Abstract
Objectives:
This study investigated the effects of post bleaching treatments to prevent restaining and the change of enamel surface microhardness after dental bleaching in vitro.
Methods:
Sixty intact human incisor teeth were stained in tea solution and randomly assigned into four groups (n=15). Then samples were bleached for two weeks (8 hours daily) by 15% carbamide peroxide. Tooth color was determined both with a spectrophotometer and visually before bleaching (T1) and immediately after bleaching (T2). Next, it was applied in group 1 fluoride (Naf 2%) gel for 2 minutes, and in group 2 a fractional CO2 laser (10 mJ, 200 Hz, 10 s), and in group 3, nanohydroxyapatite gel for 2 minutes. The bleached teeth in group 4 remained untreated (control group). Then teeth placed in tea solution again. Color examinations were repeated after various post bleaching treatments (T3) and restaining with tea (T4) and color change values recorded. The microhardness was measured at the enamel surface of samples. Data was analyzed using ANOVA, Tukey HSD test and Dunnett T3 (α = 0.05).
Results:
Directly after bleaching (ΔE T3-T2), the treatment with nanohydroxyapatite showed significantly the least color lapse in colorimetric evaluation. In experimental groups, the color change between T3 and T4 stages (ΔE T4-T3) was significantly lower than control group (P < 0.05). Different methods of enamel treatment caused a significant increase in surface microhardness compared to control group (P < 0.05).
Significance:
Application of fluoride, fractional CO2 laser and nanohydroxyapatite as post bleaching treatments are suggested for prevention of stain absorption and increasing the hardening of bleached enamel.
Keywords: Bleaching, enamel, fluoride, laser CO2, microhardness, nanohydroxy apatite.
INTRODUCTION
Although vital bleaching with carbamide peroxide (CP) is becoming more and more popular, there are some studies found surface softening, mineral loss, increased susceptibility to erosion or caries, reduced fracture stability or a decrease in abrasion resistance of bleached dental hard tissues [1-4]. It was shown that the loss of microhardness in bleached enamel could be outweighed by a remineralization period following the bleaching period [5]. No clinical studies or case reports in the literature have documented macroscopically or clinically visible damage due to vital bleaching or clinically relevant tissue destruction, it is important to minimize the risk of even minor damage in order to ensure life-long integrity of dental hard tissue. It has been shown that the loss of enamel microhardness due to carbamide peroxide gels is not limited to the enamel surface and also be detected in the subsurface within the outermost enamel layer [6, 7]. It is conceivable that a dietary component, such as tea consumed during or just after the completion of bleaching treatment may lead to staining of the bleached enamel surface [8]. There is little research available to evaluate the effect of tea, coffee, cola etc. on the color of freshly bleached enamel surface of human teeth. It was shown that the loss of microhardness in bleached enamel could be reduced when fluoride compounds were administered after the bleaching steps or during the bleaching period [9]. The literature review also does not show much study done to analyze the effect of various remineralizing or treatment agents such as nanohydroxyapatite or fractional CO2 laser applied over the freshly home bleached enamel surface of human teeth, to prevent stain absorption and incrassing surface microhardness. Several types of lasers, with different parameter settings, have been used for enamel reinforcement. The CO2 laser (9.3, 9.6, 10.3 and 10.6 µ wavelengths), however, should be considered the mainstay in enamel hardening, because the absorption bands of phosphate, carbonate and hydroxyl groups of enamel and dentin structures are within 9.0 to 11.0 µ region which coincide well with the wavelengths of the CO2 laser [10]. It was thought if the enamel of freshly bleached tooth was surface treated, so that along with the effect of reduced sensitivity, it may reduce the absorption of stains and therefore maintain the effect of bleaching for a longer time [11]. However, there is controversy regarding whether this treatment improves the porous enamel or it just rehardens the surface layer with less effect on its appearance. Some studies to date utilized conventional CO2 lasers for assisting in bleaching, and to the best of the authors’ knowledge, no one employed a fractional CO2 laser as an enamel post bleaching treatment. Hence, the aim of this study was to investigate the effects of fluoride, fractional CO2 laser irradiation and nanohydroxyapatite to prevent enamel restaining and microhardness improvment after dental bleaching.
