Abstract
Evidence from functional neuroimaging studies of emotional perception shows that when attention is focused on external features of emotional stimuli (external perceptual orienting—EPO), the amygdala is primarily engaged, but when attention is turned inwards towards one’s own emotional state (interoceptive self-orienting—ISO), regions of the salience network, such as the anterior insula (AI) and the dorsal anterior cingulate cortex (dACC), also play a major role. Yet, it is unknown if ISO boosts the contributions of AI and dACC not only to emotional ‘perception’ but also to emotional ‘memory’. To investigate this issue, participants were scanned with functional magnetic resonance imaging (fMRI) while viewing emotional and neutral pictures under ISO or EPO, and memory was tested several days later. The study yielded three main findings: (i) emotion boosted perception-related activity in the amygdala during both ISO and EPO and in the right AI exclusively during ISO; (ii) emotion augmented activity predicting subsequent memory in AI and dACC during ISO but not during EPO and (iii) high confidence memory was associated with increased amygdala–dACC connectivity, selectively for ISO encoding. These findings show, for the first time, that ISO promotes emotional memory formation via regions associated with interoceptive awareness of emotional experience, such as AI and dACC.
Keywords: interoceptive awareness, emotion, subsequent memory effects, functional magnetic resonance imaging
Introduction
Perception depends not only on physical characteristics of stimuli but also on how we process them, and emotional perception is no exception (Damasio, 1999; Phan et al., 2002; Phillips et al., 2003; Taylor et al., 2003; Damasio and Carvalho, 2013). An embodied view of emotion based on appraisal theories (James, 1894; Schachter and Singer, 1962) and human neuroscience research (Craig, 2002, 2007, 2009a; Critchley et al., 2004) has led to the distinction between two processing orientations during emotional perception: external perceptual orienting (EPO) and interoceptive self-orienting (ISO). During EPO, attention directed externally toward the arousing event causes changes in behavioral, autonomic and endocrine responses (LeDoux, 2012), independent of the awareness of these changes. During ISO, in contrast, attention is directed internally toward one’s own affective response to the arousing item or event (Lane et al., 1997; Pollatos et al., 2007; Zaki et al., 2012; see also Tsuchiya and Adolphs, 2007). An accumulating body of evidence indicates that during emotional perception, EPO and ISO orientations are mediated by distinct neural systems, whereas EPO is primarily mediated by sensory and subcortical areas, such as the amygdala (Vuilleumier et al., 2001; Pessoa et al., 2002), ISO is primarily mediated by the anterior insula (AI), particularly the right AI, and the anterior cingulate cortex (ACC), particularly the dorsal ACC (dACC) (Medford and Critchley, 2010).
A recent model (Seth, 2013; see also Singer, 2009; Gu, 2013) proposes that during emotional awareness perception, the AI and dACC generate, compare and update predictions of visceral and autonomic signals. Along these lines, the AI and dACC have been described as core components of the ‘salience network’ (Seeley et al., 2007; Menon and Uddin, 2010), which is assumed to monitor the saliency of stimuli and mediate interactions between emotional/motivational and cognitive processes (Pessoa, 2009, 2010). With respect to the processing of affective information, it has been proposed that the AI and dACC operate on signals generated during EPO by sensory-limbic regions, including the amygdala (Lane, 2008; Craig, 2009a; Damasio, 2012; Damasio and Carvalho, 2013). In this way, during ISO, the initial ‘pre-reflective’ or automatic representation of emotional states is elaborated upon, enabling the appraisal of one’s own affective response to a given stimulus. Thus, although the amygdala and other limbic regions appear generally responsive to emotional content in the environment (inside or outside of awareness; Vuilleumier et al., 2001; Dolan, 2002; Winston et al., 2003; Pessoa, 2005; Anderson, 2007), through coordination with limbic regions, AI and dACC may integrate these responses enabling a more internally oriented awareness of emotional experience.
Given that past theoretical and empirical work regarding amygdala vs AI/dACC under EPO vs ISO has focused mainly on emotional perception, it is unclear how changes in emotional processing orientation relate to memory. Emotion exerts a strong influence on memory, and the nature of emotional processing is therefore likely to determine how emotional information is encoded. This relationship is particularly important, since changes in emotional experience and the corresponding subsequent memories may influence emotional well-being (e.g., remembering happy events) (Walker et al., 2003; Kryla-Lighthall and Mather, 2009) or contribute to psychiatric disorders (depression, post-traumatic stress disorder, etc.) (Bradley and Mathews, 1988; Mathews et al., 1989; Linehan, 1993; Bradley et al., 1995; MacLeod and McLaughlin, 1995; Becker et al., 1999; Ridout et al., 2003; Hall et al., 2007; Castaneda et al., 2008; Herbener, 2008; Dere et al., 2010; Winter, 2014; Ai et al., 2015). Thus, understanding how attentional orientation modulates the impact of emotion on memory has both important theoretical and clinical implications.
In general, emotional arousal tends to enhance memory formation, an effect that has been linked to the amygdala and its interactions with medial temporal lobe (MTL) memory regions (LaBar and Cabeza, 2006; Dolcos et al., 2004, 2005). In fMRI studies of emotional memory encoding (Murty et al., 2010), emotion typically enhances activity that predicts later memory (subsequent memory effects—SMEs; Paller and Wagner, 2002) in the amygdala and MTL. In contrast, emotion effects on SMEs in AI or dACC have been rarely reported (Qin et al., 2012; Waringa and Kensingera, 2012). One possible explanation for the scarcity of AI and dACC effects in fMRI studies of emotional memory is that most of these studies investigated conditions that emphasized EPO rather than ISO, such as attending to or judging physical or semantic features of the stimuli or performing tasks unrelated to the emotional experience (Kensinger and Corkin, 2004; Kensinger and Schacter, 2006, 2008; Harvey et al., 2007; Kensinger et al., 2007; Mickley and Kensinger, 2008; Ritchey et al., 2011). Thus, it is largely unknown if AI and dACC would show emotion-related SMEs when the orienting condition emphasizes ISO rather than EPO.
In the current event-related fMRI study, we scanned participants while viewing emotional and neutral pictures under EPO (rate the brightness of each picture) or ISO (rate their own affective or bodily reactions to each picture), and tested memory for the pictures several days later. We investigated three main questions. First, we examined ‘perception-related activity’ independently of memory, expecting to replicate and extend past literature which suggests that the amygdala should show similar emotion effects during ISO and EPO with condition-related differences more likely in AI and dACC. We next turned to ‘memory-related activity’ to assess how the amygdala and AI/dACC supported emotional memory formation, as evidenced by emotion-related SMEs across the different emotional orienting conditions. As with perception-related effects, we expected the most pronounced differences between ISO and EPO to appear in AI/dACC or other regions associated with interoceptive processing. Finally, given the importance of the amygdala in both online emotional processing and emotional memory formation, and the interactions between this region and the salience network (Seeley et al., 2007; Menon and Uddin, 2010), we examined how functional connectivity between the amygdala and other regions varied as a function of emotional memory and orienting condition.
Methods
Participants
Twenty young adults participated in the study. Participants were healthy, right handed, native English speakers, with no disclosed history of neurological or psychiatric episodes. Participants gave written informed consent for a protocol approved by the Duke University Institutional Review Board. Three participants were excluded from the analysis: one due to failing to follow instructions during the EPO condition, one due to disproportionately high false alarm rates (>60%, more than 2 s.d. above group mean) and the third for insufficient number of miss trials on recognition performance across conditions. All behavioral and neuroimaging analyses were conducted on the remaining 17 participants (9 women, mean age = 22.7, s.d. = 2.5).
