Abstract
Healthy social relationships are linked to myriad positive physical and mental health outcomes, raising the question of how to enhance relationship formation and quality. Behavioral data suggest that theory of mind (ToM) may be one such process. ToM is supported by a network of brain regions including the temporo-parietal junction (TPJ), medial prefrontal cortex and precuneus (PC). However, little research has investigated how the ToM network supports healthy social relationships. Here, we investigate whether recruitment of the ToM network when thinking about the mental states of one’s romantic partner predicts the partner’s well-being. We find that selectivity in left TPJ (LTPJ) and PC for beliefs vs physical attributes of one’s partner is positively associated with partner well-being the day of and day after a meaningful encounter. Furthermore, LTPJ and PC selectivity moderated how the partner’s perception of being understood during the encounter affected their later well-being. Finally, we find the association between ToM-related neural selectivity and well-being robust to other factors related to the relationship and the encounter. Together, these data suggest that selective engagement of the neural network supporting ToM may be a key ingredient for the development and maintenance of healthy romantic relationships.
Keywords: theory of mind, well-being, relationships, temporo-parietal junction, fMRI
Introduction
Human beings are exceptionally social animals. We thrive in social relationships and wither in isolation. Specifically, the extent of social connection, or lack thereof, is associated with mortality (House et al., 1988; Holt-Lunstad et al., 2010), physical illness (Cohen et al., 1997; Uchino, 2006; Umberson et al., 2010), psychiatric illness (Kessler et al., 1985; Coyne and Downey, 1991; Matheson et al., 2013), subjective well-being (VanderWeele et al., 2012) and neurobiological, cognitive and immunological function (Eisenberger and Cole, 2012; Cacioppo and Cacioppo, 2014; Cacioppo et al., 2015). These associations are particularly robust in the context of romantic relationships (Loving and Slatcher, 2013), which for many adults are the central and most enduring social relationship. Clearly, relationships are linked to a variety of important outcomes, raising the question of how to enhance relationship formation, maintenance and quality.
One process that influences relationship quality is theory of mind (ToM), the process by which we attribute and reason about the mental states of others. Whether in the service of expressing empathy for a romantic partner (Franzoi et al., 1985; Davis and Oathout, 1987; Long and Andrews, 1990; Long, 1993; Cramer and Jowett, 2010; Cohen et al., 2012), negotiating with others at the bargaining table (Galinsky et al., 2008), or in other social contexts (Goldstein et al., 2014), ToM is associated with positive relationship outcomes, including increasing liking of and prosocial behavior toward the individual engaging in ToM.
ToM relies on a network of brain regions including temporo-parietal junction (TPJ), medial prefrontal cortex (MPFC) and precuneus (PC) (Saxe and Kanwisher, 2003; Van Overwalle, 2009; Mar, 2011; Schurz et al., 2014)—often referred to as the ToM network. However, there are little data showing that engagement of this network predicts anything about social relationships in real life, leaving the route from brain to behavior largely unmapped. Previous research has shown that the structure and function of the ToM network, in healthy and schizophrenia samples across the lifespan, are associated with ToM performance on laboratory assessments (Hooker et al., 2011; Gweon et al., 2012; Dodell-Feder et al., 2014b; Rice and Redcay, 2014), as well as self-reported and observer reported tendency to engage in perspective-taking and empathy (Hooker et al., 2008, 2010; Dodell-Feder et al., 2014b). Experience sampling methods, such as daily diary questionnaires, are a particularly useful way of getting at these relations as they provide a more ecologically valid and nuanced assessment of ToM engagement in daily life. For example, in Dodell-Feder et al. (2014a), participants completed a functional magnetic resonance imaging (fMRI) ToM task and then reported on their social behavior in a daily diary questionnaire every evening for 28 days. Results showed that neural selectivity for mental state information in bilateral TPJ and MPFC during the fMRI task predicted daily reports of ToM engagement, social motivation and enjoyment of socializing. Other studies using experience sampling methods have revealed similar brain–behavior relationships, for example, between empathy-related activity in MPFC and prosocial behavior (Masten et al., 2011; Rameson et al., 2012), and neural activity in dorsal (D)MPFC when viewing social scenes and time spent around other people (Powers et al., 2015).
Though these preliminary studies suggest that engaging the ToM network to understand others supports social behavior and relationships, many questions remained unanswered. For example, though engagement of the network may support perspective-taking, empathy and prosociality, it is unclear how this may affect social partners, and in what ways it might support healthy relationships. Though studies have shown that an individual’s neural response tracks with their own behavior (Masten et al., 2011; Rameson et al., 2012; Dodell-Feder et al., 2014a), few if any studies have examined how an individual’s neural response affects another individual’s behavior or social experience, and whether this has any impact on more global and consequential outcomes such as well-being. Furthermore, almost nothing is known about how engagement of the ToM network supports romantic relationships in particular. If recruitment of the ToM network is linked to positive relationship outcomes, it would suggest that selective engagement of ToM network during social interaction would be one way of improving the quality of relationships, allowing couples to better reap the physical and mental health benefits of having a romantic partner.
Elucidating the neural mechanisms subserving ToM in the context of relationships may be beneficial for several other reasons. Though several behavioral studies have shown that a partner’s perception of ToM engagement (e.g. perceived empathic understanding or perspective-taking) by the participant is related to relationship satisfaction (Long and Andrews, 1990; Cramer and Jowett, 2010; Cohen et al., 2012), it remains a challenge to tease apart putative ToM-related behavior from the underlying processes engaged by the participant. More specifically, partners may perceive a variety of behaviors as indicative of ToM (e.g. head nodding), when in only some instances these behaviors reflect actual engagement of brain regions that support ToM (e.g. head nodding while considering one’s partner’s perspective vs head nodding while thinking about what to eat for dessert). Only when individuals actually engage ToM-related regions would we expect an appropriately supportive and empathic response that translates to better outcomes for partners. Furthermore, the partner’s perception of ToM engagement by the individual may be unduly influenced by many non-ToM-related intrapersonal factors such as the partner’s neuroticism, self-esteem and security in one’s relationship. Beyond how social partners perceive ToM engagement, attempts to measure actual ToM ability are often hindered by behavioral measures that heavily tap non-social cognitive processes (e.g. working memory) concomitant with mental state reasoning, and/or exhibit a lack of sensitivity to individual differences (see Dodell-Feder et al., 2013 and Rice and Redcay, 2014 for discussions). Thus, neural measures may provide a clearer window into the processes that are actually engaged during meaningful encounters with one’s partner.
