Abstract
Objective
To determine if differences in coronary endothelial function are observed between asymptomatic women with type 2 diabetes (DM) and healthy controls using coronary phase contrast flow velocity MRI in response to cold pressor stress, an established endothelium-dependent vasodilatory stress.
Methods
Phase contrast flow velocity imaging of the right coronary artery (RCA) was performed in seven asymptomatic pre-menopausal women with DM and eight healthy female participants in response to the cold pressor test at 3T.
Results
There was no significant difference in percent increase in coronary flow velocity from rest to peak flow velocity between DM and controls (32% ± 22 vs. 46% ± 17, p=0.11). However, percent increase in coronary flow velocity was lower in DM than controls (-3% ± 14 vs 31% ± 30, p=0.01) during the second minute of cold pressor stress, when endothelial-mediated vasodilation should occur.
Conclusions
Asymptomatic women with DM demonstrate reduced coronary flow velocity during the second minute of cold pressor stress, indicating coronary endothelial dysfunction.
Keywords: coronary flow, coronary blood flow reserve, endothelial dysfunction, cardiac magnetic resonance
Introduction
Type 2 diabetes (DM) substantially increases the risk for cardiovascular (CV) events, particularly in women.1 CV risk in asymptomatic patients with DM is equivalent to that of patients without DM who have had a prior CV event.2-4 Specifically for premenopausal women, the diagnosis of DM heralds a dramatic change in CV risk, essentially eliminating the favorable gender differences observed before menopause. Of greater concern, women with DM are more likely to die following their first CV event than women or men without DM.5 Although there is a significant need to identify women with asymptomatic CV disease prior to the first event, most non-invasive screening tests focus on plaque-based disease, such as measurements of coronary calcium, computed tomography angiography and exercise or pharmacologic stress tests. Coronary endothelial dysfunction has been characterized in women with angina prior to the development of significant obstructive plaque, and the presence of coronary endothelial dysfunction increases the risk for CV events.6 However, these studies diagnosed coronary endothelial dysfunction during coronary angiography, a technique that would be inappropriate for screening asymptomatic women.
Non-invasive imaging techniques for measuring coronary vascular reactivity include ultrasound and cardiac magnetic resonance (CMR) strategies. Transthoracic Doppler echocardiography has been used to measure coronary flow velocity reserve in the LAD with good correlation to intracoronary Doppler flow.7 Prior studies using transthoracic Doppler have demonstrated reduced coronary flow velocity reserve in asymptomatic patients with DM8 and in patients with Syndrome X.9 However, feasibility of transthoracic Doppler is limited in patients with large body habitus and poor acoustic window. More recently, coronary vascular reactivity has been measured using quantitative myocardial perfusion CMR in response to cold pressor stress.10, 11 However, this technique requires intravenous gadolinium contrast which limits repeat measurements and is unsuitable for patients with decreased renal function.
Pharmacologically induced changes in coronary flow velocity have been measured non-invasively using phase contrast CMR at 1.5 Tesla 12-17 and validated with intracoronary Doppler.14 Phase contrast coronary CMR is technically challenging as both high spatial and temporal resolution are required for accurate flow measurement.13, 18 More recent studies have reported feasibility of coronary phase contrast CMR at 3 Tesla, which affords higher signal-to-noise that can be leveraged for increased spatial resolution.19, 20 To further improve spatial and temporal resolution, k-space sampling techniques can also be utilized with less sensitivity to motion than Cartesian methods.21 We tested a protocol using coronary phase contrast flow velocity CMR at 3 Tesla with spiral k-space sampling to determine: (1) if changes in coronary flow velocity can be measured in response to cold pressor stress, a well-established endothelium-dependent vasodilatory stress22 ; and (2) if the technique could detect significant differences in coronary flow velocity between asymptomatic women with DM and healthy controls.
