Abstract
Generalized social anxiety disorder (gSAD) is associated with aberrant anterior cingulate cortex (ACC) response to threat distractors. Perceptual load has been shown to modulate ACC activity such that under high load, when demands on processing capacity is restricted, individuals with gSAD exhibit compensatory activation to threat distractors yet under low load, there is evidence of reduced activation. It is not known if neural predictors of response to cognitive behavioral therapy (CBT), based on such emotional conflict resolution, interact with demands on controlled processes. Prior to CBT, 32 patients with gSAD completed an fMRI task involving a target letter in a string of identical targets (low perceptual load) or a target letter in a mixed letter string (high perceptual load) superimposed on fearful, angry and neutral face distractors. Whole-brain voxel-wise analyses revealed better CBT outcome was predicted by more frontopartial activity that included dorsal ACC (dACC) and insula to threat (vs neutral) distractors during high, but not low, perceptual load. Psychophysiological interaction analysis with dACC as the seed region revealed less connectivity with dorsolateral prefrontal cortex to threat distractors during high load. Results indicate patients with less regulatory capability when demands on higher-order control are great may benefit more from CBT.
Keywords: generalized social anxiety, fMRI, treatment, threat faces, brain imaging, predictors
Introduction
Generalized social anxiety disorder (gSAD) is a common psychiatric illness characterized by inappropriate, pervasive fears of potential scrutiny by others across a variety of social and/or performance situations (American Psychiatric Association, 2000; Kessler et al., 2005). ‘Gold standard’ psychotherapy for gSAD and other internalizing disorders (e.g. posttraumatic stress disorder, major depressive disorder) is cognitive behavioral therapy (CBT) (Butler et al., 2006). While generally effective, treatment response varies and many remain symptomatic after completing CBT (Heimberg et al., 1998; Davidson et al., 2004).
The neurobiology of gSAD points to dysfunction in fear circuitry evinced by consistent reports of exaggerated amygdala reactivity and abnormal prefrontal cortical activation to threat-relevant signals (Brühl et al., 2014). In CBT, techniques such as cognitive restructuring to alter maladaptive thoughts and the facing of social fears (e.g. in vivo exposures) (Heimberg, 2002) suggest individual differences in regions involved in fear processes (e.g. amygdala; LeDoux, 2000; Hariri and Whalen, 2011) and its regulation [e.g. anterior cingulate cortex (ACC), lateral prefrontal cortex; Gross, 2002; Phillips et al., 2003; Ochsner et al., 2009; Etkin, 2010] may help ‘set the stage’ in CBT outcome. For example, in posttraumatic stress disorder (PTSD), CBT success was shown to be predicted by less responsivity to masked fearful faces in amygdala and rostral ACC indicating excessive anxiety to threat prior to CBT led to poorer response (Bryant et al., 2008). In major depressive disorder (MDD), which shares neurobiological features with anxiety disorders (e.g. exaggerated amygdala and dorsal ACC response to negative stimuli; Hamilton et al., 2012), less pre-CBT subgenual ACC activity also corresponded with CBT success though, here, greater treatment response was predicted by more amygdala reactivity to negative words (Siegle et al., 2006). Thus, in MDD patients with less ability to regulate affective state yet greater reactivity to mood-congruent stimuli did better in CBT (Siegle et al., 2006).
In gSAD, a slightly different pattern has been demonstrated. Doehrmann et al. (2013) were the first to show patients with enhanced ability to regulate emotions to threat-relevant stimuli had greater symptom improvement as signified by more pre-CBT activation in secondary visual areas (dorsal and ventral occipitotemporal cortex) as well as dorso- and ventrolateral prefrontal regions, though these frontal areas emerged at a trend level.
We also observed a relationship between symptom improvement and more activation in visual areas (middle temporal and angular gyri) and prefrontal regions (e.g. medial orbitofrontal, dorsomedial frontal gyrus) (Klumpp et al., 2013). Additionally, in a study of emotion processing and sustained attentional control to emotional face distractors, symptom change in gSAD was predicted by less amygdala and more dorsal ACC activation, respectively (Klumpp et al., 2014). Collectively, data suggest gSAD patients with greater automatic emotion regulation and less emotional reactivity to socioemotional signals prior to CBT might better manage the distress evoked in CBT and consequently gain more from treatment.