Here, we investigate how recruitment of the ToM network supports romantic relationships. More specifically, we ask whether the participant’s selective engagement of the ToM network for mental vs physical information, as measured by a laboratory-based fMRI task, is related to their partner’s well-being—a construct that has significant implications for physical and mental health (Diener and Chan, 2011; Boehm and Kubzansky, 2012). We test this question by looking at the relationship between ToM-related neural activity and partner well-being after a meaningful interpersonal encounter. To address this question, individuals in romantic relationships underwent fMRI while judging statements regarding the beliefs, affective states, or physical attributes of their romantic partner, a close friend, or themselves. This design allowed us to investigate recruitment of the network using personalized stimuli that are more likely to capture ToM processes as they occur in vivo with their romantic partner. Using a 3-week daily diary questionnaire, romantic partners reported on their well-being, and how much they perceived the participant to understand their thoughts and emotions during a meaningful encounter (henceforth, ‘perceived understanding’). Measuring these constructs every day for 21 days provides an ecologically valid, reliable and stable measure of each phenomenon that is less subject to memory biases, and amenable to characterizing dynamic temporal associations between the processes of interest (Bolger et al., 2003; Myin-Germeys et al., 2009). We specifically looked at well-being in the context of meaningful encounters (e.g. a conflict or discussion of personally relevant issues) because this is, in theory, when ToM would be most important and have the most impact on the partner. Both individuals from a dyad participated in the fMRI and diary portion of the study serving as both a participant (i.e. contributing fMRI data) and partner (i.e. contributing daily diary data).
Based on neuroimaging data demonstrating positive associations between selectivity for mental state information in the ToM network and adaptive social processes (e.g. perspective-taking, empathy, prosociality) (Hooker et al., 2010; Masten et al., 2011; Gweon et al., 2012; Rameson et al., 2012; Dodell-Feder et al., 2014a), and behavioral data demonstrating a relation between ToM (i.e. the perception of being understood) and positive relationship outcomes among romantic couples (Long and Andrews, 1990; Cramer and Jowett, 2010; Cohen et al., 2012), we predicted that selectivity for mental states vs physical attributes of one’s partner would be positively associated with the partner’s well-being after a meaningful encounter. In addition, as an exploratory analysis, we investigated the relation between perceived understanding, ToM-related neural selectivity and well-being.
Methods
Participants
Fifteen couples (N = 30 individuals; 15 female; Age M = 24.9 years, s.d. = 4.3, range = 18–35) in committed romantic relationships (M relationship length = 40.9 months, s.d. = 34.3, range = 6–113) for at least 3 months participated in the study. All participants were right-handed, English speaking and had normal or corrected-to-normal vision. Exclusion criteria included head trauma, IQ < 70, use of psychotropic medication, neurological disorder, or current Axis I DSM-IV disorder as assessed with the MINI International Neuropsychiatric Interview (Sheehan et al., 1998). Of the 17 couples (N = 34) that came to the lab to participate, two couples (n = 4) were excluded for a current Axis I disorder, and one individual was excluded just prior to the scan due to an MRI contraindicator.
Of the 29 participants that contributed fMRI data, 6 were excluded for not completing at least 14 of the 21 days of the diary, and the participant whose partner had an MRI contraindicator withdrew from the study. The final sample with fMRI and daily diary data included 22 participants (11 female; Age M = 24.6 years, s.d. = 4.2, range = 18–35) with an average relationship length of 29.8 months (s.d. = 19.4, range = 10–71).
Participants were compensated financially for their time. Harvard University’s Institutional Review Board approved this study.
Relationship satisfaction
Participants completed the Relationship Assessment Scale (RAS; α = 0.73) (Hendrick, 1988), a seven-item self-report measure of relationship satisfaction in which participants answer questions using a 1 (low satisfaction) to 5 (high satisfaction) scale (e.g. ‘In general, how satisfied are you with your relationship?’). Mean RAS score was 32.1 (s.d. = 2.9, range = 26–35) indicating high relationship satisfaction in our sample.
fMRI task
In the scanner, participants judged how much they agreed with a series of statements that described either the beliefs, affective states, or physical characteristics of one of three targets: romantic partner, close friend (identified by the participant prior to scanning) or self (see Supplementary Data for a complete list of stimuli). Physical statements served as the control condition as they involved people, but did not contain mental states (i.e. beliefs or emotions). fMRI data from the self-condition were not analyzed for the purposes of this experiment and will not be discussed further. Versions of this task have been used previously to isolate ToM-related activity for the self, close others and distant others (Mitchell et al., 2006; Jenkins et al., 2008). One other study using this task has demonstrated that selectivity for mental state information is associated with social impairment in autism (Lombardo et al., 2011).
During each trial, participants saw the name of the target to be rated, a statement and the response scale (1 = not at all, 4 = definitely) concurrently on the screen during which they made their response. Statements were presented in a block design where participants rated one target on one attribute type in each block (e.g. Partner Belief). Belief, affective and physical statements were matched on number of words and Flesch reading ease level (Flesch, 1948). Participants rated a total 35 statements per condition, which were randomly presented in each block. Blocks were presented in one of two pseudo-randomized orders.
One block per condition was presented in each of seven functional runs. Each 22.5 s block consisted of five statements, which appeared on the screen for 4 s each and was separated by a 0.5 s ITI. Blocks were followed by 12 s of fixation on a center cross.
Stimuli were presented in white text on a black background with Matlab 7.6 using Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997).
fMRI task performance
We derived an accuracy score by evaluating the correspondence between the participant’s and their partner’s ratings during the task. Specifically, we calculated the absolute mean difference between the participant’s rating and partner’s rating of the same statements such that scores closer to 0 represent greater accuracy, with possible scores ranging from 0 to 3. This was performed separately for the Belief and Affective statements.
fMRI data acquisition and analysis
Data were acquired on a 3T Siemens Tim Trio scanner with a 12-channel head coil at Harvard University’s Center for Brain Science. An anatomical image was acquired using a T1-weighted multi-echo MPRAGE sequence (176 sagittal slices, 1 × 1 × 1 mm voxels). Functional data were collected with echo-planar images (126 volumes per run) in the axial plane with whole-brain coverage (40 slices, 3 × 3 × 3 mm voxels, TE/TR/flip angle = 30 ms/2560 ms/85°).
Data were preprocessed and analyzed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) in the following steps: realignment of the functional data to the mean functional image, coregistration of the anatomical image to the mean functional image, normalization to MNI-template space and smoothing with a 6 mm FWHM Gaussian kernel.
Individual participant effects were estimated using a general linear model. Effects were estimated for each condition (i.e. Partner Belief, Partner Affective, Partner Physical, Friend Belief, Friend Affective, Friend Physical). The six motion parameters estimated during realignment and session mean were included as covariates of no interest. Hemodynamic response was modeled at the onset of each condition for the block duration and convolved with the canonical hemodynamic response function. Data were high-pass filtered at 128 s. Contrasts were created within each participant for Partner Belief > Partner Physical, Partner Affective > Partner Physical, Friend Belief > Friend Physical and Friend Affective > Friend Physical.
