Abstract
AIM: To determine the safety and efficacy of endoscopic duodenal stent placement in patients with malignant gastric outlet obstruction.
METHODS: This prospective, observational, multicenter study included 39 consecutive patients with malignant gastric outlet obstruction. All patients underwent endoscopic placement of a nitinol, uncovered, self-expandable metal stent. The primary outcome was clinical success at 2 wk after stent placement that was defined as improvement in the Gastric Outlet Obstruction Scoring System score relative to the baseline.
RESULTS: Technical success was achieved in all duodenal stent procedures. Procedure-related complications occurred in 4 patients (10.3%) in the form of mild pneumonitis. No other morbidities or mortalities were observed. The clinical success rate was 92.3%. The mean survival period after stent placement was 103 d. The mean period of stent patency was 149 d and the patency remained acceptable for the survival period. Stent dysfunction occurred in 3 patients (7.7%) on account of tumor growth.
CONCLUSION: Endoscopic management using duodenal stents for patients with incurable malignant gastric outlet obstruction is safe and improved patients’ quality of life.
Keywords: Duodenal stenosis, Gastrointestinal stent, Gastric stenosis, Malignant tumors, Metallic stent
Core tip: Endoscopic management using duodenal stents for patients with incurable malignant gastric outlet obstruction is safe and improved patients’ quality of life.
INTRODUCTION
Approximately 15%-20% of patients with various types of gastrointestinal malignancies, such as gastric cancer or pancreatic cancer, develop gastric outlet obstruction (GOO) during the end stage of their disease[1]. GOO causes nausea, vomiting, and abdominal discomfort, which diminish quality of life[2]. The primary aim of palliation for these patients is relief of obstruction-related symptoms. Traditionally, GOO was treated using open surgical bypass; however this procedure has been reported to be associated with considerable morbidity and mortality[3].
Recently, endoscopic placement of self-expandable metal stents has emerged as an alternative, minimally invasive treatment in cases of malignant GOO. The reported technical success rates have ranged from 94% to 100%, and the clinical success rates have ranged from 84% to 97%[4-10]. Furthermore, stent placement allows faster resumption of food intake, usually tolerated the day after stent placement, and involves a shorter hospital stay than surgical gastrojejunostomy (GJ)[4,11,12]. Previously stent placement involved use of an over-the-wire technique under fluoroscopy. However, recent advances in devices technology have led to stent placement using a through-the-scope technique. The former technique takes much longer to perform, and the placement procedure is complicated and difficult[13].
The objective of this prospective single-arm observation study was to determine the safety and efficacy of endoscopic duodenal stent placement in patients with malignant GOO, including postoperative recurrence.
MATERIALS AND METHODS
Patients
This was a prospective, observational, multicenter study of consecutive patients with malignant GOO, including postoperative recurrence, who were referred to 3 hospitals in Japan (1 university hospital and 2 referral hospitals) for palliative treatment from April 2011 to June 2013. Surgery was contraindicated in these patients, either because the lesion was not resectable or because the patients had advanced metastatic disease. Patients who had symptoms of GOO and a Gastric Outlet Obstruction Scoring System (GOOSS)[14] score (0 = no oral intake, 1 = liquid diet, 2 = soft solid diet, 3 = low residue or normal diet) of ≤ 2 were considered for inclusion in this study. Exclusion criteria included age < 20 years; obstruction in the proximal stomach, distal small intestine, or colon; previous treatment with metal stent for the same site; and contraindications for endoscopic therapy. The study protocol was approved by the Institutional Review Boards of the Ethics Committees at each hospital and registered with the University Hospital Medical Information Network Clinical Trials Registry (ID: UMIN000005112). Written informed consent was obtained from all patients.
Procedures
The WallFlex duodenal stent (Boston Scientific Japan, Tokyo, Japan) was used to treat all patients in this study. A nitinol, uncovered, self-expandable metal stent, available in lengths of 6, 9, and 12 cm, with a body diameter of 22 mm and a flare diameter of 27 mm at the proximal and distal ends, was used. The stent delivery system had a diameter of 10 Fr and allowed stent placement through the scope.
