Abstract
Background
Sepsis, the most severe manifestation of acute infection, poses a major challenge to health care systems around the world. To date, adequate data on the incidence and mortality of sepsis in Germany have been lacking.
Methods
Nationwide case-related hospital DRG statistics for the years 2007–2013 were used to determine the in-hospital incidence and mortality of sepsis. Cases were identified on the basis of the clinical and pathogen-based ICD-10 codes for sepsis. The statistical evaluation was standardized for age and sex and carried out separately for each age group.
Results
The number of cases of sepsis rose by an average of 5.7% per year, from 200 535 in 2007 to 279 530 in 2013, corresponding to an increase in the adjusted in-hospital incidence from 256 to 335 cases per 100 000 persons per year. The percentage of patients with severe sepsis rose from 27% to 41%. The in-hospital mortality of sepsis fell over the same period by 2.7%, to 24.3%. In 2013, 67 849 persons died of sepsis in German hospitals (or died of another disease, but also had sepsis). The incidence was highest in the youngest and oldest age groups, and the in-hospital mortality rose nearly linearly with age from age 40 onward.
Conclusion
Sepsis and death from sepsis are markedly more common in Germany than previously assumed, and they are on the rise. Sepsis statistics should become a standard component of federal statistical reports on public health, as well as of hospital statistics. Preventive measures and evidence-based treatment should be implemented across the nation.
Sepsis, the most severe manifestation of acute infection, can cause multi-organ failure and ends in death in 30–50% of cases (1, 2). According to the National Center for Health Statistics, the annual incidence of sepsis in the USA rose by 7–8% per year over a period of 8 years, from 221/100 000 persons in 2000 to 377/100 000 persons in 2008 (3). Despite a continual decline in mortality (4), more than 200 000 persons died of sepsis in the USA in 2007 (5). Sepsis is thus much more common in the USA than myocardial infarction, breast cancer, or colon cancer (6, 7). In 2011, sepsis took first place on the list of the most expensive disease conditions in the USA, with annual hospital costs of $22.2 billion (8). The rising incidence of documented cases of sepsis has been attributed to demographic changes, with a larger population of elderly, multimorbid patients, and to the expansion of treatment with drugs and with invasive medical and intensive-care measures that weaken the immune response (9). Increased coding of sepsis due to increased awareness and financial incentives associated with sepsis coding has also been mentioned as a possible cause (10).
No aggregate data on sepsis are given in the health reports of the German Federal Ministry of Health (11). The reported data concern only the frequency of pathogen-based sepsis codes, even though clinical sepsis codes—R65.0! (sepsis), R65.1! (severe sepsis), and R57.2 (septic shock)—have been included in the World Health Organization’s International Classification of Diseases (ICD-10) since 2005 (the last of these three was included in 2010). The reason for including clinical codes was that a pathogen can be identified by blood culture in no more than 30–40% of patients with sepsis (1). Clinical criteria, in addition to microbiological criteria, have provided the basis for epidemiological studies of sepsis since the 1990s (2, 12).
Because of the lack of robust data for Germany, the German Sepsis Competence Network (SEPNET) carried out a point-prevalence study in 2003 to estimate the incidence of sepsis in Germany according to the abovementioned criteria (2). Data were collected only from intensive care units and concerned only patients with severe sepsis; thus, cases of sepsis without organ dysfunction, most of which were treated on regular hospital wards rather than intensive care units (ICUs), were not recorded, and their incidence could only be roughly estimated. With this limitation, the study revealed 154 000 cases of sepsis and about 60 000 deaths from sepsis in 2003. A DRG system (DRG, diagnosis related groups) was introduced in Germany in 2004; since then, German hospitals have been required to report their patients’ ICD-coded diagnoses on discharge to an administrative body that oversees the hospital reimbursement system (Institut für das Entgeltsystem im Krankenhaus, InEK). These data are then released in summarized form by the Federal Statistical Office in the form of diagnosis-related hospital statistics (DRG statistics). The purpose of our study is to use data from this source to examine secular trends in the incidence and mortality of sepsis over the years 2007–2013. The use of hospital discharge diagnoses enabled us to obtain nationwide epidemiological data on sepsis not only for intensive-care patients, but also for patients treated on regular hospital wards. Thus, the data we analyzed are not only representative for the entire country, but also take account of the many patients with sepsis (and even severe sepsis) who are treated on a regular ward rather than an ICU.
Methods
This study is based on DRG statistics compiled by the Federal Statistical Office on the basis of data from all hospitals participating in the DRG reimbursement system, according to the provisions of German law (§ 21 Krankenhausentgeltgesetz [KhEntgG]). The primary and secondary diagnoses of all hospitalized patients from 2007 to 2013 were evaluated. 27 clinical and pathogen-based ICD-10 diagnosis codes for sepsis (eBox 1) were used for case identification. The pathogen-based codes classify sepsis by the causative pathogen and do not enable any subclassification of cases by clinical severity. Clinical ICD-10 codes for sepsis (R65.0!) and severe sepsis (R65.1!) were introduced in Germany in 2005. A code for septic shock (R57.2) was added in 2010; until then, cases of septic shock had been subsumed under the code for severe sepsis. R65.0! and R65.1! can only be coded as secondary diagnoses and are defined according to the clinical consensus definition (13, 14), which distinguishes sepsis, in which there must be an infection and at least two signs of a systemic inflammatory reaction syndrome (SIRS) but no organ dysfunction, from severe sepsis, in which organ dysfunction must be present in addition to infection and at least two signs of SIRS. SIRS criteria include fever, leukocytosis, tachycardia, and tachypnea. R57.2 is the code for septic shock, i.e., severe sepsis with hypotension despite adequate volume administration. Sepsis may only be coded as the primary diagnosis when septic shock or sepsis coded with one of the pathogen-based sepsis codes was the reason for hospital admission.
eBox 1. ICD-10 sepsis codes for the identification of sepsis cases in DRG statistics.
