Abstract
Objective
This study aimed to compare the responsiveness to change of the Patient-Reported Outcomes Measurement Information System (PROMIS®) asthma impact and generic scales to a legacy scale, the Paediatric Asthma Quality of Life Questionnaire (PAQLQ).
Methods
229 child-parent dyads in public insurance programs were enrolled. PROMIS Pediatric Short Forms and the PAQLQ were used to measure health-related quality of life (HRQoL) across 4 time points (T1 to T4) over 2 years; the Asthma Control and Communication Instrument was used to measure change of asthma control; Global Rating of Change (GRC) for breathing problems and overall health were used to measure change of health status. Responsiveness was tested by comparing the changes of HRQoL with change of asthma control and health status over time using t-tests, generalized estimating equation, and relative validity approaches.
Results
The PROMIS asthma impact scale (p<0.05) and all PAQLQ scales (p’s<0.05) exhibited significant responsiveness when anchored to asthma control and health status. Several PROMIS generic scales (pain, fatigue, and mobility) also indicated adequate responsiveness.
Conclusion
The PROMIS asthma impact scale indicated similar responsiveness to the PAQLQ. Due to its brevity and responsiveness, the PROMIS asthma impact scale is useful for clinical practice or research.
Keywords: Asthma, Children, Health-Related Quality of Life, Patient-Reported Outcomes Measurement Information System (PROMIS®), Responsiveness
Introduction
According to the 2012 National Health Interview Survey, approximately 6.8 million American children under 18 years of age had asthma [1]. Poorly controlled or uncontrolled asthma can lead to psycho-social-health impairments including sleep disturbances [2], school absenteeism [3], academic underperformance [4, 5], physical activity limitations [6], and poor health-related quality of life (HRQoL) [7–9].
Although several generic and asthma-specific HRQoL scales for children with asthma are available, most scales were developed using classical test theory (CTT) [10] that likely yield significant ceiling effects in HRQoL scores [11] and limit a scale’s ability to capture the full spectrum of the HRQoL related to asthma [12]. As a result, HRQoL scales derived from CTT may not sensitively identify important changes in HRQoL that mirror an underlying change in health status (e.g., asthma control status). Alternatively, item response theory (IRT) methodology allows for selecting items that are calibrated on the same metric and cover different levels of HRQoL on the latent continuum for asthma.
The concept of responsiveness to change refers to a scale’s ability to detect changes in HRQoL over time with the same directionality between the change of HRQoL and underlying health status [13, 14]. Small but meaningful changes are important to detect, particularly to estimate minimally important differences (MIDs) for use by clinicians [15]. Developed using IRT methodology, modern HRQoL scales, such as the Patient-Reported Outcomes Measurement Information System (PROMIS®) [16], may possess a superior responsiveness to change compared with the legacy scales that are derived from CTT.
Assessing responsiveness relies on the use of meaningful anchors and an appropriate time window to observe the HRQoL change [17]. However, previous responsiveness studies for pediatric asthma scales are not ideal because most investigations are based on data collected from only two time points that did not fully capture random variation related to measurements [18, 19]. One legacy scale, the Paediatric Asthma Quality of Life Questionnaire (PAQLQ), has shown significant responsiveness within and between study participants; however, this study used a small sample (N=52) in a single 9-week period [20].
Although psychometric properties of the PROMIS pediatric asthma impact and generic scales have been reported [10, 21–24], the longitudinal validity such as responsiveness has not been evaluated in a sample of asthmatic children. The main purpose of this study was to investigate the responsiveness of the PROMIS pediatric asthma impact and generic scales in asthmatic children and to compare responsiveness with the PAQLQ [20]. Data were collected from dyads of asthmatic children and their parents within a 26-week observational window over a 2-year period. We hypothesized that for children whose asthma status has changed (either worse or better), the change in mean HRQoL scores will be statistically significant and in the expected direction.
Methods
Source of Data
This responsiveness study is based on the PROMIS Pediatric Asthma Study which was funded by the US National Institutes of Health and implemented by University of Florida (2009–2014) and St. Jude Children’s Research Hospital (2014–2015) to investigate the natural progression of health outcomes in asthmatic children.
Study Population
Study participants were child-parent dyads (N=229) recruited from the Florida Medicaid and State Children’s Health Insurance Program (SCHIP) between 2010 and 2011. Inclusion criterion for study participants were children who were between the ages of 8 and 17.9 years, had been diagnosed with asthma (ICD-9-CM: 493.1, 493.2, or 493.x), had two or more asthma-related medical visits in the previous 12 months, and had a parent who was ≥18 years old. Both children and parents who had been enrolled continuously in the Florida Medicaid or SCHIP in the past six months, were able to read and speak in English, and had internet access at home for the past six months were eligible for study participation. Potential participants were approached via mailed letter with a follow-up phone call to introduce the study. Informed consent was obtained from all individual participants included in the study. The study was approved by the University of Florida Institutional Review Board prior to data collection.