Stimuli
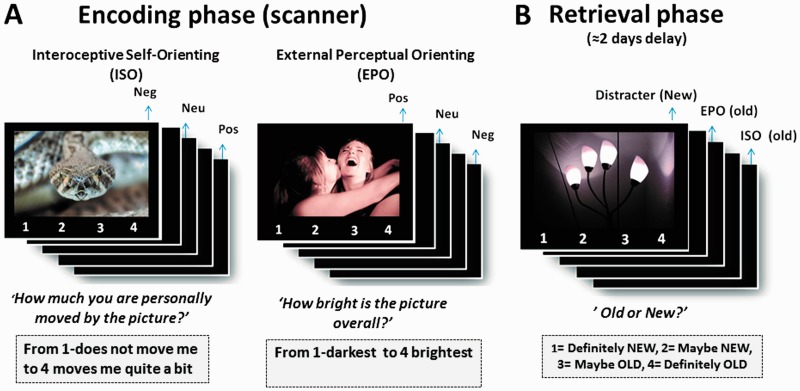
Stimuli consisted of 600 pictures selected from the International Affective Picture System (IAPS) (Lang et al., 2008) as well as from an in-house set that was used in a previous study by our laboratory (Ritchey et al., 2011). Pictures were assigned to emotional condition on the basis of a 9-point normative valence scale: negative (1–4), neutral (4–6) and positive (6–9) conditions. In accordance with the picture selection procedure, standardized valence scores were lower for negative (M = 2.85, s.d. = 0.62) than neutral pictures (M = 5.12, s.d. = 0.42; (p < .001), and higher for positive (M = 7.02, s.d. = 0.57) than neutral pictures (P < 0.001). Additionally, arousal scores (1 = calm, 9 = excited) were greater for negative (M = 5.76, s.d. = 0.47) than neutral pictures (M = 3.46, s.d. = 0.45; P < 0.001), greater for positive (M = 5.72, s.d. = 0.58) than neutral pictures (P < 0.001), and did not significantly differ between negative and positive pictures (P = 0.54). Note that for copyright reasons, the images shown in Figure 1 are not from the IAPS set but illustrate the type of pictures that participants viewed at encoding.
Fig. 1.
Schematic of the experimental design.
Procedure
Scanned paradigm
During encoding session (scanned), participants viewed 150 negative, 150 positive and 150 neutral pictures (Figure 1A). Each picture was presented for 3 s along with a 4-point scale and was followed by a jittered inter-trial interval (3 s average) (drawn from an exponential distribution). The encoding session consisted of six functional runs of incidental encoding, with each run containing an equal number of negative, positive and neutral pictures. Runs alternated between three distinct conditions (with two runs per task condition): ISO, EPO and semantic orienting. The results of the semantic processing condition are not reported in this study. The pictures within each run (75 total pictures per run, 25 of each emotion type) were pseudo-randomized so that no more than 3 pictures of the same valence appeared consecutively. The sequence of encoding orienting conditions was counterbalanced across participants, as was the assignment of stimuli to each condition and the division of pictures between old (encoding) and new (novel recognition test distractors).
Before the scan session, participants completed a short practice session in which they were familiarized with the instructions specific to each orienting condition. Condition-specific instructions were again presented at the beginning of each run. In the EPO condition, participants were instructed to rate the visual brightness of each picture. In the ISO condition, they were instructed to rate how much they were personally moved by each picture. This was further explained as follows: ‘How much are you personally moved by each picture, means to what extent (from 1 to 4) each picture makes you feel or experience an emotional reaction. This could include changes in your bodily state (e.g. breathing, heart rate) or the experience of a particular positive or negative emotion. Thus, you need to decide if each picture moves you or not, based on the presence of these feelings or bodily changes’.
Two to 3 days (mean hours = 55.06, s.d. = 11.28) after the encoding phase, participants completed a surprise recognition memory test administered in a separate computer laboratory (Figure 1B). The test included all the 450 scene pictures previously viewed in the scanner (‘old’) randomly mixed with 150 novel images (‘new’) (50 from each emotional type). Each picture was presented for 4 s and followed by a fixation screen for 500 ms. Participants indicated whether each picture was old or new using a 4-point confidence scale (1 = definitely new; 2 = probably new; 3 = probably old; 4 = definitely old).
fMRI methods
Data acquisition and preprocessing
All MRI data acquisition was collected using a 3-T GE scanner. Scanner noise was reduced with earplugs, and head motion was minimized using foam pads and a headband. Stimuli were projected onto a mirror at the back of the scanner bore, and behavioral responses were recorded using a MR-compatible four button box. High-resolution T1-weighted structural anatomical images were collected with a 24 cm field of view, 1.9 mm slice thickness, 68 slices, and 256 × 256 matrix. For our functional imaging, 34 contiguous slices parallel to the anterior and posterior commissure plane were acquired in an interleaved order using an inverse spiral sequence with a 2 s repetition time, 31 ms echo time, 24 cm field of view, 64 × 64 image matrix, 60° flip angle and 3.8 mm slice thickness. Preprocessing and statistical fMRI analyses were performed using SPM5 (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm/) software implemented in MATLAB. The first five volumes of each functional dataset were discarded from analysis for T1 equilibration effects. The functional images were slice timing corrected and motion corrected, spatially normalized to Montreal Neurological Institute (MNI) space, and spatially smoothed using an 8 mm isotropic Gaussian kernel.
fMRI analyses
A standard two-level SPM analysis was employed (Friston et al., 1995). For the first-level (fixed-effects) analysis, we modeled evoked hemodynamic responses to event types with a delta (stick) function corresponding to stimulus presentation onset convolved with a canonical hemodynamic response function within the context of the general linear model (GLM). Six main event types were included, representing all possible combinations of picture type (negative, neutral, positive) and orienting condition (ISO, EPO), along with regressors for motion and run mean. To incorporate memory, scores from the subsequent recognition phase were used to construct a parametric regressor with three levels: level 1 = subsequent new responses (i.e. misses, collapsing high/low confidence New responses); level 2 = subsequent low-confidence hits (LCHs) and level 3 = subsequent high-confidence hits (HCHs). This parametric regressor was applied to each trial type in a linear fashion, producing a set of contrast images where effects were modulated as a function of subsequent memory, in addition to a set of contrast images where the effect of memory had been removed.
For the second-level (random effect) analysis, we performed two separate 2 (orienting condition: ISO vs EPO) × 3 (picture type: negative, positive, neutral) repeated measures analysis of variance (ANOVAs), focusing on the effects of arousal, that is, emotional (both positive and negative) vs neutral pictures. To explore perception-related effects not varying by memory, the initial ANOVA was constructed using the unmodulated contrast images described above, while SMEs were examined with a corresponding ANOVA constructed with the parametrically modulated contrast images.
For the two brain regions where we predicted differences between ISO and EPO on the basis of extant literature (Craig, 2002; Critchley, 2005; Lane, 2008), namely AI and dACC, we used a region-of-interest (ROI) analysis, which we followed with a more exploratory whole-brain analysis. The bilateral AI ROI was bounded caudally at y = 0, which corresponds to the agranular insula demarcated in previous studies of subjective body or/and emotional feelings (Craig, 2002; Critchley, 2005). The dACC ROI combined the bilateral anterior and middle cingulate ROIs (Automated Anatomical Labeling atlas) within Brodmann areas 24 and 32, and was bounded between 0 < y < 33 (MNI coordinates) (Bush et al., 2000; Vogt et al., 2003; Steele and Lawrie, 2004), borders used in previous studies of emotional awareness (Lane, 2008; McRaea et al., 2008).
Unless otherwise noted, results were thresholded as follows. For the whole-brain analysis, we used a voxelwise threshold of P < .005, with a minimum cluster extent of 19 contiguous voxels. These voxelwise and cluster extent values were established via Monte Carlo simulation (Slotnick et al., 2003) to provide a whole-brain threshold that was corrected for multiple comparisons at P < 0.05. For the ROI analyses, for each of the 2 a priori ROIs defined above (dACC and AI), a false discovery rate (FDR) corrected threshold of P < 0.05 was used (small volume correction implemented in SPM), with a minimum cluster extent of 10 contiguous voxels (Benjamini and Hochberg, 1995). In both whole-brain and ROI analyses, to ensure that emotion × attentional orienting interactions (e.g. greater emotional > neutral effect in ISO than EPO) were not driven by the opposite effect in the orienting condition (e.g. greater neutral > emotional effect in EPO than ISO), contrasts were inclusively masked with a significant effect (at P < 0.05) within the condition of interest (e.g. emotional > neutral in ISO).
To examine SMEs pertaining to high confidence responses specifically (rather than those showing a parametric increase with confidence), an additional first-level model was run that included separate regressors for HCHs, LCHs and misses (M). With the exception of these separated memory regressors, other model parameters (e.g. motion and run mean regressors) were similar to the initial model. A second-level contrast based on this second model targeted regions showing SMEs within each condition based on highly confident memory responses (i.e. HCH vs miss).