If patients were suspected of having biliary obstruction, biliary drainage by insertion of a self-expandable metal stent was endoscopically performed in advance or concurrent with duodenal stent placement. Duodenal stent placement was performed with the patient under conscious sedation. A forward-viewing gastrointestinal endoscope (Olympus CF-H260AI; Olympus Medical Systems, Tokyo, Japan) or a lateral-viewing duodenoscope (Olympus TJF-260V; Olympus Medical Systems) with a working channel diameter of ≥ 3.7 mm was used depending on the site of the stricture. The oral side of the stricture was confirmed by direct endoscopic observation, and the length and shape of the stricture was assessed fluoroscopically (Figure 1). A catheter and a guidewire were passed through the stricture and the guidewire was used to position the stent delivery system into the stricture. A stent length of ≥ 2 cm longer than the stricture was selected. The stent was deployed under continuous endoscopic and fluoroscopic control. All procedures were performed by therapeutic endoscopists who performed more than 100 endoscopic procedures per year.
Figure 1.
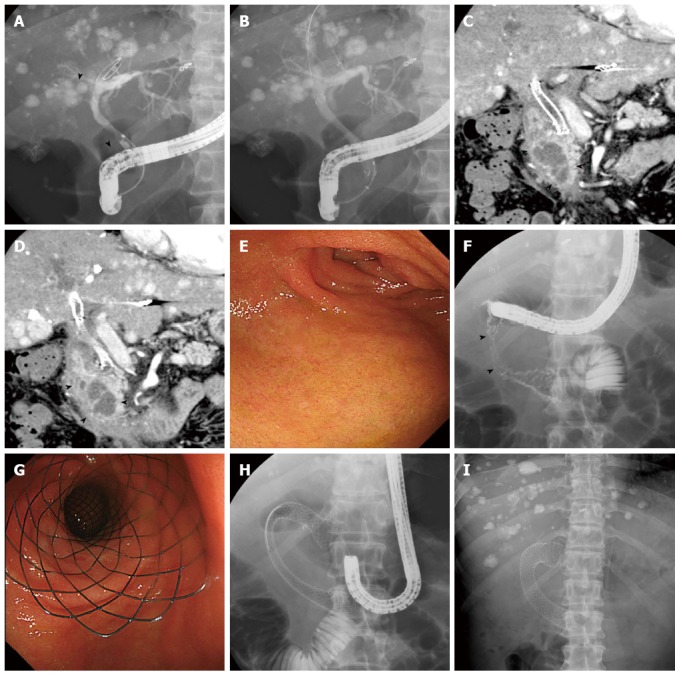
Case of hepatocellular carcinoma. A: Cholangiogram showing a biliary stricture caused by primary tumor and lymph node metastasis in the right hepatic duct and middle bile duct (arrow heads); B: A self-expandable metal stent was inserted endoscopically; C, D: Coronal sections of contrast-enhanced computed tomography images show duodenal invasion of lymph node metastasis (arrow heads); E: Endoscopic view showing the oral side of the stricture at the superior duodenal angle; F: Injection of contrast material demonstrates a stricture in the second duodenal segment (arrow heads); G, H: A nitinol metal stent was placed in the shape of the character “C” from the stomach pylorus to the third duodenal segment (G: Endoscopic view; H: X-ray); I: X-ray image taken 1 wk later shows sufficient expansion and stability of the duodenal stent.
Outcome and definitions
The primary outcome measure of this study was clinical success at 2 wk after stent placement. Clinical success was defined as improvement in the GOOSS score relative to the baseline score. Secondary outcomes included technical success, improvement in the World Health Organization (WHO) performance score, procedure-related complications, overall survival, and stent patency, which was defined as the time period between stent placement and stent dysfunction. Technical success was defined as successful stent placement and deployment at the site of stricture. The 2010 American Society for Gastrointestinal Endoscopy consensus criteria were used to define and grade complications[15].
Data collection
The following data were collected before stent placement: age, sex, medical history, malignant GOO type, clinical stage of cancer according to the TNM classification system[16] , obstruction site, GOOSS score, and WHO performance score. After stent placement, follow-up data were obtained during inpatient clinic visits when the patient was still hospitalized or during outpatient clinic visits. GOOSS scores and WHO performance scores were collected at 2 wk. The following data were collected until patient death or 18 mo postprocedure: procedure-related complications, additional therapy (chemotherapy, radiotherapy), and stent dysfunction. We confirmed stent dysfunction in a gastrointestinal contrast study and by endoscopy when GOO symptoms recurred.
