Abstract
Aberrant brain reward responses to food-related cues are an implied characteristic of human obesity; yet, findings are inconsistent. To explain these inconsistencies, we aimed to uncover endophenotypes associated with heterogeneity in attributing incentive salience to food cues in the context of other emotionally salient cues; a phenomenon described as sign- vs goal tracking in preclinical models. Data from 64 lean and 88 obese adults who were 35.5 ± 9.4 years old and predominantly women (79%) were analyzed. Participants viewed food-related, pleasant, neutral and unpleasant images while recording electroencephalograph. Late positive potentials were used to assess incentive salience attributed to the visual stimuli. Eating and affective traits were also assessed. Findings demonstrated that obese individuals, in general, do not demonstrate aberrant brain reward responses to food-related cues. As hypothesized, latent profile analysis of the late positive potential uncovered two distinct groups. ‘Sign-trackers’ showed greater responses to food-related cues (P < 0.001) but lower responses to pleasant stimuli (P < 0.001) compared with ‘goal-trackers’. There were proportionally more obese than lean ‘sign-trackers’ (P = 0.03). Obese ‘sign-trackers’ reported significantly higher levels of emotional eating and food craving (P < 0.001). By examining the heterogeneity in brain reactivity to various emotional stimuli, this translational study highlights the need to consider important neurobehavioral endophenotypes of obesity.
Keywords: obesity, reward, brain, cue-reactivity, event-related potentials, humans
Introduction
Currently, 35% of the US adult population is obese (Ogden et al., 2014). Despite health promotion efforts, the prevalence of obesity and its related comorbidities has continued to rise (Hursting et al., 2007; World Cancer Research Fund/American Institute for Cancer, 2007; Basen-Engquist and Chang, 2011). Neurobehavioral models of obesity suggest that some individuals have difficulty controlling food intake partly because they attribute excessive incentive salience to stimuli signaling food availability (Wang et al., 2004; Berridge et al., 2010; Berthoud, 2012). Incentive salience refers to the motivational properties that make a stimulus wanted (Robinson et al., 2014). Stimuli with high incentive salience capture attention, activate affective states and motivate behaviors (Berridge et al., 2010; Johnson, 2013). Although enhanced reward responses to food-related stimuli are an implied characteristic of obesity, findings in humans are inconsistent (Nijs et al. 2008; Stice et al., 2009; Nijs and Franken, 2012). We argue here that these inconsistencies may be study design related. When brain responses to food-related images are greater than to traditionally used neutral images in obese vs lean individuals, the finding is often (over)interpreted as being an abnormally high or an aberrant response to food-related stimuli. Rather, findings such as these suggest only that images of food are emotionally valued as more pleasant (greater valence and/or arousal) than neutral cues in obese vs lean individuals. Without measuring brain responses to non-food-related emotional stimuli, it is therefore impossible to know if an enhanced reward response to food is not simply representing higher sensitivity to rewards in general (Versace and Schembre, 2015). Hence, the first goal of this study was to fill this knowledge gap and assess brain responses to neutral, food-related, pleasant and unpleasant emotional images in obese and lean individuals.
The second aim of this study was to test the hypothesis that heterogeneity in brain reactivity to food-related and other intrinsically pleasant stimuli would uncover the presence of two human endophenotypes associated with individual differences in the propensity to attribute incentive salience to food-related cues. Recent preclinical findings indicate that during Pavlovian conditioning, rats show large behavioral differences in the presence of cues predicting rewards. When a discrete neutral stimulus (the ‘sign’ or conditioned stimulus) is paired with food delivery (the ‘goal’ or unconditioned stimulus), all animals learn the conditioned-unconditioned stimulus contingency, but only a subgroup (‘sign-trackers’) attributes incentive salience to the conditioned stimulus. Behaviorally, sign-trackers approach and interact with the conditioned stimulus as though it is the unconditioned stimulus itself (Tomie et al. 2008; Meyer et al., 2012). Importantly, among sign trackers, the presence of the conditioned stimulus also triggers compulsive behaviors that have striking similarities with human behaviors associated with addiction and, possibly, obesity (Tomie et al. 2008; Yager and Robinson, 2010; Lovic et al., 2011; Meyer et al., 2012; Saunders and Robinson, 2013; Robinson et al., 2015). Recent findings from our smoking cessation laboratory (Versace et al., 2012, 2014) and others (Mahler and de Wit, 2010; Styn et al., 2013) suggest that humans may also be characterized by significant differences in the propensity to attribute incentive salience to reward-related stimuli and that heterogeneity along this psychological trait influences the expression of compulsive behaviors.
In this study, we hypothesize that the human endophenotype that reflects sign-tracking would be characterized by enhanced brain responses to food-related cues (i.e. images of high fat/high sugar foods) relative to other natural rewards, whereas the human endophenotype that reflects goal tracking would be characterized by lower brain responses to food-related cues relative to other natural rewards. Showing that humans, lean and obese, may be characterized by individual differences in the propensity to attribute incentive salience to food-related stimuli relative to other reward-related stimuli could have profound theoretical and clinical significance. These findings will represent one of the first steps toward developing and testing new, and hopefully more effective and personalized, weight control interventions that aim to rebalance brain reward responses to food-related and other non-food-related intrinsic rewards.
Materials and Methods
Participants
We enrolled 152 participants (64 lean, 88 obese) into the study from the Harris county/Houston metropolitan area. Participants were included in the study if they were between the ages of 18 and 55 years, were able to speak English and had access to a telephone. Recruitment was limited to lean and obese individuals. Specifically, lean participants had to have a current measured body mass index (BMI) < 25.0 kg/m2 and a reported maximum adult weight and height that resulted in a BMI < 25.0 kg/m2. Similarly, obese participants had to have a BMI > 29.9 kg/m2.