In a departure from emotion perception, Falconer et al. (2013) examined neural predictors related to inhibitory control (Go/No Go task) in PTSD and reported symptom improvement was foretold by activation in a discrete frontostriatal network (e.g. anterior medial prefrontal cortex, orbitofrontal/inferior frontal gyrus, dorsal striatum). In contrast, less improvement was associated with activation in a widely distributed frontoparietalstriatal and cerebellar network (e.g. inferior frontal/ventrolateral prefrontal cortex/insula, precuneus, putamen, cerebellum). Results indicate PTSD patients with more efficient top-down control were likely to benefit more from CBT (Falconer et al., 2013).
While methodological differences across studies, including patient sample, may have contributed to mixed results, accumulating data suggest pre-CBT cortical and/or limbic response to relevant stimuli may serve as biomarkers in CBT outcome. For gSAD, data suggest regulatory ability is capitalized on by CBT; however, findings are largely based on studies of emotion perception. A next step would be to examine predictors related to higher-order control over threat distractors given cognitive models of social anxiety propose attentional bias to threat plays a key role in the maintenance of fears (Clark and Wells, 1995; Mogg and Bradley, 1998). A means of evaluating attentional bias relates to perceptual load theory, which proposes task-irrelevant stimuli (i.e. distractors) are subject to being processed unless attentional load capacity is exhausted (Lavie, 1995, 2000; Lavie et al., 2009). Thus, under low perceptual load, resources towards cognitive goals are available to process distractors, whereas, under high load, the restriction of attentional capacity inhibits the processing of distractors. Therefore, an advantage of the model is its ability to probe facilitatory and inhibitory processes in the context of salient, task-irrelevant distractors.
In gSAD, difficulty ‘ignoring’ sensory-driven, bottom-up threat distractors may be due in part to aberrant ACC. The ACC is part of a corticolimbic system that plays a role in the appraisal of salient stimuli, generation of adaptive responses and regulation of emotional state (Bush et al., 2000; Etkin et al., 2011). The affective, ventral-rostral region has connections with limbic (e.g. amygdala) and paralimbic (e.g. insula, orbitofrontal cortex) structures (Devinsky et al., 1995) and is primarily associated with emotional conflict resolution (Etkin et al., 2006; Egner et al., 2008; Ochsner et al., 2009) and evaluative functions (e.g. assessing salience of stimuli) (Bush et al., 2000; Etkin et al., 2011). The cognitive area instead, with interconnections to other cortical regions (e.g. lateral prefrontal cortex, supplementary motor cortex) (Devinsky et al., 1995), is more closely involved with conflict-related processes (e.g. conflict monitoring, error detection) and adaptive response to motivationally relevant information (Carter et al., 1998; Bush et al., 2000; MacDonald et al., 2000; Botvinick et al., 2001; Liu et al., 2006; Banich et al., 2009; Etkin et al., 2011).
Evidence of ACC dysfunction during emotional conflict in gSAD include observations of exaggerated rostral (rACC) activation to threat distractors, relative to healthy controls, when attentional capacity resources are restricted (high perceptual load) denoting a compensatory response in gSAD to maintain goal-directed behavior (Wheaton et al., 2014). However, when demands on control are relatively low (i.e. task is fairly easy to execute) gSAD is associated with deficient rACC recruitment to salient face distractors (Klumpp et al., 2013; Wheaton et al., 2014). These data are consistent with findings by Bishop et al. (2007) who reported an inverse relationship between trait anxiety level and recruitment in dorsal ACC, dorsolateral prefrontal cortex (PFC) and ventrolateral PFC (with a non-significant trend towards rACC) to threat face distractors when perceptual load was low. Together, when resources to process task-irrelevant stimuli are very much constrained, there may be protracted engagement of control processes in gSAD. Yet, when more resources are available to process distractors (e.g. low perceptual load), there is evidence of impoverished frontal engagement in anxious individuals.
Results suggest ACC activity is modulated by perceptual load in gSAD and that individual differences in ACC, along the lines of attenuated or compensatory neural response during conflict resolution, may interact with CBT. Based on the literature, we hypothesized greater control over task-irrelevant threat faces prior to CBT would portend better response to CBT. Accordingly, under low perceptual load, change in symptom severity was expected to correspond with more activation in areas such as dorsal ACC, lateral PFC and/or rostral medial PFC (Devinsky et al., 1995; Bush et al., 2000; Ochsner et al., 2009; Etkin et al., 2011). For high perceptual load, we hypothesized patients with greater focal activation to threat distractors in medial and/or lateral PFC would improve more after completing CBT. To explore other areas that contributed to pre-CBT response during cognitive control, significant a priori regions of interest were followed-up with psychophysiological interactions analysis.