Individual participant contrasts were submitted to second level group analyses where each participant was treated as a random effect. To isolate ToM-related neural activity for one’s partner, we conducted whole-brain one-sample t-tests for Partner Belief > Partner Physical and Partner Affective > Partner Physical. To isolate ToM-related neural activity for one’s close friend, we conducted the same analysis for Friend Belief > Friend Physical and Friend Affective > Friend Physical. Finally, we evaluated whether selectivity for mental states differed for Partner vs Friend by conducting a paired-samples t-test for (Partner Belief > Partner Physical) <> (Friend Belief>Friend Physical). These contrasts were thresholded at P < 0.001 and FWE-corrected at the cluster-level to P < 0.05 using the CorrClusTh tool (http://www-personal.umich.edu/∼nichols/JG5/CorrClusTh.m). Beta values were extracted from significant clusters in TPJ, MPFC and PC that were revealed in these contrasts, representing ToM-related neural activity for either partner or close friend, and used in all subsequent analyses. fMRI data from all participants with fMRI data (N = 29) were used to identify TPJ, MPFC and PC in order to get the best localization of brain regions recruited during Partner and Friend-related ToM. All other analyses used data from the 22 participants who had complete fMRI and daily diary data.
Daily diary
Following the scan, participants completed an online daily diary between 5 P.M. and 3 A.M. for 21 days. Questions assessed well-being as the primary outcome variable (Table 1). In accordance with previous research on well-being (e.g. Reis et al., 2000), this construct was designed to tap aspects of autonomy (e.g. ‘I felt in control of my life’), competency (e.g. ‘I was confident in my abilities’), esteem (e.g. ‘I felt good about myself today’), general life satisfaction (e.g. ‘I felt content with my life today’), clarity/certainty in one’s life (e.g. ‘Today I felt like I had a clear sense of who I am and what I want in my life’), and social satisfaction and support (e.g. ‘I felt supported,’ ‘I felt accepted’). We were specifically interested in well-being after meaningful encounters with partners in which ToM skills would be most important and most likely to have an effect on partner well-being. Thus, participants were also asked whether they had a meaningful encounter with their partner that day (i.e. ‘Did you have a meaningful or emotional interaction, disagreement, or conflict with your partner today?’). If so, participants were asked to provide information regarding the nature of the encounter, specifically the extent to which the participant would describe the encounter as a conflict or disagreement, and the extent to which the participant felt distressed about the topic of the encounter (see Supplementary Data). Finally, as a measure of perceived understanding, participants were asked to report how much they thought their partner understood their thoughts and feelings during the encounter (e.g. ‘Was your partner able to see things from your eyes or from your point of view?’). If a participant experienced more than one meaningful encounter with their partner that day, they were asked to report on the ‘most important or memorable interaction.’ All items were rated on a 5-point scale from 1 (not at all) to 5 (extremely).
Table 1.
Daily diary items
| Construct | Item | M (s.d.) [range] | α |
|---|---|---|---|
| Well-being | 3.83 (0.66) [1.88–5] | 0.95 | |
| I felt supported | |||
| I felt valued | |||
| I felt loved | |||
| I felt accepted | |||
| I felt positive or hopeful about the future | |||
| I felt a sense of meaning and purpose in my life today | |||
| I felt content with my life today | |||
| I felt as though I could achieve/complete whatever I needed or wanted to | |||
| I felt as though I had the ability to solve my own problems | |||
| I was confident in my abilities | |||
| I trusted my own judgment today | |||
| I felt like I had a good understanding of life today | |||
| I knew what to expect from life today | |||
| I felt good about myself today | |||
| I felt in control of my life | |||
| I felt as if I were free to do what I wanted/need to today | |||
| Today, I felt like I had a clear sense of who I am and what I want in life | |||
| Perceived understanding | 3.24 (1.21) [1–5] | 0.94 | |
| Was your partner able to see things from your eyes or from your point of view? | |||
| Did he/she share in your emotional experience? | |||
| Did he/she understand your feelings, experiences, or perspective on an intellectual level? | |||
| Did he/she understand your feelings, experiences, or perspective on an emotional level? |
Note: All questions were answered on a 1–5 scale.
Analysis of fMRI and daily diary data
Diary data had a hierarchical structure such that diary day was nested within participant and participant was nested within couple. To account for this structure and the non-independence between observations within participant, and participants within couple, we analyzed the fMRI and daily diary data with multilevel models (Hox, 2002) using the nlme package (Pinheiro et al., 2014) in R (R Core Team, 2013).
We tested the hypothesis that ToM-related neural selectivity for one’s partner would predict partner well-being the day of a meaningful encounter and the day after a meaningful encounter. Separate multilevel models were conducted that included neural selectivity in a single region-of-interest (ROI) identified from the whole-brain analysis for either partner (e.g. Partner Belief > Partner Physical) or friend (e.g. Friend Belief > Friend Physical) as the predictor variable. As the outcome variable, we used well-being the day of the encounter (i.e. ‘same-day’ well-being), and in separate models, well-being the day after the encounter (i.e. ‘next-day’ well-being). We examined next-day well-being for several reasons. First, it allowed us to test whether the effect of neural activity on well-being had a lasting influence that extended into subsequent days. Second, because perceived understanding is confounded with same-day well-being (i.e. well-being may influence perceived understanding rather than the reverse), it allowed us to evaluate the effect of perceived understanding on subsequent well-being. To reduce the number of tests performed, in all subsequent models, we used only data from ROIs demonstrating a significant simple relation with well-being.
We then evaluated the associations between perceived understanding, ToM-related neural selectivity and well-being. First, we tested whether similar to behavioral findings, perceived understanding predicted well-being. Second, we tested whether ToM-related selectivity was associated with perceived understanding. Finally, as an exploratory analysis, we examined whether the interaction of ToM-related selectivity and perceived understanding predicted next-day well-being. Said otherwise, we tested whether engagement of the ToM network would influence whether the partner’s perception of being understood impacted their subsequent well-being. This was tested with separate multilevel models that included as the predictors: (i) selectivity in a single ROI, (ii) perceived understanding during the encounter and (iii) the interaction between these terms. We used next-day well-being in order to evaluate the effect of perceived understanding, and its interaction with ToM-related selectivity, on subsequent well-being. When a statistical interaction was present, simple slopes analysis was performed at high and low levels of ToM-related neural selectivity (±1 s.d. of the mean) (Aiken and West, 1991).
We conducted two sets of follow-up analyses. First, we evaluated whether the simple relation between ToM-related neural selectivity for partner and well-being was influenced by factors related to the relationship or nature of the encounter. This was tested with separate models that included as the predictors: (i) selectivity in a single ROI; (ii) a single variable previously shown to impact relationship outcomes or ToM in relationships—gender (Cohen et al., 2012), relationship length (Thomas and Fletcher, 2003), conflict/disagreement during the encounter (Gable et al., 2006), or topic stress; and (iii) the interaction between these terms (e.g. ROI selectivity*relationship length). Second, we evaluated whether ToM-related neural selectivity for partner explained variance in well-being above and beyond the effect of perceived understanding, and in a separate model, general relationship satisfaction (RAS score). Next-day well-being was used in these models.
Variance inflation factors were inspected for all models with multiple predictors, and were in the acceptable range (1.00–1.50) indicating that multicollinearity was not a problem (Myers, 1990). Predictor variables were grand-mean centered in models that included an interaction term.