Recruitment was stratified by weight status with an enrollment goal of 30% men. A total of 724 were screened for eligibility via telephone or in-person screening interviews. In total, 531 individuals were excluded from the study for not meeting eligibility criteria or having met recruitment goals for obese individuals and/or women. An additional 46 individuals were eligible but were not enrolled in the study due to scheduling issues, resulting in an analytical sample of N = 152.
Procedures
The study included an eligibility screening interview and one in-person laboratory visit. Information about age, height, weight, weight loss history, medical history, including history of diagnosed psychiatric disorders, eating disorders and diet-related chronic diseases, drug use and pregnancy or lactation status was collected as part of the screening procedures. Eligible individuals were scheduled for an in-person visit.1
At the in-person visit, informed consent was obtained. Participants were told that the purpose of the study was to learn more about how attention and reaction to food-related and non-food-related images might differ between people with different weight histories. It was further explained to them that researchers wanted to know if patterns in brain activity may be related to body weight. Once consent was obtained, BMI status was confirmed by measuring weight and height in light clothing without shoes. Drug use and pregnancy status were confirmed by urine test, and participants completed a series of well-validated questionnaires about eating behaviors, mood and hedonic capacity. When the questionnaires were completed, sensors for the electroencephalographic (EEG) assessment were placed and the EEG session started. Participants were asked to passively watch a slide show of different types of images such as pictures of people and things, pictures of food, violent and erotic pictures on the computer screen. Study procedures were approved by the MD Anderson IRB.
The EEG was continuously recorded using a 129-channel Geodesic Sensor Net, amplified with an AC-coupled high input impedance (200 MΩ) amplifier (Geodesic EEG System 200; Electrical Geodesics Inc., Eugene, OR) and referenced to Cz. The sampling rate was 250 Hz, and data were filtered online by using 0.1 Hz high-pass and 100 Hz low-pass filters. Scalp impedance of each sensor was kept below 50 KΩ, as suggested by the manufacturer. After the EEG assessment, participants rated the pictures viewed during the session using a computerized version of the self-assessment manikin (see Supplementary Material) (Bradley and Lang, 1994). At the conclusion of the self-assessment manikin rating procedure, the participant was debriefed and compensated for their participation.
Picture-viewing task
The images used in the study were selected from the International Affective Picture System (IAPS) (Lang et al., 2008), from a food-related picture collection that we created downloading images from the internet and from another library of food images (Miccoli et al., 2014). The images used for the study belonged to eight categories (Erotica, Romantic, Food, Neutral, Neutral Objects, Pollution, Attack, Mutilations) with 16 pictures in each category (see Supplementary Material for the IAPS numbers). Food-related pictures were of high fat and/or high sugar foods (e.g. French fries, pizza, ice cream and doughnuts). During the EEG session, the images were presented in pseudo-random sequences (no more than two consecutive pictures of the same category). Pictures were presented for 4 s followed by 3–5 s random intertrial intervals showing a white fixation cross against a black background. The entire picture presentation lasted approximately 20 min. Each session was divided into 4 equivalent 5-min blocks separated by 30-s intervals, during which the participant was instructed to relax. During picture presentation, 25% of the slides in each category were startle probed by presenting a burst of 100 dB white noise for 50 ms between 2.5 and 3.5 s after picture onset. Since the late positive potential (LPP) peaks between 400 and 700 ms after picture onset, the presentation of the startle probes could not affect the results that we were interested in and that we present here. Stimulus presentation was controlled by a Pentium 4 computer using Psychology Tools’ E-prime software (version 1.4; Pittsburgh, PA) on a plasma screen placed approximately 1.5 m from the participant’s eyes. The images subtended approximately a 24° horizontal viewing angle.
Maladaptive eating behavior questionnaires
Weight-related eating questionnaire
The 16-item Weight-Related Eating Questionnaire (WREQ) assesses four theory-based aspects of eating behavior labeled compensatory restraint, routine restraint, susceptibility to external cues and emotional eating (Schembre et al., 2009) and has been validated in a diverse sample of males and females confirming its unbiased generalizability across gender, age, race and BMI subgroups (Schembre and Geller, 2011).
Power of food scale
The power of food scale (Lowe et al., 2009) is a 15-item questionnaire designed to assess individual differences in appetitive motivation toward highly palatable foods. The questionnaire has been used widely and demonstrates adequate internal consistency and test–retest reliability (Cappelleri et al., 2009; Lowe et al., 2009).
General food cravings questionnaire—trait
The modified, 21-item version of the General Food Cravings Questionnaire assesses ‘craving for food in general (total score)’ using four factors: preoccupation with food, loss of control, positive outcome expectancy and emotional craving (Nijs et al., 2007).
Mood and affect questionnaires
Center for epidemiologic studies depression scale
The Center for Epidemiologic Studies Depression Scale is a 20-item self-report instrument assessing the frequency of several depressive symptoms and it was originally developed for studying depressive symptomatology in the general population (Radloff, 1977).
Positive and negative affect schedule
The Positive and Negative Affect Schedule is a 20-item self-report instrument designed to measure the two primary measures of mood: positive and negative affect (Watson et al., 1988). This instrument is a reliable and valid measure of the two mood constructs (Crawford and Henry, 2004).
Snaith–Hamilton pleasure scale
The Snaith–Hamilton Pleasure Scale is a self-report measure of anhedonia that, unlike other instruments, was specifically developed to be unaffected by social class, gender, age, dietary habits or nationality (Snaith et al., 1995). The Snaith–Hamilton Pleasure Scale is a reliable and valid questionnaire to assess hedonic tone in patient and non-patient populations (Franken et al., 2007).