-
Case identification—sepsis:
A02.1 (Salmonella sepsis)
A20.0 (Bubonic plague)
A20.7 (Septicaemic plague)
A21.7 (Generalized tularemia)
A22.7 (Anthrax sepsis)
A24.1 (Acute and fulminating melioidosis)
A26.7 (Erysipelothrix sepsis)
A28.2 (Extraintestinal yersiniosis)
A32.7 (Listerial sepsis)
A39.2 (Acute meningococcaemia)
A39.3 (Chronic meningococcaemia)
A39.4 (Meningococcaemia, unspecified)
A39.1 (Waterhouse-Friderichsen syndrome)
A40.– (Streptococcal sepsis)
A41.– (Other sepsis)
A42.7 (Actinomycotic sepsis)
A48.3 (Toxic shock syndrome)
B00.7 (Disseminated herpesviral disease)
A54.8 (Other gonococcal infections)
B37.7 (Candidal sepsis)
B37.6 (Candidal endocarditis)
B49 (Unspecified mycosis, incl. fungaemia not otherwise specified)
A49.9 (Bacterial infection, unspecified, incl. bacteremia, not otherwise specified)
P36.- (Bacterial sepsis of newborn)
O75.3 (Other infection during labour)
O85 (Puerperal sepsis),
R65.0! (Systemic Inflammatory Response Syndrome [SIRS] of infectious origin without organ failure)
R65.1! (Systemic Inflammatory Response Syndrome [SIRS] of infectious origin with organ failure)
R57.2 (Septic shock)
-
Case identification—severe sepsis:
R65.1! (SIRS of infectious origin with organ failure)
-
Case identification—septic shock:
R57.2 (Septic shock)
We studied the annual numbers of inpatient cases of sepsis and in-hospital deaths from sepsis, among patients in all age groups, in Germany over the period 2007–2013. The annual population-based frequency of sepsis was directly standardized to the German population structure as of 31 December 2010 on the basis of the nationwide population data of the Federal Statistical Office for 2007–2013. For the years 2007–2010, an adjustment to the normed United States population was also carried out, in order to enable a comparison of the rates of sepsis in the two countries (eTable 1). The term “sepsis,” as used in the present report, includes cases of severe sepsis and septic shock, unless otherwise specified; the term “severe sepsis” includes cases of septic shock. Patients given more than one ICD-10 code for sepsis were only counted once, according to the most severe condition coded (septic shock > severe sepsis > sepsis). The direct costs of inpatient and outpatient treatment of patients with sepsis were estimated on the basis of data from the Federal Insurance Office (Bundesversicherungsamt, BVA), which regularly calculates the mean expenditures for patients with sepsis and its sequelae to determine the appropriate morbidity-oriented risk structure equalization. This calculation is based on a group of ICD-10 codes that the BVA considers relevant to sepsis (eBox 2); the way in which it is carried out is described in detail on the BVA website (15).
eTable 1. The in-hospital incidence of sepsis, severe sepsis, and septic shock per 100000 population per year,adjusted to the population of the United States in 2000 (e9).
| Sepsis (clinical and pathogen-basedsepsis codes) | Severe sepsis/septic shock (R65.1!, R57.2) | Septic shock (R57.2) | |
|---|---|---|---|
| 2007 | 191.26 | 46.74 | – |
| 2008 | 188.15 | 53.18 | – |
| 2009 | 196.14 | 59.86 | – |
| 2010 | 205.59 | 71.88 | 18.11 |
| 2011 | 212.79 | 79.04 | 22.25 |
| 2012 | 220.77 | 83.83 | 24.61 |
| 2013 | 236.19 | 90.89 | 26.80 |
eBox 2. Sepsis/SIRS-defining ICD codes for the reference year 2013, according to § 31 Section 2 RSAV (diseases and diagnoses for morbidity-oriented risk structure equalization, as laid down by the German Federal Insurance Office).