Study Design
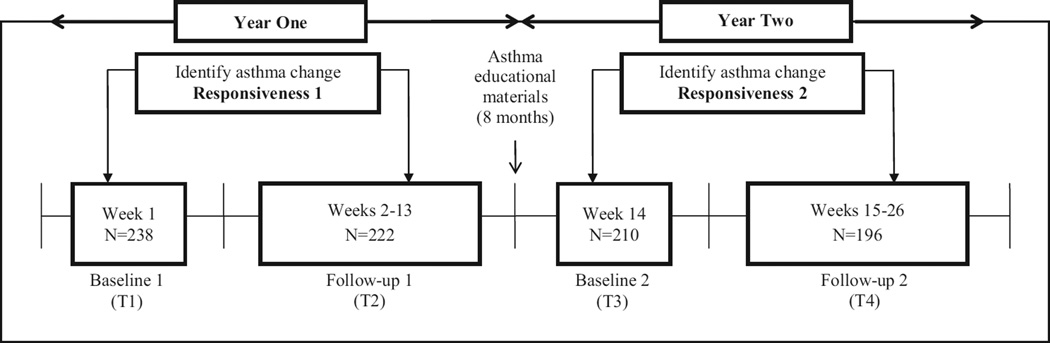
Figure 1 shows the study design. Data collection periods comprised 13 weeks of observation in the first year (Weeks 1–13) and another 13 weeks in the second year (Weeks 14–26). Two cohorts with roughly equal numbers of participants were recruited during asthma-dependent seasons where asthma flares are prevalent. Data collection for the first cohort took place in the fall (September – December) and for the second cohort in the spring (January – April) in each respective year. At baseline of the first year (T1) and the second year (T3), sociodemographic, asthma control, peak flow values, and nighttime sleep quality/quantity data were collected from parents through the secured research website. Parents were asked to report continuously on their children’s asthma control status, peak flow values, and nighttime sleep quality/quantity every week until Week 13 in the first year and Week 26 in the second year. In each year, if asthma control status changed (worse or better) from the baseline week to a specific follow-up week, then HRQoL data from the child were collected through a telephone interview. If asthma control status did not change throughout the 13 weeks, HRQoL data from the child were only collected at Week 13 and Week 26, respectively. To keep participants engaged and minimize the attrition rate, asthma educational materials on seven topics were delivered via mail and a study website during the 8-month gap between T2 and T3.
Figure 1.
The design of PROMIS Pediatric Asthma Study
Primary Measures
Anchors
Two types of anchors were used to test responsiveness: a clinical anchor to assess changes in asthma control status, and retrospective judgment anchors to assess changes in breathing problems and overall health status.
Clinical Anchor: Asthma Control Status
Asthma control status was collected using the Asthma Control and Communication Instrument (ACCI) [25, 26] which is a 12-item questionnaire completed by the parent. The ACCI includes 5 items that assess asthma control; 3 items for asthma-related acute health care; 1 item for asthma symptoms; 1 item for medication adherence; 1 item for how much asthma bothers the participant; and 1 qualitative item that asks about patient and doctor communication. The most severe response from the 5-items that measure asthma control is used to classify a child into four severity categories (mild-intermittent, mild-persistent, moderate-persistent, and severe-persistent) based on the National Heart, Lung and Blood Institute’s guidelines [27]. The ACCI was administered each week and changes in control status were classified as ‘better’ (from poor to good control), the ‘same’ (no change), and ‘worse’ (from good to poor control). The ACCI has demonstrated good psychometric properties [26] and can discriminate poor versus good asthma control status in a diverse sample of children [25].
Retrospective Judgment Anchor: Global Rating of Change
The Global Rating of Change (GRC) Index was used to assess a participant’s self-perception of the improvement or deterioration in breathing problems and overall health status over time. Administered at T2 and T4, children were asked to report if their breathing problems and overall health were better, worse, or about the same since the last time they did the survey. The change status was classified as better, the same, or worse.
HRQoL
HRQoL was measured using the PROMIS Pediatric Short Forms and the PAQLQ.
PROMIS Pediatric Short Forms
PROMIS Pediatric Short Forms [28] were developed using qualitative (i.e., focus group and cognitive debriefing) and quantitative (i.e., IRT) methodology. In this study, six domains were administered, including asthma impact (8 items), fatigue (10 items), pain interference (8 items), depressive symptoms (8 items), physical functioning – mobility (8 items), and peer relationships (8 items). Each PROMIS item used the context statement “In the past 7 days.” Responses included 5-point Likert-type categories ranging from “never” to “almost always” in the majority of domains and from “with no trouble” to “not able to do” in the physical functioning domain. Domain scores were calculated based on the scores of corresponding items and then converted to a T-score metric with a mean of 50 and a standard deviation of 10 derived from the original calibration sample of children [29]. Higher scores indicate better outcomes in the domains of physical functioning – mobility and peer relationships, and worse outcomes in the domains of asthma impact, fatigue, pain interference, and depressive symptoms. PROMIS pediatric asthma impact and generic scales have shown good measurement properties including test-retest reliability, construct validity, convergent-discriminant validity, and known-group validity [10, 21–24].
Paediatric Asthma Quality of Life Questionnaire (PAQLQ)
The PAQLQ is the most widely used scale to measure asthma-specific HRQoL for children [20]. It is a self-report scale that consists of 23 items capturing three domains: symptoms (10 items), activity limitation (5 items), and emotional function (8 items). The PAQLQ has demonstrated acceptable reliability and responsiveness to change [19, 20]. Each child was asked to report their HRQoL during the last seven days based on 7-point Likert-type response categories ranging from “not bothered at all” to “extremely bothered,” with higher scores indicating better HRQoL.
Statistical Methods
Bivariate analyses were performed to test the association between HRQoL and asthma control status by individual time points (T1, T2, T3, T4), and the associations between the change in HRQoL and the changes in the levels of respective anchors (better, the same, and worse). For the association between HRQoL and asthma control status by individual time points, two-sample t-tests were conducted for comparing the mean HRQoL domain scores between those with poor and good asthma control status. To summarize the overall effect of asthma control status on HRQoL, generalized estimating equation (GEE) was used to examine the association of asthma control status with individual HRQoL domains across four time points by accounting for clustering effects of HRQoL within individuals [30].
The change in HRQoL associated with the changes in the levels of anchors was examined using paired t-tests. The association between the mean changes in HRQoL scores between children whose asthma control status became worse compared to those who remained the same/better was tested using two-sample t-tests. The analyses were conducted based on two categories (worse and the same/better) since the use of three change categories provided no additional information as opposed to two categories (i.e. the magnitude of change in HRQoL was similar for the same/better groups). Similarly, the two-category (worse vs. the same/better) approach was performed for GRC breathing problems and overall health, respectively.
Relative validity was calculated to compare the efficiency and discriminative ability [31] between the individual domains of the PROMIS and the PAQLQ. Relative validity is calculated as a ratio of F-statistics across different domains, where the domain with the lowest F-statistic is used as the denominator and F-statistics of the other domains as the numerator. Domains with larger ratio values are considered superior to the other domains. SAS version 9.3 (SAS Institute, Inc., Cary, North Carolina, 2011) was used to conduct all analyses.
