Abstract
Background
To improve hypertension control, this cluster randomized trial evaluated the effectiveness of physician manager education about hypertension management.
Methods
After randomization at practice level, primary care physicians of the intervention arm, whose practices collaborated with a university department, participated in a three-session education on evidence-based hypertensiology and practice implementation strategies. The primary outcome was blood pressure (BP) control (ambulatory blood pressure [ABP] <130/80 mmHg) after 5 months. Secondary outcomes were changes in BP and practice routines regarding hypertension management. Following an intention-to-treat approach, data analyses included crude and adjusted generalized mixed models and sensitivity analyses. These took into account sex, age, ≥ hypertension-related disease and resistant hypertension (RH).
Results
The analysis included 103 of 169 patients from 22 practices. Overall, BP decrease was –8.2 systolic and –4.1 mmHg diastolic. The intervention had no effect on BP control (odds ratio 0.84 [95% CI 0.29–2.43]) and BP changes (interventional effect: systolic –2.48 mmHg [95% CI –7.24 to 2.29], diastolic –0.25 mmHg [95% CI 3.31 to 2.82]). Sensitivity analysis indicated effect modification in patients with RH. Intervention practices requested educational input on difficult cases, and newly implemented 3 practice strategies (14.5±2.6 versus 11.4±2.2; P=0.005).
Conclusions
After the short follow-up of 5 months, the intervention had no impact on BP control but improved the use of practice strategies.
The need to improve blood pressure (BP) control is documented in various studies (1, 2). Targeting patients, health care professionals and organizations, randomized controlled trials and cluster randomized trials (CRTs) have evaluated various interventions to improve BP control. Examples are:
patient-centered approaches (e.g. electronic reminders for self-care [3])
physician-centered strategies (including: provider-specific benchmarking reports [6], therapeutic recommendations by an external data center (7), physician-pharmacist collaborations [8–10])
separate hypertension outpatient clinics (11).
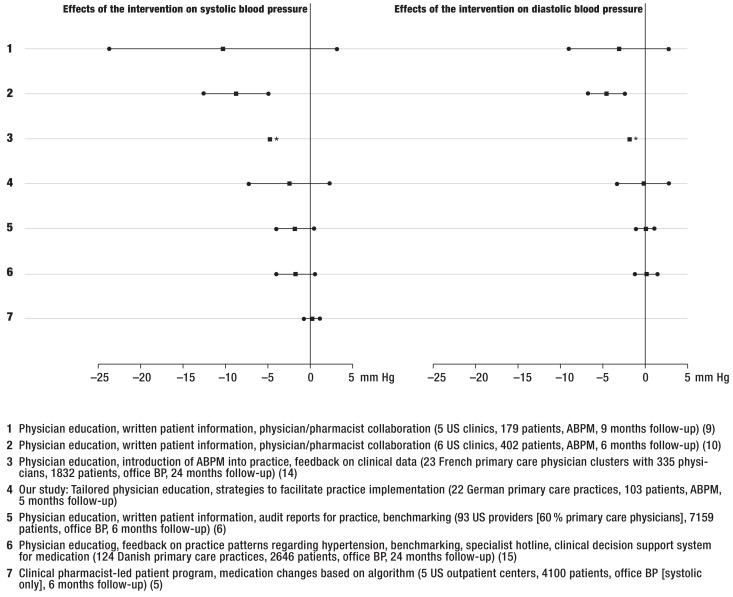
Although epidemiological data documented outcome improvements with reduced cardiovascular events even after reductions of –2 mmHg systolic BP (12), this is difficult to implement in routine care. The health services interventions mentioned above showed heterogeneous interventional effects on systolic BP (SBP) (+0.18 to –10.2 mmHg [5, 10]) and diastolic BP (DBP) (+0.04 to –4.6 mmHg [6, 9]) (Figure 1). According to a Cochrane review, best effects were achieved when educational and organizational approaches were combined (13), with a five-year follow-up demonstrating a significant decrease in mortality (11).
Figure 1.
*Cluster randomized trials about interventions to improve hypertension therapy: effects on systolic and diastolic blood pressure (in mmHg). Reference: control group.
*95% CI not reported..
US, United States; ABPM, ambulatory blood pressure monitoring; BP, blood pressure
While the majority of these interventions used rather costly external support structures, few randomized studies combined internal practice redesign with educational strategies (14, 15): Reuther et al. (2012) combined physician education about clinical management and practice organization with a clinical decision support system, benchmarking, peer audits, and a specialist hotline: After a 24-month follow-up in 2646 patients from 124 practices, the interventional effects amounted to –1.75 mmHg systolic [95% confidence interval (CI) 4.02 to 0.53] and +0.14 mmHg diastolic [95% CI: –1.16 to 1.44] (15).
We studied whether a series of three continuing medical education (CME) sessions for primary care physicians on evidence-based hypertensiology and practice redesign changed BP control rate and practice strategies. Our CRT addressed physicians in their dual role as clinicians and managers (so called “physician managers”) which is appropriate for the German health care system where the majority of practices are physician-owned. BP was measured using the gold standard ambulatory blood pressure monitoring (ABPM).