A02.1 (Salmonella sepsis)
A20.7 (Septicaemic plague)
A22.7 (Anthrax sepsis)
A26.7 (Erysipelothrix sepsis)
A32.7 (Listerial sepsis)
A39.1 (Waterhouse-Friderichsen syndrome)
A39.2 (Acute meningococcaemia)
A39.3 (Chronic meningococcaemia)
A39.4 (Meningococcaemia, unspecified)
A40.0 (Sepsis due to streptococcus, group A)
A40.1 (Sepsis due to streptococcus, group B)
A40.2 (Sepsis due to streptococcus, group D)
A40.3 (Sepsis due to Streptococcus pneumoniae)
A40.8 (Other streptococcal sepsis)
A40.9 (Streptococcal sepsis, unspecified)
A41.0 (Sepsis due to Staphylococcus aureus)
A41.1 (Sepsis due to other specified staphylococcus)
A41.2 (Sepsis due to unspecified staphylococcus)
A41.3 (Sepsis due to Haemophilus influenzae)
A41.4 (Sepsis due to anaerobes)
A41.5 (Sepsis due to other Gram-negative organisms)
A41.51 (sepsis: Escherichia coli [E. coli])
A41.52 (sepsis: Pseudomonas)
A41.58 (sepsis: other Gram-negative pathogens)
A41.8 (Other specified sepsis)
A41.9 (Sepsis, unspecified)
A42.7 (Actinomycotic sepsis)
B00.7 (Disseminated herpesviral disease)
B37.7 (Candidal sepsis)
O88.3 (Obstetric pyaemic and septic embolism)
P36.0 (Sepsis of newborn due to streptococcus, group B)
P36.1 (Sepsis of newborn due to other and unspecified streptococci)
P36.2 (Sepsis of newborn due to Staphylococcus aureus)
P36.3 (Sepsis of newborn due to other and unspecified staphylococci)
P36.4 (Sepsis of newborn due to Escherichia coli)
P36.5 (Sepsis of newborn due to anaerobes)
P36.8 (Other bacterial sepsis of newborn)
P36.9 (Bacterial sepsis of newborn, unspecified)
R57.2 (Septic shock)
R65.0 (Systemic inflammatory response syndrome [SIRS] of infectious origin without organ failure)
R65.1 (Systemic inflammatory response syndrome [SIRS] of infectious origin with organ failure)
R65.2 (Systemic inflammatory response syndrome [SIRS] of non-infectious origin without organ failure)
R65.3 (Systemic inflammatory response syndrome [SIRS] of non-infectious origin with organ failure)
R65.9 (Systemic inflammatory response syndrome [SIRS], unspecified)
Results
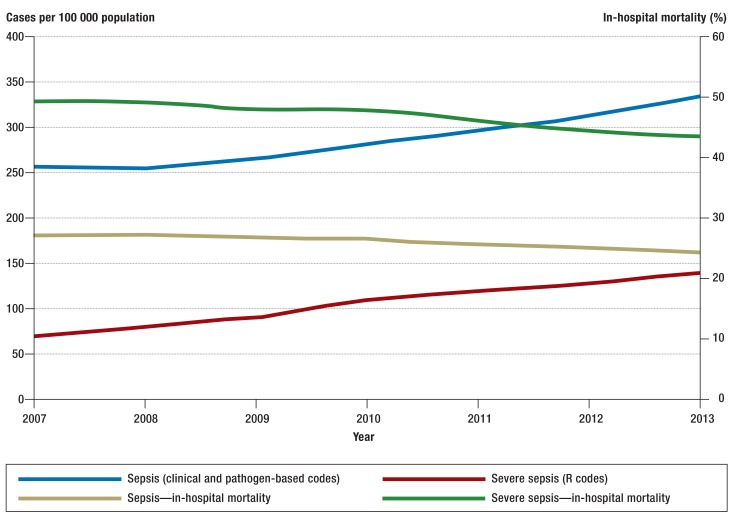
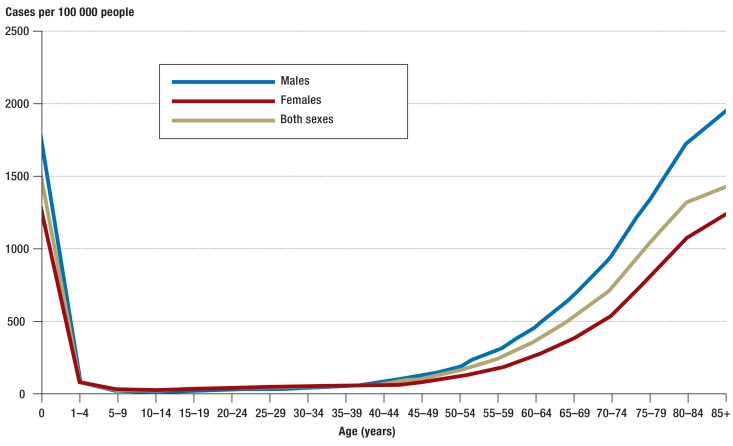
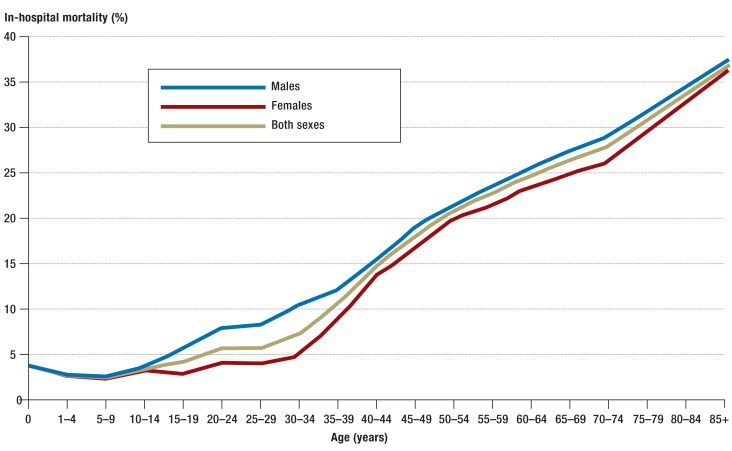
The annual number of sepsis cases counted in the German DRG statistics rose from 200 535 in 2007 to 279 530 in 2013, corresponding to an average annual increase of 5.7% (Table 1, Figure 1). Over the same period of time, the incidence of sepsis, standardized to the 2010 population structure, rose from 256 to 335 cases per 100 000 population per year. The percentage of sepsis patients among all inpatients also rose, from 1.21% to 1.54%. On the other hand, the in-hospital mortality of patients with sepsis fell from 27% to 24.3%. In 2007, 27% of all patients with sepsis had severe sepsis; this figure rose to 41% by 2013, corresponding to a doubling in the population-standardized incidence of severe sepsis from 69 to 138 cases per 100 000 persons per year. The in-hospital