Results
Description of sample
Table 1 shows the characteristics of the study sample (N=229) at the baseline of the first year. The mean age for children was 12.2 years (SD: 2.6), and the sample was predominately male (59.9%), white (58.1%) and non-Hispanic (72.5%). Parents reported that 17% of the children in the sample had ADHD and 10.5% were overweight. The mean age for the parents was 40.6 (SD: 8.7) and the majority of them were female (88.2%), white (58.1%), and non-Hispanic (74.2%). About 67% of the mother’s had some college education or above, and most families had a yearly income less than $35,000.
Table 1.
Patient Characteristics (N=229)
| Child | |
| Age, mean (SD) | 12.2 (2.6) |
| 8–11 (N=102) | 9.9 (0.8) |
| 12–14 (N=72) | 12.8 (0.8) |
| 15–17.9 (N=55) | 15.9 (1.0) |
| Female, % | 41.1% |
| Race, %* | |
| White | 58.1% |
| Black | 33.6% |
| Other | 17.0% |
| Ethnicity, % | |
| Hispanic | 27.5% |
| Non-Hispanic | 72.5% |
| Chronic Conditions, % | |
| ADHD | 17.0% |
| Overweight | 10.5% |
| Mental Health Disorder | 3.1% |
| Epilepsy | 2.6% |
| Seasonal cohort | |
| Fall (N=135) | 59% |
| Spring (N=94) | 41% |
| Parent | |
| Age, mean (SD) | 40.6 (8.7) |
| Female, % | 88.2% |
| Race, %* | |
| White | 58.1% |
| Black | 29.3% |
| Other | 15.7% |
| Ethnicity, % | |
| Hispanic | 25.8% |
| Non-Hispanic | 74.2% |
| Mother’s Education, % | |
| High school or below | 32.7% |
| Some college | 42.5% |
| College and above | 24.8% |
| Family Income, % | |
| Less than $15,000 | 20.5% |
| $15,000 to $34,999 | 44.5% |
| $35,000 to $54,999 | 24.9% |
| More than $55,000 | 10.1% |
| Marital Relationship, % | |
| Married | 51.5% |
| Divorced | 19.7% |
| Never married | 17.5% |
| Other | 11.3% |
Race over 100% due to multiple choices
Association between asthma control and HRQoL by individual time points
Table 2 shows HRQoL domain scores by asthma control status at individual time points. Generally speaking, children with good control status reported better HRQoL than children with poor control status. Specifically, mean domain scores were statistically different between children with poor and good control status for the PROMIS asthma impact domain at T1, T2, T3 and T4, respectively. Means were are also statistically different between poor and good control status on the PROMIS pain interference domain at T3; the PROMIS fatigue domain at T1; and the PROMIS mobility domain at T1 and T2. For the PAQLQ, mean domain scores were statistically different between children with poor and good asthma control status on all domains at T1, T2, T3 and T4, respectively. GEE analyses indicate children with poor asthma control status reported significantly impaired HRQoL compared to children with good asthma control across four time points in the PROMIS asthma impact (β=4.31, p<0.001) and fatigue (β=1.96, p<0.01) domains, and all domains of the PAQLQ (Supplemental Table 1).
Table 2.
Association between asthma control status and HRQoL by individual time points
| T-test | T1 | T2 | T3 | T4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PROMISa | Poor | Good | Cohen’s D ESb |
Poor | Good | Cohen’s D ESb |
Poor | Good | Cohen’s D ESb |
Poor | Good | Cohen’s D ESb |
| Asthma Impact | 51.4 | 45.2 | 0.64*** | 50.2 | 44.0 | 0.65*** | 49.6 | 43.2 | 0.69*** | 47.4 | 43.4 | 0.41* |
| Pain Interference | 48.9 | 47.7 | 0.41 | 47.2 | 44.7 | 0.27 | 48.3 | 44.6 | 0.40* | 45.8 | 44.0 | 0.19 |
| Fatigue | 50.7 | 47.5 | 0.29* | 48.9 | 45.5 | 0.27 | 49.5 | 46.7 | 0.28 | 47.4 | 45.2 | 0.20 |
| Depressive | 49.7 | 47.4 | 0.23 | 48.4 | 46.7 | 0.13 | 48.4 | 47.6 | 0.09 | 47.7 | 44.6 | 0.32 |
| Symptoms | ||||||||||||
| Mobility | 46.0 | 48.8 | −0.38** | 47.3 | 49.8 | −0.30* | 48.4 | 49.9 | −0.20 | 48.6 | 50.9 | −0.29 |
| Peer Relationships | 46.9 | 47.2 | −0.01 | 49.6 | 48.5 | 0.10 | 48.1 | 48.5 | −0.07 | 48.5 | 49.2 | −0.06 |
| PAQLQa | ||||||||||||
| Symptoms | 5.0 | 5.7 | −0.54*** | 5.4 | 6.0 | −0.53*** | 5.3 | 6.2 | −0.93*** | 5.7 | 6.3 | −0.51** |
| Activity | 5.1 | 5.8 | −0.57*** | 5.4 | 6.0 | −0.50*** | 5.5 | 6.2 | −0.62*** | 5.8 | 6.3 | −0.42* |
| Emotional | 5.4 | 6.1 | −0.55*** | 5.9 | 6.2 | −0.30* | 5.7 | 6.4 | −0.71*** | 6.1 | 6.4 | −0.34* |
: Asthma Impact, Fatigue, Pain Interference & Depressive Symptoms: higher scores for worse outcomes; other domains: higher scores for better outcomes
: Based on t-tests
p<0.001;
p<0.01;
p<0.05;
Association between HRQoL and asthma control status
Table 3 shows the association between the change in HRQoL and a specific change in asthma control status (worse and the same/better, respectively). Children whose asthma control status became worse reported a significant HRQoL deterioration on the PROMIS asthma impact domain (T1 to T2: p<0.01; T3 to T4: p<0.05), and the PAQLQ activity domain (T1 to T2: p<0.05). Unexpectedly, worsened asthma control status was associated with improved peer relationships on the PROMIS domain (T1 to T2: p<0.01). Those whose asthma remained the same/became better reported a significant HRQoL improvement on the PROMIS asthma impact domain (T1 to T2: p<0.001; T3 to T4; p<0.05), the PROMIS pain interference domain (T1 to T2: p<0.001), the PROMIS fatigue domain (T1 to T2: p<0.001; T3 to T4: p<0.05), the PROMIS mobility domain (T1 to T2: p<0.01), and all three domains of the PAQLQ (T1 to T2: p’s<0.001; T3 to T4; p’s<0.01).