Methods
Study design
This CRT involved practices from the general medicine practice network of the University of Duisburg-Essen, Germany. The cluster design was used because the intervention addressed the physician/practice level, while it aimed at improving patients BP indirectly (16). At the 2013 spring network meeting all 51 attending practices were invited to participate.
Practices were eligible if they cared for hypertensive patients and were equipped with a calibrated ABPM device. In September 2013, 24 practices that volunteered and met the inclusion criteria were randomly assigned to the intervention or control arm (1:1) (17). To blind physicians for their interventional status, participants of the control arm received the same intervention after follow-up (waiting list control).
Allocation of patients was conducted at cluster level. All participants provided informed consent. The follow-up data collection was completed in August 2014. The Ethics Commission of the Medical Faculty of the University of Duisburg-Essen issued a positive vote for the study (reference: 13–5537-BO, date: 09/09/2013). Details of the study design are published elsewhere (17).
Study participants
The practices were asked to recruit at least 10 consecutive hypertensive patients with or without prior medication and/or hypertension-related diseases fulfilling the inclusion criteria:
Age ≥ 18 years
ability to complete the study documents
uncontrolled BP according to the European hypertension guidelines: office BP readings of ≥ 140/90 mm Hg, ABP readings of ≥ 130/80 mm Hg or mean home measurements of ≥ 135/85 mm Hg in a one-week protocol with ≥ 2 measurements daily (18)
Because patients were recruited for the study prior to their ABPM, patients with white coat hypertension (ABP <130/80 mmHg) were excluded from the final analysis.
Intervention
Practices received a physician manager-focused intervention offering strategies for structured hypertension management (17). Aiming at a participatory approach, physicians were asked for their information needs. Three CME sessions combined evidence-based information and practice implementation strategies:
Presentation of BP devices and training on valid BP readings
information about diagnostic and therapeutic strategies including pharmacotherapy
approaches for managing patients with white coat, secondary, juvenile and resistant hypertension (RH)
implementation of tools to facilitate guideline adherence
The sessions were provided by four hypertension specialists with one being experienced in practice hypertension management. Physicians were free to choose the diagnostic and therapeutic regimens for their patients.
Outcomes
The primary outcome was the BP control rate (percentage of patients with an average ABP <130/80 mmHg [18]) in the intervention and the control arm after five months. Secondary outcomes were:
changes in systolic and diastolic ABP (in mmHg), and
changes in practice strategies.
ABP was measured at baseline and 5 months follow-up.
For covariate assessment, study patients completed a questionnaire on sociodemographic characteristics, lifestyle factors, adherence, and BP self-checks at baseline and follow-up. At both times, physicians provided the patients’ medical characteristics including information on antihypertensive medication (substances, dosages, daily distribution) and practice strategies used. Practice and physician characteristics (sex, age, qualifications) were obtained at baseline.
Statistical methods
Based on our pilot study, BP control rates of 35% for the control and 70% for the intervention group were considered for sample size calculation (19, 20). Assuming an equal number of clusters per study arm, an average cluster size of 5 patients and an intra-class correlation coefficient of 0.05 (16), 10 clusters per study arm were required to detect the expected proportions with a two-sided significance level of 0.05 (80% power). The sample size was calculated with R Package ’CRTSize’, function n4props (21, 22).
Following an intention-to-treat approach (23) the analysis included all study patients with an ABPM at baseline and follow-up expect those with white coat hypertension (ABPM readings <130/80 mmHg at baseline (18)). The chi-square test was used to compare BP control rates between intervention and control. Using generalized linear mixed models (GLM), crude and adjusted analyses were performed to determine the effect of the intervention on BP control with the control arm as reference. The models accounted for clustered data by random effect modeling with an unstructured covariance pattern. The effect of the intervention on BP control is described as an odds ratio (OR) with 95% confidence interval.
GLMs were also calculated for BP changes (secondary outcomes). The interventional effects on SBP and DBP were expressed as point estimates. The GLMs for the total analysis population were adjusted for sex, age, and having ≥1 hypertension-related disease as a marker for disease severity. To assess effect modification, stratified sensitivity analyses were performed for sex, age (≤61, >61), having ≥ 1 hypertension-related disease and the presence of RH (≥ 3 antihypertensive substances). The latter was a post-hoc analysis triggered by the learning content requested by the participants. Additionally, GLMs were calculated for the extended study population including patients with white coat hypertension.
Statistical analyses were performed with IBM SPSS Statistics, Version 22.0; GLMs were calculated with SAS, Version 9.4. Statistical significance was set at p<0.05. There was no correction for multiple testing.