Statistical analysis
The sample size was calculated on the basis of clinical success after stent placement. Previous reported data indicated that the clinical success rate was approximately 90%[4-10]. Consequently, we estimated that 35 patients would be required to assess the duodenal stent clinical success rate with a confidence interval of 95%, a power of 80%, and a margin of error of 10%. Furthermore, we estimated that as many as 40 patients would be required to account for possible loss of patients during follow-up.
Continuous variables were expressed as the mean, median, standard deviation, standard error, and interquartile range, whereas categorical variables were expressed as counts and percentages. The Wilcoxon signed-rank test was used to assess improvements in the GOOSS score and WHO performance score relative to the baseline scores. Kaplan-Meier analysis was used to calculate overall survival and stent patency. SAS version 9.3 (SAS Institute, Cary, NC, United States) was used to perform all statistical analyses. The significance level was set to P < 0.05.
RESULTS
Baseline characteristics
A total of 39 patients [25 men, 14 women; age (mean ± SD): 69.2 ± 13.3 years] underwent duodenal stent placement between April 2011 and June 2013. The patient characteristics are summarized in Table 1. Of the included patients, 17 (43.6%) were diagnosed as having gastric cancer. The remaining patients had pancreatic cancer (16 patients, 41.0%), duodenal cancer (2 patients, 5.1%), extrahepatic cholangiocarcinoma (1 patient, 2.6%), intrahepatic cholangiocarcinoma (1 patient, 2.6%), ampullary carcinoma (1 patient, 2.6%), and hepatocellular carcinoma (1 patient, 2.6%). A pathological diagnosis of malignancy was made for 31 patients (79.5%). Six patients (15.4%) with altered gastrointestinal anatomy had strictures in the surgical anastomosis. Sixteen patients (41.0%) had stricture in the distal stomach, whereas 17 patients (43.6%) had strictures in the duodenum. Before stent placement, 16 (41.0%), 18 (46.2%), 5 (12.8%), and 0 (0%) patients had GOOSS scores of 0, 1, 2, and 3, respectively.
Table 1.
Baseline patients and stricture characteristics n (%)
| Age, yr, mean ± SD (range) | 69.2 ± 13.3 (35-90) |
| Sex (M/F) | 25/14 |
| Primary malignancy, | |
| Gastric cancer | 1 (43.6) |
| Pancreatic cancer | 1 (41.0) |
| Duodenal carcinoma | 2 (5.1) |
| Extrahepatic cholangiocarcinoma | 1 (2.6) |
| Intrahepatic cholangiocarcinoma | 1 (2.6) |
| Ampullary carcinoma | 1 (2.6) |
| Hepatocellular carcinoma (lymph node metastasis) | 1 (2.6) |
| Clinical stage of cancer1 | |
| Stage III2 | 16 (41.0) |
| Stage IV3 | 23 (59.0) |
| Altered gastrointestinal anatomy | |
| Gastroduodenostomy after Billroth I gastrectomy | 3 (7.7) |
| Gastrojejunostomy after Billroth II gastrectomy | 1 (2.6) |
| Gastrojejunostomy after pancreatoduodenectomy | 1 (2.6) |
| Gastrojejunostomy for surgical bypass | 1 (2.6) |
| Location of stricture | |
| Distal stomach | 16 (41.0) |
| Duodenal bulb | 4 (10.3) |
| Second duodenal segment | 4 (10.3) |
| Second/third duodenal segment | 4 (10.3) |
| Third duodenal segment | 5 (12.8) |
| Anastmosis site (gastrostomy) | 6 (15.4) |
| Stricture length, mm, mean ± SD (range) | 42.6 ± 19.8 (15-93) |
| GOOSS score before stent placement | |
| 0-no oral intake | 16 (41.0) |
| 1-liquid diet | 18 (46.2) |
| 2-soft solid diet | 5 (12.8) |
| 3-low residue or normal diet | 0 |
| WHO perfomance score | |
| 0-fully active | 7 (17.9) |
| 1-cannot carry out heavy physical work | 8 (20.5) |
| 2-up and about > 50% of the day | 13 (33.3) |
| 3-up and about < 50% of the day | 9 (23.1) |
| 4-bed or chair bound all day | 2 (5.1) |
TNM classification system (ref.);
Including stage IIIB and IIIC of gastric cancer;
Including stage IVA of intrahepatic cholangiocarcinoma and hepatocellular carcinoma. GOOSS: Gastric outlet obstruction scoring system; WHO: World Health Organization.