MATERIALS AND METHODS
Sixty extracted human incisor teeth with no visible caries or structural defects on enamel surface were collected, cleaned and stored in a 0.1% thymol solution at room temperature. Ethics approval for the collection of teeth and for this in vitro study was obtained (University of Mashhad Office of Research Ethics Protocol # 910650). Teeth were stained by embedding for 7 days at room temprature in the tea solution that was prepared by immersing 2g black tea into 100 ml of boiling distilled water for 5 minutes and filtered through gauze to remove the tea from the infusion [8]. Then samples were randomly assigned into four groups (n=15). The shade was determined by one examiner with two different techniques: an objective evaluation using spectrophotometric VITA Easyshade (VITA Zahnfabrik H Rauter GmbH & Co KG, Deasyas-79704, Germany), which allowed the measurment of CIELAB color variables (L, a, b); and the subjective visual evaluation with the VITA shade guide (Table 1). Before bleaching, the teeth were polished with a fluoride free tooth paste. Then samples were bleached for two weeks (8 hours daily) with 15% carbamide peroxide (Opalescence, Ultradent Products, Inc, South Jordan, UT, USA). The measurement area of interest for shade matching was the middle one third of the facial surface of the incisor teeth. The examiner was previously calibrated for both color selection methods. Tooth color was determined both with a spectrophotometer and visually before bleaching (T1) and immediately post bleaching (T2). Afterward, samples in group 1 were exposed to fluoride (NaF 2%, Dentscare Ltda, Duo Desense, Brazil) gel for 2 minutes with a brush. In group 2, enamel was exposed to irradiation from a fractional CO2 laser (wavelength 10.6 µm, Lutronic Inc., Princeton Junction, NJ, USA). The laser operated in the dynamic mode with frequency of 200 Hz, 10 mJ of energy and power of 10 W, and the beam was adjusted to cover a square area of 8×8 mm2 in the center of teeth facial surface for 10 s per tooth. The laser’s handpiece was held manually at an approximate distance of 55 mm from the sample surface by one investigator (HM). The specimens in group 3 were subjected to nanohydroxyapatite (Sigma Aldrich, 677418 DG, USA) cream for 2 minutes with a brush. Samples in the experimental groups were treated by one of the mentioned treatments after bleaching, while the bleached teeth in group 4 remained untreated (control group). Color examinations were repeated after various treatments (T3). Then teeth placed in tea solution again and restained with tea in stage (T4) and color change values were recorded.
Table 1. Vita shade guide with 16 shades ranked from the lightest color on the left to the darkest color on the right [12, 13].
| B1 | A1 | B2 | D2 | A2 | C1 | C2 | D4 | A3 | D3 | B3 | B3,5 | B4 | C3 | A4 | C4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
For spectrophotometric evaluation, each of the tooth samples was embedded in light green colored putty silicone (Coltene, Swiss, Speedex) with approximately 10 mm thickness, to ensure standardized positioning of the labial tooth surface from the spectrophotometer probe. Putty was used so that the samples could be easily retrieved after each spectrophotometric analysis. A window was created on the labial surface of the molded silicone guide for the incisor tooth to be evaluated. The window was made using a metal device with well-formed borders and radius of 3 mm. The measurements were made by only one operator. The shade was determined using the parameters of the Easyshade device, which indicated the following values: L*, a* and b*, in which L* represents the value from 0 (black) to 100 (white) and a* and b* represent the shade, where a* is the measurement along the red-green axis and b* is the measurement along the yellow-blue axis. The shade comparison before and after various treatments is given by the differences between two shades (∆E) using the following formula:
Shade selection was carried out under similar laboratory conditions for each sample in a neutral colored room. At each examination time point, the digital shade evaluation was performed first and then visual one. For visual evaluation that is an subjective examination, the 16 shade guide tabs were arranged from highest (B1) to lowest (C4) value, making the minimum qualifying shade C2 as number 7 (seventh tab on the value ordered arrangement) (Table 1). Although this scale is not linear in the truest sense, the changes were treated as though they represented a continuous and approximately linear ranking for the purpose of analysis. The shade comparison before and after treatments and staining is given by the difference between the time evaluations shade (DSGU) as below formula:
Δ Vita shade change = Vita score (after treatment)-Vita score (before treatment)
A schematically outlay of the experiment including the bleaching and post bleaching treatment and restaining processes and color evaluations by visual and digital methods are presented in Fig. (1).
Fig. (1).
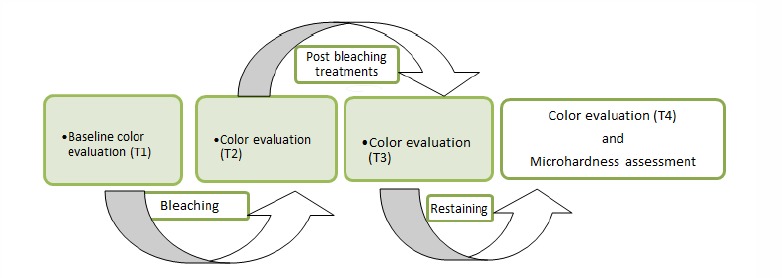
A schematically outlay of the treatment processes and evaluations.