Amygdala connectivity analyses
To investigate amygdala connectivity, we used a functionally derived seed from the right amygdala region showing significant emotion effects on SMEs (HCHs vs misses) during both ISO and EPO. In a psychophysiological interaction (PPI) analysis (Friston et al., 1997), activation time courses from an 8-mm sphere centered on this right amygdala peak (MNI: 19, 0, −23) were multiplied by the psychological variable of interest (SME contrast: HCHs > misses). For the PPI analysis, to simplify calculation of the time courses, we constructed a new GLM, in which data were concatenated across runs. This model included regressors for HCH and miss trial types for all combinations of emotion and orienting conditions, as well as confound regressors for motion and run mean. The PPI models included regressors for the physiological variable (amygdala time course), psychological variable (SME contrast), and the PPI interaction. For each participant, contrasts corresponding to the PPI psychological variable were extracted for each encoding condition and emotional condition separately. The PPI interaction variables were then entered into a two-way ANOVA with emotion and attentional orientation (EPO vs ISO) as factors.
Results
Behavioral results
Subjective EPO and ISO ratings during encoding
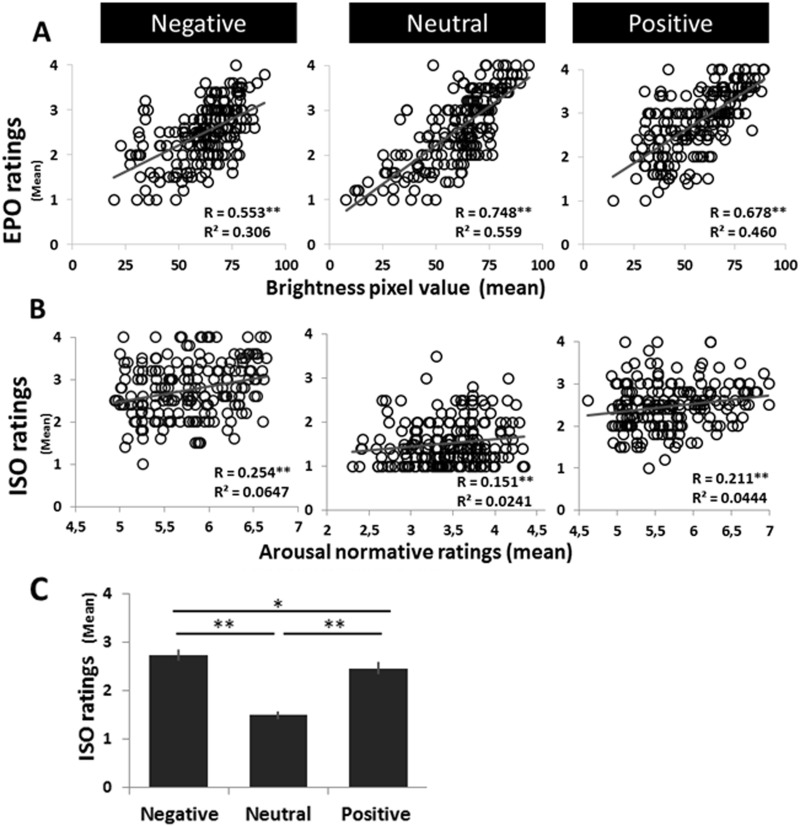
Confirming the validity of participants’ subjective ratings, brightness ratings during EPO were significantly correlated with picture luminance (average of all pixels in each image) (Figure 2A), and interoceptive ratings in the ISO condition were significantly correlated with arousal ratings from the IAPS norms (two-tailed significance tests) (Figure 2B). These group-wise correlations were obtained by plotting the (across-subject) mean EPO and ISO ratings for each image against the corresponding image luminance (mean pixel-wise value of relative luminance, calculated for each picture in MATLAB) or normative arousal value. Also, ISO ratings were higher for emotional (negative and positive) pictures than neutral pictures (F(2, 32) = 91.50, P < .0005, partial η2 = 0.85; Figure 2C), and for negative than positive pictures (t(16) = 2.80, P = 0.014, two-tailed). In sum, the results confirm the validity of subjective EPO and ISO ratings as well as the significant impact of the picture category manipulation on ISO ratings.
Fig. 2.
Behavioral encoding response data. Across images within each valence condition, significant correlations were found between (A) the picture-specific (group level) mean subjective brightness rating (from the EPO condition) and the objective picture luminance and (B) the picture-specific (group level) mean rating from the ISO condition and the picture’s normative arousal score (from IAPS and in-house, standardized database). (C) Average ISO ratings were entered into one-way ANOVAs with emotion (negative, neutral, positive) as a factor; ISO ratings also displayed a main effect of emotion (P < 0.0005), with emotional pictures (negative and positive) rated as more personally arousing than neutral pictures. Error bars denote standard error.
Recognition Memory Data
Table 1 shows hits, false alarms and corrected recognition scores (hits-false alarms). An Emotion × Orienting condition ANOVA on corrected recognition scores yielded a main effect of emotion (F(2, 32) = 13.39, P < 0.001, partial η2 = 0.46), and post hoc paired t-tests showed that negative pictures were remembered better than neutral (t(16) = 3.32, P < 0.004) and positive (t(16) = 5.15, P < 0.0005) pictures, with no difference between positive and neutral pictures (t(16) = 1.77, P = 0.100. There was also a main effect of orienting condition because pictures encoded during ISO were better remembered than pictures encoded in the EPO condition (F(1, 16) = 62.78, P < 0.001, partial η2 = 0.80). The interaction between emotion and orienting condition was not significant (F(2, 32) = 1.99, P = 0.15, partial η2 = 0.11). Finally, the false alarm rate to positive pictures was higher than of negative and neutral pictures, (F(2, 32) = 6.92, P = 0.03, partial η2 = 0.30). The absence of an interaction between orienting condition and emotion indicates that potential brain-related dissociations in emotional encoding between ISO and EPO should reflect differences in the manner through which material is encoded rather than varying degrees of encoding efficiency. Nonetheless, given that little behavioral work has been done directly comparing ISO and EPO encoding, further studies are needed to explore the mnemonic advantage of each condition with respect to emotional material.
Table 1.
Behavioral results: recognition memory
| Emotion type | Negative | Neutral | Positive |
|---|---|---|---|
| Hit rate | |||
| ISO | 0.75 (0.11) | 0.67 (0.17) | 0.66 (0.013) |
| EPO | 0.63 (0.15) | 0.47 (0.16) | 0.56 (0.15) |
| False alarm rate | 0.22 (0.16) | 0.18 (0.10) | 0.28 (0.14) |
| Correct recognition (hit rate − false alarm rate) | |||
| ISO | 0.54 (0.20) | 0.47 (0.17) | 0.39 (0.17) |
| EPO | 0.41 (0.29) | 0.29 (0.17) | 0.28 (0.16) |
Note: Data are reported as mean (s.d.). FA rates are common to both conditions.