Procedural details
Technical success was achieved in 39 patients (100%). The duodenal stent was placed at the oral side of the papilla in 23 patients (59.0%), on the papilla in 6 patients (15.4%), and at the anal side of the papilla in 10 patients (25.6%). Procedure-related complications occurred in 4 patients (10.3%) in the form of mild pneumonitis. No other morbidities or mortalities were observed.
Clinical success
Clinical success was achieved in 36 of the 39 patients (92.3%). There was no increase/decrease in the GOOSS score for 3 patients (Figure 2A). The GOOSS scores for these patients were 0, 1, and 2, respectively. Oral intake inability in a patient whose GOOSS score remained 0 was caused by progression of a peritoneal carcinomatosis. At inclusion, the mean GOOSS score was 0.69 (Figure 2B). After 14 d of duodenal stent placement, the mean GOOSS score significantly improved to 2.21 (P < 0.0001). On the other hand, there was no significant difference in the WHO performance score before and after duodenal stenting (mean, 1.77 vs 1.95, P = 0.57).
Figure 2.
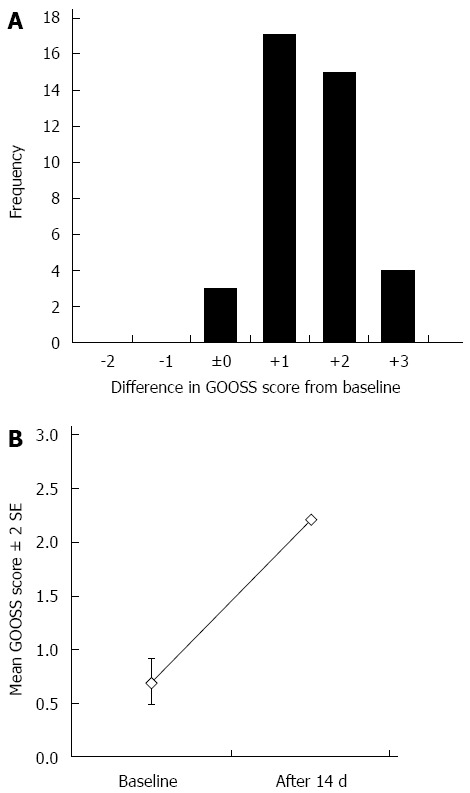
Differences between the Gastric Outlet Obstruction Scoring System score at baseline and at 14 d after stent placement are shown (A) and the mean Gastric Outlet Obstruction Scoring System scores at baseline and after 14 d are shown (B). P-value of the Wilcoxon signed-rank test comparison was < 0.0001. GOOSS: Gastric Outlet Obstruction Scoring System.
Survival
After duodenal stenting, 15 patients (38.5%) received chemotherapy as an additional treatment of malignancy, and 1 patient (2.6%) received chemoradiation. Thirty-eight patients died during and 1 was alive at the end of the follow-up period. The median survival period of the 39 patients was 50 d, and the mean period was 103 d (Table 2). Four patients survived > 200 d, 2 survived > 300 d, 1 survived > 400 d, and 1 survived > 500 d.
Table 2.
Procedure details and study outcomes n (%)
| Technical success | 39 (100) |
| Relationship of the papilla and stent placement site | |
| Oral side of the papilla | 23 (59.0) |
| On the papilla | 6 (15.4) |
| Anal side of the papilla | 10 (25.6) |
| Procedure related complication | |
| Aspiration pneumonitis | 4 (10.3) |
| Bleeding 0 | 0 |
| Perforation 0 | 0 |
| Cholangitis 0 | 0 |
| Motality disorder 0 | 0 |
| Clinical success | 36 (92.3) |
| Additional treatment of malignancy after stent placement | |
| Chemotherapy | 15 (38.5) |
| Chemoradiation | 1 (2.6) |
| Survival after stent placement, d | |
| Median (IQR) | 50.0 (25.0-152.0) |
| mean ± SE | 102.9 ± 18.2 |
| Stent patency, d | |
| Median | Not available |
| mean ± SE | 149.6 (7.2) |
| Stent dysfunction | |
| Stent ingrowth | 2 (5.1) |
| Stent overgrowth | 1 (2.6) |
| Stent compression | 0 |
| Stent migration | 0 |
| Food impaction | 0 |
IQR: Interquartile range.