EEG data reduction
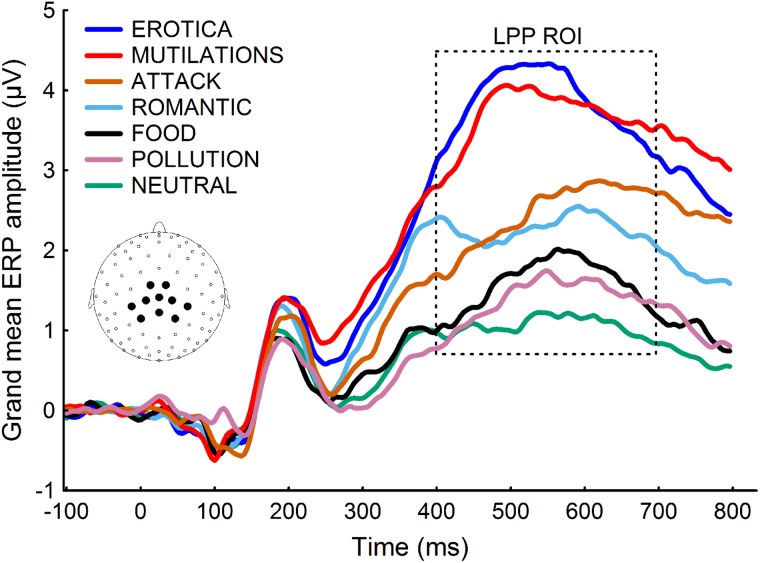
After EEG data collection, a 30-Hz low-pass filter was applied, EEG traces were visually inspected and channels contaminated by artifacts for more than 50% of the recording time (∼2% of the channels) were interpolated using spherical splines. Eye blinks were then corrected by using a spatial filtering method as implemented in BESA ver. 5.1.8.10 (MEGIS Software GmbH, Gräfelfing, Germany). After eye blink correction, the EEG data were transformed to the average reference and segmented into 900-ms segments starting 100 ms before onset of the picture. Baseline was defined as the 100-ms interval preceding the picture. Artifacts affecting sensors within each trial were identified using the following criteria: EEG amplitude above 100 or below –100 μV; absolute voltage difference between any two data points within the segment larger than 100 μV; voltage difference between two contiguous data points above 25 μV and less than 0.5 μV variation for more than 100 ms. A segment was excluded from the subsequent averages if more than 10% of the sensors within the segment were contaminated by artifacts. Overall, fewer than 5% of the segments were excluded. At the end of this process, the average event-related potentials (ERP) were calculated at each scalp site for each category. In line with standard procedures (Cuthbert et al., 2000; Schupp et al., 2000; Keil et al., 2002; Bradley, 2009; Lang and Bradley, 2010; Versace et al., 2011; Liu et al., 2012; Minnix et al., 2013), we used the amplitude of the LPP as a measure of motivational salience. The LLP for each category was calculated by averaging the voltage recorded between 400 and 700 ms after picture onset from 10 central and parietal sensors. This group of sensors covers the area where the LPP differences between neutral and emotional pictures peak (see inset of Figure 1 for electrode location). A preliminary analysis showed that the amplitude of the LPPs for neutral stimuli depicting objects or people was comparable; hence we decided to collapse the two neutral categories together.
Fig. 1.
The time course of the ERP grand averages for each category of stimuli demonstrates a significant main effect of image category (F(6, 145) = 55.84, P < 0.0001; Wilks’ λ = 0.30). The waveforms represent grand averages from 10 electrodes (see inset for electrode location). The box indicates the time window used to calculate the LPP amplitude for each picture category.
Statistical analyses
Characterization of brain reward responses to emotional stimuli in lean and obese
Analysis of variance (ANOVA) was used to achieve the first objective. We used the amplitude of the LPP as dependent variable in a multivariate ANOVA (Vasey and Thayer, 1987) with BMI status (obese vs lean) as a between-subjects factor and image category (i.e. erotica, romantic, food, neutral, pollution, attack and mutilations) as a within-subject factor. Significant main effects and interactions were followed up by post-hoc pairwise comparisons Bonferroni corrected to control for type I error rate.
Heterogeneity in brain reactivity to food-related and other intrinsically pleasant stimuli
The second objective of this study was to test the hypothesis that human sign- and goal-tracking endophenotypes can be determined on the basis of specific patterns of brain activity evoked by food-related and other intrinsically pleasant stimuli. To achieve this objective, first we used latent profile analysis (LPA) to classify individuals into clusters based on their brain responses to the stimuli presented during the experiment (i.e. food-related, pleasant, neutral and unpleasant) and then we examined the nature of the between clusters differences using a three-way ANOVA with cluster and BMI status as between-subjects factors and image category as within-subjects factor (erotica, romantic, food-related, neutral, pollution, attack, mutilations). Before LPA, we standardized the LPP values to account for individual variation in absolute voltage amplitude using ipsatization (Hicks, 1970). LPA was applied on the ipsatized data using maximum likelihood estimation to derive model parameters (Muthén and Muthén, 1998-2007). Only the LPP values were entered into LPA. Best fit was determined by Bayesian information criterion (BIC) values, Lo–Mendell–Rubin (LMR) likelihood ratio tests and entropy values (Nylund et al., 2007). Lower BIC values usually indicate better models. LMR likelihood ratio tests provide P values to evaluate whether a model with k − 1 profiles should be rejected in favor of a model with k profiles. Entropy values higher than 0.8 indicate well-defined profiles. To avoid the problem of local maxima (i.e. chance selection of a suboptimal solution), we conducted the analyses for each model with 500 random sets of start values and increased the default to 100 iterations for these random starts and retained the 50 best solutions for final stage optimization (McLachlan and Peel, 2000) to ensure that the best log likelihood value was adequately replicated.