fMRI results
Emotion effects on perception-related activity (picture vs baseline)
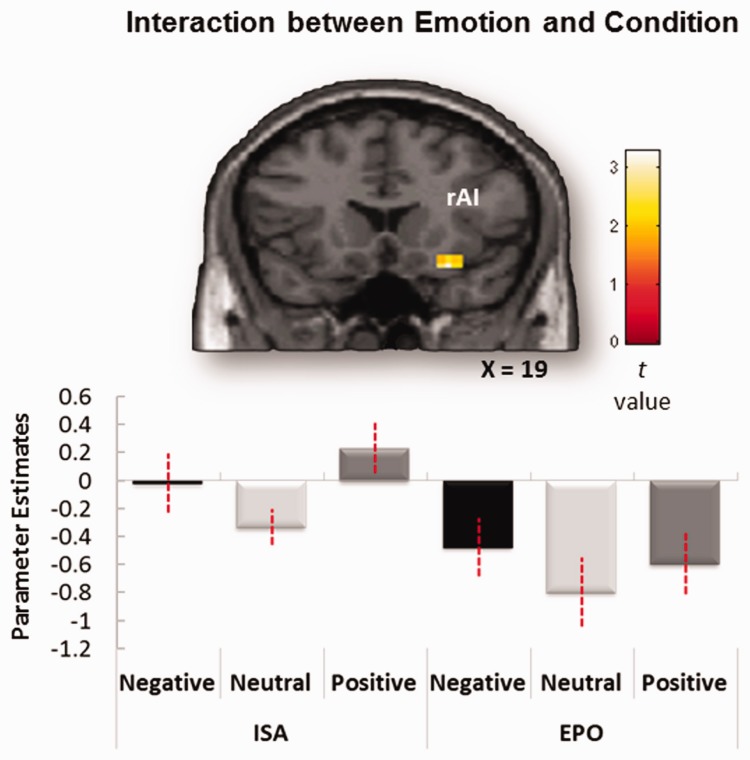
Table 2 lists regions showing significant main effects of emotion and regions showing emotion × condition interactions. As expected, one of the regions showing a main effect of emotion was the amygdala. This finding is consistent with evidence that the amygdala is sensitive to emotion regardless of attentional manipulations (Dolan, 2002; Winston et al., 2003; Critchley, 2009; Vuilleumier and Huang, 2009). AI also showed a main effect of emotion and also a marginally significant emotion × orienting condition interaction in which emotion effects were larger during the ISO than during EPO (SVCFDR P = 0.064, k = 12) (Figure 3). Although this interaction was only trending toward significance, it is consistent with other evidence that ISO augments the contribution of AI to emotional processing (Lane et al., 1997; Critchley et al., 2002, 2004; Phan et al., 2004; Pollatos et al., 2007; Zaki et al., 2012; Satpute et al., 2013). Unexpectedly, the dACC showed only the main effect of emotion but did not show a significant interaction between emotion and orienting condition. Finally, no brain region showed larger emotion effects for EPO than ISO.
Table 2.
Perception-related activity: main effects of Emotion and Emotion × Attentional orienting interactions
| Regions | Hem | BA | MNI xyz coordinates | t | Z | voxels | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main effect of emotion | ||||||||||||||||
| Amygdala | R | – | 26 | 0 | −27 | 3.70 | 3.70 | 38 | ||||||||
| AI ROI | L | – | −30 | 26 | 8 | 6.17 | 5.65 | 92 | ||||||||
| L | 13 | −41 | 19 | 0 | 5.83 | 5.38 | – | |||||||||
| R | 47 | 34 | 26 | 4 | 5.26 | 4.92 | 39 | |||||||||
| dACC ROI | L | – | −8 | 19 | 46 | 8.27 | 7.17 | 15 | ||||||||
| R | 32 | 11 | 19 | 46 | 5.01 | 4.71 | 11 | |||||||||
| Inferior frontal gyrus | R | 47 | 30 | 34 | −11 | 5.78 | 5.34 | 129 | ||||||||
| R | 47 | 34 | 26 | 4 | 5.26 | 4.92 | – | |||||||||
| R | – | 45 | 8 | 30 | 8.77 | 7.50 | 450 | |||||||||
| R | – | 45 | 30 | 23 | 8.53 | 7.34 | – | |||||||||
| R | – | 34 | 0 | 49 | 6.29 | 5.74 | – | |||||||||
| Inferior occipital gyrus | L | – | −23 | −86 | −4 | 22.49 | >8 | 6564 | ||||||||
| R | – | 23 | −86 | −4 | 22.42 | >8 | – | |||||||||
| R | – | 26 | −86 | 23 | 18.19 | >8 | – | |||||||||
| Emotion × Attentional orienting interactions | ||||||||||||||||
| Greater emotion effects during ISO than EPO | ||||||||||||||||
| AI ROI* | R | – | 30 | 19 | −19 | 3.27 | 3.18 | 12 | ||||||||
| Greater emotion effects during EPO than ISO | ||||||||||||||||
| No regions | ||||||||||||||||
Note: Up to three local maxima set 8 mm apart are reported for each cluster. All regions with the exception of the two a priori ROIs result from contrasts using a whole-brain extent threshold of P < 0.005 and a cluster extent of 19 voxels to correct for multiple comparisons (see Methods). ROI results (AI ROI and dACC ROI) were FDR corrected at a threshold of P < 0.05, while (*) denotes a trend level finding (Pfdrcorrected < 0.1). Coordinates are in MNI space. Abbreviations: Amy, amygdala; BA, Brodmann area; Hem, hemisphere; L, left; R, right.
Fig. 3.
Emotion × Orienting condition interaction in emotional–perception activity. Activity in the right AI-inferior frontal gyrus (IFG) cluster shows a trend-level emotional effect for the ISO vs EPO condition (SVCFDR < 0.064, k = 12; see Table 2 for coordinates). Mean parameter estimates averaged across all the voxels in the AI-IFG region for each participant are plotted for each task condition.
Emotion effects on SMEs (subsequently remember vs forgotten)
Regions showing main effects of emotion are listed in Table 3, and regions showing interactions between emotion and orienting condition, in Table 4. We performed SME analyses on main effects; one analysis used a parametric regressor, which identified encoding activity that predicted gradual increases from misses to LCHs to HCHs, whereas the other analysis focused on HCHs. The linear SME analysis identified inferior frontal and ventral parietal (BA39) regions, whereas the high confidence-related SME analysis identified the amygdala. These findings are consistent with the fMRI literature on emotional memory, where such regions often show SMEs (Murty et al., 2010).
Table 3.
Memory-related activity: main effects of emotion
| Regions | Hem | BA | xyz coordinates | t | Z | voxels | ||
|---|---|---|---|---|---|---|---|---|
| Linear SME regressor (HCHs > LCHs > misses) | ||||||||
| Inferior frontal gyrus | R | 46 | 53 | 30 | 23 | 4.23 | 4.04 | 59 |
| R | 45 | 56 | 30 | 8 | 3.46 | 3.35 | – | |
| R | 44 | 45 | 8 | 30 | 3.57 | 3.45 | 26 | |
| R | – | 53 | 19 | 27 | 2.67 | 2.62 | – | |
| Inferior parietal lobule | L | 39 | −49 | −64 | 8 | 3.48 | 3.36 | 25 |
| L | 39 | −53 | −75 | 15 | 3.09 | 3.01 | – | |
| High confidence-related SME regressor (HCH > misses) | ||||||||
| Amygdala | R | 19 | 0 | −23 | 4.86 | 4.71 | 33 | |
| R | 8 | 0 | −19 | 3.42 | 3.37 | – | ||
| R | 8 | −15 | −19 | 3.33 | 3.28 | – | ||
Note: Up to three local maxima set 8 mm apart are reported for each cluster. All regions result from contrasts using a whole-brain extent threshold of P < 0.005 and a cluster extent of 19 voxels to correct for multiple comparisons (see Methods). Coordinates are in MNI space. Abbreviations: Amy, amygdala; BA, Brodmann area; Hem, hemisphere; L, left; R, right.
Table 4.