mortality of patients with severe sepsis fell from 49.5% to 43.6% (Table 1). The number of cases of septic shock per year rose from 22 326 in 2010 to 33 815 in 2013. The in-hospital mortality of severe septic shock fell slightly (by 2.2%) over this interval and was 58.8% in 2013. The annual incidence of sepsis over the entire period (2007–2013) was 1556/100 000 in neonates and 30/100 000 in persons aged 10 to 14. The annual incidence of sepsis rose steadily with age, reaching a second peak among persons aged 85 and above, in whom it was 1434/100 000 (Figure 2). Likewise, in-hospital mortality among patients with sepsis and among patients with severe sepsis also rose with age, reaching maximum values of 36.5% and 60.3%, respectively (Figure 3, eTable 2).
Table 1. Hospital incidence and mortality rates for sepsis, severe sepsis, and septic shock in Germany, 2007–2013*1.
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | |
|---|---|---|---|---|---|---|---|
| Sepsis, including severe sepsis and septic shock | |||||||
| Cases | 200535 | 201985 | 214615 | 230952 | 240470 | 256918 | 279530 |
| Deaths | 54169 | 54829 | 56992 | 61068 | 61243 | 63419 | 67849 |
| Adjusted rate per 100000 population | 256 | 255 | 267 | 283 | 296 | 311 | 335 |
| In-hospital mortality (%) | 27.0 | 27.1 | 26.6 | 26.4 | 25.5 | 24.7 | 24.3 |
| Severe sepsis, including septic shock (R65.1!, R57.2*2) | |||||||
| Cases | 53722 | 62374 | 71642 | 87973 | 96558 | 105130 | 115421 |
| Deaths | 26606 | 30712 | 34269 | 42084 | 44513 | 46666 | 50349 |
| Adjusted rate per 100000 persons | 69 | 79 | 89 | 107 | 119 | 127 | 138 |
| In-hospital mortality (%) | 49.5 | 49.2 | 47.8 | 47.8 | 46.1 | 44.4 | 43.6 |
| Septic shock (R57.2) | |||||||
| Cases | – | – | – | 22326 | 27151 | 30688 | 33815 |
| Deaths | – | – | – | 13616 | 16143 | 18024 | 19891 |
| Adjusted rate per 100000 persons | – | – | – | 27 | 33 | 37 | 40 |
| In-hospital mortality (%) | – | – | – | 61.0 | 59.5 | 58.7 | 58.8 |
*1Standardized to the 2010 population structure. DRG statistics of the Federal Statistical Office
*2Up to and including 2009, the code for severe sepsis (R65.1!) also included all cases with septic shock. These two entities began to be coded separately in 2010, when a new code for septic shock was introduced (R57.2)
Figure 1.
Incidence rate per 100 000 population, standardized to the German population structure in 2010, and in-hospital mortality of sepsis and severe sepsis (including septic shock) in Germany, 2007–2013
Figure 2.
In-hospital incidence of sepsis per 100 000 persons per year, by age group and sex, in the period 2007–2013 (clinical and pathogen-based sepsis codes)
Figure 3.
In-hospital mortality of patients with sepsis, by age group and sex, in the period 2007–2013 (clinical and pathogen-based sepsis codes)
eTable 2. The in-hospital incidence of sepsis, severe sepsis, and septic shock per 100000 population per year in Germany, 2007–2013, by age group.