Questionnaires
Questionnaire results were analyzed using a cluster-by-BMI status multivariate ANOVA. Significant main effects and interactions were followed up by post-hoc pairwise comparisons Bonferroni corrected to control for type I error rate.
Results
Sample characteristics
Table 1 lists the characteristics of the sample. Obese participants (N = 88) were comparable to lean participants (N = 64) in every aspect except for age and race (P < 0.001). When including age and race as covariates in our analyses, results did not change. The addition of sex as a covariate also did not change our results. As such, we present findings from the unadjusted analyses.
Table 1.
Participant characteristics by BMI status
| Characteristic | All | Lean | Obese | P value |
|---|---|---|---|---|
| (n = 64) | (n = 88) | |||
| Age (y) | 35.5 ± 9.4 | 30.4 ± 8.6 | 39.2 ± 8.2 | <0.001 |
| Women | 78.9% | 76.6% | 80.7% | 0.593 |
| Hispanic | 17.1% | 21.9% | 13.6% | 0.183 |
| Race | <0.001 | |||
| Black | 46.1% | 17.2% | 67.0% | |
| White | 32.9% | 43.8% | 25.0% | |
| Asian | 17.1% | 29.7% | 8.0% | |
| Other | 2.6% | 6.3% | 0% | |
| Not reported | 1.3% | 3.1% | 0% |
Note. P values estimated by independent t-tests and chi-square analyses.
Comparison of ERPs to food-related and other emotional stimuli in lean and obese individuals
Figure 1 shows the time course of the ERP grand averages for each stimulus category and the time region of interest (400–700 ms) used in the subsequent statistical analyses. The MANOVA led to a significant main effect of image category (P < 0.001).
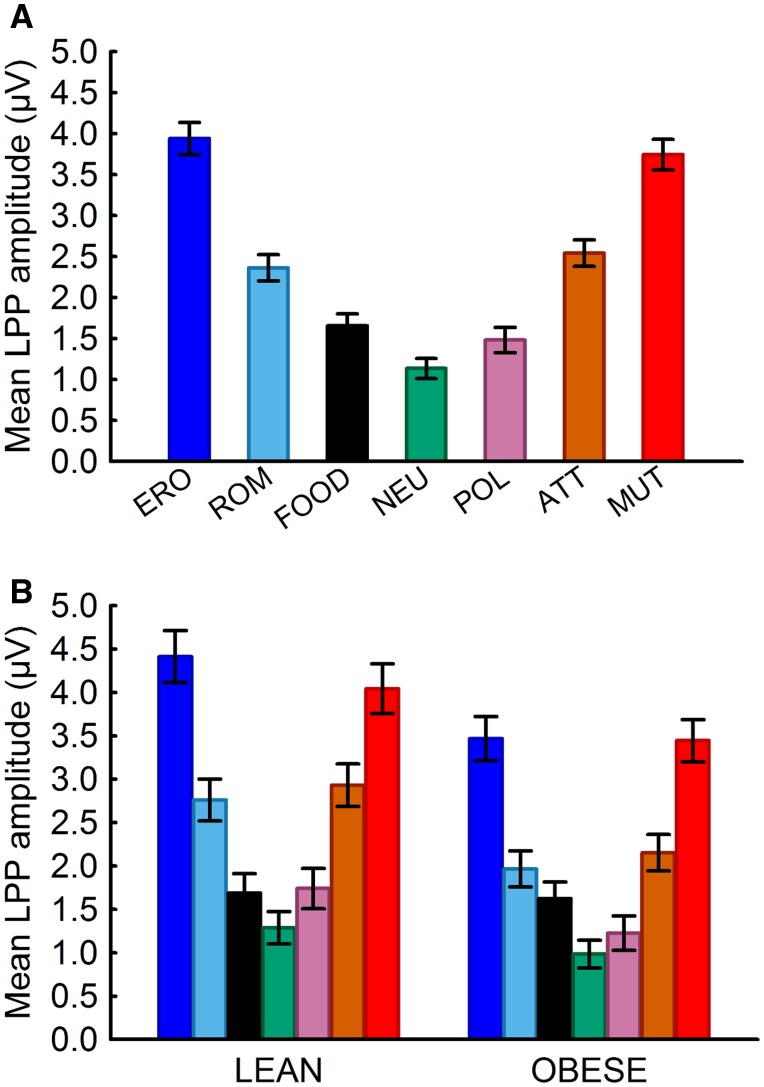
In the sample as a whole, the amplitude of the LPP increased as a function of the motivational salience of the images (Figure 2A). The LPP evoked by food-related images was significantly larger than the LPP evoked by neutral images (P < 0.05) but significantly smaller (all P < 0.002) than the LPP evoked by all other emotional images except ‘pollution’.
Fig. 2.
(A) LPPs (400–700 ms post stimulus onset) evoked at centroparietal sensors increased as a function of the positive and negative motivational salience of the images in the sample as a whole. Note: ERO, erotica; ROM, romance; NEU, neutral; POL, pollution; ATT, attack; MUT, mutilations. (B) Overall, lean and obese individuals had similar response patterns across all image categories (Fint(6, 145) = 1.26, Pint = 0.28; Wilks’ λ = 0.95). Pairwise comparisons further reveal that obese individuals more so than lean individuals have greater brain reward responses to food-related cues relative to neutral cues (P < 0.001 and P = 0.061, respectively) but that brain responses evoked by food-related images are lower than or do not exceed responses to low arousing, pleasant images in lean (P < 0.001) or obese individuals (P = 0.12).