Microhardness Assessment
After completing color measurements, the crowns were separated from the roots and the teeth were embedded in epoxy resin, and their labial surfaces were polished. A micro Vickers hardness tester (Matsuzawa, model MHT2, Japan) was utilized under a load of 300 g and a dwell time of 5 seconds for microhardenss measurement, placing its indenter at the labial enamel surface, and repeated at three ponits with 100 micron distance from each other on the surface.
Statistical Analysis
Normal distribution of the data and homogeneity of variances were confirmed by Kolmogrov-Smirnov and Levene’s tests, respectively. A one-way ANOVA was performed to compare the color change (∆E & ΔV) obtained from the two measurements at T1 to T4 time points between the groups, followed by Tukey HSD test for pairwise comparisons. The between group differences in microhardness were also determined by ANOVA and Dunnett T3 tests at the enamel surface. The statistical analysis was performed using Statistical Package for the Social Sciences (version 16.0, SPSS Inc, Chicago, Ill) at the 95% confidence interval of the difference.
RESULTS
The color changes at each tested time for both types of color assessment are given in Table 2. The statistical analysis was performed seperately for each kind of color evaluation (digital with spectrophotometer and visual). The digital evaluation revealed in all samples a greater color shade change compared with the visual assessment, at each tested time and for all the post bleaching treatments used. Figs. (2-5) illustrate the color change achived with each post bleaching treatment methods for both evaluation methods up to tea restaining after the bleaching procedure.
Table 2.
Mean values of shade changes (Δ-Values) ± standard deviations for all groups and at each tested time
| Type of post bleaching surface treatment | Shade evaluation | Post bleaching ΔE,ΔV (T2-T1) | After surface treatment ΔE,ΔV (T3-T2) |
After tea restaining ΔE,ΔV(T4-T3) |
After tea restaining with baseline ΔE,ΔV(T4-T1) |
|---|---|---|---|---|---|
| Group 1 (Fluoride) | Digital | 11.80 ± 6.1 a | 5.17 ± 3.7a | 3.71 ± .34a | 12.13 ± .38a |
| Visual | 5.06 ± 2.9A | .10 ± .31A | .76 ± .35A | 5.40 ± .27A | |
| Group 2 (Fractional CO2 laser) | Digital | 11.19 ± 5.3 a | 4.54 ± .30a | 3.07 ± .14a | 8.49 ± .50ab |
| Visual | 5.30 ± 2.4A | .36 ± 0.2A | 2.20 ± .38A | 3.46 ± .40B | |
| Group 3 (Nanohydroxyapatite) | Digital | 11.20 ± 4.9 a | 3.26 ± .19a | 2.73 ± 1.6a | 12.13 ± .58a |
| Visual | 5.03 ± 3.9A | .53 ± .29A | .43 ± .03A | 4.06 ± .41B | |
| Group 4 (Control) | Digital | 11.57 ± 7.7 a | - | 8.30 ± .42b | 6.71 ± .58b |
| Visual | 5.60 ± 3.5A | - | 3.40 ± .38A | 2.8 ± .048B |
a,A - There is no significant statistic differences between similar characters.
Small letter for digital evaluation and capital letter for visual evaluation.
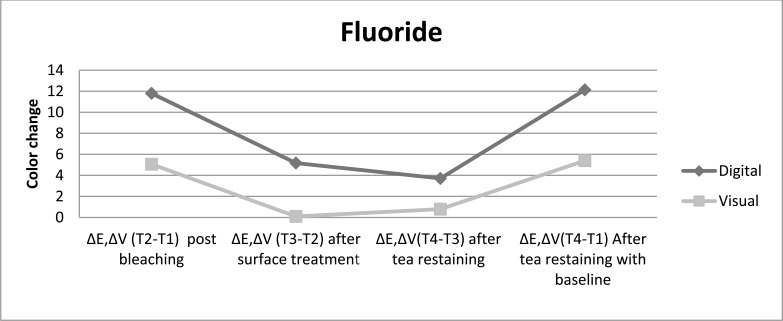
Fig. (2).
The color change achived with fluoride as post bleaching treatment is presented for both color evaluation methods up to restaining after bleaching procedure.
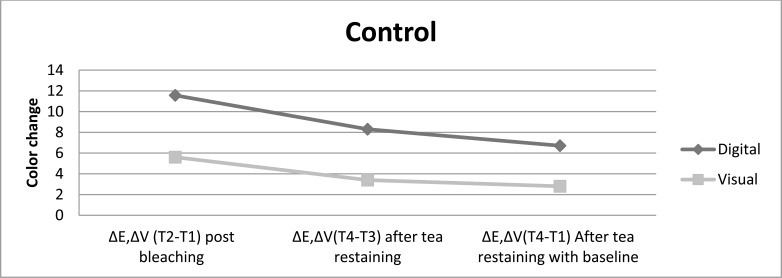
Fig. (5).