Methods
Study Participants
This study was approved by the University of Texas Southwestern Institutional Review Board and all participants provided written informed consent. Seven asymptomatic pre-menopausal women with DM and eight pre-menopausal overweight but otherwise healthy female participants were recruited. Women with DM were required to have a hemoglobin A1c below 10 within three months of the study. Participants were excluded if they had chest pain, a history of coronary artery disease, heart failure, hypertension, stroke, lung disease, smoking or cocaine use. Participants were excluded if they were taking oral contraceptives or any medications that would affect vascular function, or if they had a contraindication to MR imaging.
CMR Technique
CMR imaging was performed using a 3 Tesla MR scanner (Achieva, Philips, Netherlands) equipped with a 40 mT/m maximum gradient strength and 200 mT/m/msec slew rate. Images were acquired with a 6-element cardiac receiver coil with the subject in the supine position. Retrospective electrocardiographic gating was utilized. Initial scout images were acquired in three orthogonal planes using a balanced turbo field echo (B-TFE) sequence. Axial B-TFE images of the heart were obtained through the proximal right coronary artery (RCA). An imaging plane was selected perpendicular to the proximal RCA at a distance 2-3 cm from the ostium for phase contrast imaging.
Velocity-encoded phase contrast imaging of the coronary arteries has previously been described.13, 14, 16 In our study, phase contrast flow velocity imaging of the proximal RCA was performed using a VCG triggered end-expiratory breath-hold (11-15 sec) and spiral k-space sampling. Additional parameters included: echo time 3.5 msec, repetition time 34 msec, flip angle 20°, field of view 256 × 256 mm2, matrix size 312 × 312, spatial resolution 0.8 × 0.8 × 7 mm3, temporal resolution 44 msec, spiral interleaves 11, velocity encode (VENC) 35 cm/sec, readout time less than 40 msec. In each flow imaging series, at least 14 phases of data were acquired and reconstructed into velocity maps with corresponding magnitude images.
Study Protocol & Cold Pressor Test
Three series of baseline RCA flow were obtained for each subject. Next, the cold pressor test was performed with the subject remaining supine in the MR scanner by immersing the subject's left hand in a magnet-compatible ice-water bath (50% ice, 50% water) for 3 minutes.23 During the cold pressor test, two additional series of RCA flow were initiated after the 1st and 2nd minute of cold stress. The ice-water bath was removed after 3 minutes of cold stress and two additional series of recovery flow were acquired at 1 minute and 10 minutes post-stress (Figure 1). Peripheral blood pressure and heart rate were continuously monitored throughout the experiment at 30 second intervals using an automated brachial blood pressure cuff rated MR compatible at 3T. Repeated flow measurements were obtained in 12 participants to evaluate reproducibility of peak RCA flow velocity.
Figure 1.
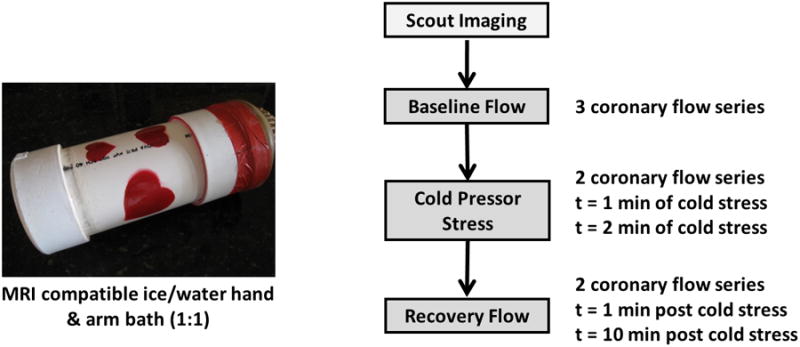
Study protocol for measurement of coronary flow velocity in response to cold pressor stress.