By weight status, Figure 2B shows that both lean and obese individuals had similar response patterns across all categories (Pint = 0.28). Considering the theoretical relevance of this interaction, we decided to explore the reactivity patterns in each the two groups, even in the absence of a significant finding. Importantly in both groups, on average, food-related stimuli prompted low amplitude LPPs, more similar to low arousing than high arousing emotional stimuli. Uncorrected pairwise comparisons showed that among lean individuals, food-related cues elicit responses at a magnitude similar to neutral cues (P = 0.061) and lower than low arousing pleasant stimuli (P < 0.001), whereas among obese individuals, food-related cues elicit responses at a magnitude greater than neutral cues (P < 0.001) and more similar to low arousing stimuli (P = 0.12).
Latent profile analysis
We extracted up to five profiles using LPA. The fit indices for the 2–5-profile solutions are reported in Table 2. As expected, the values for BIC continued to decrease with the addition of profiles (Petras and Masyn, 2009). All models reported very good classification quality (entropy > 0.8); however, the likelihood ratio test indicated that the 2-profile solution is significantly better than the 1-profile solution. The LPA algorithm assigned 32% of the sample (n = 49) to Cluster 1 and 68% (n = 103) to Cluster 2.
Table 2.
Fit indices for LPA
| K profiles | LL | BIC | Profile proportions | Entropy | LMR |
|---|---|---|---|---|---|
| 2 | −561.36 | 1188.03 | 1: 32% | 0.877 | 0.008 |
| 2: 68% | |||||
| 3 | −521.48 | 1133.39 | 1: 45% | 0.853 | 0.127 |
| 2: 29% | |||||
| 3: 26% | |||||
| 4 | −489.10 | 1093.75 | 1: 41% | 0.895 | 0.117 |
| 2: 26% | |||||
| 3: 22% | |||||
| 4: 11% | |||||
| 5 | −459.55 | 1059.77 | 1: 6% | 0.914 | 0.333 |
| 2: 24% | |||||
| 3: 25% | |||||
| 4: 38% | |||||
| 5: 7% |
Note. k, number of latent profiles in the model; LL, model loglikelihood; BIC, Bayesian information criterion; LMR, Lo–Mendell–Rubin likelihood ratio test.
Comparison of ERPs to food-related and other emotional stimuli by cluster assignment
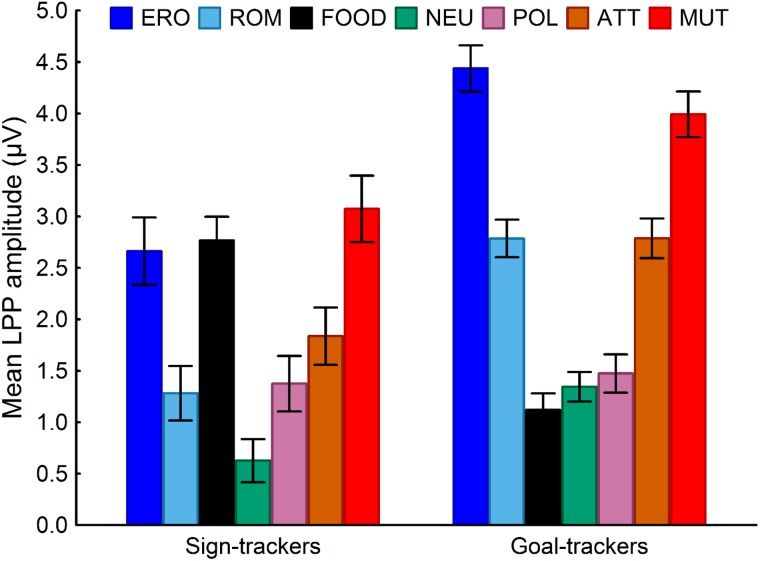
Participants assigned to Cluster 1 vs Cluster 2, as depicted in Figure 3, were characterized by significantly higher LPP responses to food-related stimuli (P < 0.001) and significantly blunted LPP responses to both high and low arousing pleasant stimuli (P < 0.001). No other between-cluster difference was significant (all P values > 0.40). From now on, we will refer to individuals in Cluster 1 as ‘sign-trackers’ and individuals in Cluster 2 as ‘goal-trackers’. Sign trackers showed LPP responses to food-related stimuli that were of the same magnitude of the LPP responses to other emotional highly arousing stimuli, whereas goal trackers showed LPP responses to food-related stimuli that were comparable to those evoked by neutral images. Within the two clusters, obese and lean individuals did not differ in their reactivity to any stimulus category (Pint = 0.67). Finally, the classification based on brain reactivity profiles showed significant predictive validity as far as separating lean from obese individuals: both clusters included obese and lean individuals, but, based on chi-square analysis, obese individuals were classified as sign trackers, with a significantly higher probability than lean individuals (P < 0.05). The higher proportion of obese individuals classified as sign trackers (40% vs 23% of the lean sample) can explain why, when only BMI is taken into account, obese individuals seem to show higher reactivity to food-related cues relative to lean individuals.
Fig. 3.
Brain reactivity to food and other emotional stimuli in sign trackers and goal trackers. The amplitude of the LPP to emotional stimuli uncovers endophenotypes associated with individual differences in the propensity to attribute incentive salience to reward-related stimuli: sign trackers react more to food-related stimuli than to pleasant stimuli (P < 0.005), goal trackers have the opposite pattern (P < 0.001). Note: ERO, erotica; ROM, romance; NEU, neutral; POL, pollution; ATT, attack; MUT, mutilations.
Interaction of cluster and BMI on maladaptive eating behaviors and mood questionnaires.