Method
Participants
All participants provided written informed consent as approved by the local Institutional Review Board at the University of Illinois at Chicago (UIC) (n = 22) and University of Michigan (UM) (n = 10). Data on a portion of the University of Michigan participants (n = 9) were published previously (Wheaton et al., 2014). Participants were recruited through the Mood and Anxiety Disorders Program (UIC) or Anxiety Disorders Treatment Clinic (UM) by means of flyers posted throughout the communities, newspaper and internet advertisement. Interested participants completed a phone screen followed by a psychiatric evaluation during which time participants reviewed the consent form. After attaining consent, participants met with a clinician trained in the Structured Clinical Interview for DSM-IV (‘SCID-IV’; First et al., 1995) and clinician-administered measures. The participant’s medical history was reviewed by a Board Certified physician and during the evaluation, participants completed self-report measures. Diagnosis was based on the SCID-IV and the clinician-administered Liebowitz Social Anxiety Scale (‘LSAS’; Liebowitz, 1987) was used to assess symptom severity. The self-reported Spielberger State-Trait Anxiety Inventory (Spielberger, 1983) and Beck Depression Inventory (Beck et al., 1996) were used to evaluate general anxiety and depression levels, respectively. Participants were compensated $15 per hour for their time.
Inclusion/exclusion criteria
All participants were between 18 and 55 years of age, free of major medical or neurologic illness as confirmed by a Board Certified physician. As long as gSAD was the primary complaint, participants with a comorbid disorder were not excluded. All participants were free of psychotropic medications and none were receiving concurrent psychotherapy. None of the participants tested positive for drugs.
Exclusion criteria included contraindications to magnetic resonance imaging (e.g. pregnancy, claustrophobia, non-removable ferrous objects), current substance abuse or dependence (within 6 months of study), or any history of major psychiatric illness (e.g. bipolar disorder, psychotic disorder, pervasive developmental disorder).
Treatment
Within a week of the first fMRI scan, patients began once-weekly sessions of manualized individual CBT for 12 weeks, which comprised psycho-education, strategies to reduce negative beliefs (e.g. cognitive restructuring), in vivo exposures to fears and relapse prevention (Hope et al., 2006). A CBT-trained licensed clinical psychologist or post-doctoral clinical psychologist conducted treatment. The clinicians were supervised by a licensed clinical psychologist with expertise in CBT and clinical trials. The Clinical Global Impression-Improvement (CGI-I; Busner and Targum, 2007), comprising a 7-point scale (1 = very much improved, 7 = worsening symptoms), was used to determine whether a patient responded to treatment and remission was defined as patients who achieved an LSAS score of ≤30 (Mennin et al., 2002; Liebowitz et al., 2005).
fMRI task
During fMRI, participants viewed a string of six letters superimposed on a task-irrelevant face distractor and were asked to identify target letters (N or X). Specifically, they were instructed as follows: ‘For the following task we want you to look at the letters on each face. Press the first button (Index Finger) if there is an X present. Press the second button (Middle Finger) if there is an N present. Make your decisions as quickly and accurately as possible. Please guess if you're not sure.’
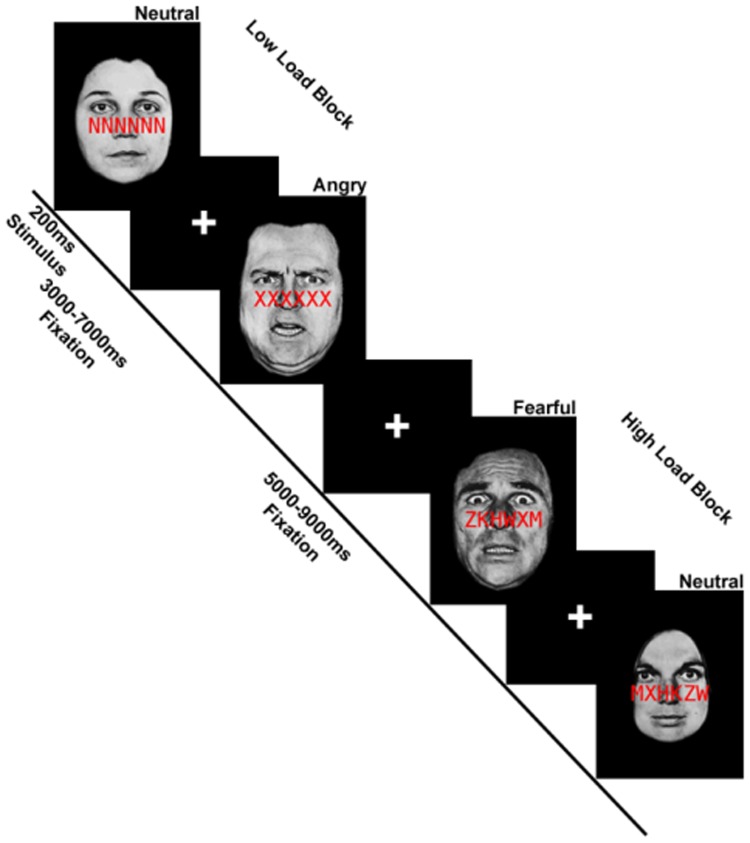
In low perceptual load trials, the string was comprised entirely of target letters; under high perceptual load, the string included a single target letter and five non-target letters (H, K, M, W, Z) arranged in randomized order. Distractor faces were from a standardized set of photographs and consisted of fearful, angry and neutral expressions from eight different individuals (Eckman and Friesen, 1976). Our paradigm was adapted from Bishop et al. (2007) wherein task-irrelevant fearful faces were contrasted with task-irrelevant neutral faces to examine the effects of anxiety level on threat distractors under low and high load. In addition to fearful expressions as distractors, we