Results
fMRI task—behavioral results
Analysis of behavioral ratings during the fMRI task showed that the difference between how participant’s rated their partner and how their partner rated themselves was minimal (Belief Statements: M = 0.65, s.d. = 0.12, range = 0.37–0.92; Affective Statements: M = 0.71, s.d. = 0.17, range = 0.43–0.97). These data indicate high participant accuracy in inferring the beliefs and emotions of their partner.
fMRI results
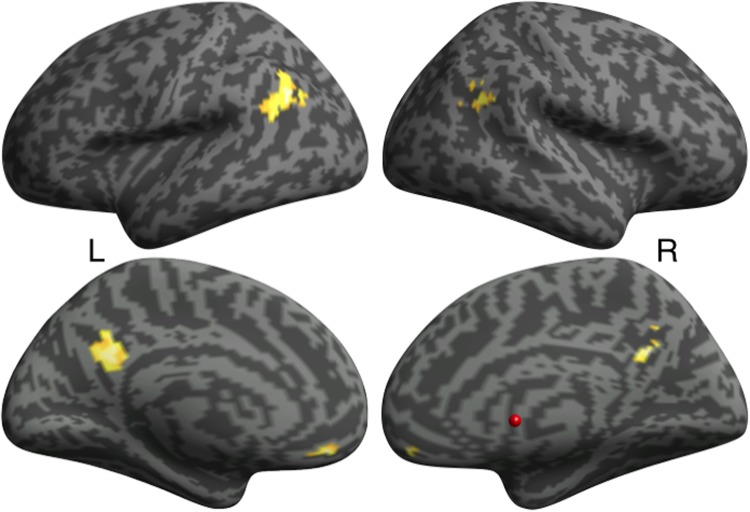
Consistent with prior reports on the neural bases of ToM, Partner Belief > Partner Physical revealed clusters of activation in bilateral TPJ, PC and ventral (V)MPFC (Table 2; Figure 1). No clusters identified from Partner Affective > Partner Physical survived correction for multiple comparisons. Similarly, Friend Belief > Friend Physical revealed clusters of activity in bilateral TPJ and PC, in addition to DMPFC, left posterior superior temporal sulcus and right cerebellum (Supplementary Data). For Friend Affective > Friend Physical, a cluster in PC emerged. Given that the Affective > Physical contrast for Partner and Friend failed to recruit the expected ROIs, we dropped these data from the diary analysis.
Table 2.
fMRI results for Partner Belief > Partner Physical
| Region | R/L | MNI coordinates (x y z) | t-Value (peak voxel) | Cluster size (voxels) |
|---|---|---|---|---|
| Ventral medial prefrontal cortex/orbital frontal cortex | — | 0 41 −20 | 6.99 | 52 |
| Temporo-parietal junction | L | −45 −58 28 | 5.35 | 146 |
| Precuneus | R | 3 −55 31 | 5.33 | 140 |
| Temporo-parietal junction | R | 48 −55 28 | 4.62 | 55 |
Note: Voxel-level P < 0.001 FWE-corrected at the cluster-level to P < 0.05.
Fig. 1.
fMRI results for Partner Belief > Partner Physical. Voxel-level P < 0.001, FWE-corrected at the cluster-level to P < 0.05. R, right; L, left.
No differences were observed when comparing belief selectivity for Partner vs Friend [i.e. (Partner Belief > Partner Physical)<> (Friend Belief > Friend Physical)].
Behavioral data from the daily diary
Diary compliance was high and all but one participant completed 20–21 days of the diary (days completed: M = 20.5, s.d. = 0.9, range = 17–21). Participants reported a total of 86 meaningful encounters with their partners across the 21 days (M encounters per participant = 4, s.d. = 3, range = 1–10). Cronbach’s alpha indicated excellent internal consistency for the well-being and perceived understanding constructs (Table 1). On average, participants engaged with their partners about minimally distressing topics (M = 2.0, s.d. = 1.0). During the encounter, participants reported little conflict (M = 2.1, s.d. = 1.4), and a moderate amount of perceived understanding from their partners (M = 3.2, s.d. = 1.2).
Analysis of fMRI and daily diary data
Does ToM-related neural selectivity predict partner well-being the day of and the day after a meaningful encounter?
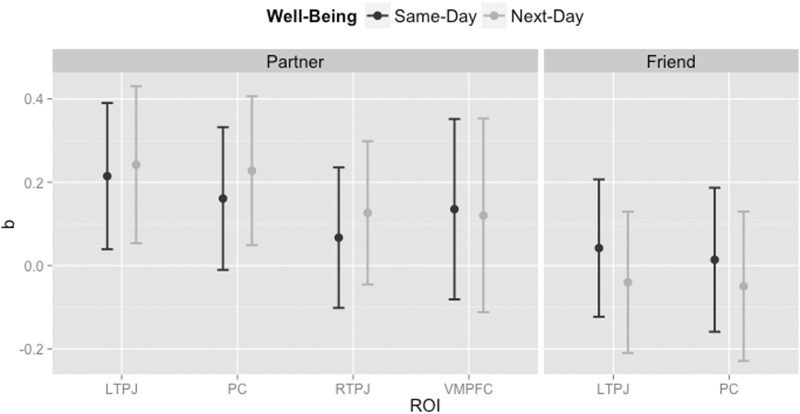
Selectivity for belief vs physical information for one’s partner in left TPJ (LTPJ) was positively associated with partner well-being the day of a meaningful encounter (b = 0.21, SE = 0.08, P = 0.02) and the day after a meaningful encounter (b = 0.24, SE = 0.08, P = 0.02) (Figure 2). Similarly, PC selectivity for belief vs physical information for one’s partner predicted partner well-being at a trend level on the same-day of the encounter (b = 0.16, SE = 0.08, P = 0.06) and significantly the day after the encounter (b = 0.23, SE = 0.08, P = 0.02). These effects were not observed for the Friend condition: selectivity in LTPJ and PC when evaluating beliefs (vs physical information) about one’s friend did not predict the partner’s same-day or next-day well-being (Ps > 0.58; Figure 2). Finally, Partner-related selectivity for belief information in RTPJ and VMPFC was not associated with same-day or next-day well-being (Ps > 0.13; Figure 2).
Fig. 2.
Coefficients and 95% confidence intervals from the multilevel models testing the simple relation between ToM-related selectivity for partner/friend and same-day/next-day well-being.
What is the relation between perceived understanding, ToM-related neural selectivity and well-being?
Behavioral studies have revealed that among romantic partners, increased perceived understanding is associated with positive outcomes, such as relationship satisfaction (Long and Andrews, 1990; Cramer and Jowett, 2010; Cohen et al., 2012). We found that, similar to these other findings, perceived understanding was positively associated with same-day well-being (b = 0.22, SE = 0.04, P < 0.001) and next-day well-being at a trend level (b = 0.10, SE = 0.06, P = 0.08).
One possibility is that ToM-related selectivity is associated with behaviors that affect how much the partner perceives the participant to understand them, which might explain why both factors are associated with well-being. We tested this idea with an additional model using ToM-related neural selectivity as the predictor variable and perceived understanding as the outcome variable. LTPJ selectivity did not predict perceived understanding (b = 0.22, SE = 0.17, P = 0.23); PC selectivity predicted perceived understanding at only a trend level (b = 0.33, SE = 0.16, P = 0.07). Thus, it seems as though ToM-related selectivity does not necessarily track with how much the partner perceives understanding from the participant.