Consistent with our ERP findings, significant cluster-by-BMI interactions were detected for WREQ Emotional Eating (Pint = 0.035) and total Food Craving (Pint = 0.016) as well as two subscales of Food Craving: Loss of Control (Pint = 0.038) and Positive Outcome Expectancy (Pint = 0.010). Post-hoc analyses of the significant interaction effects indicated that obese individuals classified as sign trackers (high responders to food-related cues; see Figure 3) have a greater propensity for maladaptive eating behavior as evidenced by higher scores for emotional eating (P < 0.001) and total food cravings (P = 0.001) as reflected by greater feelings of loss of control (P < 0.001) and positive outcome expectancies (P = 0.007).
Analyses conducted on the mood questionnaires did not yield any significant main effect or interaction.
Discussion
Enhanced reward responses to food-related stimuli are an implied characteristic of obesity, but when tested empirically, this hypothesis is not consistently supported (Nijs et al., 2008; Stice et al., 2009; Nijs and Franken, 2012). We have suggested two plausible reasons for these inconsistencies (Versace and Schembre, 2015). The first is methodological; selecting the most appropriate control condition (i.e. other pleasant stimuli) to evaluate reactivity to food-related stimuli. The second is due to the heterogeneity of brain reward responses to food-related stimuli vs other pleasant stimuli; a neurobehavioral phenomenon only recently described in humans that we think reflects two endophenotypes associated with individual differences in the propensity to attribute incentive salience to rewards and reward-related cues. While some individuals (the ‘goal-trackers’) react to food-related cues as though they are neutral stimuli, others (the ‘sign-trackers’) react to food-related images as though they are highly arousing pleasant stimuli. The observation of these endophenotypes is consistent with our current research and with others’ in both human and animal models (Mahler and de Wit, 2010; Versace et al., 2012, 2014; Styn et al., 2013; Robinson et al., 2015). We hypothesized that both lean and obese individuals would demonstrate sign- and goal-tracking patterns of cue reactivity. Our findings supported this hypothesis; within goal- and sign-tracking groups, lean and obese individuals had comparable brain reactivity patterns (as evidenced by a non-significant three way interaction; P = 0.67). We also observed that a higher proportion of obese individuals was classified as sign trackers (40% of the obese vs 23% of the lean sample) suggesting that the sign-tracking endophenotype is more common among obese individuals. We think these findings can explain why, when we only considered BMI as a between-subjects variable, obese individuals showed somewhat higher reactivity to food-related cues than lean individuals (Figure 2B). Small differences in the proportion of sign- and goal trackers recruited into previous studies can also explain the inconsistent findings between lean and obese individuals in the previously published literature (Nijs et al., 2008; Stice et al., 2009; Nijs and Franken, 2012; Hendrikse et al., 2015). Collectively, our results challenge the general belief that obesity is characterized by the abnormally high attribution of incentive salience to highly palatable food cues, highlighting the importance of considering alternative study paradigms that incorporate other emotionally relevant stimuli rather than neutral stimuli as a ‘control’ condition and demonstrating the need to consider important neurobehavioral endophenotypes of obesity.
As mentioned above, there were proportionately more obese than lean ‘sign-trackers’ in our sample, and these individuals reported greater susceptibility to maladaptive eating than their lean ‘sign-tracker’ counterparts. Our observation that a substantial proportion of lean individuals in our sample were also classified as ‘sign-trackers’ suggests that neurobehavioral processes, other than enhanced brain reward responses to food-related cues, such as trait impulsivity, affect regulation expectancies, genetics or the environment (Stice et al., 2009), likely play a significant role in models of obesity. Significant within-group variability in brain activity to salient emotional cues has been observed in other contexts (e.g. substance users vs non-users) though results have not been published widely (Volkow, personal communication). In the context of obesity, the observation of incongruences between behavior and weight status have been described as early as the 1970s [‘latent obesity’ among lean individuals (Meyer and Pudel, 1977)]. More recently, other models of dual reward prediction, including preparatory/anticipatory vs consummatory responses, have been applied to the context of obesity (Stice et al., 2009); however, we are unaware of literature examining within-group heterogeneity in reward responses to food cues in lean and obese individuals. As such, these data lay the foundation for future work to determine the neural correlates underlying sign-tracking vs goal tracking, to explore variability in reward responses to food intake (i.e. consummatory responses) vs food-related cues (i.e. anticipatory responses) and to shift our focus to potentially more influential neurobehavioral processes that interact with or operate independently from approach responses to food cues and food intake (Herman and Polivy, 2008).
Despite the previously noted strengths of this study, it is not without its limitations. Our sample was gender imbalanced. Previous research has shown differences in response to food cues between males and females (Geliebter et al., 2013; Atalayer et al., 2014). Though we did not have the sample size to stratify our analyses by sex, we found no difference in the proportion of men and women classified as sign trackers and goal trackers (P = 0.52) and, as previously stated, the inclusion of sex into our analyses did not influence our results. Additionally, this study focused only on the brain responses to food cues not responses to food intake or on consummatory behavior. We are therefore limited to interpreting our findings within the context of cue-related responses. Lastly, without longitudinal data, we are unable to make causal inferences regarding the role of sign-tracking on the development of obesity.
In conclusion, our results clearly indicate that, under standard laboratory conditions, obese individuals do not respond to the presence of food-related stimuli with abnormally high brain responses. On average, obese and lean individuals reacted similarly to both emotional and food-related images. Furthermore, our procedure identified a subgroup of individuals, lean and obese, who were characterized by high reactivity to food-related cues and blunted reactivity to pleasant stimuli. This observation is consistent with preclinical models of ‘sign-tracking’ and ‘goal-tracking’, highlighting the two important endophenotypes corresponding to high vs low propensity to attribute incentive salience to food-related cues relative to other pleasant stimuli that might increase susceptibility to overeating and, ultimately, obesity. This is the first time that such an endophenotype of obesity has been observed in humans. Future work will be dedicated to exploring the underlying neural correlates and behavioral traits (e.g. impulsivity and cognitive control) of lean and obese ‘sign-trackers’ vs ‘goal-trackers’ in an effort to develop new and more effective weight control that incorporate strategies aimed to reduced brain reward responses to food-related stimuli while increasing reward activity to other intrinsically pleasant rewards.