included angry face distractors, as anger and fear have shown differential effects in emotion processing circuitry (Whalen et al., 2001). The experiment involved two image acquisition runs, each comprising 12 blocks of 5 trials. A mixed block/event-related design was employed whereby perceptual load (low vs high) varied across blocks and facial expression (fearful, angry, neutral) varied within blocks on a trial-by-trial basis. Images were presented for 200 ms followed by a fixation cross presented for 1800 ms; responses were made via button press. Within blocks, trials were separated by a jittered interstimulus interval lasting 2–6 s; trials between blocks were separated by 4–8 s (Figure 1).
Fig. 1.
Schematic of an exemplar of low perceptual load and high perceptual load blocks superimposed on task-irrelevant faces in the functional magnetic resonance imaging (fMRI) paradigm. Adapted from Bishop et al. (2007).
fMRI data acquisition and preprocessing
This study was conducted on two 3 Tesla GE Signa System (General Electric; Milwaukee, WI) scanners, all using the same standard radiofrequency coil. All scanning was performed with blood oxygen-level dependent (BOLD)-sensitive whole-brain fMRI using the same GE software (LX 8.3, Neuro-optimized gradients). At the University of Michigan (n = 10) images were acquired with a gradient-echo reverse spiral acquisition with the following parameters: TR = 2 s, TE = 25 ms, flip angle = 77°, field of view = 24 × 24 cm2, acquisition matrix 64 × 64; 30 axial, 5-mm-thick slices with no gap. At the University of Illinois at Chicago (n = 22) images were acquired using a gradient-echo echo-planar imaging sequence with the following parameters: TR = 2s, TE = 25 ms, flip angle = 90°, field of view = 22 x 22 cm2, acquisition matrix 64 x 64; 44 axial, 3-mm-thick slices with no gap. For anatomical localization, a high-resolution, T1-weighted volumetric anatomical scan was acquired.
Data from all participants met criteria for quality with minimal motion correction (movements were < 3 mm and < 3 degrees rotation in any one direction) and the first four volumes from each run were discarded to allow for T1 equilibration effects. Conventional preprocessing steps were used in Statistical Parametric Mapping (SPM8) software package (Wellcome Trust Centre for Neuroimaging, London www.fil.ion.ucl.ac.uk/spm). Briefly, images were temporally corrected to account for differences in slice time collection, spatially realigned to the first image of the first run, normalized to a Montreal Neurological Institute (MNI) template, resampled to 2 × 2 × 2 mm3 voxels and smoothed with an 8-mm isotropic Gaussian kernel.
fMRI analyses
A general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128-s high-pass filter. Nuisance regressors comprising six motion parameters were included to correct for motion artifacts. Blocks of low and high perceptual load were modeled separately based on task-irrelevant face type (fearful, angry, neutral) resulting in six regressors (fearful low, fearful high, angry low, angry high, neutral low, neutral high), the effects of which were estimated for each voxel for each participant and taken to the second level for random effects analysis.
To assess predictors of CBT response, contrasts of interest from gSAD pre-treatment scans were entered into a whole-brain analysis of covariance, regressing LSAS change (ΔPreTx – PostTx) while initial severity (LSASPreTx) and scanner site were controlled for as regressors of no interest. Consistent with prior fMRI studies of gSAD concerning whole-brain analysis (Amir et al., 2005; Evans et al., 2008; Gentili et al., 2009; Phan et al., 2013; Klumpp et al., 2014), significance was set at P < 0.001 (uncorrected) with a cluster extent threshold at least 10 contiguous voxels (volume ≥ 80 mm3). This type of joint intensity and cluster size threshold is within the recommended threshold to attain a balance between Type I and Type II errors (Lieberman and Cunningham, 2009). Frontal region(s) implicated in cognitive control that emerged from whole-brain analysis were followed-up with PsychoPhysiological Interactions (PPI) analysis (Friston et al., 1997) as implemented in SPM8 to evaluate regions functionally connected with baseline activation predictive of symptom change.