Table 3.
Association between change in asthma control status and HRQoL
| Asthma Control Change: T1 to T2 | |||||||
| Worse | Same/Better |
Worse vs. Same/Better |
|||||
| PROMISa |
Mean Changeb |
Cohen’s D Effect Size |
SRMc |
Mean Changeb |
Cohen’s D Effect Size |
SRMc |
Relative Validityd |
| Asthma Impact | 3.36** | 0.41 | 0.42 | −3.23*** | −0.30 | −0.41 | 1.14*** |
| Pain Interference | 0.14 | <0.01 | <0.01 | −2.64*** | −0.27 | −0.32 | 0.23* |
| Fatigue | 2.02 | 0.20 | 0.22 | −3.20*** | −0.28 | −0.34 | 0.57*** |
| Depressive | −0.51 | −0.92 | <0.01NS | ||||
| Symptoms | −0.05 | −0.07 | −0.09 | −0.11 | |||
| Mobility | −1.36 | −0.15 | −0.16 | 1.90** | 0.26 | 0.28 | 0.39** |
| Peer Relationships | 3.81** | 0.38 | 0.41 | 1.11 | 0.14 | 0.14 | 0.20* |
| PAQLQa | |||||||
| Symptoms | −0.26 | −0.20 | −0.21 | 0.53*** | 0.39 | 0.49 | 0.99*** |
| Activity | −0.32* | −0.27 | −0.28 | 0.51*** | 0.38 | 0.46 | 1*** |
| Emotional | −0.07 | −0.07 | −0.08 | 0.45*** | 0.35 | 0.43 | 0.49** |
| Asthma Control Change: T3 to T4 | |||||||
| Worse | Same/Better |
Worse vs. Same/Better |
|||||
| PROMISa |
Mean Changeb |
Cohen’s D Effect Size |
SRMc |
Mean Changeb |
Cohen’s D Effect Size |
SRMc |
Relative Validityd |
| Asthma Impact | 2.78* | 0.31 | 0.33 | −1.39* | −0.13 | −0.19 | 0.82** |
| Pain Interference | −0.61 | −0.06 | −0.07 | −0.72 | −0.07 | −0.10 | <0.01NS |
| Fatigue | −0.80 | −0.07 | −0.10 | −1.67* | −0.15 | −0.22 | 0.04NS |
| Depressive | −2.00 | −1.53 | <0.01NS | ||||
| Symptoms | −0.19 | −0.22 | −0.14 | −0.18 | |||
| Mobility | 0.78 | 0.10 | 0.10 | 0.43 | 0.06 | 0.06 | <0.01NS |
| Peer Relationships | −0.10 | <0.01 | <0.01 | 0.60 | 0.06 | 0.06 | 0.02NS |
| PAQLQa | |||||||
| Symptoms | −0.27 | −0.28 | −0.26 | 0.31** | 0.27 | 0.35 | 1*** |
| Activity | −0.13 | −0.13 | −0.12 | 0.27** | 0.24 | 0.36 | 0.54* |
| Emotional | −0.21 | −0.22 | −0.23 | 0.22** | 0.22 | 0.33 | 0.83** |
: Asthma Impact, Fatigue, Pain Interference, & Depressive Symptoms: higher scores for worse outcomes; other domains: higher scores for better outcomes
: Based on paired t-tests
: Standardized response mean
: PAQLQ domain with the highest significant t-statistic was used to calculate relative validity
p<0.001;
p<0.01;
p<0.05;
NS: non-significant relative validity
Table 3 further shows the difference in the change of HRQoL scores between those with worse and the same/better asthma control status. From T1 to T2, the mean domain score difference was statistically significant for the PROMIS asthma impact (p<0.001), pain interference (p<0.05), fatigue (p<0.001), mobility (p<0.01), and peer relationships domains (p<0.05). The comparisons were also statistically significant on all three domains of the PAQLQ: symptom (p<0.001), activity (p<0.001) and emotional (p<0.01) domains. From T3 to T4, the mean domain score change was statistically significant on the PROMIS asthma impact domain (p<0.01), and the PAQLQ symptom (p<0.001), activity (p<0.05) and emotional (p<0.01) domains.
Association between HRQoL and GRC Breathing Problems
Table 4 shows the association between the change in HRQoL and a specific change in breathing problems by GRC (worse and the same/better, respectively). Children whose breathing problems became worse reported a significant HRQoL deterioration on the PROMIS asthma impact domain (T1 to T2: p<0.05; T3 to T4: p<0.01), the PROMIS mobility domain (T1 to T2: p<0.05) and the PAQLQ symptom domain (T1 to T2 and T3 to T4: p’s<0.05). Those whose breathing problems remained the same or became better reported significant HRQoL improvement on the PROMIS asthma impact (T1 to T2: p<0.01), pain interference (T1 to T2: p<0.001), fatigue (T1 to T2: p<0.01; T3 to T4: p<0.05), depressive symptoms (T3 to T4: p<0.001), mobility (T1 to T2: p<0.01), and peer relationships (T1 to T2: p<0.01) domains, and all PAQLQ domains (T1 to T2: p<0.001; T3 to T4: p<0.001 for symptom and activity domains and p<0.01 for emotional domain).
Table 4.