The color change achived without any post bleaching treatment is presented for both color evaluation methods up to restaining after bleaching procedure.
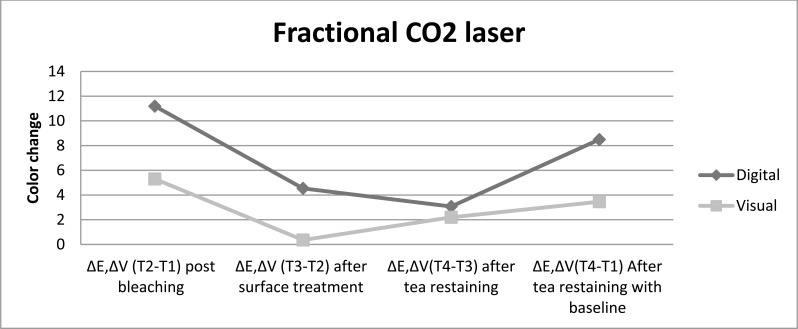
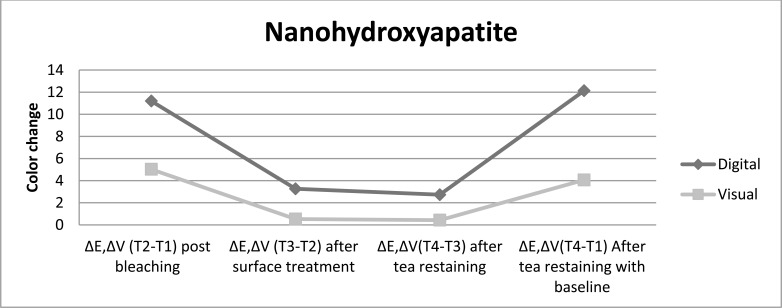
Post bleaching treatment with nanohydroxyapatite showed the lowest shade change after restaining, followed by treatment with fractional CO2 laser and then treatment with fluoride, while without any treatment displayed the highest color lapse, independent of the color evaluation method. The digital evaluation showed color change post bleaching treatment with fluoride > fractional CO2 laser > nanohydroxyapatite>control just after surface treatments, whereas the visual evaluation showed nanohydroxyapatite > fractional CO2 laser > fluoride > control after different surface treatments.
Digital Evaluation
All post bleaching treatments showed significance difference color change in comparison with control group after surface treatment and restaining (P<0.05). Nanohydroxyapatite treatment showed whiter color and resistance to color change after restaining, then fractional CO2 laser and fluoride. In comparison to restaining with baseline indicated that all post bleaching treatments had significantly lighter color than without any treatment. It was also done pairwise analysis of the post bleaching methods tested for each assessment time. In contrast to control group, different treatments bring significant color change after post bleaching surface treatment and tea restaining. There was no significant difference among surface treatment methods and tea restaining in experimental groups in opposes to control group.
Visual Evaluation
Various post bleaching surfeace treatments showed no significance difference color change in comparison with control group after surface treatment and restaining (P>0.05). However the most color change toward the darker shade was related to control group after restaining. Fluoride-treated samples had higher resistance to stain absrpotion than other treatment methods. Pairwise analysis of the post bleaching methods tested for each evaluation time showed significant difference color change post bleaching versus after surface treatment and tea restaining (P<0.05). The color change after surface treatment versus after tea restaining, for all post bleaching methods performed similarly.
Microhardness Assessment
The results of the microhardness measurements are provided in Table 3. The one-way ANOVA displayed significant differences in microhardness at the enamel surface among the experimental groups. Further analysis with Dunnett T3 test revealed that surface microhardness was significantly greater in various post bleaching surface treatment methods as compared with the control group (P<0.05). No significant difference was found in enamel surface microhardness of fluoride, fractional CO2 laser, or nanohydroxyapatite post bleaching treatments (Table 3) (P>0.05.).
Table 3.
Comparison of microhardness at the enamel surface among the groups
| Type of post bleaching surface treatment | Enamel Surface | ||
|---|---|---|---|
| Mean | SD | Pairwise comparisons | |
| Group 1 (Fluoride) | 446.58 | 53.32 | a |
| Group 2 (Fractional CO2 laser) | 429.04 | 29.04 | a |
| Group 3 (Nanohydroxyapatite) | 470.20 | 53.82 | a |
| Group 4 (Control) | 387.70 | 21.25 | b |
| P-value | 0.000* | ||
*Statistically significant difference at P<0.05.