Image Analysis
Velocity and magnitude images were transferred to a remote workstation and analyzed using QFLOW (v. 4.1.6, Medis, Leesburg, VA). Cross-sectional contours of the RCA lumen were manually traced on each magnitude image during diastole by two trained observers (CM and AC) masked to study group. Identical tracings were automatically applied to the corresponding velocity maps so that peak RCA flow velocity (cm/sec) during diastole could be measured.14 Images acquired during systole were not evaluated due to excessive coronary motion.24
Calculated Variables
Mean arterial pressure (MAP) was estimated using the following formula:
Rate-pressure product was defined as the product of heart rate and mean arterial pressure. Coronary flow velocity was defined as the peak RCA flow velocity measured by phase contrast imaging during diastole. The change in coronary flow velocity after cold pressor stress was calculated as percent increase in coronary flow velocity from baseline to peak coronary flow during the two minutes after stress14 and by percent increase in coronary flow velocity from baseline to the first minute, and baseline to the 2nd minute after cold pressor stress. Coronary vascular resistance was estimated by dividing mean arterial pressure by peak diastolic RCA flow velocity.
Statistical Analysis
Data are expressed as mean plus or minus standard deviation. Measurement differences between baseline and cold stress were analyzed by means of a t test for paired data (baseline and cold stress) or independent t test between groups for the 1st and 2nd minute of the cold stress. Coefficients of variability were calculated to evaluate inter-observer and intra-observer reproducibility of coronary flow velocity measurements. A p value of less than 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics of our study participants are presented (Table 1). Compared to healthy controls, women with DM had higher body mass index (p=0.03), greater waist circumference (p=0.02), and greater systolic and diastolic blood pressures at rest (p=0.02 and p=0.03, respectively). Only one DM patient was on insulin while the others were on oral medications. All 15 participants successfully tolerated the cold pressor test. Diastolic RCA phase contrast flow images were successfully acquired in 100% of participants at baseline and during cold stress (Figure 2). Recovery flow images were not obtained in one subject due to early termination of the MRI study related to claustrophobia. Coefficients of variability for measures of peak coronary flow velocity were 5.0% for inter-observer variability and 2.0% for intra-observer variability.
Table 1. Baseline characteristics of the study participants.
| Variable | Diabetes (n=7) | Control (n=8) | P |
|---|---|---|---|
| Age (years) | 43 ± 5 | 35 ± 7 | 0.05 |
| Body mass index (kg/m2) | 35 ± 9 | 25 ± 2 | 0.03 |
| Waist circumference (cm) | 102 ± 18 | 84 ± 10 | 0.02 |
| Resting heart rate (bpm) | 68 ± 5 | 62 ± 7 | 0.07 |
| Resting systolic BP (mmHg) | 117 ± 7 | 102 ± 9 | 0.02 |
| Resting diastolic BP (mmHg) | 75 ± 4 | 65 ± 5 | 0.03 |
| Ethnicity | |||
| Caucasian, n (%) | 1 (14%) | 3 (38%) | 0.03 |
| African-American, n (%) | 4 (58%) | 2 (25%) | 0.02 |
| Hispanic, n (%) | 1 (14%) | 2 (25%) | 0.14 |
| Asian, n (%) | 1 (14%) | 1 (12%) | 0.67 |
| Hemoglobin A1c | 7.4 ± 1.2 | -- | -- |
BP: blood pressure
Figure 2.
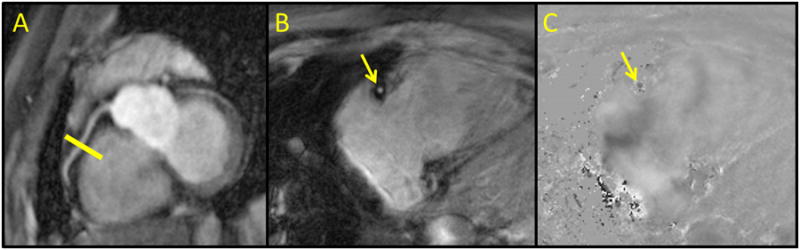
Localization and phase contrast images of the proximal RCA. Non-contrast balanced turbo field image (A) of the proximal RCA (white arrows) with selection of the imaging plane for phase contrast imaging (yellow line). Representative magnitude (B) and velocity (C) phase contrast images through the RCA at mid-diastole.