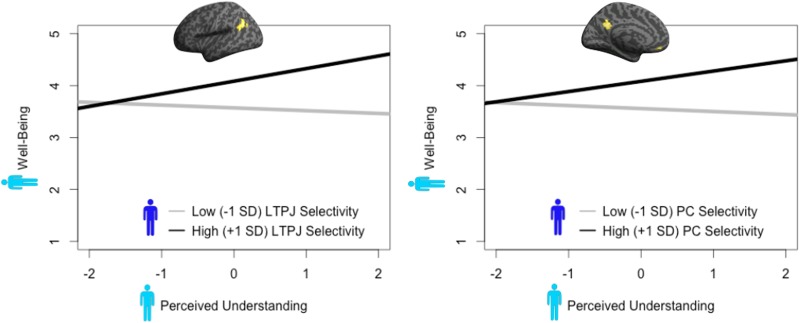
Another possibility is that perceived understanding is most strongly associated with well-being under certain conditions. For example, given the positive association between ToM-related selectivity and adaptive social behaviors, it is possible that perceived understanding maximally contributes to well-being when participants exhibit high neural selectivity for belief information. Thus, we conducted an exploratory analysis in which we tested whether well-being the day after an encounter was predicted by the interaction of LTPJ/PC selectivity and perceived understanding. Said otherwise, we tested whether the relation between perceived understanding and well-being depended on the selectivity of these brain regions. Consistent with this notion, we found that next-day well-being was predicted by the interaction of LTPJ and perceived understanding (interaction term b = 0.15, SE = 0.05, P = 0.006). Simple slopes analysis revealed a positive relation between perceived understanding during the encounter and next day well-being in the case of high LTPJ selectivity (b = 0.24, SE = 0.07, P = 0.001) (Figure 3), and a non-significant negative relationship between perceived understanding during the encounter and next day well-being in the case of low LTPJ selectivity (b =−0.05, SE = 0.07, P = 0.47).
Fig. 3.
Interaction effects of neural selectivity and perceived understanding in predicting well-being. Simple slopes analysis demonstrated the statistical interaction between how much the partner perceived being understood by the participant and participant’s level of LTPJ(left)/PC(right) selectivity in predicting the partner’s day-after-encounter well-being. In the case of high neural selectivity for beliefs, we find a significant positive relationship between the partner’s perception of being understood by the participant and the partner’s subsequent well-being. No relationship between the partner’s perception of being understood by the participant and the partner’s subsequent well-being was observed in the case of low neural selectivity for beliefs. Dark blue figure: participant; light blue figure: partner.
A similar interaction was found between partner-related ToM selectivity in PC and next-day well-being (interaction term b = 0.10, SE = 0.05, P = 0.05), such that perceived understanding was positively associated with next-day well-being only in the case of high PC selectivity (b = 0.20, SE = 0.08, P = 0.02), but not low PC selectivity (b = −0.06, SE = 0.09, P = 0.51) (Figure 3).
Follow-up analyses
Given the complexity of interpersonal relationships, there are many factors outside of ToM-related neural selectivity that may impact a partner’s well-being or alter the associations demonstrated above. Indeed, other research has demonstrated that factors including gender (Cohen et al., 2012), relationship length (Thomas and Fletcher, 2003) and emotional valence of the encounter (Gable et al., 2006) are important in this regard. Thus, we conducted follow-up analyses to examine whether the relationship between ToM-related neural activity and well-being was influenced by these other factors. This was tested with models that separately evaluated whether same-day/next-day well-being was predicted by the interaction of neural selectivity in LTPJ/PC and gender, conflict/disagreement during the encounter, topic distress, or relationship length. Results showed that the associations between partner-related ToM activity in LTPJ/PC and same-day/next-day well-being did not differ by gender (interaction term Ps > 0.35) nor was it influenced by relationship length (interaction term Ps > 0.41), conflict/disagreement during the encounter (interaction term Ps > 0.07), or topic distress (interaction term Ps > 0.16).
We also investigated whether ToM-related neural selectivity accounted for variance in well-being above and beyond behavioral variables known to influence relationship quality and/or well-being: perceived understanding and general relationship satisfaction. This was tested in separate models that included as the predictors LTPJ or PC selectivity and perceived understanding or RAS score, and next-day well-being as the outcome variable. These models allowed us to investigate the unique effects of one variable (e.g. ToM-related selectivity) net of the effect of the other variable (e.g. perceived understanding). In the model that included LTPJ selectivity and perceived understanding, only LTPJ was significantly associated with well-being (b = 0.22, SE = 0.08, P = 0.02); perceived understanding was not (b = 0.09, SE = 0.05, P = 0.11). In the model that included LTPJ selectivity and relationship satisfaction, LTPJ selectivity was significantly associated with well-being (b = 0.21, SE = 0.08, P = 0.03); relationship satisfaction was associated with well-being at only a trend level (b = 0.07, SE = 0.03, P = 0.06). Similarly, in the model that included PC selectivity and perceived understanding, only PC selectivity was associated with well-being (b = 0.20, SE = 0.08, P = 0.03); perceived understanding was not (b = 0.07, SE = 0.06, P = 0.20). In the model that included PC selectivity and general relationship satisfaction, both factors significantly accounted for variance in well-being (PC: b = 0.21, SE = 0.08, P = 0.02; relationship satisfaction: b = 0.08, SE = 0.03, P = 0.04). Thus, ToM-related neural selectivity in LTPJ and PC appeared to account for variance in well-being even after controlling for the effects of perceived understanding and relationship satisfaction.
Finally, we conducted an additional analysis to evaluate how partner-related ToM activity in LTPJ and PC may translate to increased partner well-being. Although ToM-related selectivity does not appear to be related to perceived understanding, one possibility is that selectivity in these regions tracks with objective understanding of the partner’s mental states, which in turn may influence partner well-being. We tested this idea by evaluating whether selectivity for one’s partner in LTPJ and PC was associated with belief accuracy on the scanner task. We found that neither LTPJ nor PC selectivity was associated with accuracy in inferring partner beliefs during the fMRI task (LTPJ: r = 0.27, P = 0.23; PC: r = 0.04, P = 0.84).
Discussion
We find that the extent of neural selectivity for belief vs physical information in LTPJ and PC when thinking about one’s partner positively predicted the partner’s well-being the day of and day after a meaningful encounter. These associations were revealed only when using relationship-specific personalized stimuli (i.e. Partner statements, but not Friend statements) in which the elicited neural response was consistent with the real-life behavioral context in which that neural response might occur. Follow-up analyses revealed that the relation between ToM-related activity and partner well-being did not differ by gender, nor was it affected by relationship length or by the nature of the encounter (i.e. topic distress, conflict/disagreement). Furthermore, LTPJ and PC selectivity accounted for variance in partner well-being above and beyond the effect of other factors including the extent to which the partner felt understood during the encounter and general relationship satisfaction.