Memory-related activity: Emotion × Attentional orienting interactions
| Regions | Hem | BA | MNI xyz coordinates | t | Z | voxels | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Greater emotion effect during ISO than EPO | |||||||||||||||
| AI ROI | L | 13 | −34 | 8 | −11 | 3.61 | 3.49 | 85 | |||||||
| L | 13 | −34 | 15 | 0 | 3.29 | 3.20 | – | ||||||||
| L | – | −30 | 23 | 8 | 3.02 | 2.95 | – | ||||||||
| R | 13 | 38 | 15 | 0 | 3.24 | 3.15 | 82 | ||||||||
| R | 13 | 45 | 8 | −4 | 3.21 | 3.12 | – | ||||||||
| R | 13 | 34 | 15 | −11 | 2.73 | 2.67 | – | ||||||||
| dACC ROI | L | 24 | −4 | 19 | 27 | 2.9 | 2.83 | 102 | |||||||
| R | 32 | 4 | 30 | 30 | 2.74 | 2.69 | – | ||||||||
| R | 32 | 8 | 19 | 34 | 2.73 | 2.68 | – | ||||||||
| Dorsal/dorsomedial frontal cortex | R | 9 | 41 | 30 | 38 | 3.83 | 3.68 | 37 | |||||||
| R | 9 | 49 | 4 | 34 | 3.64 | 3.51 | 164 | ||||||||
| R | 8 | 19 | 19 | 49 | 3.56 | 3.44 | – | ||||||||
| R | 6 | 15 | 8 | 65 | 3.42 | 3.31 | – | ||||||||
| R | 8 | 4 | 26 | 53 | 3.09 | 3.01 | 20 | ||||||||
| Ventrolateral prefrontal cortex | R | 47 | 45 | 41 | −8 | 3.41 | 3.30 | 19 | |||||||
| Substantia nigra | R | SN | 8 | −11 | −15 | 3.7 | 3.57 | 55 | |||||||
| L | – | −15 | −11 | −4 | 3.05 | 2.97 | – | ||||||||
| L | – | −8 | −4 | −11 | 2.86 | 2.79 | – | ||||||||
| Striatum | R | Put | 30 | −19 | −4 | 4.06 | 3.89 | 36 | |||||||
| R | 38 | −11 | −15 | 2.91 | 2.84 | – | |||||||||
| R | – | 15 | 11 | 4 | 3.21 | 3.12 | 37 | ||||||||
| R | – | 15 | 0 | 4 | 2.92 | 2.86 | – | ||||||||
| R | Put | 15 | 11 | −8 | 2.73 | 2.67 | – | ||||||||
| Inferior parietal lobule | R | 30 | −49 | 61 | 3.55 | 3.43 | 90 | ||||||||
| Superior temporal gyrus | R | 60 | −53 | 15 | 3.36 | 3.26 | 25 | ||||||||
| R | 53 | −49 | 15 | 3.29 | 3.20 | – | |||||||||
| Greater emotion effect on SMEs during EPO than ISO | |||||||||||||||
| No regions | |||||||||||||||
Note: Up to three local maxima set 8 mm apart are reported for each cluster. All regions with the exception of the two a priori ROIs result from contrasts using a whole-brain extent threshold of P< 0.005 and a cluster extent of 19 voxels to correct for multiple comparisons (see Methods). ROI results (AI ROI and dACC ROI) were FDR corrected at a threshold of P< 0.05. Coordinates are in MNI space. Abbreviations: SN, substantia nigra; Put, putamen; BA, Brodmann area; Hem, hemisphere; L, left; R, right.
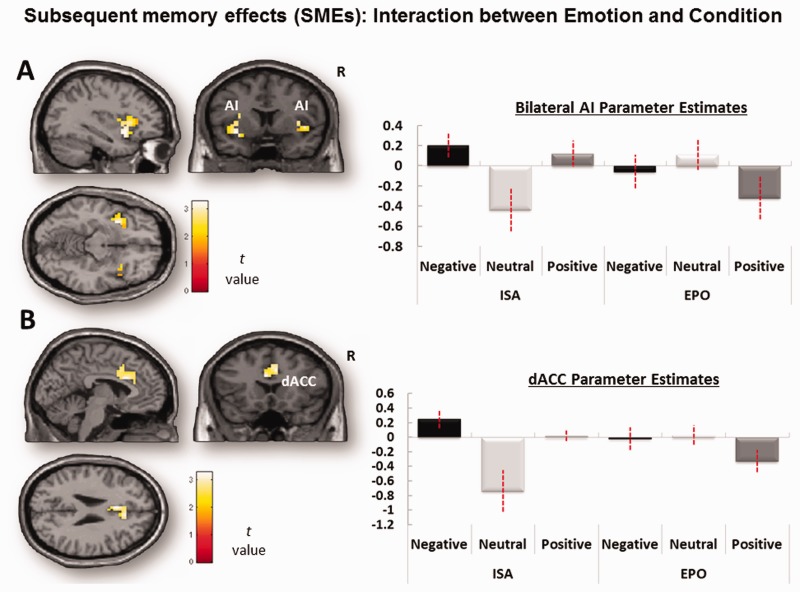
Turning to interactions between emotion and orienting condition (Table 4), every region identified in this interaction showed larger emotion effects during ISO than EPO, with no region showing the opposite pattern. In both the AI and the dACC ROIs (Figure 4), there was a significant influence of emotion on SMEs during ISO but not EPO, with stronger effects for the AI than dACC (Table 4). For the whole-brain analysis, greater emotion effects on SMEs during ISO than EPO were also found in right dorsal/dorsomedial frontal, substantia nigra (SN), striatum and right parietotemporal regions. In sum, confirming our prediction, we found for the first time that both core components of the salience network (particularly the AI) contributed to emotional memory formation when attentional orientation was directed internally toward self-awareness of the emotional state, but not when it is was directed externally to properties of the stimuli.
Fig. 4.
ROI analysis for memory-related Emotion × Orienting condition interaction. Mean parameter estimates, extracted and averaged across all the voxels in each ROI for each participant, are plotted for each task condition. (A) Bilateral AI (SVCFDR < 0.05, k > 10 contiguous voxels) and (B) dACC regions showing emotional SMEs in the ISO condition than in the EPO condition (SVCFDR < 0.05, k > 10 contiguous voxels; see Table 4 for coordinates). Error bars denote standard error.
Functional connectivity of the amygdala
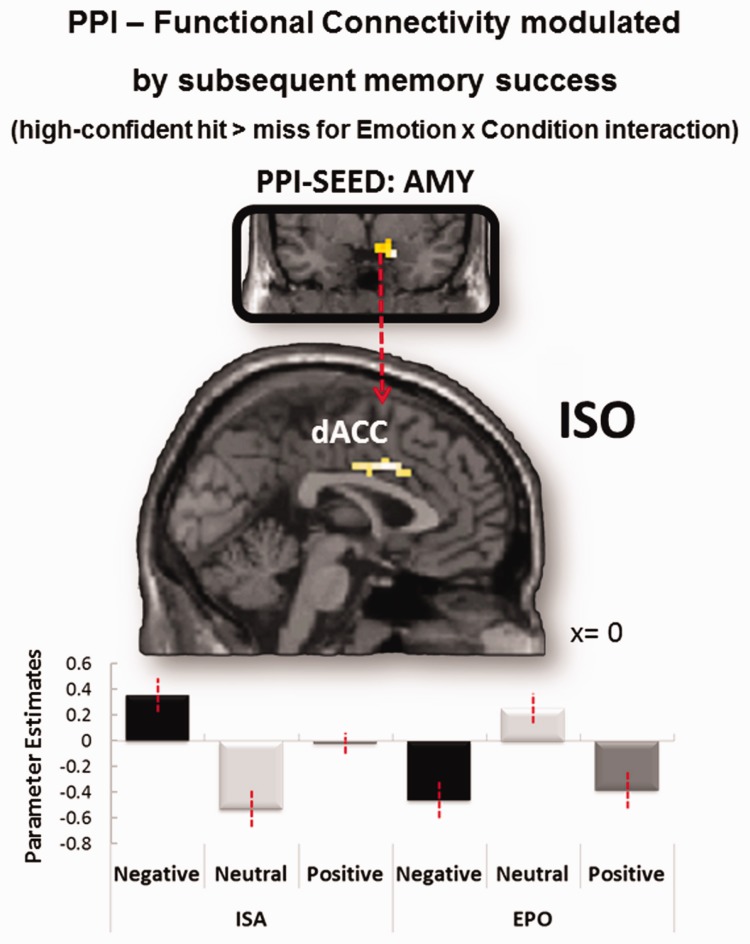
Finally, we performed a PPI analysis to investigate the effects of emotion on memory-related amygdala connectivity during ISO and EPO. As a seed, we used the right amygdala region previously identified as showing a main effect of emotion on SMEs (Table 3). Within the amygdala seed region, we extracted the representative time course for voxels that showed any effect across an omnibus F-test at P < 0.05. The fact that this region showed similar activation patterns during ISO and EPO is important because it prevents a potential confound between differences in seed connectivity and seed activity. Given that activity effects were found in high confidence-related SMEs (Table 3), we used the same SME contrast (HCHs vs misses) in the PPI analysis. As illustrated in Figure 5A, we found a significant interaction between emotion and orienting condition in dACC, where SME-related connectivity with the right amygdala was stronger for emotional than neutral pictures for ISO but not EPO. No other regions survived the whole-brain threshold. Unexpectedly, we did not find amygdala connectivity differences in AI. In sum, PPI results showed that during ISO but not during EPO, amygdala interactions with dACC mediated emotion effects on encoding activity predicting subsequent high confidence memory.