| Age (years) | Sepsis (clinical and pathogen-based sepsis codes) | Severe sepsis / septic shock (R65.1!, R57.2) | Septic shock (R57.2) |
|---|---|---|---|
| 0 | 1556.16 | 146.8 | 16.74 |
| 1–4 | 79.89 | 8.5 | 1.82 |
| 5–9 | 37.02 | 3.84 | 0.9 |
| 10–14 | 29.72 | 4.31 | 1.14 |
| 15–19 | 42.55 | 8.55 | 2.57 |
| 20–24 | 43.78 | 9.99 | 2.94 |
| 25–29 | 49.56 | 11.33 | 3.63 |
| 30–34 | 56.85 | 14.5 | 4.87 |
| 35–39 | 63.36 | 19.46 | 6.68 |
| 40–44 | 75.87 | 27.31 | 10.4 |
| 45–49 | 108.86 | 41.54 | 14.87 |
| 50–54 | 165.07 | 65.65 | 24.36 |
| 55–59 | 249.74 | 100.74 | 37.46 |
| 60–64 | 364.94 | 147.75 | 53.82 |
| 65–69 | 532.9 | 210.6 | 77.44 |
| 70–74 | 733.74 | 295.1 | 99.73 |
| 75–79 | 1049.26 | 419.59 | 133.1 |
| 80–84 | 1332.94 | 514.71 | 163.77 |
| 85+ | 1434.28 | 517.01 | 143.12 |
Men suffered from sepsis up to 1.8 times more commonly than women; likewise, the incidence of severe sepsis was up to twice as high among men as among women, depending on the age group. In 2013, the Federal Insurance Office estimated the mean expenditure per case of sepsis at €27 467.92. On this basis, we calculate the overall cost of the inpatient care of sepsis patients, and subsequent outpatient care, at roughly €7.7 billion in 2013.
Discussion
We analyzed the German DRG statistics to obtain a nationwide, representative estimate of the in-hospital incidence and mortality of sepsis. From 2007 to 2013, the annual number of cases of sepsis rose to 335 cases per 100 000 persons, while the mortality of the condition fell by 2.7%, to 24.3%. Both the incidence and the mortality of sepsis were markedly age- and sex-dependent. An evaluation of routine data in the USA in the last decade yielded an incidence of 377 cases per 100 000 persons, with annual increases ranging from from 8.2% to 17.8% (3, 5, 16). Meanwhile, the annual incidence of severe sepsis in Spain is reported to have risen by 8.6% annually to a rate of 85/100 000 (17), while the incidence of severe sepsis in the United Kingdom (not including Scotland) rose by 43% in 10 years (18). In Scandinavia, the incidence of hospital admission via the emergency room because of community-acquired sepsis is estimated at 731/100 000, with severe sepsis accounting for 60% of this figure (19). Meaningful comparisons of figures across studies from different countries are difficult because of widely varying study designs and definitions of sepsis. The use of less restrictive definitions than those mandated by the German coding guidelines elevates the apparent incidence of sepsis and, in most cases, lowers its apparent mortality (20). The incidence of sepsis found in the present study, adjusted to the structure of the United States population (as per the 2000 census), is 236/100 000 and thus lower than the American figure of 377/100 000 (3) (eTable 2).
Reasons for the rising incidence of sepsis include the following:
Biological and medical factors associated with the aging of the population. Susceptibility to infection and sepsis is increased by underlying comorbidities such as cancer, hepatic cirrhosis, diabetes, and HIV/AIDS, and by harmful behaviors such as excessive alcohol consumption, smoking, and lack of exercise (4, 21– 26).
While medical progress undeniably saves lives, it has also led to an increase in treatments that diminish immune competence and break down natural defensive barriers, thereby making some patients more susceptible to infection and sepsis—particularly neonates and very elderly patients (27). Sepsis-associated multi-organ failure was first described in the 1970s; in the pre–intensive care era, sepsis of this degree of severity led to death within a few hours. Sepsis can also arise, however, as a complication of treatment in intensive care. In the United States (which, along with Germany, has the highest number of intensive-care beds per capita in the world), 29.2% of Medicare-insured patients are treated in an ICU in the last three months of their lives (28). Such patients are obviously much more likely to suffer from sepsis than the general population.
The increased coding of sepsis is yet another reason for an (apparent) rise in its incidence. The clarification of the definitions of sepsis (13) and the ensuing implementation of these definitions in the (SIRS and) sepsis codes of the ICD-9/10 system have made sepsis easier to code and have created a financial incentive to the coding of sepsis in a number countries, including Germany (10, 29). This might well produce a variant of the “Will Rogers phenomenon” that has been observed in oncology (i.e., spurious changes in outcome statistics brought about by a reassignment of diagnoses) (30). Yet sepsis rates are also reported to have risen in registry studies, which are unaffected by coding incentives (31). Ever since the DRG system was introduced in Germany, the coding of sepsis, SIRS, neutropenia, and bacteremia has been strictly regulated to prevent overcoding especially in less severe cases. Special coding guideline 103a, issued in 2004, specified how codes were to be correctly applied; the thoroughly revised guideline 103d, in effect since 2005, also contains instructions for the coding of SIRS. The supplementary instructions on SIRS coding issued by the German Institute of Medical Documentation and Information (Deutsches Institut für Medizinische Dokumentation und Information, DIMDI) are supposed to be followed as well (32). The clinical sepsis codes may only be assigned if at least two out of four SIRS criteria are met. To guard against false coding, there is a rule in Germany, in effect since 2007, that code R65.0! may only be accepted for billing purposes if at least two SIRS criteria are met in cases with positive cultures or organ complications, or if all four SIRS criteria are met in cases with negative cultures. On the other hand, about 12% of patients with microbiologically and clinically unequivocal, severe sepsis meet only one SIRS criterion, or none at all (33); this regulation therefore tends to promote the undercoding, rather than the overcoding, of sepsis. In general, the exclusive examination of ICD-10–based sepsis codes that are assigned on discharge can yield estimates of the frequency of sepsis that are lower, by a factor of 2.3 to 6, than those derived from a prospective evaluation of patients’ hospital charts (19, 34). Some cases of sepsis are coded wrongly or not at all (35, 36). Some pathogen-based codes, however, are not unequivocally linked to sepsis, and thus some of the patients who receive these codes (probably a very small fraction) do not, in fact, have sepsis. It is hard to weigh the relative contributions of demographic changes, widened treatment options, and coding effects to the observed increase in incidence. An age-independent increase in the incidence of sepsis remains visible after the annual figures are adjusted for changes in population structure. Clearly, then, the latter two factors—i.e., coding effects and the profusion of modern treatments that increase the risk of infection—are playing a major role in the rising incidence of sepsis now being observed in industrialized countries. The figures of the present study presumably still underestimate the overall incidence of sepsis in Germany, as up to 12% of persons with sepsis are not inpatients, but rather become ill and die in nursing institutions or at home (37).