Endnote
Participants were requested to arrive for their in-person visit in a comfortably full state. Fullness was confirmed by the Satiety Labeled Intensity Magnitude (Cardello et al., 2005) in an effort to measure hedonic vs homeostatic responses to food-related stimuli. In the event participants reported being at least ‘slightly hungry’, a snack was provided. The snack was provided to 14 participants.
Supplementary Material
Acknowledgements
Study protocols were implemented by Drs Versace’s and Schembre’s research teams that included Danika Dirba, Troy Gilchrist, Kristin Cortese, Jennifer Ng, Aurelija Slapin and Kimberly Claiborne. This research has been previously presented as posters at the annual meetings of the Society for Psychophysiological Research and The Obesity Society in 2014. Drs Versace, Kypriotakis, Basen-Engquist and Schembre report no biomedical financial interests or potential conflicts of interest.
Funding
This work was supported by the NIH/NCI under award number P30CA016672, the Chandler Cox Foundation, a University of Texas MD Anderson Cancer Center Basic Science Institutional Research Grant, the Center for Energy Balance in Cancer Prevention and Survivorship and the Duncan Family Institute Bionutrition Research Core.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Atalayer D., Pantazatos S.P., Gibson C.D., et al. (2014). Sexually dimorphic functional connectivity in response to high vs. low energy-dense food cues in obese humans: an fMRI study. NeuroImage, 100, 405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basen-Engquist K., Chang M. (2011). Obesity and cancer risk: recent review and evidence. Current Oncology Reports, 13(1), 71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Ho C.Y., Richard J.M., Difeliceantonio A.G. (2010). The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Research, 1350, 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H.-R. (2012). The neurobiology of food intake in an obesogenic environment. Proceedings of the Nutrition Society, 71(04), 478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M., Lang P.J. (1994). Measuring emotion: the self-assessment semantic differential manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25(I), 49–59. [DOI] [PubMed] [Google Scholar]
- Bradley M.M. (2009). Natural selective attention: orienting and emotion. Psychophysiology, 46(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelleri J.C., Bushmakin A.G., Gerber R.A., et al. (2009). Evaluating the power of food scale in obese subjects and a general sample of individuals: development and measurement properties. International Journal of Obesity, 33(8), 913–22. [DOI] [PubMed] [Google Scholar]
- Cardello A.V., Schutz H.G., Lesher L.L., Merrill E. (2005). Development and testing of a labeled magnitude scale of perceived satiety. Appetite, 44(1), 1–13. [DOI] [PubMed] [Google Scholar]
- Crawford J.R., Henry J.D. (2004). The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. The British Journal of Clinical Psychology/the British Psychological Society, 43(Pt 3), 245–65. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Schupp H.T., Bradley M.M., Birbaumer N., Lang P.J. (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. [DOI] [PubMed] [Google Scholar]
- Franken I.H.A., Rassin E., Muris P. (2007). The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS). Journal of Affective Disorders, 99, 83–9. [DOI] [PubMed] [Google Scholar]
- Geliebter A., Pantazatos S.P., McOuatt H., Puma L., Gibson C.D., Atalayer D. (2013). Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behavioural Brain Research, 243(1), 91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrikse J.J., Cachia R.L., Kothe E.J., McPhie S., Skouteris H., Hayden M.J. (2015). Attentional biases for food cues in overweight and individuals with obesity: a systematic review of the literature. Obesity Reviews, 16, 424–32. [DOI] [PubMed] [Google Scholar]
- Herman C.P., Polivy J. (2008). External cues in the control of food intake in humans: the sensory-normative distinction. Physiology and Behavior, 94(5), 722–8. [DOI] [PubMed] [Google Scholar]
- Hicks L.E. (1970). Some properties of ipsative, normative, and forced-choice normative measures. Psychological Bulletin, 74(3), 167–84. [Google Scholar]
- Hursting S.D., Lashinger L.M., Colbert L.H., et al. (2007). Energy balance and carcinogenesis: underlying pathways and targets for intervention. Current Cancer Drug Targets, 7(5), 484–91. [DOI] [PubMed] [Google Scholar]
- Johnson A.W. (2013). Eating beyond metabolic need: how environmental cues influence feeding behavior. Trends in Neurosciences, 36(2), 101–9. [DOI] [PubMed] [Google Scholar]
- Keil A., Bradley M.M., Hauk O., Rockstroh B., Elbert T., Lang P.J. (2002). Large-scale neural correlates of affective picture processing. Psychophysiology, 39(5), 641–9. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M. (2010). Emotion and the motivational brain. Biological Psychology, 84(3), 437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida, Gainesville, FL. [Google Scholar]
- Liu Y., Huang H., McGinnis-Deweese M., Keil A., Ding M. (2012). Neural substrate of the late positive potential in emotional processing. Journal of Neuroscience, 32(42), 14563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovic V., Saunders B.T., Yager L.M., Robinson T.E. (2011). Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behavioural Brain Research, 223(2), 255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M.R., Butryn M.L., Didie E.R., et al. (2009). The power of food scale. A new measure of the psychological influence of the food environment. Appetite, 53(1), 114–8. [DOI] [PubMed] [Google Scholar]
- Mahler S.V., de Wit H. (2010). Cue-reactors: individual differences in cue-induced craving after food or smoking abstinence. PLoS One, 5(11), 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan G., Peel D. (2000). Finite Mixture Models. New York: Wiley. [Google Scholar]