In addition, the association between the perception of being understood by the partner and their well-being the day after an encounter depended on the extent of the participant’s LTPJ and PC selectivity. Specifically, we observed a positive association between partner-perceived understanding and partner well-being only in the case of high neural selectivity. That is, greater perception of being understood by the partner was associated with greater reports of their subsequent well-being only when participants demonstrate greater recruitment of LTPJ and PC for their partner’s belief vs physical attributes. No relation was observed between perceived understanding and subsequent well-being in the case of low neural selectivity.
Taken together, we interpret these findings to mean that individuals who exhibit greater selective engagement of LTPJ and PC when thinking about their partner’s beliefs vs physical attributes are able to enact more adaptive interpersonal processes or strategies in the context of meaningful interpersonal interactions that would benefit from ToM-related processes. These processes may include perspective-taking, empathy and acting altruistically—abilities which have previously been shown to correlate with neural selectivity in ToM-related regions (Hooker et al., 2008, 2010; Masten et al., 2011; Rameson et al., 2012; Waytz et al., 2012; Dodell-Feder et al., 2014a,b)—and other important processes for social functioning that may include validation and demonstrations of social support. This in turn may contribute to increased feelings of being understood, and subsequent social connection and reward—an association demonstrated in prior neuroimaging work (Morelli et al., 2014)—which carries concomitant effects for well-being on the part of the recipient.
Interestingly, despite how much the partner perceives the participant to understand their thoughts and feelings in the moment, our findings suggest that only individuals who exhibit high selective engagement of LTPJ and PC for belief information during the scanner task, and possibly during meaningful encounters, may deploy strategies that have positive, measurable and longer-lasting consequences for their partner. Individuals who fail to recruit these regions or do so less selectively are presumably using a different strategy during meaningful encounters with their partner that may not involve perspective-taking, empathy or other processes that are associated with activity in the ToM network. And, even though the partner may perceive understanding from the participant in the moment, whatever strategy or interpersonal process being used fails to carry more enduring positive benefits for the partner. This would suggest that a key ingredient to interactions that have positive outcomes for one’s partner involves the selective engagement of the ToM network for belief information.
Nevertheless, our findings do not directly speak to how partner-related ToM activity in LTPJ or PC translates to social behavior and positive partner outcomes. Based on prior neuroimaging work (e.g. Dodell-Feder et al., 2014a), we have speculated above that individuals who selectively engage these regions during interpersonal encounters are more likely or able to engage in accurate mental state attribution, and use that information to enact adaptive social behavior (e.g. empathy, validation, social support). This notion should be considered within the context of several limitations. For one, these data are correlational preventing us from making strong claims about the effect of selectivity of the ToM network on positive relationship outcomes. Additionally, there exists a potential discrepancy between neural processes and behavioral performance in the scanner vs in vivo social interaction. Said otherwise, our scanner measures of ToM-related processes (neural and behavioral) may be a poor proxy for how or whether these processes occur during actual meaningful encounters such as those reported in the daily diary. Though we have shown that the relation between LTPJ/PC and well-being does not appear to be influenced by variables related to the encounter (e.g. topic distress, conflict/disagreement) or relationship (e.g. satisfaction, length), other factors not measured here, related to the encounter or the participants, may alter how or whether these brain regions are recruited during encounters. In a similar vein, even if the neural processes isolated here did approximate what occurs during actual social encounters, with the current data, we cannot say with certainty what processes or behaviors (e.g. perspective-taking, prosocial acts) accompany ToM-related selectivity. We addressed one possibility for how selectivity for partner-related belief vs physical states may translate to improved well-being, namely through increased belief accuracy as measured on the scanner task. However, we failed to find a relation between LTPJ or PC selectivity and belief accuracy. As discussed, it is possible that behavioral performance on the fMRI task is a poor proxy for accuracy in inferring one’s partner’s mental states in the context of in vivo social interaction. Our measure also assumes that the partner is highly insightful and can accurately report on his/her belief states, which may not be the case. Another possibility is that our neural measure may instead reflect the tendency to spontaneously process belief information, as opposed to the accuracy of the inference. Indeed, prior fMRI research has also implicated LTPJ and PC in the spontaneous consideration of mental states across a variety of paradigms including those involving passive viewing of ambiguous animations (Schurz et al., 2014) and moral judgment (Bzdok et al., 2012).
It also remains unclear as to why only LTPJ and PC, but not other ToM-related regions, showed a relation with well-being. Though recent meta-analytic data implicate LTPJ and PC as part of a core network of regions, including RTPJ and MPFC, that are reliably activated during ToM across tasks and stimulus presentation modality (Schurz et al., 2014), findings from prior neuroimaging work has suggested specialization of function among these regions (Perner et al., 2006; Saxe and Powell, 2006; Van Overwalle, 2009; Schurz et al., 2013). As argued and demonstrated by others, ToM is complex construct, reliant upon multiple component and complimentary cognitive processes that are implemented in different brain regions and subnetworks (Schaafsma et al., 2014; Spunt and Adolphs, 2014). The mapping of LTPJ, PC and other regions that underlie ToM to the specific cognitive processes and social behaviors they support, however, remains to be elucidated.
We note that strong conclusions about the differences in the predictive power of Partner- vs Friend-related ToM, and LTPJ/PC vs other regions in the network, however, cannot be made. We did not observe any neural differences between Partner- and Friend-related ToM in the whole-brain analyses suggesting that a similar network is recruited when thinking about the mental states of two very close others. Other research suggests that aspects of the ToM network including LTPJ and MPFC are indeed sensitive to the target, for example, the target’s closeness/perceived similarity to the participant (Mitchell et al., 2005; Saxe and Wexler, 2005; Mitchell et al., 2006), social relevance (Krienen et al., 2010), or the participant’s idiosyncratic knowledge of a target (Welborn and Lieberman, 2014). The high degree of closeness, relevance and knowledge that participant’s theoretically had of their romantic partners and close friends may account for the lack of neural differences. However, this finding does not preclude the existence of important neural differences among the ROIs in how they process belief information about romantic partners vs close friends, and more sensitive analyses such as multi-voxel pattern analyses, may reveal such differences. Examination of the large and overlapping confidence intervals on the estimates of the relation between brain and well-being also suggests that the effect of different ROIs on partner well-being may not be significantly different. The same is true for Partner- vs Friend-related ToM. We would hypothesize that a similar relation between ToM-related activity and well-being would exist for close friends had we measured the friend’s well-being; however, additional data would be needed to evaluate this idea.
The findings of the current study may carry implications for clinical science. Many forms of psychopathology, namely schizophrenia and autism spectrum disorders, are associated with marked deficits in social ability (Chung et al., 2013; Savla et al., 2013), including the ability to form and maintain social relationships (American Psychiatric Association, 2013). Taken with other findings (Hooker et al., 2011; Lombardo et al., 2011; Dodell-Feder et al., 2014a,b), the link between selective engagement of aspects of the ToM network and positive partner outcomes demonstrated here suggests that the pathophysiology of social dysfunction may be linked, in part, with disruption to the ToM network. If so, the ToM network would represent a neurobiological target for intervention.