We found only a 0.45% average annual decline in the in-hospital mortality rate of sepsis for our overall patient cohort during the period of observation. The corresponding figure for in-hospital mortality from severe sepsis was an average annual decline of 0.98%, which can be compared with the greater average annual declines, in the range of 1.3% to 2%, that were found in studies from the USA and Australia (5, 16, 31, 38). It is hard to tell whether the in-hospital mortality rate of sepsis has apparently declined faster in the USA than in Germany because of a coding artifact (i.e., upcoding of less severe cases with a lower risk of death) or because of better early recognition and treatment of sepsis. Whichever of these may be true, the incidence of postoperative sepsis is used as a mandatory, official quality indicator for all American hospitals (39), and a number of large hospital chains in the USA have included a reduction of mortality from sepsis among their quality goals (40). It is noteworthy that the in-hospital mortality of patients with severe sepsis, in absolute terms, is 10–15% lower in the USA and Australia than in Germany and elsewhere in Europe. This is probably due to differences in the severity of illness (e1) and in the length of hospital stay: hospitalizations are 10–14 days shorter in the USA and Australia than in Germany (2, 5, 31).
It is well known that many patients with sepsis die in the first weeks and months following their discharge from the hospital (e2). Better early recognition of sepsis and the resulting timely initiation of treatment (e3), as well as improved care of sepsis patients in the emergency room and the ICU, are considered to be the main reasons for the declining mortality that has been measured in registry studies and clinical studies (31, e4). In the USA, recommendations issued by the National Quality Forum (39) have led in recent years to the introduction of sepsis protocols in private and public hospitals, in accordance with the guidelines of the Surviving Sepsis Campaign, with reported reductions of up to 50% in mortality from sepsis (e5, e6). Germany still lacks any comparable nationwide recommendations for the prevention and early recognition of sepsis. Another proposed reason for the lower documented mortality from sepsis in the USA is the increased documentation of less severe cases (10), although improved survival has been documented in the USA and Australia even in mechanically ventilated sepsis patients and those with organ failure (e7).
Conclusion
The “burden of disease” in Germany that is accounted for by sepsis has grown over the period of observation of this study. Two reasons for this are the aging of the population and the expansion of modern, advanced medical interventions to persons at the extremes of age. The degree to which altered coding practices have contributed to the increase is unclear. What we now need, to prevent a further rise in the incidence of sepsis and to improve its treatment, is a trans-sectoral approach that exploits all of the available potential for prevention, early diagnosis, acute treatment, and treatment of long-term sequelae.
Such an approach should include vaccinating persons in high-risk groups, improving the early detection of sepsis in the outpatient setting and in all clinical inpatient departments as a prerequisite for timely antimicrobial treatment and circulatory support, making effective anti-infectious drugs widely available, developing adjunctive, immune-modulating drugs against sepsis, and working out suitable rehabilitation plans for survivors who suffer from the underappreciated long-term consequences of sepsis.
To gauge the progress that will be made (as we hope) in these areas over time, the monitoring of key sepsis statistics based on the clinical and pathogen-based ICD-10 codes should become a permanent component of federal health reporting in Germany and should be used by hospitals as a guide to quality-improving measures. This is already being done voluntarily for sepsis in the framework of the Quality Medicine Initiative (Initiative Qualitätsmedizin, IQM) (e8).
Key Messages.
The in-hospital incidence of sepsis in Germany rose over the period 2007 to 2013 by an average of 5.7% per year, to 335 per 100 000 persons per year in 2013.
Sepsis is particularly common in neonates and the elderly. The mortality of sepsis rises nearly linearly with age from 40 years onward.
Over the period of observation, patients with sepsis constituted an increasing percentage of all hospitalized patients (from 1.21% to 1.54%).
Mortality remains high: 43.6% for severe sepsis and 58.8% for septic shock.