Association between the change in Global Rating of Change breathing problems and HRQoL
| GRC Breathing Change: T1 to T2 | |||||||
| Worse | Same/Better |
Worse vs. Same/Better |
|||||
| PROMISa |
Mean Changeb |
Cohen’s D Effect Size |
SRMc |
Mean Changeb |
Cohen’s D Effect Size |
SRMc |
Relative Validityd |
| Asthma Impact | 3.17* | 0.44 | 0.42 | −2.06** | −0.19 | −0.23 | 0.50** |
| Pain Interference | 1.66 | 0.19 | 0.18 | −2.44*** | −0.26 | −0.32 | 0.37* |
| Fatigue | 2.90 | 0.33 | 0.31 | −2.49** | −0.22 | −0.26 | 0.44** |
| Depressive | 0.31 | −1.01 | 0.04NS | ||||
| Symptoms | 0.03 | 0.04 | −0.10 | −0.12 | |||
| Mobility | −2.79* | −0.48 | −0.43 | 1.61** | 0.22 | 0.24 | 0.55** |
| Peer Relationships | 2.10 | 0.26 | 0.29 | 1.91** | 0.22 | 0.23 | <0.01NS |
| PAQLQa | |||||||
| Symptoms | −0.46* | −0.35 | −0.38 | 0.45*** | 0.36 | 0.44 | 1*** |
| Activity | −0.41 | −0.33 | −0.32 | 0.40*** | 0.31 | 0.36 | 0.69*** |
| Emotional | −0.22 | −0.17 | −0.20 | 0.40*** | 0.32 | 0.40 | 0.49** |
| GRC Breathing Change: T3 to T4 | |||||||
| Worse | Same/Better |
Worse vs. Same/Better |
|||||
| PROMISa |
Mean Changeb |
Cohen’s D Effect Size |
SRMc |
Mean Changeb |
Cohen’s D Effect Size |
SRMc |
Relative Validityd |
| Asthma Impact | 6.74** | 0.60 | 0.73 | −1.07 | −0.11 | −0.15 | 0.90*** |
| Pain Interference | 0.95 | 0.08 | 0.10 | −0.90 | −0.09 | −0.13 | 0.05NS |
| Fatigue | 0.11 | 0.01 | 0.01 | −1.60* | −0.15 | −0.21 | 0.04NS |
| Depressive | 2.79 | −2.35*** | 0.31* | ||||
| Symptoms | 0.24 | 0.26 | −0.22 | −0.29 | |||
| Mobility | −2.05 | −0.20 | −0.23 | 0.86 | 0.12 | 0.12 | 0.13NS |
| Peer Relationships | −0.84 | −0.10 | −0.11 | 0.57 | 0.06 | 0.07 | 0.02NS |
| PAQLQa | |||||||
| Symptoms | −0.78* | −0.54 | −0.49 | 0.26*** | 0.25 | 0.33 | 1*** |
| Activity | −0.60 | −0.42 | −0.47 | 0.25*** | 0.25 | 0.33 | 0.77*** |
| Emotional | −0.58 | −0.39 | −0.45 | 0.18** | 0.20 | 0.28 | 0.78*** |
: Asthma Impact, Fatigue, Pain Interference, & Depressive Symptoms: higher scores for worse outcomes; other domains: higher scores for better outcomes
: Based on paired t-tests
: Standardized response mean
: PAQLQ domain with the highest significant t-statistic was used to calculate relative validity
p<0.001;
p<0.01;
p<0.05;
NS: non-significant relative validity
Table 4 also shows the difference in the change of HRQoL scores between those with worse and the same/better breathing problems as reported on the GRC. From T1 to T2, comparisons were statistically significant on the PROMIS asthma impact (p<0.01), pain interference (p<0.05), fatigue (p<0.01), and mobility (p<0.01) domains. Comparisons were also statistically significant in all three PAQLQ domains: symptom (p<0.001), activity (p<0.001) and emotional (p <0.01). From T3 to T4, the comparisons were statistically significant on the PROMIS asthma impact (p<0.001) and depressive symptoms domains (p<0.05), and all three PAQLQ domains (p’s<0.001).
Association between HRQoL and GRC Overall Health
Table 5 shows the association between the change in HRQoL and a specific change in overall health reported on the GRC (worse and the same/better, respectively). Children whose overall health became worse reported a significant HRQoL deterioration on the PROMIS fatigue (T1 to T2: p<0.01), and peer relationships (T1 to T2: p<0.01) domains. Those whose overall health remained the same or became better reported a significant HRQoL improvement on all PROMIS and PAQLQ domains from T1 to T2, on the PROMIS fatigue and depressive symptoms (T3 to T4: p’s<0.05) domains, and on all three domains of the PAQLQ (T3 to T4: p’s<0.01).
Table 5.