Changes in coronary flow velocity for women with DM and healthy controls in response to cold pressor stress are also depicted in Figure 3. A significant and similar hemodynamic response to the cold pressor test was observed in both women with DM and healthy controls, as represented by changes in the rate pressure product from baseline: at 1-min of cold pressor stress, 40% ± 26 and 46% ± 25 (p=0.76); and at 2-min of cold pressor stress, 28% ± 18 and 23% ± 16 (p=0.41). There was no difference in baseline coronary flow velocity between women with DM and healthy controls (DM, 18.5 cm/s ± 6.6 vs. controls, 16.9 cm/s ± 6.4, p=0.65) or in peak percent increase in coronary flow velocity (DM, 32% ± 22 vs. controls, 46% ± 17, p=0.11). However, when comparing changes in coronary flow velocity specifically during the second minute of cold stress, a significant difference was observed between women with DM and healthy controls (-3% ± 14 vs 31% ± 30, p=0.01) (Figure 4). Upon further review, differences in coronary flow velocity at the second minute of cold stress correlated with changes in coronary vascular resistance between women with DM and healthy controls (18% ± 25 vs -9% ± 16, p = 0.02), not mean arterial pressure (Figure 4).
Figure 3.
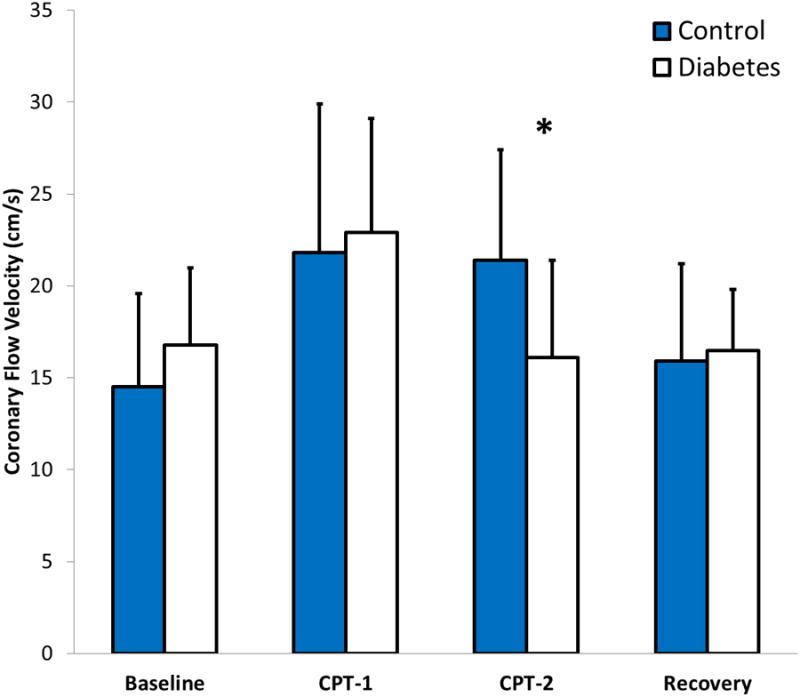
Changes in peak coronary flow velocity in response to the cold pressor test. Asterisk (*) indicates p<0.05. Error bars indicate the 95% confidence interval. CPT-1: first minute of cold pressor stress, CPT-2: second minute of cold pressor stress
Figure 4.