Fig. 5.
Functional connectivity modulated by subsequent memory success (HCH > miss) as a function of Emotion and Orienting condition. In dACC, functional connectivity with the right amygdala increased for emotion-related SMEs in the ISO vs EPO condition (i.e. an emotion × condition interaction) surviving to whole-brain analysis at P < 0.005, uncorrected and extent threshold of 19 contiguous voxels; dACC global maxima = MNI: 0, 8, 34, Z-value = 3.29, cluster extent = 38 voxels. Mean parameters are plotted for each task condition.
Discussion
In fMRI studies of emotional ‘perception’, EPO typically emphasizes the role of the amygdala whereas ISO additionally involves processes supported by salience network regions such as AI and dACC. In this study, we investigated whether a similar dissociation between EPO and ISO could be found for emotional ‘memory’. The study yielded three main findings. First, consistent with the emotional perception literature (Phelps and LeDoux, 2005; Vuilleumier and Huang, 2009; Tamietto and de Gelder, 2010; LeDoux, 2012), the amygdala showed significant emotion effects on perception-related activity during both ISO and EPO conditions, whereas AI showed a trend for these effects during the ISO but not EPO (Figure 3). Second, for the first time, we found a similar dissociation in the memory domain, whereas the amygdala showed emotion effects on SMEs during both ISO and EPO, and AI (and to a lesser extent dACC) showed these effects exclusively during ISO (Figure 4). Finally, in an analysis of functional connectivity, we observed that the enhancing effect of emotion on memory formation was selectively mediated by increased coupling between the amygdala and dACC—a relationship specific to ISO (Figure 5A). These three findings are discussed in separate sections below.
Perception-related activity
The dissociation we found between the right amygdala, where emotion effects were independent of attentional orientation, and AI, where emotion effects were larger for ISO than EPO, is broadly consistent with the literature describing the function of these regions. Functional neuroimaging studies of emotional perception have shown that the amygdala tracks arousal independently of attentional orientation, voluntary attention and even conscious awareness (Winston et al., 2003). In contrast, available evidence indicates that the contributions of AI to emotional processing depend on self-awareness of the aroused experience (Critchley et al., 2002, 2004; Phan et al., 2004). This characteristic of the AI is consistent with contributions of AI to awareness of visceral responses, which provides a substrate for subjective feelings (Craig, 2009a). For example, right AI activity has been linked to interoceptive awareness of heartbeats (Harrison et al., 2010; Zaki et al., 2012).
Unexpectedly, we did not find greater activity for ISO than EPO in dACC, another component of the salience network associated with interoceptive awareness of emotion (Critchley et al., 2004; Pollatos et al., 2007; Zaki et al., 2012). In our study, dACC showed a significant main effect of emotion but not a significant interaction between emotion and attentional orientation (Table 2). Although we predicted an interaction, there is evidence in the literature that some dACC regions may respond similar to ISO and EPO. For example, a meta-analysis of automatic fear conditioning and conscious appraisal of threat, which could be respectively classified as EPO and ISO conditions, found some overlapping activity within the dACC (Mechias et al., 2010). At any rate, it is clear that further research is required to distinguish between dACC regions that are sensitive or insensitive to the ISO vs EPO manipulation.
Although no emotion-related effects were observed for EPO vs ISO, it is important to note that the specific nature of the task used to induce external orienting is likely to influence the present results. Externally oriented tasks in studies using emotional stimuli have been shown to generate activity in higher order perceptual processing regions (Sato et al., 2001; Pessoa et al., 2002; Vuilleumier et al., 2003; Sarkheil et al., 2013), particularly when emotionally salient stimulus dimensions are relevant. Thus, with respect to both active perception and later memory, it may be beneficial for future studies to investigate differences between different external orienting tasks while also comparing these effects to conditions that focus on the internally oriented experience of emotion.
Memory-related activity
The main result of this study was finding that the differences between the amygdala and salience network previously reported in the emotional perception domain occur also in the emotional memory domain, whereas the amygdala showed emotion effects on SMEs during both ISO and EPO, AI and dACC showed these effects exclusively during ISO (Figure 4). It is noteworthy that in addition to AI and dACC, other components of the salience network such as SN and ventral striatum (Seeley et al., 2007) also showed the same effect. A few previous emotional memory fMRI studies found AI and dACC activations under conditions that could be interpreted as emphasizing ISO (Canli et al., 2002; see also Canli et al., 1999; Qin et al., 2012; Waringa and Kensingera, 2012). However, this study provides the first clear evidence that the contributions of AI and dACC to emotional memory occur for ISO but not for EPO and are dissociable from the pattern displayed by the amygdala.
The current finding linking AI and dACC to emotional memory formation is consistent with evidence from non-human animals showing that, in addition to processing interoceptive signals, the AI and dACC mediate consolidation of affective associations. For example, one study showed that immediately following an inhibitory avoidance task, injections of glucocorticoid receptor agonists into rodent AI enhanced later memory for aversive associations (Miranda et al., 2008; Fornari et al., 2012). In related work, administration of memory disrupting β-adrenergic receptor antagonists to the AI reduced memory related to aversive environmental contexts (Miranda et al., 2011), while a separate study found that inhibitory stimulation of dACC in primates prevented spontaneous recovery of aversive memories (Klavir et al., 2012). Moreover, the amygdala, AI and prelimbic cortex (the rodent analog of human dACC; Milad and Quirk, 2012) are synchronized during aversive learning in rodents, showing increases in activity corresponding to neuronal firing in basolateral amygdala (Uematsu et al., 2014). Taken together, extant animal research shows that salience network regions are an important substrate for more basic arousal-related memory processes in non-human animals. In humans, the present findings indicate that they may play a distinct but related role in processing and integrating autonomic/somatosensory markers into long-term representations of individual events.
Memory-related amygdala connectivity
Finally, our third finding was that the enhancing effect of emotion on memory formation during ISO was associated with increased coupling between the amygdala and dACC. Amygdala connectivity is known to play an important role in emotional learning and memory (Hermans et al., 2014). For example, a recent fMRI study of classical conditioning showed that amygdala–dACC interactions predicted the consolidation of acquired fear associations (Feng et al., 2014). Also, conditioning studies in rodents have shown that coupling between the amygdala and prelimbic cortex plays a role in associative fear or aversive memory formation (Burgos-Robles et al., 2009; Tan et al., 2010, 2011; Uematsu et al., 2014; see also for primates: Livneh and Paz, 2012; Klavir et al., 2013). However, it is difficult in humans and impossible in rodents to determine the extent of ISO involvement in these conditioning paradigms. To our knowledge, this is the first study demonstrating that amygdala connectivity with dACC contributes to emotional memory under ISO but not under EPO.
Conclusion
This study explored how internally oriented attention to affective or bodily reactions during emotional experience influences memory encoding. We observed that AI was preferentially recruited for emotional perception during ISO, as compared with EPO. Critically, both AI and dACC, the core components of the salience network (along with other regions of this network), were found to support subsequent memory success for ISO encoding. Finally, although the amygdala supported emotional memory in both conditions, PPI analyses revealed that the formation of particularly strong memories during ISO was associated with selective coupling between the amygdala and dACC. These results provide additional evidence that orientation can greatly influence emotional processing, and most importantly, they suggest that partly distinct mechanisms of emotional memory formation may accompany internally oriented awareness of the affective response. As this area of research is explored further, it will be necessary to consider factors like individual variation in emotional responsivity (emotional awareness trait, somatic response, etc.) to obtain a fuller picture of the relationship between interoceptive awareness and memory.
Acknowledgements
We would like to thank Kerry Townsend and Jeffrey Brooks for their assistance with data collection.
Funding
This work was supported by a grant from the National Institute on Aging (R01 AG34580) awarded to R.C. and the Foundation for Science and Technology of Portugal scholarship (SFRH/BD/68769/2010) awarded to C.P.V.
Conflict of interest. None declared.