Stent patency
During the follow-up period, 3 patients (7.7%) experienced stent dysfunction, which was caused by tumor growth in all 3 patients. No patient had stent migration (Table 2). The mean period of stent patency was 149 d (Figure 3). Stent patency was > 200 d in 2 patients, > 300 d in 2 patients, > 400 d in 1 patient, and > 500 d in 1 patient. All 6 patients with altered gastrointestinal anatomy died without recurrence of GOO symptoms, and the maximum period of stent patency was 77 d. All 3 patients with stent dysfunction underwent reintervention involving stent-in-stent placement of the duodenal stent, and all of them died without experiencing recurrent stent dysfunction. The stent patency periods after reintervention were 41, 73, and 88 d, respectively.
Figure 3.
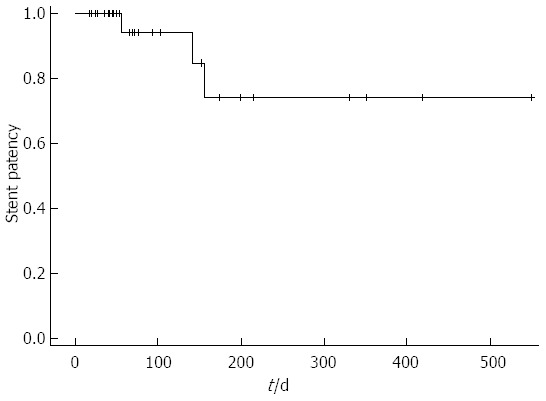
Kaplan-Meier curve showing stent patency.
DISCUSSION
We prospectively evaluated outcomes of endoscopic management using a nitinol metal stent in patients with malignant GOO. In this study, the clinical success rate was 92.3%. Recent prospective multicenter studies have reported similar clinical success rates ranging from 85% to 91%[17-21]. Our study showed that the stent patency period was acceptable for the patient survival period.
Endoscopic stent placement or surgical GJ are commonly used palliative treatments for malignant GOO. It has been reported that patients who have undergone stent placement require a shorter time to achieve oral intake and a shorter hospital stay than those who have undergone surgical GJ[4,11,12]. In a previous study, better physical health scores were obtained 1 mo after stent placement than after laparoscopic GJ[22], whereas fewer recurrent obstructive symptoms were associated with surgical GJ than with stent placement. The SUSTENT study results suggest that GJ should be primarily considered for patients with an expected survival of ≥ 2 mo and that stent placement should be primarily considered for patients with a shorter anticipated survival[23]. However, in this study, some patients, even those with unresectable malignancies, survived longer after chemotherapy. Moreover, long-term stent patencies were also observed. Further investigation to determine if duodenal stenting should be performed in patients with prognoses expected to be ≥ 2 mo is necessary. The major late complication associated with duodenal stents was obstruction, which was caused by tumor ingrowth or overgrowth. However, reintervention for obstructed duodenal stents is noninvasive and effective[24]. In addition, in this study, reintervention to correct an obstructed duodenal stent was technically and clinically successful. Patients at the end stage of cancer do not tolerate invasive procedures, and their life expectancy is relatively short; thus, stent placement is preferred for such patients.
In a previous report of uncovered stents for malignant GOO, approximately 19% of patients had tumor ingrowths[25]. If the origin of obstructive malignancies was intraluminal, such as gastric cancer and duodenal cancer, then tumor ingrowth was often induced by uncovered stents. Extraluminal malignancies have a low risk of causing tumor ingrowth[26,27]. In a prospective cohort study[9] and a randomized controlled trial[10] of covered vs uncovered metal stents for malignant GOO, there were no differences in the clinical success rate and stent patency but there was a difference in the pattern of late stent failure. In addition, stent migration occurred more frequently for covered stents than for uncovered stents, and restenosis caused by tumor ingrowth occurred more frequently for uncovered stents[9,10]. Similarly, in the present study using uncovered metal stents, there was no stent migration, and only stent dysfunction due to tumor growth was observed.
The currently available duodenal stents are the braided nitinol metal type[8,17,18,20]. Some studies have examined radial force as an expanding force and axial force as a force for recovery to a straight position for various structures and materials in biliary[28] and esophageal[29] metal stents but not in duodenal stents. In esophageal stents, braided nitinol metal stents were reported to have a lower radial force and a higher axial force than non-braided stents[29]. Because the axial force decreases with an increase in stent length[28], kinking and intestinal damage may be prevented by selecting a long stent in the duodenum (Figure 1). Duodenal stents of various structures and materials should be examined to reduce stent dysfunction.