Association between the change in Global Rating of Change overall health and HRQoL
| GRC Overall Health Change: T1 to T2 | |||||||
| Worse | Same/Better |
Worse vs. Same/Better |
|||||
| PROMISa |
Mean Changeb |
Cohen’s D Effect Size |
SRMc |
Mean Changeb |
Cohen’s D Effect Size |
SRMc |
Relative Validityd |
| Asthma Impact | 1.78 | 0.22 | 0.24 | −1.57* | −0.14 | −0.16 | 0.25NS |
| Pain Interference | 3.11 | 0.38 | 0.38 | −2.36*** | −0.25 | −0.30 | 0.88** |
| Fatigue | 7.53** | 0.83 | 0.87 | −2.69*** | −0.24 | −0.29 | 2.24*** |
| Depressive | 3.47 | −1.29* | 0.62* | ||||
| Symptoms | 0.31 | 0.47 | −0.13 | −0.16 | |||
| Mobility | −0.68 | −0.14 | −0.09 | 1.11* | 0.16 | 0.17 | 0.12NS |
| Peer Relationships | −4.47** | −0.50 | −0.73 | 2.66*** | 0.32 | 0.32 | 1.40*** |
| PAQLQa | |||||||
| Symptoms | −0.22 | −0.19 | −0.17 | 0.37*** | 0.30 | 0.35 | 0.51* |
| Activity | −0.38 | −0.31 | −0.23 | 0.35*** | 0.28 | 0.32 | 0.72** |
| Emotional | −0.38 | −0.26 | −0.30 | 0.37*** | 0.32 | 0.39 | 1** |
| GRC Overall Health Change: T3 to T4 | |||||||
| Worse | Same/Better |
Worse vs. Same/Better |
|||||
| PROMISa |
Mean Changeb |
Cohen’s D Effect Size |
SRMc |
Mean Changeb |
Cohen’s D Effect Size |
SRMc |
Relative Validityd |
| Asthma Impact | 5.08 | 0.47 | 0.44 | −0.58 | −0.06 | −0.08 | 0.36* |
| Pain Interference | −2.38 | −0.20 | −0.22 | −0.51 | −0.05 | −0.07 | 0.04NS |
| Fatigue | −2.23 | −0.16 | −0.23 | −1.32* | −0.12 | −0.18 | 0.01NS |
| Depressive | −2.62 | −1.63* | 0.01NS | ||||
| Symptoms | −0.21 | −0.21 | −0.16 | −0.20 | |||
| Mobility | −1.62 | −0.17 | −0.14 | 0.70 | 0.09 | 0.10 | 0.06NS |
| Peer Relationships | −1.62 | −0.18 | −0.19 | 0.59 | 0.06 | 0.07 | 0.04NS |
| PAQLQa | |||||||
| Symptoms | −0.93 | −0.73 | −0.57 | 0.23** | 0.22 | 0.28 | 1*** |
| Activity | −0.68 | −0.59 | −0.44 | 0.22** | 0.21 | 0.29 | 0.69*** |
| Emotional | −0.78 | −0.60 | −0.58 | 0.17** | 0.17 | 0.25 | 0.99*** |
: Asthma Impact, Fatigue, Pain Interference, & Depressive Symptoms: higher scores for worse outcomes; other domains: higher scores for better outcomes
: Based on paired t-tests
: Standardized response mean
: PAQLQ domain with the highest significant t-statistic was used to calculate relative validity
p<0.001;
p<0.01;
p<0.05;
NS: non-significant relative validity
Table 5 also shows the difference in the change of HRQoL scores between those with worse and the same/better overall health reported on the GRC. From T1 to T2, comparisons were statistically significant in the PROMIS pain interference (p<0.01), fatigue (p<0.001), depressive symptoms (p<0.05) and peer relationships (p<0.001) domains, and the PAQLQ symptom (p<0.05), activity (p<0.01), and emotional (p<0.01) domains. From T3 to T4, the comparisons were statistically significant on the PROMIS asthma impact domain (p<0.05) and all three PAQLQ (p’s<0.001) domains.
Relative Validity
Relative validity among individual domains of the PROMIS and PAQLQ related to individual anchors is reported in Tables 3–5. Using asthma control status as an anchor, the PROMIS asthma impact domain exhibited the highest relative validity (RV: 244.14) compared with other domains from T1 to T2. In contrast, the PAQLQ symptoms domain exhibited the highest relative validity from T3 to T4 (RV: 1860). Using GRC breathing problems as an anchor, the PAQLQ symptoms domain had highest relative validity both from T1 to T2 (RV: 1493) and from T3 to T4 (RV: 50.12). Using GRC overall health as an anchor, the PROMIS fatigue domain exhibited the highest relative validity from T1 to T2 (RV: 19.36), and the PAQLQ symptoms domain had the highest relative validity from T3 to T4 (RV: 109.20).
Discussion
The PROMIS Pediatric Asthma Study was designed to observe the natural course of pediatric asthma over 26 weeks within a two-year study period. Using a pediatric asthma cohort, we discovered the responsiveness as part of longitudinal validity for the PROMIS pediatric asthma impact and generic scales. The complex design feature similar to the PROMIS Pediatric Asthma Study has not been fully established in other HRQoL studies especially in pediatric asthma populations.
This study specifically found that children with worsened asthma control status, breathing problems, and overall health tended to report deteriorating HRQoL on both the PROMIS and PAQLQ scales; children with stable or improved asthma control status, breathing problems, and overall health reported improvements in HRQoL. The PROMIS asthma impact and several generic domains (such as pain interference, fatigue, and mobility) could significantly distinguish between the worse and the same/better asthma control, breathing problem and overall health groups, particularly between T1 and T2. Further, the PROMIS asthma impact domain was able to consistently discriminate between the worse and same/better change groups across all three anchors between T3 and T4.
Literature is sparse in assessing responsiveness to change for pediatric asthma HRQoL scales [32]. Our results are similar to that of Seid et al [33] who demonstrated responsiveness of the PedsQL generic and asthma impact scales reported by 252 asthmatic children between baseline and at 3-months. Using asthma symptom frequency as an anchor, the authors found that the PedsQL exhibited significant differences between those whose symptom frequency improved compared with those who remained stable in overall, psychosocial, social, and asthma domains. However, this study collected HRQoL at only two time points 3 months apart from each participant, and did not report the time/season of year that data were collected. Indeed, asthma is a season-dependent condition and few studies have carefully selected an appropriate observational window to test the responsiveness. Raat et al. demonstrated responsiveness for the PAQLQ in 238 Dutch children but only for children whose asthma symptoms became worse [19]. In contrast, our PROMIS Pediatric Asthma Study was designed to assess the dynamic change in asthma control status and corresponding change in HRQoL from each participant in two asthma-dependent seasons (e.g., Fall and Spring) across 4 time points among children whose asthma status became worse and improved, thus denoting a robust study design.
Although the PROMIS and PAQLQ domains did respond to change, the magnitude of change was more significant from T1 to T2 than from T3 to T4 (Tables 3–5). This could be attributed to children’s cognitive growth for interpreting the meaning of items over time, or could possibly be due to the provision of the asthma educational component between T2 and T3 to improve asthma management skills. Conducting cognitive appraisal would likely provide more insight regarding this finding. We also observed a more significant amount of change in the majority of domains from participants in the same/better groups than those in the worse group particularly from T1 to T2. This finding is similar to Kheir et al [34] where asthmatic adults whose asthma improved over a 3-month period reported more significant amounts of change in HRQoL on the Asthma Quality of Life Questionnaire (AQLQ) than those who reported a worsening of asthma symptoms. Juniper et al [35] found mixed magnitudes of change on the GRC in asthmatic adults that either deteriorated or improved across 8-weeks. Since these two studies focused on adults, it is hard to draw inferences for the pediatric asthma population. Nevertheless, the findings derived from this and previous studies imply that the asymmetrical responsiveness in PROs related to the same/better and worse health status may influence the estimation of the clinically importance meaning [36].