Different letters indicate significant differences at P<0.05.
DISCUSSION
The present study was undertaken to evaluate the effect of fluoride, fractional CO2 laser irradiation and nanohydroxyapatite to prevent enamel restaining and improvement of microhardness after dental bleaching. Although it was utilized conventional CO2 lasers for assisting in bleaching, no one to date employed a fractional CO2 laser as an enamel post bleaching treatment and assessing their effects on enamel stain absorption and microhardness. For all groups the same bleaching agent (15% carbamide peroxide, two weeks, 8 hours daily) was used, making the comparison effect of the post bleaching treatment methods easier. Bleaching restores normal color to a tooth by decolorization of stain with a powerful oxidizing or reducting agent and it has been shown that bleaching agents may alter both enamel morphology and mineral content. The eight-hour daily period of bleaching was chosen to simulate the condition of wearing bleaching tray overnight [8]. Fluoride gel and nanohydroxyapatite were used in the study for surface treatment, just after bleaching procedure. Fluoride gel and nanohydroxyapatite enhance the remineralization of enamel surface, which had been demineralized after bleaching [14]. In present study, CO2 laser was used as post bleaching treatment. We attempted to select parameters that were as closely as possible to those suggested by Esteves-Oliveria et al. [15] which were shown to cause as much as 81% caries inhibition in vitro without any damage to enamel surface. It seems that fractional CO2 laser following absorption, there would be a high temperature increase in the surface and near the surface enamel layers, which results in structural and chemical alterations of enamel including decomposition of organic matrix, reduction of carbonate content, and fusion and recrystallization of hydroxyapatite crystals [10, 16, 17].
After bleaching and surface treatments, samples were stored in tea for 10 minutes surely represents a long period as compared with the shorter contact of the teeth during tea intake. Specimens were exposed for 10 minutes to get the maximum effect of stain. The reason to use tea as a solution for staining of the teeth was, that tea was proved to have a higher capacity to stain teeth than other staining solutions [18]. Attin showed that application of tea solution directly after bleaching with 10% CP, changes the color of enamel towards the darker side [8]. He also advocated the application of fluoride over the bleached enamel surface; to improve the remineralization to reduce sensitivity [19]. Nanohydroxyapatite enhances remineralization of enamel surfaces by occluding the surface microporosities, which results in prevention stain absorption and increasing surface microhardness [20]. Bleaching caused a color shift towards the blue direction within the color space and lightened the color of the teeth. The observed tooth color change was mainly due to a shift of the L* value towards the positive side (more brightness) and the b* value towards the negative side (less yellow and more blue). The a* value also decreased (less red and more green) after bleaching, but it had only a minor influence on the total tooth color change (ΔE*) [21]. It is believed that a ∆E (discrepancy between two hues) exceeding 3.3 units and ΔV grater than 1 shade tab indicate color mismatching, because it would be clinically visible in any site by independent observers. After bleaching the digital color difference between baseline measurement and bleaching stage (∆ET2-T1) was greater than 3.3 units and the visual color change between baseline measurement and bleaching stage (∆VT2-T1) was greater than 4 shade tabs in the four groups, indicating the creation of clinically visible whiter teeth. There was no statistical difference in value of ∆ET2-T1 and ∆VT2-T1 among the groups which was a necessary condition of samples for proper comparison of treatment effects on bleached enamel. Following post bleaching treatment regimen (T3) which included fluoride, fractional CO2 laser, and nanohydroxyapatite, a more decrease in degree of lightness was observed. The color change between the bleaching and treatment stages (∆ET3-T2) was insignificantly greater in group 3 (nanohydroxyapatite) compared to groups 2 (fractional CO2 laser) and group 1 (fluoride), while (∆VT3-T2) was not significant among study groups. It should be mentioned that the group 4 (control) was not calorimetrically and visually evaluated at T3, as it was not exposed to any treatment. After tea restaining (T4), the three tested groups (Groups 1-3) showed singnificantly lighter shade in comparison to group 4, that did not show significant differences among themselves in T3 (surface treatments) and T4 (tea restaining) stages (∆E, ΔVT4-T3). This could be the consequence of similar effect of post bleaching treatments on the bleached enamel. When the digitaly assessment color change between baseline (T1) and final (T4) examinations were compared among the study groups, it was found that the laser groups exhibited a significantly lower color difference compared to fluoride (group 1) and nanohydroxyapatite (group 3) groups. The ∆ET4-T1 in digitaly method evaluation was 12.13 in both groups 1 and 3 (fluoride and nanohydroxyapatite), which were statistically comparable to each other and both were significantly higher than those of the group 2 (fractional CO2 laser) and control (group 4) (8.49 and 6.71, respectively). This was possibly due to the creation of microscopic pores within the structure of laser-irradiated enamel, which enhanced stain absorption immediately after bleaching and the result was to some extent similar to control group. It should be noted that the digitaly color change between baseline (T1) and final (T4) examinations was higher than 3.3 units in the study groups, meaning that enamel appearance at the end of the post bleaching therapy and restaining is different from bleached enamel surface alone when observed with the naked eye. In contrast, the visually (∆VT4-T1) corresponding color change was significantly higher in the fluoride-treated than other groups that are in agreement of previous study [22].