There were some limitations in the present study because we did not conduct a comparative investigation. First, we did not compare metal stent types, such as covered vs uncovered metal stents, the structure and material used, or the stent length. Second, stent placement techniques, such as the over-the-wire technique under fluoroscopy and the through-the-scope technique, were not compared. Third, we did not compare endoscopic stenting for malignant GOO with other endoscopic management approaches, such as endoscopic GJ. Van Hooft et al[30] reported a prospective multicenter study of endoscopic GJ that used a magnetic anastomotic device and transanastomotic deployment of stents. In an animal study, Itoi et al[31] reported an endoscopic ultrasonography-guided GJ technique using a double-balloon enteric tube and a bilateral reflected metal stent. Stent dysfunction may be less likely to occur in routes that avoid the malignant obstruction section than in routes that include the malignant obstruction section. A randomized controlled trial of endoscopic duodenal stenting vs endoscopic GJ for malignant GOO is required.
In conclusion, this prospective multicenter study showed that placement of a nitinol, uncovered, self-expandable metal stent in patients with incurable malignant GOO was safe and improved their quality of life.
ACKNOWLEDGMENTS
The authors thank Rintaro Mikata, Shin Yasui, Kiyofumi Ishii, Sadahiro Itoh, Hiroshi Ohyama, Dai Sakamoto, Yuto Watanabe, Masato Nakamura, Ryousaku Azemoto, Yu Yoshida, and Toru Wakamatsu for their contributions in the collection of data.
COMMENTS
Background
Gastric outlet obstruction (GOO) was treated using open surgical bypass; however this procedure has been reported to be associated with considerable morbidity and mortality. Recently, endoscopic placement of self-expandable metal stents has emerged as an alternative, minimally invasive treatment in cases of malignant GOO.
Research frontiers
Patients of GOO underwent endoscopic placement of a nitinol, uncovered, self-expandable metal stent. The primary outcome was clinical success at 2 wk after stent placement that was defined as improvement in the Gastric Outlet Obstruction Scoring System (GOOSS) score relative to the baseline.
Innovations and breakthroughs
Endoscopic stent placement or surgical gastrojejunostomy (GJ) are commonly used palliative treatments for malignant GOO. This study showed that the stent patency period was acceptable for the patient survival period. At inclusion, the mean GOOSS score was 0.69. After 14 d of duodenal stent placement, the mean GOOSS score significantly improved to 2.21 (P < 0.0001). Endoscopic management using duodenal stents for patients with incurable malignant gastric outlet obstruction is safe and improved patients’ quality of life.
Applications
Surgery was contraindicated in these patients, either because the lesion was not resectable or because the patients had advanced metastatic disease.
Terminology
Endoscopic management using duodenal stents for patients with incurable malignant gastric outlet obstruction is safe and improved patients’ quality of life.
Peer-review
The reviewed paper is very well organized, performed, and written research on actual topic. This article is very interesting and innovating and deserves publication.
Footnotes
Institutional review board statement: This study was conducted under approval our ethical committee.
Clinical trial registration statement: Medical Information Network Clinical Trial Registry (ID: UMIN000005112).
Informed consent statement: All the treatment procedures were performed after obtaining the informed consent in writing from the patients.
Conflict-of-interest statement: The authors have no other disclosures.