Interestingly, the PROMIS asthma domain showed an equal but not superior responsiveness to the PAQLQ domains. The 8 items in the PROMIS asthma impact Short Form were selected from an IRT-calibrated item bank (17 items). Scales comprising a smaller number of items may not capture broader levels of the HRQoL latent traits than the individual domains of PAQLQ that comprises more items with more complete information. Yeatts et al [10] revealed that administering all 17-items in the PROMIS asthma impact item pool produced more precision than the Short Forms, implying that using all items for comparison may yield superior responsiveness to the PAQLQ. Given the comparable responsiveness between the PROMIS asthma impact Short Form and the PAQLQ asthma scales, the selection of scales for use depends upon the research or clinical purpose. If the purpose is to screen patients’ overall asthma impact, the use of the PROMIS asthma impact Short Form would be acceptable. However, if the purpose is to identify patients’ specific impact of asthma (e.g., physical or emotional domains), the use of PAQLQ may be appropriate. It is also important to note that several PROMIS generic domains (i.e. fatigue) possess satisfactory responsiveness that may be used alongside the asthma-specific scales.
Several limitations deserve notation. First, our study focuses on a Medicaid and SCHIP population who have exhibited a higher prevalence of children with asthma related to lower social economic status [37]; generalizability of our findings to other populations should be further investigated. Second, our sample is restricted to children and parents who were English-speaking only and different results may be observed in other non-English-speaking populations. Third, we used asthma control status and GRCs as anchors to evaluate responsiveness. Future studies may include biological or objective clinical anchors (e.g., lung functioning by FEV1 or peak flow values) to test responsiveness for HRQoL scales. In addition, asthma control status was reported via parent-proxy rather than by the child. Although using a parent-proxy may not be ideal [38], the ACCI probes information of asthma medications and flares of which parents may be more aware, particularly for younger children. Although previous studies have indicated that there were no significant differences between parents and adolescents in reporting of asthma medication use [39, 40], discrepancies between parent and younger children reports for asthma control status have not been examined.
Conclusion
In sum, the asthma impact, pain interference, fatigue, and mobility domains of the PROMIS pediatric scales are responsive to change related to the change in health status in asthmatic children. The responsiveness of the PROMIS pediatric asthma impact domains was specifically comparable to the PAQLQ. This serves an important foundation for estimating MIDs in the PROMIS and PAQLQ scales, which can facilitate the use of HRQoL information in clinical decision-making.
Supplementary Material
Acknowledgments
The authors would like to thank Elizabeth Shenkman, PhD and Caprice Knapp, PhD, who helped with study design and conceptualization; and Pey-Shen Wen, PhD and Nammi Ketheeswaran, MPH, who assisted with data collection and project coordination
Funding Sources:
National Institutes of Health U01 AR052181 (Howell, Thompson, Gross, Reeve, DeWalt, Huang) and American Lebanese Syrian Associated Charities (Howell, Huang).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bloom B, Cohen RA, Freeman G. Summary health statistics for US Children: national health interview survey, 2011. Vital and health statistics Series 10, Data from the National Health Survey. 2012:1–88. [PubMed] [Google Scholar]
- 2.Koinis-Mitchell D, Kopel SJ, Boergers J, et al. Asthma, allergic rhinitis, and sleep problems in urban children. J Clin Sleep Med. 2015;11:101–110. doi: 10.5664/jcsm.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean BB, Calimlim BM, Kindermann SL, et al. The impact of uncontrolled asthma on absenteeism and health-related quality of life. J Asthma. 2009;46:861–866. doi: 10.3109/02770900903184237. [DOI] [PubMed] [Google Scholar]
- 4.Diette GB, Markson L, Skinner EA, et al. Nocturnal asthma in children affects school attendance, school performance, and parents' work attendance. Arch Pediatr Adolesc Med. 2000;154:923–928. doi: 10.1001/archpedi.154.9.923. [DOI] [PubMed] [Google Scholar]
- 5.Okelo SO, Wu AW, Krishnan JA, et al. Emotional quality-of-life and outcomes in adolescents with asthma. J Pediatr. 2004;145:523–529. doi: 10.1016/j.jpeds.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 6.O’Byrne P, Bateman E, Bousquet J, et al. Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention. 2013 Updated 2009. [Google Scholar]
- 7.Everhart RS, Fiese BH. Asthma severity and child quality of life in pediatric asthma: a systematic review. Patient Educ Couns. 2009;75:162–168. doi: 10.1016/j.pec.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 8.King S, Chambers CT, Huguet A, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152:2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Schmier JK, Manjunath R, Halpern MT, et al. The impact of inadequately controlled asthma in urban children on quality of life and productivity. Ann Allergy Asthma Immunol. 2007;98:245–251. doi: 10.1016/S1081-1206(10)60713-2. [DOI] [PubMed] [Google Scholar]
- 10.Yeatts KB, Stucky B, Thissen D, et al. Construction of the Pediatric Asthma Impact Scale (PAIS) for the Patient-Reported Outcomes Measurement Information System (PROMIS) J Asthma. 2010;47:295–302. doi: 10.3109/02770900903426997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenzik KM, Tuli SY, Revicki DA, et al. Comparison of 4 pediatric health-related quality-of-life instruments: a study on a medicaid population. Med Decis Making. 2014;34:590–602. doi: 10.1177/0272989X14529846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams R, Rosier M, Campbell D, et al. Assessment of an asthma quality of life scale using item-response theory. Respirology. 2005;10:587–593. doi: 10.1111/j.1440-1843.2005.00754.x. [DOI] [PubMed] [Google Scholar]