The incidence of sepsis is probably rising for multiple reasons: the aging of the population, the increasing use of invasive and immunosuppressive treatments in ever older patients, and the increasing awareness of sepsis, which has an effect on DRG coding.
eTable 3. Cases and in-hospital mortality of sepsis, severe sepsis, and septic shock in Germany, 2007–2013, by age group.
| Age(years) | Number of casesSepsis | In-hosp. mortality Sepsis (%) | Number of cases Severe sepsis | In-hosp. mortality Severe sepsis (%) | Number of cases Septic shock | In-hosp. mortality Septic shock (%) |
|---|---|---|---|---|---|---|
| 0 | 73568 | 3.9 | 6940 | 17.6 | 451 | 39.5 |
| 1–4 | 15316 | 2.8 | 1630 | 15.9 | 198 | 33.3 |
| 5–9 | 9320 | 2.6 | 968 | 15.5 | 126 | 31.0 |
| 10–14 | 8135 | 3.6 | 1181 | 16.0 | 176 | 25.0 |
| 15–19 | 12627 | 4.4 | 2536 | 15.1 | 418 | 25.8 |
| 20–24 | 14851 | 5.8 | 3388 | 18.8 | 566 | 31.3 |
| 25–29 | 17204 | 5.8 | 3933 | 18.7 | 717 | 29.1 |
| 30–34 | 19185 | 7.4 | 4892 | 21.4 | 955 | 32.1 |
| 35–39 | 22684 | 10.7 | 6968 | 25.7 | 1,263 | 37.6 |
| 40–44 | 34315 | 14.8 | 12353 | 29.8 | 2,509 | 43.4 |
| 45–49 | 52904 | 18.2 | 20189 | 33.9 | 4,161 | 45.4 |
| 50–54 | 71858 | 21.0 | 28580 | 37.2 | 6,280 | 48.1 |
| 55–59 | 95527 | 22.9 | 38532 | 39.7 | 8,311 | 52.1 |
| 60–64 | 118009 | 24.7 | 47778 | 42.4 | 10,521 | 54.6 |
| 65–69 | 168208 | 26.4 | 66476 | 44.8 | 12,557 | 56.9 |
| 70–74 | 240913 | 28.0 | 96891 | 46.8 | 19,300 | 59.5 |
| 75–79 | 244562 | 30.7 | 97798 | 50.7 | 18,844 | 64.2 |
| 80–84 | 214275 | 33.5 | 82742 | 55.3 | 15,251 | 69.4 |
| 85 + | 191544 | 36.5 | 69045 | 60.3 | 11,376 | 76.3 |
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Reinhart has served as a paid consultant for Adrenomed and has a personal relationship with InflaRx.
Prof. Welte has served as a paid consultant for Astellas, AstraZeneca, Basilea, Bayer, MSD, Novartis, and Pfizer and has been paid for carrying out clinical studies related to the topic of this article on behalf of AstraZeneca, Basilea, Bayer, MSD, Novartis, and Pfizer.
This study was partly supported by an Integrated Research and Treatment Center (Integriertes Forschungs- und Behandlungszentrum, IFB) called Sepsis and Its Consequences (Sepsis und Sepsisfolgen). The IFB receives research funds from the German Federal Ministry of Education and Research, project no. 01 E0 1002.
Dr. Dennler, Ms. Fleischmann, Prof. Hartmann, Dr. Hartog, Mr. Heublein and Mr. Thomas-Rüddel state that they have no conflict of interest.
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369 doi: 10.1056/NEJMc1312359. [DOI] [PubMed] [Google Scholar]
- 2.Engel C, Brunkhorst FM, Bone HG, et al. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med. 2007;33:606–618. doi: 10.1007/s00134-006-0517-7. [DOI] [PubMed] [Google Scholar]
- 3.Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011:1–8. [PubMed] [Google Scholar]
- 4.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med. 2014;42:625–631. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 6.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 7.National Cancer Institute. Cancer Statistics. www.cancer.gov/statistics/find. (last accessed on 2 October 2015)
- 8.Torio CM, Andrews RM. National inpatient hospital costs: The most expensive conditions by payer, 2011: Statistical Brief #160. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. 2013 [Google Scholar]
- 9.Angus DC, Pereira CA, Silva E. Epidemiology of severe sepsis around the world. Endocr Metab Immune Disord Drug Targets. 2006;6:207–212. doi: 10.2174/187153006777442332. [DOI] [PubMed] [Google Scholar]
- 10.Rhee C, Gohil S, Klompas M. Regulatory mandates for sepsis care-reasons for caution. N Engl J Med. 2014;370:1673–1676. doi: 10.1056/NEJMp1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gesundheitberichtserstattung des Bundes. www.gbe-bund.de. (last accessed on 16 February 2015) [Google Scholar]
- 12.Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis—current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 13.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 14.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 15.Bundesversicherungsamt. So funktioniert der Morbiditätsorientierte Risikostrukturausgleich. www.bundesversicherungsamt.de/fileadmin/redaktion/Risikostrukturausgleich/Wie_funktioniert_Morbi_RSA.pdf. (last accessed on 25 March 2015)
- 16.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 17.Bouza C, Lopez-Cuadrado T, Saz-Parkinson Z, Amate-Blanco JM. Epidemiology and recent trends of severe sepsis in Spain: a nationwide population-based analysis (2006-2011) BMC Infect Dis. 2014;14 doi: 10.1186/s12879-014-0717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: Secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care. 2006;10 doi: 10.1186/cc4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henriksen DP, Laursen CB, Jensen TG, Hallas J, Pedersen C, Lassen AT. Incidence rate of community-acquired sepsis among hospitalized acute medical patients-a population-based survey. Crit Care Med. 2015;43:13–21. doi: 10.1097/CCM.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 20.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 21.Bello S, Menendez R, Torres A, et al. Tobacco smoking increases the risk for death from pneumococcal pneumonia. Chest. 2014;146:1029–1037. doi: 10.1378/chest.13-2853. [DOI] [PubMed] [Google Scholar]