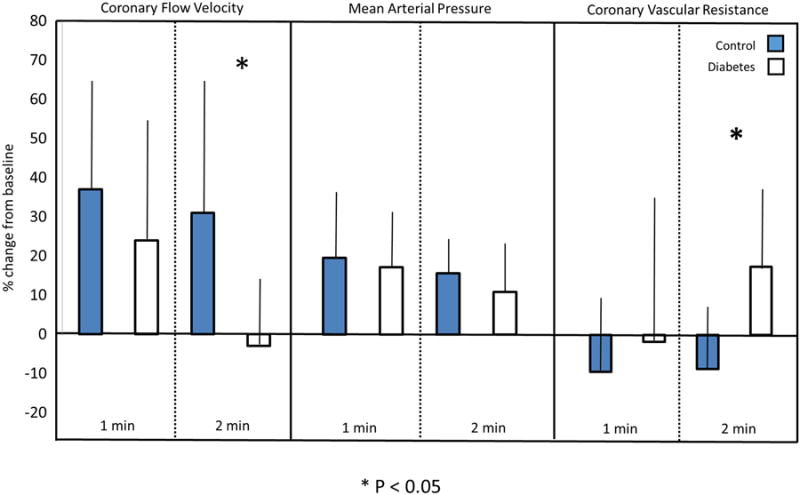
Impaired coronary flow velocity in women with DM after cold stress corresponds with increased coronary vascular resistance. Asterisk (*) indicates p <0.05. Error bars indicate the 95% confidence interval.
Discussion
To our knowledge, this is the first study demonstrating the feasibility of coronary phase contrast flow velocity CMR at 3 Tesla for measuring changes in coronary flow velocity during the cold pressor test. Cold pressor stress induced similar peak increases in coronary flow velocity in women with DM and controls. However, women with DM demonstrated reduced flow velocity during the second minute of the cold pressor stress when endothelial-mediated vasodilation should occur. Thus, asymptomatic women with DM have evidence of coronary endothelial dysfunction measurable using a non-invasive, CMR technique without the use of intravenous contrast or pharmacologic stress.
Previous studies have shown evidence for impaired myocardial flow reserve and coronary flow velocity reserve in DM. For example, Yokoyama et al.25 used N13-ammonia positron emission tomography (PET) to demonstrate reduced myocardial flow reserve in response to pharmacologic stress among 25 asymptomatic, normotensive patients with DM compared to 12 healthy controls. QuPrior et al.26 also used N13-ammonia PET to show reduced myocardial flow reserve in response to endothelium-dependent cold pressor stress among patients with insulin resistance and DM compared to insulin-sensitive controls. These data are concordant with our observation of attenuated endothelium-dependent coronary flow velocity in asymptomatic women with DM at the 2nd minute of cold stress, which corresponds to the interval of maximum stimulated sympathetic nerve activity and endothelial-dependent pressor response.27 While PET is considered the gold standard for quantification of absolute myocardial blood flow, this technique is limited by the injection of radiopharmaceuticals and exposure to ionizing radiation, making it a less suitable technique for screening asymptomatic patients. Using transthoracic Doppler echocardiography, Kawata et al. 8 further demonstrated reduced coronary flow velocity reserve in response to adenosine stress among a cohort of asymptomatic patients with DM compared to controls. While the phase-contrast CMR technique has been validated against intracoronary Doppler flow with good correlation,7 the feasibility of transthoracic coronary Doppler flow is limited in some patients with large body habitus and poor acoustic windows. However, both the inter-observer and intra-observer variability of transthoracic coronary Doppler flow are similar to those reported in our study using phase contrast coronary CMR.
Phase contrast coronary imaging can be performed during breath-hold or by employing a respiratory gating technique.13, 15 However, both techniques are subject to error from through-plane motion of the coronary artery throughout the cardiac cycle. While prior studies have described strategies for correcting phase contrast coronary flow velocities to account for through-plane vessel motion,28 these strategies were not applied in our study. Rather, we selected peak coronary flow velocity measurements during end-diastole, during which time the RCA motion is minimal. This reporting method is similar to that used for intracoronary Doppler flow measurement.29 Further, a 3D spiral k-space acquisition technique was utilized in our study to improve motion sensitivity.30 Utilizing a spiral readout technique, we were able to achieve readout times (less than 40 msec) much shorter than the average diastolic rest period, typically around 80-100 msec. A readout time shorter than this can only be achieved using a spiral technique. In spiral k-space acquisitions, the center of k-space is oversampled thereby allowing for phase correction between lines of acquired data. Bornert et al. previously demonstrated that 3 Tesla coronary MR angiography with a spiral acquisition technique yielded higher signal-to-noise and less motion when compared to Cartesian schemes.31 Importantly, our measurement of peak diastolic coronary flow velocity demonstrated a consistent measurable increase in response to cold pressor stress with measurement reproducibility at baseline and after stress. Although as a group, women with DM had a significant decrease percent change in coronary flow velocity in the 2nd minute, there is enough inter individual variability to suggest this technique may potentially differentiate those at lower and higher risk (Figure 3).