- 13.Deyo RA, Inui TS. Toward clinical applications of health status measures: sensitivity of scales to clinically important changes. Health Serv Res. 1984;19:275–289. [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt GH, Deyo RA, Charlson M, et al. Responsiveness and validity in health status measurement: a clarification. J Clin Epidemiol. 1989;42:403–408. doi: 10.1016/0895-4356(89)90128-5. [DOI] [PubMed] [Google Scholar]
- 15.Patrick DL, Deyo RA. Generic and disease-specific measures in assessing health status and quality of life. Med Care. 1989;27(Suppl.):S217–S232. doi: 10.1097/00005650-198903001-00018. [DOI] [PubMed] [Google Scholar]
- 16.Ader DN. Developing the Patient-Reported Outcomes Measurement Information System (PROMIS) Med Care. 2007;45(Suppl.):S1–S2. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Revicki D, Hays RD, Cella D, et al. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61:102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Marks GB, Dunn SM, Woolcock AJ. An evaluation of an asthma quality of life questionnaire as a measure of change in adults with asthma. J Clin Epidemiol. 1993;46:1103–1111. doi: 10.1016/0895-4356(93)90109-e. [DOI] [PubMed] [Google Scholar]
- 19.Raat H, Bueving HJ, de Jongste JC, et al. Responsiveness, longitudinal- and cross-sectional construct validity of the Pediatric Asthma Quality of Life Questionnaire (PAQLQ) in Dutch children with asthma. Qual Life Res. 2005;14:265–272. [PubMed] [Google Scholar]
- 20.Juniper EF, Guyatt GH, Feeny DH, et al. Measuring quality of life in children with asthma. Qual Life Res. 1996;5:35–46. doi: 10.1007/BF00435967. [DOI] [PubMed] [Google Scholar]
- 21.DeWitt EM, Stucky BD, Thissen D, et al. Construction of the eight-item patient-reported outcomes measurement information system pediatric physical function scales: built using item response theory. J Clin Epidemiol. 2011;64:794–804. doi: 10.1016/j.jclinepi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai JS, Stucky BD, Thissen D, et al. Development and psychometric properties of the PROMIS® pediatric fatigue item banks. Qual Life Res. 2013;22:2417–2427. doi: 10.1007/s11136-013-0357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varni JW, Magnus B, Stucky BD, et al. Psychometric properties of the PROMIS® pediatric scales: precision, stability, and comparison of different scoring and administration options. Qual Life Res. 2014;23:1233–1243. doi: 10.1007/s11136-013-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varni JW, Stucky BD, Thissen D, et al. PROMIS Pediatric Pain Interference Scale: an item response theory analysis of the pediatric pain item bank. J Pain. 2010;11:1109–1119. doi: 10.1016/j.jpain.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okelo SO, Eakin MN, Patino CM, et al. The Pediatric Asthma Control and Communication Instrument asthma questionnaire: for use in diverse children of all ages. J Allergy Clin Immunol. 2013;132:55–62. doi: 10.1016/j.jaci.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patino CM, Okelo SO, Rand CS, et al. The Asthma Control and Communication Instrument: a clinical tool developed for ethnically diverse populations. J Allergy Clin Immunol. 2008;122:936–943. e6. doi: 10.1016/j.jaci.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Asthma Education and Prevention Program. Bethesda, MD: U.S. Dept. of Health and Human Services, National Institutes of Health, National Heart, Lung and Blood Institute; Third Expert Panel on the Management of Asthma. 2008., Guidelines for the diagnosis and management of asthma: summary report 2007. [Google Scholar]
- 28.Irwin DE, Stucky B, Langer MM, et al. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual Life Res. 2010;19:595–607. doi: 10.1007/s11136-010-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irwin DE, Stucky BD, Thissen D, et al. Sampling plan and patient characteristics of the PROMIS pediatrics large-scale survey. Qual Life Res. 2010;19:585–594. doi: 10.1007/s11136-010-9618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diggle P, Heagerty P, Liang K-Y, et al. Analysis of longitudinal data. Oxford University Press; 2002. [Google Scholar]
- 31.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Wilson SR, Rand CS, Cabana MD, et al. Asthma outcomes: quality of life. J Allergy Clin Immunol. 2012;129(Suppl.):S88–S123. doi: 10.1016/j.jaci.2011.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seid M, Limbers CA, Driscoll KA, et al. Reliability, validity, and responsiveness of the pediatric quality of life inventory (PedsQL) generic core scales and asthma symptoms scale in vulnerable children with asthma. J Asthma. 2010;47:170–177. doi: 10.3109/02770900903533966. [DOI] [PubMed] [Google Scholar]
- 34.Kheir NM, Emmerton L, Shaw JP. Assessing the responsiveness of the Asthma Quality of Life Questionnaire with pharmaceutical care. Pharm World Sci. 2008;30:322–328. doi: 10.1007/s11096-007-9179-y. [DOI] [PubMed] [Google Scholar]
- 35.Juniper EF, Guyatt GH, Willan A, et al. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47:81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 36.Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: differences between improvement and worsening. Qual Life Res. 2002;11:207–221. doi: 10.1023/a:1015276414526. [DOI] [PubMed] [Google Scholar]
- 37.Zahran HS, Bailey CM, Qin X, et al. Assessing asthma severity among children and adults with current asthma. J Asthma. 2014;51:610–617. doi: 10.3109/02770903.2014.892966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eiser C, Morse R. Can parents rate their child's health-related quality of life? Results of a systematic review. Qual Life Res. 2001;10:347–357. doi: 10.1023/a:1012253723272. [DOI] [PubMed] [Google Scholar]
- 39.Bender B, Wamboldt FS, O'Connor SL, et al. Measurement of children's asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol. 2000;85:416–421. doi: 10.1016/s1081-1206(10)62557-4. [DOI] [PubMed] [Google Scholar]
- 40.Bender BG, Bartlett SJ, Rand CS, et al. Impact of interview mode on accuracy of child and parent report of adherence with asthma-controller medication. Pediatrics. 2007;120:e471–e477. doi: 10.1542/peds.2006-3457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.