Results
Practice and physician characteristics
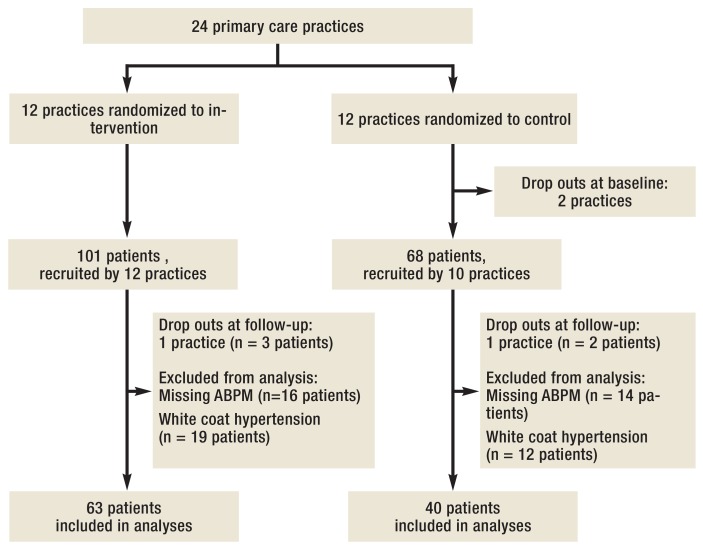
24 of the 51 invited practices volunteered and met the inclusion criteria (47.1%). They were randomly assigned to the intervention (n=12) or the waiting list control arm (n=12) and recruited 169 patients: intervention arm, n=101, control arm, n=68 (Figure 2). Four practices dropped out during data collection (1 physician died, 3 practices discontinued due to lack of interest). In key characteristics, these did not differ from participating ones. Also, physicians from both study arms were comparable (Table 1, eTable 1).
Figure 2.
Flowchart
of study patients
Table 1. Baseline characteristics of participating patients.
| Study patient | Intervention (n=63) | Control (n =40) | p* |
|---|---|---|---|
| Age, years, mean ± SD | 58.7 ± 13.5 | 63.4 ± 13.4 | 0.111 |
| Male, n (%) | 34 (54.0) | 24 (60.0) | 0.548 |
| Married, n (%) | 40 (64.5) | 25 (62.5) | 0.836 |
| Employed, n (%) | 30 (48.4) | 16 (40.0) | 0.406 |
| ≥1 burden in daily life, n (%) | 37 (59.7) | 25 (62.5) | 0.776 |
| – Stress at work, n (%) | 27 (43.5) | 8 (20.0) | 0.014 |
| – Concerns about family issues, n (%) | 10 (16.1) | 10 (25.0) | 0.271 |
| – Physically strenuous work, n (%) | 13 (21,0) | 5 (12.5) | 0.273 |
| – Overtime at work, n (%) | 8 (12.9) | 6 (15.0) | 0.764 |
| – Noise, dust, gases, fumes, n (%) | 10 (16.1) | 4 (10.0) | 0.380 |
| – Concerns about job security, n (%) | 6 (9.7) | 5 (12.5) | 0.748 |
| – Rotating shifts/night shift, n (%) | 8 (12.9) | 3 (7.5) | 0.521 |
| Poor subjective health status, n (%) | 25 (40.3) | 20 (50.0) | 0.337 |
| BMI, kg/m², mean ± SD | 30.9 ± 6.0 | 30.8 ± 6.6 | 0.986 |
| Regular physical activity, n (%) | 28 (45.9) | 14 (35.9) | 0.323 |
| Smoker, n (%) | 17 (27.9) | 5 (12.8) | 0.076 |
| Years since first hypertension diagnosis, mean ± SD | 8.6 ± 8.3 | 9.5 ± 8.8 | 0.612 |
| Regular BP self-checks, n (%) | 37 (62.7) | 25 (62.5) | 0.983 |
| Receives antihypertensive(s), n (%) | 61 (96.8) | 37 (92.5) | 0.374 |
| Number of antihypertensive agents, mean ± SD | 2.7 ± 1.7 | 2.8 ± 1.6 | 0.858 |
| Resistant hypertension, n (%) | 28 (44.4) | 24 (60.0) | 0.124 |
| Diagnosis of ≥1 hypertension-related secondary disease and/or diabetes mellitus type 2, n (%) | 34 (54.0) | 24 (60.0) | 0.548 |
| – Diabetes mellitus type 2, n (%) | 23 (37.1) | 17 (45.9) | 0.385 |
| – Coronary heart disease, n (%) | 8 (12.7) | 8 (20.0) | 0.319 |
| – Chronic renal insufficiency, n (%) | 6 (9.8) | 6 (16.2) | 0.361 |
| – Cardiac insufficiency, n (%) | 4 (6.5) | 5 (12.8) | 0.302 |
| – Stroke, n (%) | 6 (9.7) | 2 (5.1) | 0.480 |
| Mental disorder, n (%) | 18 (28.6) | 8 (21.1) | 0.402 |
| Secondary hypertension, n (%) | 7 (11.3) | 5 (13.5) | 0.758 |
Percentages are reported for valid cases.SD, standard deviation; BMI, body mass index; BP, blood pressure
*Data of the intervention and the control arm were compared using chi-square tests for categorical variables and t-tests in independent samples for continuous variables
eTable 1. Baseline characteristics of participating physicians.