References
- Ai H., Opmeer E.M., Veltman D.J., et al. (2015). Brain activation during emotional memory processing associated with subsequent course of depression. Neuropsychopharmacology , 40, 2454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A.K. (2007). Feeling emotional: the amygdala links emotional perception and experience. Social Cognitive and Affective Neuroscience , 2(2), 71–2. [Google Scholar]
- Becker E.S., Roth W.T., Andrich M., Margraf J. (1999). Explicit memory in anxiety disorders. Journal of Abnormal Psychology, 108(1), 153–63. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society , 57(1), 289–300. [Google Scholar]
- Bradley B.P., Mathews A. (1988). Memory bias in recovered clinical depressives. Cognition & Emotion, 2(3), 235–45. [Google Scholar]
- Bradley B.P., Mogg K., Williams R. (1995). Implicit and explicit memory for emotion-congruent information in clinical depression and anxiety. Behaviour Research and Therapy, 33(7), 755–70. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A., Vidal-Gonzalez I., Quirk G.J. (2009). Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. The Journal of Neuroscience , 29(26), 8474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences , 4(6), 215–22. [DOI] [PubMed] [Google Scholar]
- Canli T., Desmond J.E., Zhao Z., Gabrieli J.D.E. (2002). Sex differences in the neural basis of emotional memories. Proceedings of the National Academy of Sciences of the United States of America , 99(16), 10789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T., Zhao Z.U.O., Desmond J.E., Glover G., Gabrieli J.D.E. (1999). fMRI identifies a network of structures correlated with retention of positive and negative emotional memory. Psychobiology , 27(4), 441–52. [Google Scholar]
- Castaneda A.E., Tuulio-Henriksson A., Marttunen M., Suvisaari J., Lönnqvist J. (2008). A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. Journal of Affective Disorders , 106(1), 1–27. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience , 3(8), 655–66. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2007). Interoception and emotion: a neuroanatomical perspective. In: Lewis M., Haviland-Jones J., Barret L., editors. Handbook of Emotion, New York: Guildford Press. [Google Scholar]
- Craig A.D. (2009a). How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience , 10(1), 59–70. [DOI] [PubMed] [Google Scholar]
- Critchley H.D. (2005). Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology , 493(1), 154–66. [DOI] [PubMed] [Google Scholar]
- Critchley H.D. (2009). Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. International Journal of Psychophysiology , 73(2), 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D., Mathias C.J., Dolan R.J. (2002). Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron , 33(4), 653–63. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Ohman A., Dolan R.J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience , 7(2), 189–95. [DOI] [PubMed] [Google Scholar]
- Damasio A. (2012). Self Comes to Mind: Constructing the Conscious Brain. Nek York: Vintage Books. [Google Scholar]
- Damasio A., Carvalho G.B. (2013). The nature of feelings: evolutionary and neurobiological origins. Nature Reviews Neuroscience , 14(2), 143–52. [DOI] [PubMed] [Google Scholar]
- Damasio A.R. (1999). The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: A Harvest Books. [Google Scholar]
- Dere E., Pause B.M., Pietrowsky R. (2010). Emotion and episodic memory in neuropsychiatric disorders. Behavioural Brain Research, 215(2), 162–71. [DOI] [PubMed] [Google Scholar]
- Dolan R.J. (2002). Emotion, cognition, and behavior. Science , 298(5596), 1191–4. [DOI] [PubMed] [Google Scholar]
- Dolcos F., LaBar K.S., Cabeza R. (2004). Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron , 42(5), 855–63. [DOI] [PubMed] [Google Scholar]
- Dolcos F., LaBar K.S., Cabeza R. (2005). Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proceedings of the National Academy of Sciences of the United States of America , 102(7), 2626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P., Feng T., Chen Z., Lei X. (2014). Memory consolidation of fear conditioning: bi-stable amygdala connectivity with dorsal anterior cingulate and medial prefrontal cortex. Social Cognitive and Affective Neuroscience , 9, 1730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornari R.V., Wichmann R., Atucha E., Desprez T., Eggens-Meijer E., Roozendaal B. (2012). Involvement of the insular cortex in regulating glucocorticoid effects on memory consolidation of inhibitory avoidance training. Frontiers in Behavioral Neuroscience, 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. (1997). Psychophysiological and modulatory interactions in neuroimaging. NeuroImage , 6(3), 218–29. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J., Poline J.P., Frith C.D., Frackowiak R.S.J. (1995). Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping , 2, 189–210. [Google Scholar]
- Gu X., Hof P.R., Friston K.J., Fan J. (2013). Anterior insular cortex and emotional awareness. The Journal of Comparative Neurology , 3388, 3371–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J., Harris J.M., McKirdy J.W., Johnstone E.C., Lawrie S.M. (2007). Emotional memory in schizophrenia. Neuropsychologia, 45(6), 1152–9. [DOI] [PubMed] [Google Scholar]
- Harrison N.A., Gray M.A., Gianaros P.J., Critchley H.D. (2010). The embodiment of emotional feelings in the brain. The Journal of Neuroscience , 30(38), 12878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P.O., Fossati P., Lepage M. (2007). Modulation of memory formation by stimulus content: specific role of the medial prefrontal cortex in the successful encoding of social pictures. Journal of Cognitive Neuroscience , 19(2), 351–62. [DOI] [PubMed] [Google Scholar]
- Herbener E.S. (2008). Emotional memory in schizophrenia. Schizophrenia Bulletin, 34(5), 875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E.J., Battaglia F.P., Atsak P., de Voogd L.D., Fernández G., Roozendaal B. (2014). How the amygdala affects emotional memory by altering brain network properties. Neurobiology of Learning and Memory , 112, 2–16. [DOI] [PubMed] [Google Scholar]
- James W. (1894). Discussion: the physical basis of emotion. Psychological Review , 1(5), 516–29. [DOI] [PubMed] [Google Scholar]
- Kensinger E.A., Corkin S. (2004). Two routes to emotional memory: distinct neural processes for valence and arousal. Proceedings of the National Academy of Sciences of the United States of America , 101(9), 3310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger E.A., Schacter D.L. (2006). Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. The Journal of Neuroscience , 26(9), 2564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger E.A., Schacter D.L. (2008). Neural processes supporting young and older adults’ emotional memories. Journal of Cognitive Neuroscience , 20(7), 1161–73. [DOI] [PubMed] [Google Scholar]
- Kensinger E.A., Garoff-Eaton R.J., Schacter D.L. (2007). How negative emotion enhances the visual specificity of a memory. Journal of Cognitive Neuroscience , 19(11), 1872–87. [DOI] [PubMed] [Google Scholar]
- Klavir O., Genud-Gabai R., Paz R. (2012). Low-frequency stimulation depresses the primate anterior-cingulate-cortex and prevents spontaneous recovery of aversive memories. The Journal of Neuroscience , 32(25), 8589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavir O., Genud-Gabai R., Paz R. (2013). Functional connectivity between amygdala and cingulate cortex for adaptive aversive learning. Neuron , 80(5), 1290–300. [DOI] [PubMed] [Google Scholar]
- Kryla-Lighthall N., Mather M. (2009). The role of cognitive control in older adults’ emotional well-being. In: Berngtson V., Gans D., Putney N., Silverstein M., editors. Handbook of Theories of Aging, 2nd edn New York, Springer Publishing, 323–44. [Google Scholar]
- LaBar K.S., Cabeza R. (2006). Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience , 7(1), 54–64. [DOI] [PubMed] [Google Scholar]
- Lane R.D. (2008). Neural substrates of implicit and explicit emotional processes: a unifying framework for psychosomatic medicine. Psychosomatic Medicine , 70(2), 214–31. [DOI] [PubMed] [Google Scholar]
- Lane R.D., Fink G.R., Chau P.M., Dolan R.J. (1997). Neural activation during selective attention to subjective emotional responses. Neuroreport , 8(18), 3969–72. [DOI] [PubMed] [Google Scholar]
- Lang P. J., Bradley M. M., Cuthbert B. N. (2008). International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical report A-8. University of Florida. [Google Scholar]
- LeDoux J. (2012). Rethinking the emotional brain. Neuron , 73(4), 653–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan M. (1993). Cognitive-Behavioral Treatment of Borderline Personality Disorder. New York, Guilford Press. [Google Scholar]
- Livneh U., Paz R. (2012). Amygdala-prefrontal synchronization underlies resistance to extinction of aversive memories. Neuron , 75(1), 133–42. [DOI] [PubMed] [Google Scholar]