In our study, it was observed that groups 1, 2, and 3 showed the lower change in color (ΔE*) after immersing in tea solution, compared to without surface treated teeth samples (group 4). This could be due to the remineralizing effect of Fluoride, fractional CO2 laser, and nanohydroxyapatite, which tend to prevent the stain absorption more effectively after surface treatment. The current study is the first attempt to prove that fractional CO2 laser irradiation, and nanohydroxyapatite is successful to prevent the stain absorption of home bleached enamel. This may have important clinical implications because the altered color of bleached enamel may produce an esthetic concern even more than many years after bleaching procedure. In the study, the lowest microhardness values were observed in group 4 (VMH =387.70) and the highest ones were found in group 3 (VMH =470.20) at surface enamel layer. Surface microhardness of the specimens in group 1 and 2 were VMH= 446.58 and VMH= 429.04 respectively. In the present study, bleaching alone lead to a microhardness decrease during the bleaching procedure in comparison to post bleached treatment ones. The decrease in microhardness might be attributed to the impact of the bleaching gels themselves. Several aspects of bleaching gels might influence enamel surface hardness, such as the peroxide contained, the thickening agent carbopol or the pH of the bleaching agent [4, 23, 24]. However, the bleaching agent was adjusted to a pH of 6.5, so that the pH of the bleaching gel could have not contributed significantly to enamel demineralization in the present study [25]. In previous studies, utilizing non-fluoridated carbamide peroxide gels, reverse of surface alterations of bleached enamel could be observed in determinations conducted after post-bleaching periods of 3 weeks or 3 months [26, 27]. It might be speculated that the enamel acquisition of fluoride, as observed after application of fluoridated carbamide peroxide bleaching gels [28], might have supported and accelerated rehardening in the post-bleaching treatments in the present study. The present study, control group in oppose to experimental groups shows significantly the least hardness after bleaching, therefore post-bleaching treatments of enamel surfaces is supported by the use of fluoride, fractional CO2 laser, and nanohydroxyapatite is needed for reverse of enamel hardness. Esteves-Oliveria et al. [29] indicated that CO2 laser irradiation was capable to reharden previously softened enamel in vitro. Known fractional photothermolysis may have potential advantages in dentistry, as it is possible to predetermine the exact area of irradiation, and the laser itself irradiates multiple spots within the target area with no need for mannual scanning of the surface [30]. The increased microhardness of the softened enamel following laser irradiation has been attributed to the ultrastructural changes including crystal size growth and recrystallization of porous enamel as a result of high temperature rise in the surface [29, 31]. However, we did not observe possible detrimental influences on enamel morphology such as surface cracking and melted areas. Furthermore, the study design did not include groups that irradiated by laser after using fluoride or nanohydroxyapatite and this could be considered a limitation of the present study. It is possible that laser irradiation produce chemical and structural alterations that facilitate fluoride or nanohydroxyapatite deposition, thus can improve the color and mechanical properties of bleached enamel. The fractional CO2 laser is not commercially designed for dental applications. Further improvement in design and accessories are required to make it more comfortable for dental applications. Further studies are suggested on the potential benefits of the fractional CO2 laser in other fields of dentistry and their effects on the temperature increase in the underlying tissues and surface morphology of sound bleached enamel. Also the reminalization effect of saliva was neglected in the study, since it was the lack of using artificial saliva in order to simulate oral human.
CONCLUSION
Within the limitations of this in vitro study, the following conclusion were drawn:
Application of fluoride, fractional CO2 laser or nanohydroxyapatite was more effective in restoring the appearance of bleached enamel and increasing hardness in compared to without treatment.
Stain absorption was reduced and tooth surfaces showed more color stability with surface treatment of fluoride, nanohydroxyapatite, and fractional CO2 laser.
This study provides a solution to reduce stain absorption by the application of fluoride, nanohydroxyapatite, and fractional CO2 laser which along with increasing microhardness in the period immediately after bleaching, when the surface is still demineralized.
Fig. (3).