| Physicians | Intervention (n = 12) | Control (n = 12) | p* |
|---|---|---|---|
| Age, years, mean ± SD | 52.1 ± 12.3 | 51.6 ± 6.7 | 0.903 |
| Male, n (%) | 8 (66.7) | 9 (75.0) | 1.000 |
| Degree, n (%) [multiple responses] | |||
| Primary care physician (BC), n (%) | 8 (66.7) | 6 (50.0) | 0.408 |
| Internal medicine (BC), n (%) | 3 (25.0) | 4 (33.3) | 1.000 |
| Internal and general medicine (BC), n (%) | 1 (8.3) | 4 (33.3) | 0.317 |
| Self-employed, n (%) | 11 (91.7) | 12 (100.0) | 1.000 |
| Time since medical license, years, mean ± SD | 24.6 ± 8.8 | 23.5 ± 7.9 | 0.770 |
Percentages are reported for valid cases.SD, standard deviation; BC, Board Certified\
*Data of the intervention and the control arm were compared using chi-square tests for categorical variables and t-tests in independent samples for continuous variables
Physicians of the intervention arm requested the following input:
Management of difficult cases (42.9%, n=6 of 14 requests) and/or complex medication regimens (28.6%, n=4);
Standardized BP measurements including reliability and validity of new devices (21.4%, n=3);
Motivational strategies to assure patients’ adherence (7.1%, n=1).
Patient characteristics
ABPM documentation at baseline and follow-up was available for 134 patients (79.3%). Excluding 31 patients (23.1%) with white coat hypertension, the final analysis included 103 patients: intervention arm, n=63; control arm, n=40. Characteristics of patients excluded were similar to those included. Of the 7.7 patients (range 1–11) per practice initially recruited, 5.2 patients (range 1–8) were included in the final analysis.
Patient baseline characteristics were similar in both groups, except for more work-related stress in the intervention arm (43.5% (n=27) versus 20.0% (n=8), P=0.014) (Table 1, eTable 1).
On average, patients with RH (n=52) were older (P=0.001), were taking more antihypertensives (P<0.001), had a longer history of hypertension (P<0.001), and a higher prevalence of coronary heart disease (P<0.001) than those without RH (Table 2).
Table 2. Characteristics of patients with resistant hypertension.
| Baseline characteristics | Intervention (n = 28) | Control (n = 24) | p*1 |
| Age, years, mean ± SD | 63.6 ± 16.7 | 66.9 ± 11.3 | 0.421 |
| Male, n (%) | 18 (64.3) | 15 (62.5) | 0.894 |
| Poor subjective health status, n (%) | 14 (50.0) | 15 (62.5) | 0.366 |
| Overweight, n (%) | 21 (84.0) | 19 (90.5) | 0.673 |
| Regular physical activity, n (%) | 15 (53.6) | 10 (41.7) | 0.392 |
| Smoker, n (%) | 8 (29.6) | 2 (8.3) | 0.168 |
| Years since first diagnosis of hypertension, mean ± SD | 14.1 ± 8.4 | 12.1 ± 7.6 | 0.414 |
| Regular BP self-checks, n (%) | 20 (74.1) | 17 (70.8) | 0.796 |
| Diagnosis of ≥1 hypertension-related secondary disease and/or diabetes mellitus type 2, n (%) | 18 (64.3) | 18 (75.0) | 0.404 |
| Follow-up: Changes in patients’ hypertension management | Intervention (n=28) | Control (n=24) | p*1 |
| Examination of the upper-arm cuff of patients’ BP monitor, n (%) | 23 (82.1) | 9 (37.5) | 0.001 |
| Prescription for BP monitor, n (%) | 13 (46.4) | 2 (8.3) | 0.003 |
| Optimization of psychiatric treatment, n (%) | 7 (25.0) | 0 (0) | 0.011 |
| Supervision of BP self-checks, n (%) | 24 (85.7) | 14 (58.3) | 0.026 |
| Mean number of follow-up appointments per patient, mean ± SD | 5,4 ± 2,5 | 4,6 ± 2,4 | 0,275 |
| Referral to a hypertensiology center, n (%) | 3 (11,1) | 1 (4,2) | 0,612 |
| Evaluation for secondary hypertension (renal insufficiency, pheochromocytoma, Conn syndrome, sleep apnea), n (%) | 21 (77.8) | 19 (79.2) | 0.904 |
| Medication | Intervention (n=28) | Control (n=24) | p*1 |
| Number of antihypertensive agents at baseline, mean ± SD | 4.2 ± 1.2 | 3.8 ± 1.0 | 0.225 |
| Number of antihypertensive agents at follow-up, mean ± SD | 4.5 ± 1.2 | 4.0 ± 1.0 | 0.072 |
| Change in antihypertensive agents, mean ± SD | +0.3 ± 0.8 | +0.2 ± 0.3 | 0.231 |
| p*2 | 0.036 | 0.083 |
Percentages are reported for valid cases.