- MacLeod C., McLaughlin K. (1995). Implicit and explicit memory bias in anxiety: a conceptual replication. Behaviour Research and Therapy, 33(1), 1–14. [DOI] [PubMed] [Google Scholar]
- Mathews A., Mogg K., May J., Eysenck M. (1989). Implicit and explicit memory bias in anxiety. Journal of Abnormal Psychology, 98(3), 236–40. [DOI] [PubMed] [Google Scholar]
- McRaea K., Reimana E.M., Forta C.L., Chene K., Lane R.D. (2008). Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal-dependent. NeuroImage , 41(2), 648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias M.L., Etkin A., Kalisch R. (2010). A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. NeuroImage , 49(2), 1760–8. [DOI] [PubMed] [Google Scholar]
- Medford N., Critchley H.D. (2010). Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure & Function , 214(5–6), 535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function , 214(5–6), 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley K.R., Kensinger E.A. (2008). Emotional valence influences the neural correlates associated with remembering and knowing. Cognitive, Affective, & Behavioral Neuroscience , 8(2), 143–52. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Quirk G.J. (2012). Fear extinction as a model for translational neuroscience: ten years of progress. Annual Review of Psychology , 63, 129–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M.I., Quirarte G.L., Rodriguez-Garcia G., McGaugh J.L., Roozendaal B. (2008). Glucocorticoids enhance taste aversion memory via actions in the insular cortex and basolateral amygdala. Learning & Memory , 15(7), 468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M.I., Sabath E., Nunez-Jaramillo L., Purón-Sierra L. (2011). β-Adrenergic receptors in the insular cortex are differentially involved in aversive vs. incidental context memory formation. Learning & Memory , 18(8), 502–7. [DOI] [PubMed] [Google Scholar]
- Murty V.P., Ritchey M., Adcock R.A., LaBar K.S. (2010). fMRI studies of successful emotional memory encoding: a quantitative meta-analysis. Neuropsychologia , 48(12), 3459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller K.A., Wagner A.D. (2002). Observing the transformation of experience into memory. Trends in Cognitive Sciences , 6(2), 93–102. [DOI] [PubMed] [Google Scholar]
- Pessoa L. (2005). To what extent are emotional visual stimuli processed without attention and awareness? Current Opinion in Neurobiology, 15(2), 188–96. [DOI] [PubMed] [Google Scholar]
- Pessoa L. (2009). How do emotion and motivation direct executive control? Trends in Cognitive Sciences, 13(4), 160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. (2010). Emergent processes in cognitive-emotional interactions. Dialogues in Clinical Neuroscience , 12(4), 433–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., McKenna M., Gutierrez E., Ungerleider L.G. (2002). Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences of the United States of America , 99(17), 11458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Taylor S.F., Welsh R.C., Ho S.H., Britton J.C., Liberzon I. (2004). Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. NeuroImage , 21(2), 768–80. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Wager T., Taylor S.F., Liberzon I. (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage , 16(2), 331–48. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron , 48(2), 175–87. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. (2003). Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry , 54(5), 504–14. [DOI] [PubMed] [Google Scholar]
- Pollatos O., Gramann K., Schandry R. (2007). Neural systems connecting interoceptive awareness and feelings. Human Brain Mapping , 28(1), 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Hermans E.J., van Marle H.J.F., Fernández G. (2012). Understanding low reliability of memories for neutral information encoded under stress: alterations in memory-related activation in the hippocampus and midbrain. The Journal of Neuroscience , 32(12), 4032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout N., Astell A., Reid I., Glen T., O’Carroll R. (2003). Memory bias for emotional facial expressions in major depression. Cognition & Emotion, 17(1), 101–22. [DOI] [PubMed] [Google Scholar]
- Ritchey M., LaBar K.S., Cabeza R. (2011). Level of processing modulates the neural correlates of emotional memory formation. Journal of Cognitive Neuroscience , 23(4), 757–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkheil P., Goebel R., Schneider F., Mathiak K. (2013). Emotion unfolded by motion: a role for parietal lobe in decoding dynamic facial expressions. Social Cognitive and Affective Neuroscience, 8(8), 950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W., Kochiyama T., Yoshikawa S., Matsumura M. (2001). Emotional expression boosts early visual processing of the face: ERP recording and its decomposition by independent component analysis. Neuroreport, 12(4), 709–14. [DOI] [PubMed] [Google Scholar]
- Satpute A.B., Shu J., Weber J., Roy M., Ochsner K.N. (2013). The functional neural architecture of self-reports. Biological Psychiatry , 73(7), 631–8. [DOI] [PubMed] [Google Scholar]
- Schachter S., Singer J.E. (1962). Cognitive, social, and physiological determinants of emotional state. Psychological Review, 69, 379–99. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience , 27(9), 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A.K. (2013). Interoceptive inference, emotion, and the embodied self. Trends in Cognitive Sciences , 17(11), 565–73. [DOI] [PubMed] [Google Scholar]
- Singer T., Critchley H.D., Preuschoff K. (2009). A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences , 13(8), 334–40. [DOI] [PubMed] [Google Scholar]
- Slotnick S.D., Moo L.R., Segal J.B., Hart J. (2003). Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research , 17(1), 75–82. [DOI] [PubMed] [Google Scholar]
- Steele J.D., Lawrie S.M. (2004). Segregation of cognitive and emotional function in the prefrontal cortex: a stereotactic meta-analysis. NeuroImage , 21(3), 868–75. [DOI] [PubMed] [Google Scholar]
- Tamietto M., de Gelder B. (2010). Neural bases of the non-conscious perception of emotional signals. Nature Reviews Neuroscience , 11(10), 697–709. [DOI] [PubMed] [Google Scholar]
- Tan H., Lauzon N.M., Bishop S.F., Bechard M.A., Laviolette S.R. (2010). Integrated cannabinoid CB1 receptor transmission within the amygdala-prefrontal cortical pathway modulates neuronal plasticity and emotional memory encoding. Cerebral Cortex , 20(6), 1486–96. [DOI] [PubMed] [Google Scholar]
- Tan H., Lauzon N.M., Bishop S.F., Chi N., Bechard M., Laviolette S.R. (2011). Cannabinoid transmission in the basolateral amygdala modulates fear memory formation via functional inputs to the prelimbic cortex. The Journal of Neuroscience , 31(14), 5300–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.F., Phan K.L., Decker L.R., Liberzon I. (2003). Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage , 18(3), 650–9. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N., Adolphs R. (2007). Emotion and consciousness. Trends in Cognitive Sciences , 11(4), 158–67. [DOI] [PubMed] [Google Scholar]
- Uematsu A., Kitamura A., Iwatsuki K., Uneyama H., Tsurugizawa T. (2014). Correlation between activation of prelimbic cortex, the basolateral amygdala, and agranular insular cortex during taste memory formation. Cerebral Cortex, 25(9), 2719–28. [DOI] [PubMed] [Google Scholar]
- Vogt B.A., Berger G.R., Derbyshire S.W.G. (2003). Structural and functional dichotomy of human midcingulate cortex. European Journal of Neuroscience , 18(11), 3134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P., Huang Y.M. (2009). Emotional attention: uncovering the mechanisms of affective biases in perception. Current Directions in Psychological Science , 18(3), 148–52. [Google Scholar]
- Vuilleumier P., Armony J.L., Driver J., Dolan R.J. (2001). Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron , 30(3), 829–41. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Armony J.L., Driver J., Dolan R.J. (2003). Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nature Neuroscience, 6(6), 624–31. [DOI] [PubMed] [Google Scholar]
- Walker W.R., Skowronski J.J., Thompson C.P. (2003). Life is pleasant—and memory helps to keep it that way!. Review of General Psychology, 7(2), 203–10. [Google Scholar]
- Waringa J.D., Kensingera E.A. (2012). How emotion leads to selective memory: neuroimaging evidence. Neuropsychologia , 49(7), 1831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston J., O’Doherty J., Dolan R. (2003). Common and distinct neural responses during direct and incidental processing of multiple facial emotions. NeuroImage , 20(1), 84–97. [DOI] [PubMed] [Google Scholar]
- Winter D., Elzinga B., Schmahl C. (2014). Emotions and memory in borderline personality disorder. Psychopathology, 47(2), 71–85. [DOI] [PubMed] [Google Scholar]
- Zaki J., Davis J.I., Ochsner K.N. (2012). Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage , 62(1), 493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]