Data sharing statement: I share data in the group of us.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 23, 2016
First decision: February 18, 2016
Article in press: March 14, 2016
P- Reviewer: Shiryajev YN S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Lillemoe KD, Cameron JL, Hardacre JM, Sohn TA, Sauter PK, Coleman J, Pitt HA, Yeo CJ. Is prophylactic gastrojejunostomy indicated for unresectable periampullary cancer? A prospective randomized trial. Ann Surg. 1999;230:322–328 discussion 328-330. doi: 10.1097/00000658-199909000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monson JR, Donohue JH, McIlrath DC, Farnell MB, Ilstrup DM. Total gastrectomy for advanced cancer. A worthwhile palliative procedure. Cancer. 1991;68:1863–1868. doi: 10.1002/1097-0142(19911101)68:9<1863::aid-cncr2820680902>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Bozzetti F, Bonfanti G, Audisio RA, Doci R, Dossena G, Gennari L, Andreola S. Prognosis of patients after palliative surgical procedures for carcinoma of the stomach. Surg Gynecol Obstet. 1987;164:151–154. [PubMed] [Google Scholar]
- 4.Jeurnink SM, van Eijck CH, Steyerberg EW, Kuipers EJ, Siersema PD. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol. 2007;7:18. doi: 10.1186/1471-230X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JH, Song HY, Shin JH, Choi E, Kim TW, Jung HY, Lee GH, Lee SK, Kim MH, Ryu MH, et al. Metallic stent placement in the palliative treatment of malignant gastroduodenal obstructions: prospective evaluation of results and factors influencing outcome in 213 patients. Gastrointest Endosc. 2007;66:256–264. doi: 10.1016/j.gie.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Mutignani M, Tringali A, Shah SG, Perri V, Familiari P, Iacopini F, Spada C, Costamagna G. Combined endoscopic stent insertion in malignant biliary and duodenal obstruction. Endoscopy. 2007;39:440–447. doi: 10.1055/s-2007-966327. [DOI] [PubMed] [Google Scholar]
- 7.Graber I, Dumas R, Filoche B, Boyer J, Coumaros D, Lamouliatte H, Legoux JL, Napoléon B, Ponchon T. The efficacy and safety of duodenal stenting: a prospective multicenter study. Endoscopy. 2007;39:784–787. doi: 10.1055/s-2007-966594. [DOI] [PubMed] [Google Scholar]
- 8.van Hooft JE, Uitdehaag MJ, Bruno MJ, Timmer R, Siersema PD, Dijkgraaf MG, Fockens P. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointest Endosc. 2009;69:1059–1066. doi: 10.1016/j.gie.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Lee KM, Choi SJ, Shin SJ, Hwang JC, Lim SG, Jung JY, Yoo BM, Cho SW, Kim JH. Palliative treatment of malignant gastroduodenal obstruction with metallic stent: prospective comparison of covered and uncovered stents. Scand J Gastroenterol. 2009;44:846–852. doi: 10.1080/00365520902929849. [DOI] [PubMed] [Google Scholar]
- 10.Kim CG, Choi IJ, Lee JY, Cho SJ, Park SR, Lee JH, Ryu KW, Kim YW, Park YI. Covered versus uncovered self-expandable metallic stents for palliation of malignant pyloric obstruction in gastric cancer patients: a randomized, prospective study. Gastrointest Endosc. 2010;72:25–32. doi: 10.1016/j.gie.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 11.Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol. 2007;42:283–290. doi: 10.1007/s00535-006-2003-y. [DOI] [PubMed] [Google Scholar]
- 12.Ly J, O’Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc. 2010;24:290–297. doi: 10.1007/s00464-009-0577-1. [DOI] [PubMed] [Google Scholar]
- 13.Maetani I, Ukita T, Nambu T, Shigoka H, Omuta S, Endo T, Takahashi K. Comparison of ultraflex and niti-s stents for palliation of unresectable malignant gastroduodenal obstruction. Dig Endosc. 2010;22:83–89. doi: 10.1111/j.1443-1661.2010.00942.x. [DOI] [PubMed] [Google Scholar]
- 14.Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol. 2002;97:72–78. doi: 10.1111/j.1572-0241.2002.05423.x. [DOI] [PubMed] [Google Scholar]
- 15.Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A, Petersen BT, Petrini JL, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Paleri V, Mehanna H, Wight RG. TNM classification of malignant tumours 7th edition: what’s new for head and neck? Clin Otolaryngol. 2010;35:270–272. doi: 10.1111/j.1749-4486.2010.02141.x. [DOI] [PubMed] [Google Scholar]
- 17.van Hooft JE, van Montfoort ML, Jeurnink SM, Bruno MJ, Dijkgraaf MG, Siersema PD, Fockens P. Safety and efficacy of a new non-foreshortening nitinol stent in malignant gastric outlet obstruction (DUONITI study): a prospective, multicenter study. Endoscopy. 2011;43:671–675. doi: 10.1055/s-0030-1256383. [DOI] [PubMed] [Google Scholar]