SD, standard deviation; BP, blood pressure
*1Comparison of intervention and control arm using chi-square tests for categorical variables and t-tests in independent samples for continuous variables
*2Comparison of baseline and follow-up data using McNemar tests for categorical variables and paired t-tests for continuous variables)
Blood pressure
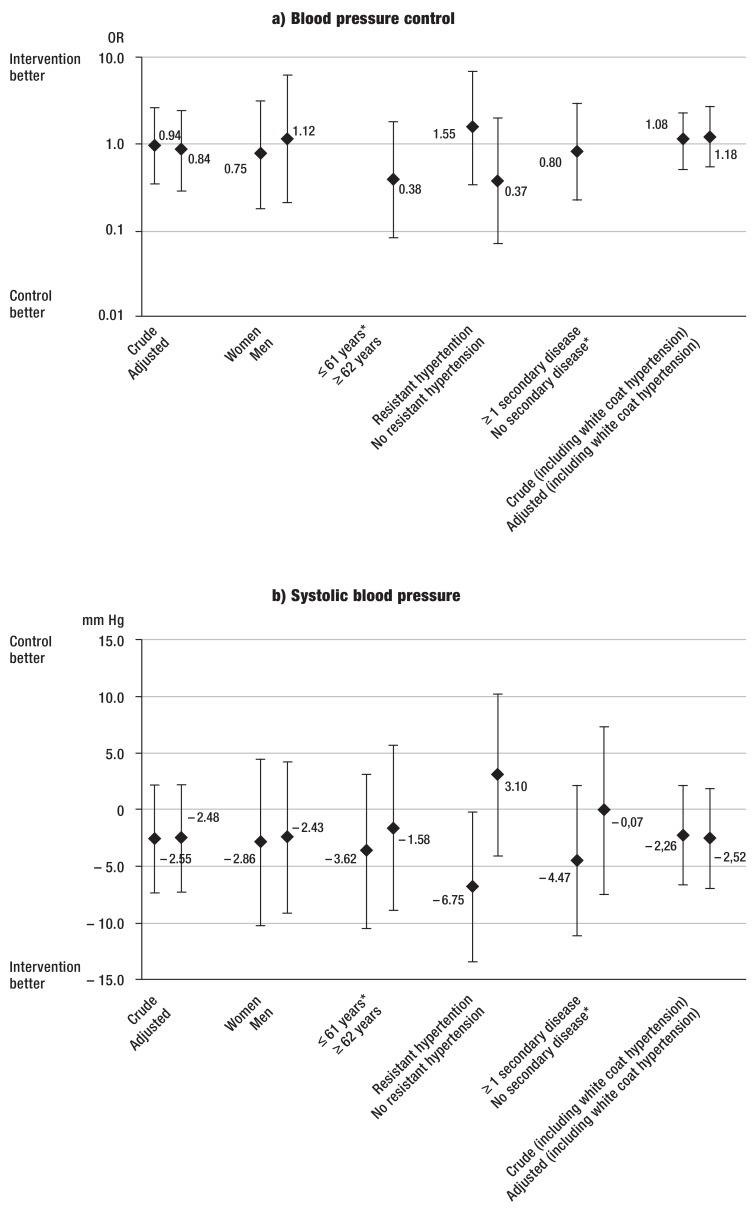
The intervention had no effect on BP control or BP changes. However, after five months, ABP was controlled in 19.4% of the patients (20 of 103 patients), without a difference between study arms (intervention: n=12, 19%; control: n=8, 20%; P=0.905) (Table 3, eTable 2). The crude OR for the intervention’s effect on BP control was 0.94 (95% CI: [0.34; 2.58]), the adjusted OR was 0.84 [0.29; 2.43].
Table 3. Blood pressure control rates and average ambulatory blood pressure values for the analysis population and patients with and without resistant hypertension, stratified by intervention status.
| Baseline, mean ± SD | 5 months follow-up, mean ± SD | Difference | p*1 | |||||
|---|---|---|---|---|---|---|---|---|
| ABP, mmHg | SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP |
| Study population (N=103) | 146.7 ± 12.1 | 84.7 ± 9.1 | 138.5 ± 13.1 | 80.6 ± 10.0 | – 8.2 ± 11.8 | – 4.1 ± 7.5 | <0.001 | <0.001 |
| Intervention (n=63) | 147.2 ± 11.8 | 85.1 ± 8.4 | 137.9 ± 13.5 | 80.9 ± 10.4 | – 9.3 ± 11.9 | – 4.2 ± 7.9 | <0.001 | <0.001 |
| Control (n=40) | 146.1 ± 12.7 | 83.9 ± 10.2 | 139.4 ± 12.7 | 80.1 ± 9.5 | – 6.7 ± 11.7 | – 3.8 ± 6.9 | 0.001 | 0.001 |
| p*2 | 0.651 | 0.512 | 0.571 | 0.674 | 0.274 | 0.815 | ||
| With resistant hypertension(N=52) | 148.1 ± 11.3 | 83.8 ± 10.7 | 140.6 ± 13.6 | 79.7 ± 11.0 | – 7.5 ± 12.1 | – 4.1 ± 7.9 | <0.001 | <0.001 |
| Intervention (n=28) | 148.1 ± 10.3 | 85.6 ± 11.0 | 137.6 ± 14.1 | 79.8 ± 12.2 | – 10.5 ± 11.9 | – 5.8 ± 8.7 | <0.001 | 0.002 |
| Control (n=24) | 148.1 ± 12.7 | 81.8 ± 10.2 | 144.1 ± 12.5 | 79.5 ± 9.6 | – 4.0 ± 11.6 | – 2.3 ± 6.4 | 0.105 | 0.093 |
| p*2 | 0.992 | 0.213 | 0.088 | 0.933 | 0.053 | 0.113 | ||
| Without resistant hypertension (N=51) | 145.3 ± 12.8 | 85.5 ± 7.1 | 136.3 ± 12.4 | 81.5 ± 9.0 | – 9.0 ± 11.6 | – 4.0 ± 7.2 | <0.001 | <0.001 |
| Intervention (n=35) | 146.4 ± 13.0 | 48.8 ± 5.6 | 138.1 ± 13.2 | 81.8 ± 8.8 | – 8.3 ± 11.9 | – 3.0 ± 7.1 | <0.001 | 0.018 |
| Control (n=16) | 143.0 ± 12.3 | 87.0 ± 9.6 | 132.3 ± 9.5 | 80.8 ± 9.5 | – 10.7 ± 11.0 | – 6.2 ± 7.0 | 0.002 | 0.003 |
| p*2 | 0.380 | 0.394 | 0.126 | 0.724 | 0.136 | 0.513 | ||
ABP, ambulatory blood pressure; SD; standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure
*1Comparison of baseline and follow-up data using McNemar tests for categorical variables and paired t-tests for continuous variables;
*2Comparison of intervention and control data using chi-square tests for categorical variables and t-tests in independent samples for continuous variables
eTable 2. Blood pressure control rates and average ambulatory blood pressure values for the study population and patients with and without resistant hypertension, stratified by intervention status.
