kaufman A. C., Strittmatter S. M. Metabotropic glutamate receptor 5 couples cellular prion protein to intracellular signalling in Alzheimer's disease. Brain. 2016;139, Part 2:526–546. doi: 10.1093/brain/awv356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Raka F., Di Sebastiano A. R., Kulhawy S. C., et al. Ca2+/calmodulin-dependent protein kinase II interacts with group I metabotropic glutamate and facilitates receptor endocytosis and ERK1/2 signaling: role of β-amyloid. Molecular Brain. 2015;8, article 21 doi: 10.1186/s13041-015-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jin D., Xue B., Mao L., Wang J. Q. Metabotropic glutamate receptor 5 upregulates surface NMDA receptor expression in striatal neurons via CaMKII. Brain Research. 2015;1624:414–423. doi: 10.1016/j.brainres.2015.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen H.-H., Liao P.-F., Chan M.-H. MGluR5 positive modulators both potentiate activation and restore inhibition in NMDA receptors by PKC dependent pathway. Journal of Biomedical Science. 2011;18(1, article 19) doi: 10.1186/1423-0127-18-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alagarsamy S., Saugstad J., Warren L., Mansuy I. M., Gereau R. W., IV, Conn P. J. NMDA-induced potentiation of mGluR5 is mediated by activation of protein phosphatase 2B/calcineurin. Neuropharmacology. 2005;49(supplement 1):135–145. doi: 10.1016/j.neuropharm.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alagarsamy S., Marino M. J., Rouse S. T., Gereau R. W., IV, Heinemann S. F., Conn P. J. Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nature Neuroscience. 1999;2(3):234–240. doi: 10.1038/6338. [DOI] [PubMed] [Google Scholar]
- 152.Alagarsamy S., Rouse S. T., Junge C., et al. NMDA-induced phosphorylation and regulation of mGluR5. Pharmacology Biochemistry and Behavior. 2002;73(2):299–306. doi: 10.1016/S0091-3057(02)00826-2. [DOI] [PubMed] [Google Scholar]
- 153.Bortolotto Z. A., Collett V. J., Conquet F., Jia Z., Van Der Putten H., Collingridge G. L. The regulation of hippocampal LTP by the molecular switch, a form of metaplasticity, requires mGlu5 receptors. Neuropharmacology. 2005;49(supplement 1):13–25. doi: 10.1016/j.neuropharm.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 154.Pietraszek M., Gravius A., Schäfer D., Weil T., Trifanova D., Danysz W. mGluR5, but not mGluR1, antagonist modifies MK-801-induced locomotor activity and deficit of prepulse inhibition. Neuropharmacology. 2005;49(1):73–85. doi: 10.1016/j.neuropharm.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 155.Gravius A., Pietraszek M., Schmidt W. J., Danysz W. Functional interaction of NMDA and group I metabotropic glutamate receptors in negatively reinforced learning in rats. Psychopharmacology. 2006;185(1):58–65. doi: 10.1007/s00213-005-0249-3. [DOI] [PubMed] [Google Scholar]
- 156.Hsu J.-C., Cheng S.-J., Yang H.-W., et al. Bidirectional synaptic plasticity induced by conditioned stimulations with different number of pulse at hippocampal CA1 synapses: roles of N-methyl-D-aspartate and metabotropic glutamate receptors. Synapse. 2011;65(8):795–803. doi: 10.1002/syn.20906. [DOI] [PubMed] [Google Scholar]
- 157.Goo M. S., Scudder S. L., Patrick G. N. Ubiquitin-dependent trafficking and turnover of ionotropic glutamate receptors. Frontiers in Molecular Neuroscience. 2015;8, article 60 doi: 10.3389/fnmol.2015.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Widagdo J., Anggono V. Ubiquitin signals the demise of AMPA receptors. Oncotarget. 2015;6(18):15718–15719. doi: 10.18632/oncotarget.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Widagdo J., Chai Y., Ridder M., et al. Activity-dependent ubiquitination of GluA1 and GluA2 regulates AMPA receptor intracellular sorting and degradation. Cell Reports. 2015;10(5):783–795. doi: 10.1016/j.celrep.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schwarz L. A., Hall B. J., Patrick G. N. Activity-dependent ubiquitination of GluA1 mediates a distinct AMPA receptor endocytosis and sorting pathway. Journal of Neuroscience. 2010;30(49):16718–16729. doi: 10.1523/JNEUROSCI.3686-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lussier M. P., Nasu-Nishimura Y., Roche K. W. Activity-dependent ubiquitination of the AMPA receptor subunit GluA2. Journal of Neuroscience. 2011;31(8):3077–3081. doi: 10.1523/jneurosci.5944-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lussier M. P., Herring B. E., Nasu-Nishimura Y., et al. Ubiquitin ligase RNF167 regulates AMPA receptor-mediated synaptic transmission. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(47):19426–19431. doi: 10.1073/pnas.1217477109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lin A., Man H.-Y. Endocytic adaptor epidermal growth factor receptor substrate 15 (Eps15) is involved in the trafficking of ubiquitinated α-amino-3-hydroxy-5-methyl-4- isoxazolepropionic acid receptors. Journal of Biological Chemistry. 2014;289(35):24652–24664. doi: 10.1074/jbc.M114.582114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rodrigues E. M., Scudder S. L., Goo M. S., Patrick G. N. Aβ-induced synaptic alterations require the E3 ubiquitin ligase Nedd4-1. The Journal of Neuroscience. 2016;36(5):1590–1595. doi: 10.1523/jneurosci.2964-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]