Limitations
Although we did not independently validate our phase contrast technique against the gold standard technique of intracoronary Doppler flow, prior studies have reported significant agreement between coronary phase contrast flow velocity imaging with intracoronary Doppler flow14 and phantom flow models.19, 20 In addition, the plane chosen for through-plane phase contrast velocity mapping of the RCA was determined using a single localization view of the RCA. Since the RCA was not interrogated in multiple planes, the velocity mapping imaging plane may not have been optimally perpendicular to flow, thus contributing to measurement variability. In future studies, the optimal location for phase contrast imaging of the RCA can be determined by empirically quantifying periods of low motion to improve measurement reproducibility.32 Because we could not reliably detect small cross-sectional area changes of the RCA in response to cold pressor stress due to limitations in spatial resolution, we could not confirm whether changes in peak coronary flow velocity were primarily attributable to a lack of vasodilation or an increase in microvascular resistance. In future studies, coronary flow velocity imaging could be performed along with high resolution vessel wall imaging at baseline and during peak stress to assess cross-sectional area changes.33 Finally, in this pilot study, we did not match on body size, blood pressure, or age. These factors may impact resting coronary flow velocity and cardiac preload, and thereby impact coronary flow reserve. However, the objective of this study was to prove the feasibility of detecting differences in coronary flow velocity between groups using MRI. Therefore, we tried to compare two groups anticipated to demonstrate a difference in coronary flow velocity to cold pressor stress. As such, larger studies with matched baseline characteristics are necessary to establish the full utility and prognostic value of coronary phase contrast flow velocity imaging among asymptomatic women with DM.
Conclusions
In conclusion, our results demonstrate the feasibility of coronary phase contrast flow velocity CMR at 3 Tesla to detect changes in coronary flow velocity in response to the cold pressor test in healthy controls and asymptomatic women with DM. Women with DM demonstrate reduced flow velocity during the second minute of cold pressor stress, the interval when maximum endothelial-mediated vasodilation should occur. Thus, asymptomatic women with DM have evidence of coronary endothelial dysfunction measurable using a non-invasive imaging technique without the use of intravenous contrast, ionizing radiation or pharmacologic stress.
Acknowledgments
We would like to thank Dr. Ivan Dimitrov and Dr. Andrew Kontak for assistance with design and testing of the CMR protocol.
Funding Sources: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number UL1TR001105. Alice Chang was supported by an AHA Fellow to Faculty award and the Office of Women's Health Research (Building Interdisciplinary Careers in Women's Health award K12HD065987)
Footnotes
Conflict of Interest Statement: None Declared
References
- 1.Hu G, Jousilahti P, Qiao Q, Katoh S, Tuomilehto J. Sex differences in cardiovascular and total mortality among diabetic and non-diabetic individuals with or without history of myocardial infarction. Diabetologia. 2005;48:856–61. doi: 10.1007/s00125-005-1730-6. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from Coronary Heart Disease in Subjects with Type 2 Diabetes and in Nondiabetic Subjects with and without Prior Myocardial Infarction. The New England journal of medicine. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Howard B, Smith S, Jr, Eckel R, Redberg R, Bonow RO. Prevention Conference VI: Diabetes and Cardiovascular Disease: Executive Summary: Conference Proceeding for Healthcare Professionals From a Special Writing Group of the American Heart Association. Circulation. 2002;105:2231–9. doi: 10.1161/01.cir.0000013952.86046.dd. [DOI] [PubMed] [Google Scholar]