Taken together, the current findings highlight the importance of the neurocognitive system supporting ToM in relation to positive outcomes in romantic relationships. Overall, these findings suggest that individuals who engage aspects of the neural network supporting ToM may enact more adaptive social behavior that have measurable real-world consequences for their partner, underscoring the positive benefits of interacting with individuals who engage in ToM.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Acknowledgements
The authors would like to thank Meghan Brady and Matthew Abrams for assistance in recruitment and data collection, the Institute for Quantitative Social Science at Harvard University for assistance with data analysis, the staff at the Center for Brain Science at Harvard University for assistance with fMRI data collection, Sara Masland and Dianne M. Hezel for comments on an earlier draft of the manuscript, and the participants. The authors would also like to apologize to their romantic partners for instances in which they have failed to recruit LTPJ and PC during meaningful encounters.
Funding
This project was completed, in part, as an Honor’s Thesis project by Matthew G. Yung, and was supported by a grant from the Center for Brain Science at Harvard University and the Psychology Department at Harvard University.
References
- Aiken L.S., West S.G. (1991). Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA: Sage. [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders : DSM-5. Washington, DC: American Psychiatric Association. [Google Scholar]
- Boehm J.K., Kubzansky L.D. (2012). The heart’s content: the association between positive psychological well-being and cardiovascular health. Psychological Bulletin, 138(4), 655–91. [DOI] [PubMed] [Google Scholar]
- Bolger N., Davis A., Rafaeli E. (2003). Diary methods: capturing life as it is lived. Annual Review of Psychology, 54, 579–616. [DOI] [PubMed] [Google Scholar]
- Brainard D.H. (1997). The Psychophysics Toolbox. Spatial Vision, 10(4), 433–6. [PubMed] [Google Scholar]
- Bzdok D., Schilbach L., Vogeley K., et al. (2012). Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Structure & Function, 217(4), 783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo J.T., Cacioppo S. (2014). Older adults reporting social isolation or loneliness show poorer cognitive function 4 years later. Evidence-Based Nursing, 17(2), 59–60. [DOI] [PubMed] [Google Scholar]
- Cacioppo J.T., Cacioppo S., Capitanio J.P., Cole S.W. (2015). The neuroendocrinology of social isolation. Annual Review of Psychology, 66, 733–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y.S., Barch D., Strube M. (2013). A meta-analysis of mentalizing impairments in adults with schizophrenia and autism spectrum disorder. Schizophrenia Bulletin, 40(3), 602–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Doyle W.J., Skoner D.P., Rabin B.S., Gwaltney J.M., Jr (1997). Social ties and susceptibility to the common cold. JAMA, 277(24), 1940–4. [PubMed] [Google Scholar]
- Cohen S., Schulz M.S., Weiss E., Waldinger R.J. (2012). Eye of the beholder: the individual and dyadic contributions of empathic accuracy and perceived empathic effort to relationship satisfaction. Journal of Family Psychology, 26(2), 236–45. [DOI] [PubMed] [Google Scholar]
- Coyne J.C., Downey G. (1991). Social factors and psychopathology: stress, social support, and coping processes. Annual Review of Psychology, 42, 401–25. [DOI] [PubMed] [Google Scholar]
- Cramer D., Jowett S. (2010). Perceived empathy, accurate empathy and relationship satisfaction in heterosexual couples. Journal of Social and Personal Relationships, 27(3), 327–49. [Google Scholar]
- Davis M.H., Oathout H.A. (1987). Maintenance of satisfaction in romantic relationships: empathy and relational competence. Journal of Personality and Social Psychology, 53(2), 397–410. [Google Scholar]
- Diener E., Chan M.Y. (2011). Happy people live longer: subjective well-being contributes to health and longevity. Applied Psychology: Health and Well-Being, 3(1), 1–43. [Google Scholar]
- Dodell-Feder D., DeLisi L.E., Hooker C.I. (2014a). Neural disruption to theory of mind predicts daily social functioning in individuals at familial high-risk for schizophrenia. Social Cognitive and Affective Neuroscience, 9(12), 1914–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodell-Feder D., Lincoln S.H., Coulson J.P., Hooker C.I. (2013). Using fiction to assess mental state understanding: a new task for assessing theory of mind in adults. PLoS One, 8(11), e81279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodell-Feder D., Tully L.M., Lincoln S.H., Hooker C.I. (2014b). The neural basis of theory of mind and its relationship to social functioning and social anhedonia in individuals with schizophrenia. Neuroimage Clinical, 4, 154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Cole S.W. (2012). Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nature Neuroscience, 15(5), 669–74. [DOI] [PubMed] [Google Scholar]
- Flesch R. (1948). A new readability yardstick. The Journal of Applied Psychology, 32(3), 221–33. [DOI] [PubMed] [Google Scholar]
- Franzoi S.L., Davis M.H., Young R.D. (1985). The effects of private self-consciousness and perspective taking on satisfaction in close relationships. Journal of Personality and Social Psychology, 48(6), 1584–94. [DOI] [PubMed] [Google Scholar]
- Gable S.L., Gonzaga G.C., Strachman A. (2006). Will you be there for me when things go right? Supportive responses to positive event disclosures. Journal of Personality and Social Psychology, 91(5), 904–17. [DOI] [PubMed] [Google Scholar]
- Galinsky A.D., Maddux W.W., Gilin D., White J.B. (2008). Why it pays to get inside the head of your opponent: the differential effects of perspective taking and empathy in negotiations. Psychological Science, 19(4), 378–84. [DOI] [PubMed] [Google Scholar]
- Goldstein N.J., Vezich I.S., Shapiro J.R. (2014). Perceived perspective taking: when others walk in our shoes. Journal of Personality and Social Psychology, 106(6), 941–60. [DOI] [PubMed] [Google Scholar]
- Gweon H., Dodell-Feder D., Bedny M., Saxe R. (2012). Theory of mind performance in children correlates with functional specialization of a brain region for thinking about thoughts. Child Development, 83(6), 1853–68. [DOI] [PubMed] [Google Scholar]
- Hendrick S.S. (1988). A generic measure of relationship satisfaction. Journal of Marriage and the Family, 50(1), 93–8. [Google Scholar]
- Holt-Lunstad J., Smith T.B., Layton J.B. (2010). Social relationships and mortality risk: a meta-analytic review. PLoS Medicine, 7(7), e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C.I., Bruce L., Lincoln S.H., Fisher M., Vinogradov S. (2011). Theory of mind skills are related to gray matter volume in the ventromedial prefrontal cortex in schizophrenia. Biological Psychiatry, 70(12), 1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C.I., Verosky S.C., Germine L.T., Knight R.T., D’Esposito M. (2008). Mentalizing about emotion and its relationship to empathy. Social Cognitive and Affective Neuroscience, 3(3), 204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C.I., Verosky S.C., Germine L.T., Knight R.T., D’Esposito M. (2010). Neural activity during social signal perception correlates with self-reported empathy. Brain Research, 1308, 100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House J.S., Landis K.R., Umberson D. (1988). Social relationships and health. Science, 241(4865), 540–5. [DOI] [PubMed] [Google Scholar]