| ABP <130/80 mmhg | Baseline, n (%) | 5 months follow-up, n (%) | Difference | p*1 |
|---|---|---|---|---|
| Overall (N=103) | 0 (0.0) | 20 (19.4) | + 19.4 | <0.001 |
| Intervention (n=63) | 0 (0.0) | 12 (19.0) | + 19.0 | 0.001 |
| Control (n=40) | 0 (0.0) | 8 (20.0) | + 20.0 | 0.008 |
| p*2 | 0.905 | |||
| With resistant hypertension (N=52) | 0 (0.0) | 10 (19.2) | + 19.2 | 0.002 |
| Intervention (n=28) | 0 (0.0) | 6 (21.4) | + 21.4 | 0.031 |
| Control (n=24) | 0 (0.0) | 4 (16.7) | + 16.7 | 0.125 |
| p*2 | 0.736 | |||
| Without resistant hypertension (N=51) | 0 (0.0) | 10 (19.6) | + 19.6 | 0.002 |
| Intervention (n=35) | 0 (0.0) | 6 (17.1) | + 17.1 | 0.031 |
| Control (n=16) | 0 (0.0) | 4 (25.0) | + 25.0 | 0.046 |
| p*2 | 0.705 | |||
ABP, ambulatory blood pressure
*1Comparison of baseline and follow-up data using McNemar tests for categorical variables and paired t-tests for continuous variables
*2Comparison of intervention and control data using chi-square tests for categorical variables and t-tests in independent samples for continuous variables
Mean ABP was 147/85 mmHg (±12.1 systolic/±9.1 diastolic) initially and decreased to 139/81 mmHg (±13.1/±10.0) at follow-up without differences between study arms (Table 3, eTable 2). BP decreased by 9.3/4.2 mmHg (±11.9/±7.9) in the intervention and by 6.7/3.8 mmHg (±11.7/±6.9) in the control arm. The intervention had no effect on the change in BP: SBP –2.55 mmHg [-7.28; 2.19] in the crude model, –2.48 mmHg SBP [-7.24; 2.29] in the adjusted model, and likewise DBP –0.28 mmHg [–3.29; 2.73] and –0.25 mm Hg [-3.31; 2.82]) (eFigure b, c). Inclusion of patients with white coat hypertension barely changed these estimates (eFigure a, b, c).
eFIGURE.
Effects of intervention on blood pressure: crude and adjusted estimates
a) Odds ratios and 95% confidence intervals for blood pressure control (ambulatory blood pressure <130/80 mmHg).
b) Changes in systolic blood pressure (in mmHg) and 95% confidence intervals.
c) Changes in diastolic blood pressure (in mmHg) and 95% confidence intervals.
a–c) Crude and adjusted estimates. The models were adjusted for sex, age, and the presence of ≥ 1 hypertension-related disease and/or diabetes mellitus, unless it was stratified for one of these parameters.
*Model did not converge.
The post-hoc sensitivity analysis indicated some effect modification for SBP when stratifying by RH: The adjusted SBP change was –6.75 mmHg [-13.36; -0.13] (eFigure b). There was no indication for effect modification when stratifying for sex, age, and having ≥1 hypertension-related disease (eFigure a, b, c).
Changes in practice management and medication
At baseline, intervention and control practices used a similar number of hypertension management strategies (11.0±2.8 versus 10.8±1.5, P=0.859), which increased by three strategies in intervention practices (14.5±2.6 versus 11.4±2.2, P=0.005). At the patient level, the following practice strategies were newly applied in the intervention group: supervision of BP self-checks (82.3%, n=51), diagnostics for renal disease (77.4%, n=48), prescription of a BP monitor (41.9%, n=26), optimized psychiatric treatment (15.9%, n=10). Intervention patients received more follow-up appointments than control patients (7.0±9.7 versus 3.9±2.4). At follow-up, the average number of antihypertensives equaled between the study arms (both 3.2, intervention ±1.6, control ±1.3), only RH patients of the intervention arm were taking 0.5 agents more (4.5±1.2 versus 4.0±1.0, P=0.072) (Table 2).