- 22.Williams PT. Inadequate exercise as a risk factor for sepsis mortality. PLoS One. 2013 doi: 10.1371/journal.pone.0079344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danai PA, Moss M, Mannino DM, Martin GS. The epidemiology of sepsis in patients with malignancy. Chest. 2006;129:1432–1440. doi: 10.1378/chest.129.6.1432. [DOI] [PubMed] [Google Scholar]
- 24.Galbois A, Aegerter P, Martel-Samb P, et al. Improved prognosis of septic shock in patients with cirrhosis: a multicenter study. Crit Care Med. 2014;42:1666–1675. doi: 10.1097/CCM.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 25.Mrus JM, Braun L, Yi MS, Linde-Zwirble WT, Johnston JA. Impact of HIV/AIDS on care and outcomes of severe sepsis. Critical Care. 2005;9:R623–R630. doi: 10.1186/cc3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Critical Care. 2009;13 doi: 10.1186/cc7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedrich I, Simm A, Kotting J, Tholen F, Fischer B, Silber RE. Cardiac surgery in the elderly patient. Dtsch Arztebl Int. 2009;106:416–422. doi: 10.3238/arztebl.2009.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finfer S, Vincent JL. Critical care—an all-encompassing specialty. N Engl J Med. 2013;369:669–670. doi: 10.1056/NEJMe1304035. [DOI] [PubMed] [Google Scholar]
- 29.Levy MM, Rhodes A, Phillips GS, et al. Surviving sepsis campaign: association between performance metrics and outcomes in a 75-year study. Crit Care Med. 2015;43:3–12. doi: 10.1097/CCM.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 30.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604–1608. doi: 10.1056/NEJM198506203122504. [DOI] [PubMed] [Google Scholar]
- 31.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 32.DIMDI. Definition SIRS, sepsis, schwere sepsis und septic shock. www.dimdi.de/static/de/klassi/faq/icd-10/icd-10-gm/maticd-sirs-def-2007-1007.pdf. (last accessed on 25 March 2015)
- 33.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372:1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 34.Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 35.Iwashyna TJ, Angus DC. Declining case fatality rates for severe sepsis: good data bring good news with ambiguous implications. JAMA. 2014;311:1295–1297. doi: 10.1001/jama.2014.2639. [DOI] [PubMed] [Google Scholar]
- 36.Whittaker SA, Mikkelsen ME, Gaieski DF, Koshy S, Kean C, Fuchs BD. Severe sepsis cohorts derived from claims-based strategies appear to be biased toward a more severely ill patient population. Crit Care Med. 2013;41:945–953. doi: 10.1097/CCM.0b013e31827466f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melamed A, Sorvillo FJ. The burden of sepsis-associated mortality in the United States from 1999 to 2005: an analysis of multiple-cause-of-death data. Crit Care Med. 2009;13 doi: 10.1186/cc7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000-2007) Chest. 2011;140:1223–1231. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 39.National Quality Forum. Severe sepsis and septic shock. Management bundle www.qualityforum.org. (last accessed on 25 March 2015)
- 40.CDC Sepsis. Improving survival. www.cdc.gov/sepsis/survival/index.html. (last accessed on 1 August 2015)
- e1.Levy MM, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis. 2012;12:919–924. doi: 10.1016/S1473-3099(12)70239-6. [DOI] [PubMed] [Google Scholar]
- e2.Pavon A, Binquet C, Kara F, et al. Profile of the risk of death after septic shock in the present era: an epidemiologic study. Crit Care Med. 2013;41:2600–2609. doi: 10.1097/CCM.0b013e31829a6e89. [DOI] [PubMed] [Google Scholar]
- e3.Lilly CM. The ProCESS trial–a new era of sepsis management. N Engl J Med. 2014;370:1750–1751. doi: 10.1056/NEJMe1402564. [DOI] [PubMed] [Google Scholar]
- e4.Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e5.Kaiser Permanente. Sepsis prevention. http://businesshealth.kaiserpermanente.org/featured-stories/sepsis-prevention/ (last accessed on 25 March 2015) [Google Scholar]
- e6.Miller RR, 3rd, Dong L, Nelson NC, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188:77–82. doi: 10.1164/rccm.201212-2199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e7.Walkey AJ, Lagu T, Lindenauer PK. Trends in sepsis and infection sources in the United States. A population-based study. Ann Am Thorac Soc. 2015;12:216–220. doi: 10.1513/AnnalsATS.201411-498BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e8.Qualitätsmedizin I G-IQI. German Inpatient Quality Indicators Version 4.2 Band 1: Definitionshandbuch für das Datenjahr 2015. https://opus4.kobv.de/opus4-tuberlin/frontdoor/index/index/docId/7007. (last accessed on 1 November 2015)
- e9.Center for Disease Control and Prevention. Age Standardization and Population Counts. www.cdc.gov/nchs/tutorials/NHANES/NHANESAnalyses/agestandardization/age_standardization_intro.htm. (last accessed on 1 November 2015)