- Meyer J.E., Pudel V.E. (1977). Experimental feeding in man: a behavioral approach to obesity. Psychosomatic Medicine, 39(3), 153–7. [DOI] [PubMed] [Google Scholar]
- Meyer P.J., Lovic V., Saunders B.T., et al. (2012). Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One, 7(6), e38987(1-15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miccoli L., Delgado R., Rodriguez-Ruiz S., Guerra P., Garcia-Mamol E., Fernandez-Santaella M.C. (2014). Meet OLAF, a good friend of the IAPS! The Open Library of Affective Foods: a tool to investigate the emotional impact of food in adolescents. PLoS One, 9(12), 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnix J.A., Versace F., Robinson J.D., et al. (2013). The late positive potential (LPP) in response to varying types of emotional and cigarette stimuli in smokers: a content comparison. International Journal of Psychophysiology, 89(1), 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L.K., Muthén B.O. (1998-2007). Mplus User’s Guide, Fifth Edition Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nijs I.M., Franken I.H. (2012). Attentional processing of food cues in overweight and obese individuals. Current Obesity Reports, 1(2), 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijs I.M., Franken I.H., Muris P. (2008). Food cue-elicited brain potentials in obese and healthy-weight individuals. Eating Behaviors, 9(4), 462–470. [DOI] [PubMed] [Google Scholar]
- Nijs I.M.T., Franken I.H.A., Muris P. (2007). The modified trait and state food-cravings questionnaires: development and validation of a general index of food craving. Appetite, 49(1), 38–46. [DOI] [PubMed] [Google Scholar]
- Nylund K.L., Asparouhov T., Muthén B.O. (2007). Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Structural Equation Modeling: A Multidisciplinary Journal, 14(4), 535–69. [Google Scholar]
- Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. (2014). Prevalence of childhood and adult obesity in the United States, 2011–2012. The Journal of the American Medical Association, 311(8), 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petras H., Masyn K. (2009). General growth mixture analysis with antecedents and consequences of change. In: Piquero A.R., Weisburd D., editors. Handbook of Quantitative Criminology, pp. 69–100. New York: Springer Science. [Google Scholar]
- Radloff L.S. (1977). The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Robinson M.J.F., Burghardt P.R., Patterson C.M., et al. (2015). Individual differences in cue-induced motivation and striatal systems in rats susceptible to diet-induced obesity. Neuropsychopharmacology, 40(9), 2113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T.E., Yager L.M., Cogan E.S., Saunders B.T. (2014). On the motivational properties of reward cues: individual differences. Neuropharmacology, 76(Part B), 450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders B.T., Robinson T.E. (2013). Individual variation in resisting temptation: implications for addiction. Neuroscience and Biobehavioral Reviews, 37(9), 1955–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schembre S.M., Greene G., Melanson K. (2009). Development and validation of a weight-related eating questionnaire. Eating Behaviors, 10(2), 119–24. [DOI] [PubMed] [Google Scholar]
- Schembre S.M., Geller K.S. (2011). Psychometric properties and construct validity of the weight-related eating questionnaire in a diverse population. Obesity, 19(12), 2336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp H.T., Cuthbert B.N., Bradley M.M., Cacioppo J.T., Ito T., Lang P.J. (2000). Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology, 37(2), 257–61. [PubMed] [Google Scholar]
- Snaith R.P., Hamilton M., Morley S., Humayan A., Hargreaves D., Trigwell P. (1995). A scale for the assessment of hedonic tone. The Snaith-Hamilton Pleasure Scale. British Journal of Psychiatry, 167, 99–103. [DOI] [PubMed] [Google Scholar]
- Stice E., Spoor S., Ng J., Zald D.H. (2009). Relation of obesity to consummatory and anticipatory food reward. Physiology and Behavior, 97(5), 551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styn M.A., Bovbjerg D.H., Lipsky S., Erblich J. (2013). Cue-induced cigarette and food craving: a common effect? Addictive Behaviors, 38(3), 1840–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A., Grimes K.L., Pohorecky L.A. (2008). Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Research Reviews, 58, 121–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasey M.W., Thayer J.F. (1987). The continuing problem of false positives in repeated measures ANOVA in psychophysiology: a multivariate solution. Psychophysiology, 24(4), 479–86. [DOI] [PubMed] [Google Scholar]
- Versace F., Engelmann J.M., Robinson J.D., et al. (2014). Prequit fMRI responses to pleasant cues and cigarette-related cues predict smoking cessation outcome. Nicotine and Tobacco Research, 16(6), 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F., Lam C.Y., Engelmann J.M., et al. (2012). Beyond cue reactivity: blunted brain responses to pleasant stimuli predict long term smoking abstinence. Addiction Biology, 17(6), 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F., Minnix J.A., Robinson J.D., Lam C.Y., Brown V.L., Cinciripini P.M. (2011). Brain reactivity to emotional, neutral and cigarette-related stimuli in smokers. Addiction Biology, 16, 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F., Schembre S. (2015). “Obesogenic” oversimplification. Obesity Reviews, 16(8), 702–03. [DOI] [PubMed] [Google Scholar]
- Wang G.J., Volkow N.D., Telang F., et al. (2004). Exposure to appetitive food stimuli markedly activates the human brain. NeuroImage, 21(4), 1790–97. [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS Scales. Journal of Personality and Social Psychology, 54, 1063–70. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer. (2007). Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: AICR. [Google Scholar]
- Yager L.M., Robinson T.E. (2010). Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behavioural Brain Research, 214(1), 30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.