- Hox J.J. (2002). Multilevel Analysis : Techniques and Applications. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Jenkins A.C., Macrae C.N., Mitchell J.P. (2008). Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proceedings of the National Academy of Sciences of the United States of America, 105(11), 4507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Price R.H., Wortman C.B. (1985). Social factors in psychopathology: stress, social support, and coping processes. Annual Review of Psychology, 36, 531–72. [DOI] [PubMed] [Google Scholar]
- Krienen F.M., Tu P.C., Buckner R.L. (2010). Clan mentality: evidence that the medial prefrontal cortex responds to close others. Journal of Neuroscience, 30(41), 13906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M.V., Chakrabarti B., Bullmore E.T., Consortium M.A., Baron-Cohen S. (2011). Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. Neuroimage, 56(3), 1832–8. [DOI] [PubMed] [Google Scholar]
- Long E.C.J. (1993). Perspective-taking differences between high- and low-adjustment marriages: Implications for those in intervention. The American Journal of Family Therapy, 21(3), 248–59. [Google Scholar]
- Long E.C.J., Andrews D.W. (1990). Perspective taking as a predictor of marital adjustment. Journal of Personality and Social Psychology, 59(1), 126–31. [Google Scholar]
- Loving T.J., Slatcher R.B. (2013). Romantic Relationships and Health. New York: Oxford University Press. [Google Scholar]
- Mar R.A. (2011). The neural bases of social cognition and story comprehension. Annual Review of Psychology, 62, 103–34. [DOI] [PubMed] [Google Scholar]
- Masten C.L., Morelli S.A., Eisenberger N.I. (2011). An fMRI investigation of empathy for ‘social pain’ and subsequent prosocial behavior. Neuroimage, 55(1), 381–8. [DOI] [PubMed] [Google Scholar]
- Matheson S.L., Vijayan H., Dickson H., Shepherd A.M., Carr V.J., Laurens K.R. (2013). Systematic meta-analysis of childhood social withdrawal in schizophrenia, and comparison with data from at-risk children aged 9-14 years. Journal of Psychiatric Research, 47(8), 1061–8. [DOI] [PubMed] [Google Scholar]
- Mitchell J.P., Banaji M.R., Macrae C.N. (2005). The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience, 17(8), 1306–15. [DOI] [PubMed] [Google Scholar]
- Mitchell J.P., Macrae C.N., Banaji M.R. (2006). Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron, 50(4), 655–63. [DOI] [PubMed] [Google Scholar]
- Morelli S.A., Torre J.B., Eisenberger N.I. (2014). The neural bases of feeling understood and not understood. Social Cognitive and Affective Neuroscience, 9(12), 1890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R.H. (1990). Classical and Modern Regression with Applications. Belmont, CA: Duxbury Press. [Google Scholar]
- Myin-Germeys I., Oorschot M., Collip D., Lataster J., Delespaul P., van Os J. (2009). Experience sampling research in psychopathology: opening the black box of daily life. Psychological Medicine, 39(9), 1533–47. [DOI] [PubMed] [Google Scholar]
- Pelli D.G. (1997). The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision, 10(4), 437–42. [PubMed] [Google Scholar]
- Perner J., Aichhorn M., Kronbichler M., Staffen W., Ladurner G. (2006). Thinking of mental and other representations: the roles of left and right temporo-parietal junction. Social Neuroscience, 1(3–4), 245–58. [DOI] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., DebRoy S., Sarkar D., R Core Team (2014). nlme: Linear and Nonlinear Mixed Effects Models. [Google Scholar]
- Powers K.E., Chavez R.S., Heatherton T.F. (2015). Individual differences in response of dorsomedial prefrontal cortex predict daily social behavior. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, ISBN 3-900051-07-0, http://www.R-project.org/.
- Rameson L.T., Morelli S.A., Lieberman M.D. (2012). The neural correlates of empathy: experience, automaticity, and prosocial behavior. Journal of Cognitive Neuroscience, 24(1), 235–45. [DOI] [PubMed] [Google Scholar]
- Reis H.T., Sheldon K.M., Gable S.L., Roscoe J., Ryan R.M. (2000). Daily well-being: the role of autonomy, competence, and relatedness. Personality and Social Psychology Bulletin, 26(4), 419–35. [Google Scholar]
- Rice K., Redcay E. (2014). Spontaneous mentalizing captures variability in the cortical thickness of social brain regions. Social Cognitive and Affective Neuroscience, 10(3), 327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savla G.N., Vella L., Armstrong C.C., Penn D.L., Twamley E.W. (2013). Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophrenia Bulletin, 39(5), 979–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. (2003). People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind.” Neuroimage, 19(4), 1835–42. [DOI] [PubMed] [Google Scholar]
- Saxe R., Powell L.J. (2006). It’s the thought that counts: specific brain regions for one component of theory of mind. Psychological Science, 17(8), 692–9. [DOI] [PubMed] [Google Scholar]
- Saxe R., Wexler A. (2005). Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia, 43(10), 1391–9. [DOI] [PubMed] [Google Scholar]
- Schaafsma S.M., Pfaff D.W., Spunt R.P., Adolphs R. (2014). Deconstructing and reconstructing theory of mind. Trends in Cognitive Sciencesh, 19(2), 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M., Aichhorn M., Martin A., Perner J. (2013). Common brain areas engaged in false belief reasoning and visual perspective taking: a meta-analysis of functional brain imaging studies. Frontiers in Human Neuroscience, 7, 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M., Radua J., Aichhorn M., Richlan F., Perner J. (2014). Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews, 42, 9–34. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., et al. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59(Suppl. 20), 22-33; quiz 34-57. [PubMed] [Google Scholar]
- Spunt R.P., Adolphs R. (2014). Validating the Why/How contrast for functional MRI studies of Theory of Mind. Neuroimage, 99, 301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Fletcher G.J. (2003). Mind-reading accuracy in intimate relationships: assessing the roles of the relationship, the target, and the judge. Journal of Personality and Social Psychology, 85(6), 1079–94. [DOI] [PubMed] [Google Scholar]
- Uchino B.N. (2006). Social support and health: a review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine, 29(4), 377–87. [DOI] [PubMed] [Google Scholar]
- Umberson D., Crosnoe R., Reczek C. (2010). Social relationships and health behavior across life course. Annual Review of Sociology, 36, 139–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. (2009). Social cognition and the brain: a meta-analysis. Human Brain Mapping, 30(3), 829–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele T.J., Hawkley L.C., Cacioppo J.T. (2012). On the reciprocal association between loneliness and subjective well-being. American Journal of Epidemiology, 176(9), 777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waytz A., Zaki J., Mitchell J.P. (2012). Response of dorsomedial prefrontal cortex predicts altruistic behavior. Journal of Neuroscience, 32(22), 7646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welborn B.L., Lieberman M.D. (2014). Person-specific theory of mind in medial pFC. Journal of Cognitive Neuroscience, 27(1), 1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.