Discussion
After five months, this CRT of physician manager education on hypertension management showed no effect of the intervention on BP control rates and changes of ABP. However, outcome-relevant blood pressure improvements were observed in both study arms (12). Intervention practices were more likely to newly implement various hypertension management strategies. In patients with RH, the post-hoc sensitivity analysis showed an effect of the intervention of –6.75 mmHg in SBP. These results are of high interest with regard to the development and implementation of practice hypertension management strategies. Nevertheless, four aspects need to be discussed in detail.
First, the improvement in both study arms (–8.2 mmHg systolic, –4.1 mmHg diastolic) suggests a bias due to study participation. Although we used a waiting list control strategy, study participation including the ABPMs and questionnaires likely influenced the physicians’ and patients’ awareness of hypertension in both study arms equally, leading to an underestimation of the intervention’s effects. This is supported by the fact that BP improved even in patients with long-standing hypertension.
Second, the results of the sensitivity analyses imply that physicians in the intervention group benefited from the education about managing patients with RH. This result is explained by the participatory approach chosen: following the physicians’ requests, the CME sessions provided information on diagnostic and therapeutic strategies for RH. However, as our study was not specifically designed for this subgroup, future studies are needed. Given that RH is associated with a 1.34-fold higher risk for all-cause mortality, a 2.11-fold higher risk for nephropathy and a 1.47-fold higher risk for cardiovascular diseases compared to patients without RH (24), our finding suggests that an intervention with input on how to manage RH bears an enormous public health potential.
Third, the BP changes observed in our study are within the range of those observed in other CRTs (Figure 1). Like ours, all studies saw improvements in BP within their study populations, but with inconsistent interventional effects (5, 6, 9, 10, 14, 15).
Fourth, physician managers of the intervention arm did not explicitly request organizational input, yet they newly applied practice strategies between baseline and follow-up. Unlike other CRTs, we focused on rather easy-to-implement internal practice redesign strategies which were selected by choice of the respective physician manager. A longer follow-up period might have shown an impact of these implementations on BP.
Limitations and Strengths
The primary outcome chosen was difficult to obtain in a patient sample such as ours in the rather short follow-up time, so that a combined outcome (BP control and/or a pre-defined BP reduction) and a longer follow-up (e.g. 12 months) are reasonable for future studies. The drop-out rate of 21% was within the range described in other CRTs addressing hypertension (11–33%) (6– 10). Cluster drop-outs and patients lacking a follow-up ABPM necessitate larger oversampling to assure study power. In addition, incentives for patients and physicians might help to increase follow-up rates. A major strength of our study is the cluster design which is well known for its proximity to real-life. Using a participatory approach, we tailored the intervention to the physician managers’ needs. Finally, the main outcomes were measured by ABPM which is considered the most valid noninvasive BP measurement method.
Conclusion
Our educational intervention showed no effect on BP, yet improved the application of practice strategies. Our finding of an interventional effect in the subgroup of patients with RH warrants further investigation. A larger CRT focusing on these cardiovascular high-risk patients is planned.
Key Messages.
This educational intervention was tailored to primary care physicians’ information needs in hypertensiology: participants were especially interested in learning how to manage difficult cases.
Addressing uncontrolled hypertension, this study included patients with long-standing hypertension: on average, hypertension was diagnosed 9 years prior.
Study participation led to outcome-relevant improvements in both study arms, but there was no greater improvement in the intervention arm.
The education improved the application of hypertension management strategies in intervention practices.
A post-hoc sensitivity analysis suggests an interventional effect of –6.75 systolic in patients with resistant hypertension; this finding is in line with the education contents as requested by participating physicians and warrants further investigation..
Acknowledgments
We thank Anna Mitchell, MD, Associate Professor, Department for Nephrology, Excellence Center for Hypertension, University Hospital Essen, Deputy Chair of the German Hypertension League; Heike Bruck, MD, Associate Professor, Chief Physician, Clinic for Internal Medicine, Helios Clinic Krefeld; and Ulrich Tholl, MD, Chief Physician, Department for Renal Diseases and Hypertension, St.-Antonius-Hospital Kleve, German Hypertension League, for supporting our CME sessions on hypertension management. We are also grateful to Claudia Ose, M.Sc. and Dr. rer. physiol. Ulrike Krahn, Dipl.-Stat., both Center for Clinical Trials Essen (ZKSE) and Institute for Medical Informatics, Biometry and Epidemiology (IMIBE), University Hospital Essen, for their input on statistical issues. Our thanks also go to the participating patients, physicians, and medical assistants for their dedication.