- 4.Schramm TK, Gislason GH, Kober L, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008;117:1945–54. doi: 10.1161/CIRCULATIONAHA.107.720847. [DOI] [PubMed] [Google Scholar]
- 5.Sowers JR. Diabetes mellitus and cardiovascular disease in women. Arch Intern Med. 1998;158:617–21. doi: 10.1001/archinte.158.6.617. [DOI] [PubMed] [Google Scholar]
- 6.von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–5. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 7.Hozumi T, Yoshida K, Akasaka T, et al. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol. 1998;32:1251–9. doi: 10.1016/s0735-1097(98)00389-1. [DOI] [PubMed] [Google Scholar]
- 8.Kawata T, Daimon M, Hasegawa R, et al. Effect on coronary flow velocity reserve in patients with type 2 diabetes mellitus: comparison between angiotensin-converting enzyme inhibitor and angiotensin II type 1 receptor antagonist. Am Heart J. 2006;151:798.e9–15. doi: 10.1016/j.ahj.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Lanza GA, Buffon A, Sestito A, et al. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008;51:466–72. doi: 10.1016/j.jacc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 10.Fairbairn TA, Motwani M, Mather AN, et al. Cardiac MR imaging to measure myocardial blood flow response to the cold pressor test in healthy smokers and nonsmokers. Radiology. 2014;270:82–90. doi: 10.1148/radiol.13122345. [DOI] [PubMed] [Google Scholar]
- 11.Ritter CO, Kowalski M, Weng AM, Beer M, Hahn D, Kostler H. Quantitative myocardial perfusion imaging with a MR cold pressor test. Magn Reson Med. 2012;67:246–50. doi: 10.1002/mrm.22941. [DOI] [PubMed] [Google Scholar]
- 12.Edelman RR, Manning WJ, Gervino E, Li W. Flow velocity quantification in human coronary arteries with fast, breath-hold MR angiography. J Magn Reson Imaging. 1993;3:699–703. doi: 10.1002/jmri.1880030503. [DOI] [PubMed] [Google Scholar]
- 13.Hofman MB, van Rossum AC, Sprenger M, Westerhof N. Assessment of flow in the right human coronary artery by magnetic resonance phase contrast velocity measurement: effects of cardiac and respiratory motion. Magn Reson Med. 1996;35:521–31. doi: 10.1002/mrm.1910350411. [DOI] [PubMed] [Google Scholar]
- 14.Hundley WG, Lange RA, Clarke GD, et al. Assessment of Coronary Arterial Flow and Flow Reserve in Humans With Magnetic Resonance Imaging. Circulation. 1996;93:1502–8. doi: 10.1161/01.cir.93.8.1502. [DOI] [PubMed] [Google Scholar]
- 15.Davis CP, Liu PF, Hauser M, Gohde SC, von Schulthess GK, Debatin JF. Coronary flow and coronary flow reserve measurements in humans with breath-held magnetic resonance phase contrast velocity mapping. Magn Reson Med. 1997;37:537–44. doi: 10.1002/mrm.1910370410. [DOI] [PubMed] [Google Scholar]
- 16.Hundley WG, Hillis LD, Hamilton CA, et al. Assessment of Coronary Arterial Restenosis With Phase-Contrast Magnetic Resonance Imaging Measurements of Coronary Flow Reserve. Circulation. 2000;101:2375–81. doi: 10.1161/01.cir.101.20.2375. [DOI] [PubMed] [Google Scholar]
- 17.Schiemann M, Bakhtiary F, Hietschold V, et al. MR-based coronary artery blood velocity measurements in patients without coronary artery disease. Eur Radiol. 2006;16:1124–30. doi: 10.1007/s00330-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 18.Hofman MB, Wickline SA, Lorenz CH. Quantification of in-plane motion of the coronary arteries during the cardiac cycle: implications for acquisition window duration for MR flow quantification. J Magn Reson Imaging. 1998;8:568–76. doi: 10.1002/jmri.1880080309. [DOI] [PubMed] [Google Scholar]