- 18.Kim YW, Choi CW, Kang DH, Kim HW, Chung CU, Kim DU, Park SB, Park KT, Kim S, Jeung EJ, et al. A double-layered (comvi) self-expandable metal stent for malignant gastroduodenal obstruction: a prospective multicenter study. Dig Dis Sci. 2011;56:2030–2036. doi: 10.1007/s10620-011-1566-5. [DOI] [PubMed] [Google Scholar]
- 19.Costamagna G, Tringali A, Spicak J, Mutignani M, Shaw J, Roy A, Johnsson E, De Moura EG, Cheng S, Ponchon T, et al. Treatment of malignant gastroduodenal obstruction with a nitinol self-expanding metal stent: an international prospective multicentre registry. Dig Liver Dis. 2012;44:37–43. doi: 10.1016/j.dld.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 20.van den Berg MW, Haijtink S, Fockens P, Vleggaar FP, Dijkgraaf MG, Siersema PD, van Hooft JE. First data on the Evolution duodenal stent for palliation of malignant gastric outlet obstruction (DUOLUTION study): a prospective multicenter study. Endoscopy. 2013;45:174–181. doi: 10.1055/s-0032-1326077. [DOI] [PubMed] [Google Scholar]
- 21.Tringali A, Didden P, Repici A, Spaander M, Bourke MJ, Williams SJ, Spicak J, Drastich P, Mutignani M, Perri V, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc. 2014;79:66–75. doi: 10.1016/j.gie.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Mehta S, Hindmarsh A, Cheong E, Cockburn J, Saada J, Tighe R, Lewis MP, Rhodes M. Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surg Endosc. 2006;20:239–242. doi: 10.1007/s00464-005-0130-9. [DOI] [PubMed] [Google Scholar]
- 23.Jeurnink SM, Steyerberg EW, van Hooft JE, van Eijck CH, Schwartz MP, Vleggaar FP, Kuipers EJ, Siersema PD. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490–499. doi: 10.1016/j.gie.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 24.Park JC, Park JJ, Cheoi K, Chung H, Lee H, Shin SK, Lee SK, Lee YC. Clinical outcomes of secondary stent-in-stent self-expanding metal stent placement for primary stent malfunction in malignant gastric outlet obstruction. Dig Liver Dis. 2012;44:999–1005. doi: 10.1016/j.dld.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Nassif T, Prat F, Meduri B, Fritsch J, Choury AD, Dumont JL, Auroux J, Desaint B, Boboc B, Ponsot P, et al. Endoscopic palliation of malignant gastric outlet obstruction using self-expandable metallic stents: results of a multicenter study. Endoscopy. 2003;35:483–489. doi: 10.1055/s-2003-39661. [DOI] [PubMed] [Google Scholar]
- 26.Telford JJ, Carr-Locke DL, Baron TH, Tringali A, Parsons WG, Gabbrielli A, Costamagna G. Palliation of patients with malignant gastric outlet obstruction with the enteral Wallstent: outcomes from a multicenter study. Gastrointest Endosc. 2004;60:916–920. doi: 10.1016/s0016-5107(04)02228-x. [DOI] [PubMed] [Google Scholar]
- 27.Kim GH, Kang DH, Lee DH, Heo J, Song GA, Cho M, Yang US. Which types of stent, uncovered or covered, should be used in gastric outlet obstructions? Scand J Gastroenterol. 2004;39:1010–1014. doi: 10.1080/00365520410003146. [DOI] [PubMed] [Google Scholar]
- 28.Isayama H, Nakai Y, Toyokawa Y, Togawa O, Gon C, Ito Y, Yashima Y, Yagioka H, Kogure H, Sasaki T, et al. Measurement of radial and axial forces of biliary self-expandable metallic stents. Gastrointest Endosc. 2009;70:37–44. doi: 10.1016/j.gie.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 29.Hirdes MM, Vleggaar FP, de Beule M, Siersema PD. In vitro evaluation of the radial and axial force of self-expanding esophageal stents. Endoscopy. 2013;45:997–1005. doi: 10.1055/s-0033-1344985. [DOI] [PubMed] [Google Scholar]
- 30.van Hooft JE, Vleggaar FP, Le Moine O, Bizzotto A, Voermans RP, Costamagna G, Devière J, Siersema PD, Fockens P. Endoscopic magnetic gastroenteric anastomosis for palliation of malignant gastric outlet obstruction: a prospective multicenter study. Gastrointest Endosc. 2010;72:530–535. doi: 10.1016/j.gie.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Itoi T, Itokawa F, Uraoka T, Gotoda T, Horii J, Goto O, Moriyasu F, Moon JH, Kitagawa Y, Yahagi N. Novel EUS-guided gastrojejunostomy technique using a new double-balloon enteric tube and lumen-apposing metal stent (with videos) Gastrointest Endosc. 2013;78:934–939. doi: 10.1016/j.gie.2013.09.025. [DOI] [PubMed] [Google Scholar]