Funding Source
The study was kindly supported by the Ministry of Innovation, Science and Research, North-Rhine Westphalia, Germany which provided a general grant for teaching and research at the Institute. The source of funding did not influence the study design, analyses, interpretation of results or the decision on publication.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Bramlage P, Böhm M, Volpe M, et al. A global perspective on blood pressure treatment and control in a referred cohort of hypertensive patients. J Clin Hypertens (Greenwich) 2010;12:666–677. doi: 10.1111/j.1751-7176.2010.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labeit AM, Klotsche J, Pieper L, et al. Changes in the prevalence, treatment and control of hypertension in Germany? A clinical-epidemiological study of 50.000 primary care patients. PloS One. 2012;7 doi: 10.1371/journal.pone.0052229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logan AG, Irvine MJ, McIsaac WJ, et al. Effect of home blood pressure telemonitoring with self-care support on uncontrolled systolic hypertension in diabetics. Hypertension. 2012;60:51–57. doi: 10.1161/HYPERTENSIONAHA.111.188409. [DOI] [PubMed] [Google Scholar]
- 4.Heisler M, Hofer TP, Klamerus ML, et al. Study protocol: The Adherence and Intensification of Medications (AIM) study—a cluster randomized controlled effectiveness study. Trials. 2010;11 doi: 10.1186/1745-6215-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heisler M, Hofer TP, Schmittdiel JA, et al. Improving blood pressure control through a clinical pharmacist outreach program in patients with diabetes mellitus in 2 high-performing health systems: The adherence and intensification of medications cluster randomized, controlled pragmatic trial. Circulation. 2012;125:2863–2872. doi: 10.1161/CIRCULATIONAHA.111.089169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hennessy S, Leonard CE, Yang W, et al. Effectiveness of a two-part educational intervention to improve hypertension control: A cluster-randomized trial. Pharmacotherapy. 2006;26:1342–1347. doi: 10.1592/phco.26.9.1342. [DOI] [PubMed] [Google Scholar]
- 7.Lüders S, Schrader J, Schmieder RE, Smolka W, Wegscheider K, Bestehorn K. Improvement of hypertension management by structured physician education and feedback system: cluster randomized trial. Eur J Cardiovasc Prev Rehabil. 2010;17:271–279. doi: 10.1097/HJR.0b013e328330be62. [DOI] [PubMed] [Google Scholar]
- 8.Roumie CL, Elasy TA, Greevy R, et al. Improving blood pressure control through provider education, provider alerts, and patient education. Ann Intern Med. 2006;145 doi: 10.7326/0003-4819-145-3-200608010-00004. [DOI] [PubMed] [Google Scholar]
- 9.Carter BL, Bergus GR, Dawson JD, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens (Greenwich) 2008;10:260–271. doi: 10.1111/j.1751-7176.2008.07434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169:1996–2002. doi: 10.1001/archinternmed.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hypertension Detection and Follow-Up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. Hypertension Detection and Follow-up Program Cooperative Group. JAMA. 1979;242:2562–2571. [PubMed] [Google Scholar]
- 12.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 13.Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD005182.pub4. CD005182. [DOI] [PubMed] [Google Scholar]
- 14.Pouchain D, Lièvre M, Huas D, et al. Effects of a multifaceted intervention on cardiovascular risk factors in high-risk hypertensive patients: the ESCAPE trial, a pragmatic cluster randomized trial in general practice. Trials. 2013;14 doi: 10.1186/1745-6215-14-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reuther LO, Paulsen MS, Andersen M, et al. Is a targeted intensive intervention effective for improvements in hypertension control? A randomized controlled trial. Fam Pract. 2012;29:626–632. doi: 10.1093/fampra/cms031. [DOI] [PubMed] [Google Scholar]
- 16.Campbell MJ, Donner A, Klar N. Developments in cluster randomized trials and statistics in medicine. Stat Med. 2007;26:2–19. doi: 10.1002/sim.2731. [DOI] [PubMed] [Google Scholar]
- 17.Weltermann B, Viehmann A, Kersting C. Hypertension management in primary care: study protocol for a cluster randomized controlled trial. Trials. 2015;16 doi: 10.1186/s13063-015-0627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 19.Weltermann B, Schlomann H, Mousa Doost S, Gesenhues S. Hypertension management program improves blood pressure control in primary care. Circulation. 2009;120 [Google Scholar]
- 20.Weltermann B, Mousa Doost S, Schlomann H, Gesenhues S. Hypertonie-Management zur Primärprävention: Welche soziobiographischen und medizinischen Faktoren beeinflussen die Qualität der Blutdruckkontrolle? Dtsch Med Wochenschr. 2010;135 [Google Scholar]
- 21.Donner A, Klar N. 1st edition. London: Wiley; 2000. Design and analysis of cluster randomized trials in health research. [Google Scholar]
- 22.Rotondi MA. CRTSize: Sample size estimation functions for cluster randomized trials R package version 0.4. http://CRAN.R-project.org/package=CRTSize. (last accessed on 29 September 2015) [Google Scholar]
- 23.Campbell MJ. Cluster randomized trials in general (family) practice research. Stat Methods Med Res. 2000;9:81–94. doi: 10.1177/096228020000900202. [DOI] [PubMed] [Google Scholar]
- 24.Muntner P, Davis BR, Cushman WC, et al. Treatment-resistant hypertension and the incidence of cardiovascular disease and end-stage renal disease: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertension. 2014;64:1012–1021. doi: 10.1161/HYPERTENSIONAHA.114.03850. [DOI] [PubMed] [Google Scholar]