- 19.Johnson K, Sharma P, Oshinski J. Coronary artery flow measurement using navigator echo gated phase contrast magnetic resonance velocity mapping at 3.0 T. J Biomech. 2008;41:595–602. doi: 10.1016/j.jbiomech.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lotz J, Doker R, Noeske R, et al. In vitro validation of phase-contrast flow measurements at 3 T in comparison to 1.5 T: precision, accuracy, and signal-to-noise ratios. J Magn Reson Imaging. 2005;21:604–10. doi: 10.1002/jmri.20275. [DOI] [PubMed] [Google Scholar]
- 21.Hennig J. K-space sampling strategies. European radiology. 1999;9:1020–31. doi: 10.1007/s003300050788. [DOI] [PubMed] [Google Scholar]
- 22.Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation. 1988;77:43–52. doi: 10.1161/01.cir.77.1.43. [DOI] [PubMed] [Google Scholar]
- 23.Maroules CD, Chang AY, Kontak A, Dimitrov I, Kotys M, Peshock RM. Measurement of coronary flow response to cold pressor stress in asymptomatic women with cardiovascular risk factors using spiral velocity-encoded cine MRI at 3 Tesla. Acta Radiol. 2010;51:420–6. doi: 10.3109/02841851003645736. [DOI] [PubMed] [Google Scholar]
- 24.Marcus JT, Smeenk HG, Kuijer JP, Van der Geest RJ, Heethaar RM, Van Rossum AC. Flow profiles in the left anterior descending and the right coronary artery assessed by MR velocity quantification: effects of through-plane and in-plane motion of the heart. J Comput Assist Tomogr. 1999;23:567–76. doi: 10.1097/00004728-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Yokoyama I, Momomura S, Ohtake T, et al. Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1997;30:1472–7. doi: 10.1016/s0735-1097(97)00327-6. [DOI] [PubMed] [Google Scholar]
- 26.QuPrior JO, Quiñones MJ, Hernandez-Pampaloni M, et al. Coronary Circulatory Dysfunction in Insulin Resistance, Impaired Glucose Tolerance, and Type 2 Diabetes Mellitus. Circulation. 2005;111:2291–8. doi: 10.1161/01.CIR.0000164232.62768.51. [DOI] [PubMed] [Google Scholar]
- 27.Victor RG, Leimbach WN, Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension. 1987;9:429–36. doi: 10.1161/01.hyp.9.5.429. [DOI] [PubMed] [Google Scholar]
- 28.Keegan J, Gatehouse PD, Yang GZ, Firmin DN. Spiral phase velocity mapping of left and right coronary artery blood flow: correction for through-plane motion using selective fat-only excitation. J Magn Reson Imaging. 2004;20:953–60. doi: 10.1002/jmri.20208. [DOI] [PubMed] [Google Scholar]
- 29.Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. Journal of the American College of Cardiology. 1999;33:1756–824. doi: 10.1016/s0735-1097(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 30.Botnar RM, Kim WY, Bornert P, Stuber M, Spuentrup E, Manning WJ. 3D coronary vessel wall imaging utilizing a local inversion technique with spiral image acquisition. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2001;46:848–54. doi: 10.1002/mrm.1268. [DOI] [PubMed] [Google Scholar]
- 31.Bornert P, Stuber M, Botnar RM, et al. Direct comparison of 3D spiral vs. Cartesian gradient-echo coronary magnetic resonance angiography. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2001;46:789–94. doi: 10.1002/mrm.1258. [DOI] [PubMed] [Google Scholar]
- 32.Suever JD, Watson PJ, Eisner RL, Lerakis S, O'Donnell RE, Oshinski JN. Time-resolved analysis of coronary vein motion and cross-sectional area. Journal of magnetic resonance imaging : JMRI. 2011;34:811–5. doi: 10.1002/jmri.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makowski MR, Botnar RM. MR imaging of the arterial vessel wall: molecular imaging from bench to bedside. Radiology. 2013;269:34–51. doi: 10.1148/radiol.13102336. [DOI] [PubMed] [Google Scholar]