The color change achived with fractional CO2 laser as post bleaching treatment is presented for both color evaluation methods up to restaining after bleaching procedure.
Fig. (4).
The color change achived with nanohydroxyapatite as post bleaching treatment is presented for both color evaluation methods up to restaining after bleaching procedure.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the research chancellor of Mashhad University of Medical Sciences for providing this research with financial support (grant number 910650). The results described in this paper have been taken from a DDS, MS post graduate student thesis (# 516).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Grobler S.R., Majeed A., Moola M.H. Effect of various tooth-whitening products on enamel microhardness. SADJ. 2009;64(10):474–479. [PubMed] [Google Scholar]
- 2.Lee K.H., Kim H.I., Kim K.H., Kwon Y.H. Mineral loss from bovine enamel by a 30% hydrogen peroxide solution. J. Oral Rehabil. 2006;33(3):229–233. doi: 10.1111/j.1365-2842.2004.01360.x. [DOI] [PubMed] [Google Scholar]
- 3.Attin T., Müller T., Patyk A., Lennon A.M. Influence of different bleaching systems on fracture toughness and hardness of enamel. Oper. Dent. 2004;29(2):188–195. [PubMed] [Google Scholar]
- 4.Basting R.T., Rodrigues A.L., Jr, Serra M.C. The effect of 10% carbamide peroxide, carbopol and/or glycerin on enamel and dentin microhardness. Oper. Dent. 2005;30(5):608–616. [PubMed] [Google Scholar]
- 5.Attin T., Schmidlin P.R., Wegehaupt F., Wiegand A. Influence of study design on the impact of bleaching agents on dental enamel microhardness: a review. Dent. Mater. 2009;25(2):143–157. doi: 10.1016/j.dental.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Akal N., Over H., Olmez A., Bodur H. Effects of carbamide peroxide containing bleaching agents on the morphology and subsurface hardness of enamel. J. Clin. Pediatr. Dent. 2001;25(4):293–296. [PubMed] [Google Scholar]
- 7.Attin T., Vollmer D., Wiegand A., Attin R., Betke H. Subsurface microhardness of enamel and dentin after different external bleaching procedures. Am. J. Dent. 2005;18(1):8–12. [PubMed] [Google Scholar]
- 8.Attin T., Manolakis A., Buchalla W., Hannig C. Influence of tea on intrinsic colour of previously bleached enamel. J. Oral Rehabil. 2003;30(5):488–494. doi: 10.1046/j.1365-2842.2003.01097.x. [DOI] [PubMed] [Google Scholar]
- 9.Lewinstein I., Fuhrer N., Churaru N., Cardash H. Effect of different peroxide bleaching regimens and subsequent fluoridation on the hardness of human enamel and dentin. J. Prosthet. Dent. 2004;92(4):337–342. doi: 10.1016/j.prosdent.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Souza-Gabriel A.E., Colucci V., Turssi C.P., Serra M.C., Corona S.A. Microhardness and SEM after CO(2) laser irradiation or fluoride treatment in human and bovine enamel. Microsc. Res. Tech. 2010;73(11):1030–1035. doi: 10.1002/jemt.20827. [DOI] [PubMed] [Google Scholar]
- 11.Suge T., Ishikawa K., Kawasaki A., Yoshiyama M., Asaoka K., Ebisu S. Effects of fluoride on the calcium phosphate precipitation method for dentinal tubule occlusion. J. Dent. Res. 1995;74(4):1079–1085. doi: 10.1177/00220345950740040801. [DOI] [PubMed] [Google Scholar]
- 12.Wang W., Zhu Y., Li J., Liao S., Ai H. Efficacy of cold light bleaching using different bleaching times and their effects on human enamel. Dent. Mater. J. 2013;32(5):761–766. doi: 10.4012/dmj.2013-109. [DOI] [PubMed] [Google Scholar]
- 13.Hahn P., Schondelmaier N., Wolkewitz M., Altenburger M.J., Polydorou O. Efficacy of tooth bleaching with and without light activation and its effect on the pulp temperature: an in vitro study. Odontology. 2013;101(1):67–74. doi: 10.1007/s10266-012-0063-4. [DOI] [PubMed] [Google Scholar]
- 14.ten Cate J.M., van Loveren C. Fluoride mechanisms. Dent. Clin. North Am. 1999;43(4):713–742, vii. [PubMed] [Google Scholar]
- 15.Esteves-Oliveira M., Zezell D.M., Meister J., Franzen R., Stanzel S., Lampert F., Eduardo C.P., Apel C. CO2 Laser (10.6 microm) parameters for caries prevention in dental enamel. Caries Res. 2009;43(4):261–268. doi: 10.1159/000217858. [DOI] [PubMed] [Google Scholar]
- 16.McCormack S.M., Fried D., Featherstone J.D., Glena R.E., Seka W. Scanning electron microscope observations of CO2 laser effects on dental enamel. J. Dent. Res. 1995;74(10):1702–1708. doi: 10.1177/00220345950740101201. [DOI] [PubMed] [Google Scholar]
- 17.Wu C.C., Roan R.T., Chen J.H. Sintering mechanism of the CaF2 on hydroxyapatite by a 10.6-l microm CO2 laser. Lasers Surg. Med. 2002;31(5):333–338. doi: 10.1002/lsm.10124. [DOI] [PubMed] [Google Scholar]
- 18.Leard A., Addy M. The propensity of different brands of tea and coffee to cause staining associated with chlorhexidine. J. Clin. Periodontol. 1997;24(2):115–118. doi: 10.1111/j.1600-051X.1997.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 19.Attin T., Kielbassa A.M., Schwanenberg M., Hellwig E. Effect of fluoride treatment on remineralization of bleached enamel. J. Oral Rehabil. 1997;24(4):282–286. doi: 10.1046/j.1365-2842.1997.d01-291.x. [DOI] [PubMed] [Google Scholar]
- 20.da Costa Soares M.U., Araújo N.C., Borges B.C., da Silva S.W., Sobral A.P. Impact of remineralizing agents on enamel microhardness recovery after in-office tooth bleaching therapies. Acta Odontol. Scand. 2013;71(2):343–348. doi: 10.3109/00016357.2012.681119. [DOI] [PubMed] [Google Scholar]
- 21.Singh R.D., Ram S.M., Shetty O., Chand P., Yadav R. Efficacy of casein phosphopeptide-amorphous calcium phosphate to prevent stain absorption on freshly bleached enamel: An in vitro study. J. Conserv. Dent. 2010;13(2):76–79. doi: 10.4103/0972-0707.66715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attin T., Betke H., Schippan F., Wiegand A. Potential of fluoridated carbamide peroxide gels to support post-bleaching enamel re-hardening. J. Dent. 2007;35(9):755–759. doi: 10.1016/j.jdent.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Basting R.T., Rodrigues A.L., Jr, Serra M.C. The effects of seven carbamide peroxide bleaching agents on enamel microhardness over time. J. Am. Dent. Assoc. 2003;134(10):1335–1342. doi: 10.14219/jada.archive.2003.0047. [DOI] [PubMed] [Google Scholar]
- 24.Basting R.T., Rodrigues Júnior A.L., Serra M.C. The effect of 10% carbamide peroxide bleaching material on microhardness of sound and demineralized enamel and dentin in situ. Oper. Dent. 2001;26(6):531–539. [PubMed] [Google Scholar]
- 25.Ten Cate JM. In vitro studies on the effects of fluoride on de- and remineralization. Journal of Dental Research. 1990;69:614–9. doi: 10.1177/00220345900690S120. [DOI] [PubMed] [Google Scholar]
- 26.Türkun M., Sevgican F., Pehlivan Y., Aktener B.O. Effects of 10% carbamide peroxide on the enamel surface morphology: a scanning electron microscopy study. J. Esthet. Restor. Dent. 2002;14(4):238–244. doi: 10.1111/j.1708-8240.2002.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues J.A., Marchi G.M., Ambrosano G.M., Heymann H.O., Pimenta L.A. Microhardness evaluation of in situ vital bleaching on human dental enamel using a novel study design. Dent. Mater. 2005;21(11):1059–1067. doi: 10.1016/j.dental.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Attin T., Albrecht K., Becker K., Hannig C., Wiegand A. Influence of carbamide peroxide on enamel fluoride uptake. J. Dent. 2006;34(9):668–675. doi: 10.1016/j.jdent.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Esteves-Oliveira M., Pasaporti C., Heussen N., Eduardo C.P., Lampert F., Apel C. Rehardening of acid-softened enamel and prevention of enamel softening through CO2 laser irradiation. J. Dent. 2011;39(6):414–421. doi: 10.1016/j.jdent.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Poosti M., Ahrari F., Moosavi H., Najjaran H. The effect of fractional CO2 laser irradiation on remineralization of enamel white spot lesions. Lasers Med. Sci. 2014;29(4):1349–1355. doi: 10.1007/s10103-013-1290-9. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki K., Tanaka Y., Takagi O. Crystallographic analysis of demineralized human enamel treated by laser-irradiation or remineralization. Arch. Oral Biol. 2000;45(9):797–804. doi: 10.1016/S0003-9969(00)00034-0. [DOI] [PubMed] [Google Scholar]