To illustrate the magnitude and direction of activation, parameter estimates of peak activation (β weights, arbitrary units [a.u.]) were extracted from spherical (10-mm diameter) regions of interest from each participant and submitted to post hoc Pearson’s correlations (2-tailed with alpha level at .05) and scatter plots in the Statistical Package for the Social Sciences (SPSS) (Chicago, IL version 22).
Results
Participants
Thirty-two patients with gSAD (75% female) had a mean age of 25.4 ± 5.1 years. Self-reported onset of SAD was classified in terms childhood (31.3%), adolescence (43.8%) and adult (15.6%) as many patients described onset (e.g. ‘since I can remember’, ‘around High School’) as opposed to a particular age. Data was missing for three patients; nevertheless, findings are consistent with reports of an early onset in SAD (e.g., before 25 years; Wittchen et al., 1999). At the start of treatment, patients had an average symptom severity (LSAS total score) of 74.3 ± 14.9, which is consistent with a clinical cutoff of ≥60 for gSAD (Heimberg et al., 1999; Mennin et al., 2002). With regard to comorbidity, two patients in the University of Michigan cohort had concurrent specific phobia and one had generalized anxiety disorder. In the University of Illinois at Chicago sample, nine patients had generalized anxiety disorder; two, panic disorder; two, major depressive disorder; four, dysthymia; one, specific phobia; one, posttraumatic stress disorder; and one adjustment disorder. Six patients had more than one comorbid disorder. Despite concurrent depression for a portion of patients, average level of depression was in the mild range 16.7 ± 10.6 (Beck et al., 1996) and state and trait anxiety averaged 48.3 ± 12.1 and 53.2 ± 9.6, respectively. Regarding race/ethnicity, 54.5% self-identified as Caucasian, 18.3% as Asian, 22.7% as Hispanic and 4.5% as African American.
Treatment effects on social anxiety severity
After completing all 12 weeks of CBT, gSAD symptom severity, as assessed with LSAS, significantly decreased from an average of 74.3 ± 14.9 to 47.9 ± 20.4 [(31) t = 7.1, P < 0.001] as did depression level (16.7 ± 10.6 to 3.5 ± 3.5) [(31) t = 7.0, P < 0.002]. Based on the CGI-I, 69% of the gSAD group (22 of 32 patients) were considered to be ‘Responders’ as they were rated to be ‘very much improved’ or ‘much improved’ (CGI-I score of 1 or 2) while 10 of 32 patients had a CGI-I score of >2 post-treatment and were thus considered ‘Non-Responders’. Remission rate was lower as approximately 16% (5 of 32 patients) reached remission based on LSAS and about 84% (27 of 32) did not attain an LSAS score of ≤ 30 (Mennin et al., 2002; Liebowitz et al., 2005).
Behavioral performance
To confirm the perceptual load manipulation was effective, overall accuracy and response time were evaluated. Planned comparisons with two-tailed t-tests revealed higher accuracy for the low (93.8 ± 8.2%) relative to high load condition (66.0 ± 10.0%), [t(31) = 15.7, P < 0.001]. Additionally, reaction time (RT) for accurate trials was faster in the low (814.5 ± 105.8 ms) compared to high load condition (1183.9 ms ± 180.6 ms) [t(31) = 16.3, P < 0.001]. See Table 1 for all behavioral results.
Table 1.
Means and standard deviations for accuracy and reaction times (ms) by load condition for face distractor type
| Condition | Accuracy | Reaction time |
|---|---|---|
| High load fear | 63.8 ± 13.5 | 1220.8 ± 208.2 |
| High load angry | 70.8 ± 10.4 | 1168.5 ± 177.3 |
| High load neutral | 63.6 ± 10.6 | 1162.3 ± 187.5 |
| Low load fear | 93.9 ± 8.0 | 816.1 ± 109.0 |
| Low load angry | 94.1 ± 8.2 | 806.3 ± 108.3 |
| Low load neutral | 93.3 ± 9.3 | 821.0 ± 117.6 |
fMRI results
Fearful (vs Neutral) Distractors: High Perceptual Load
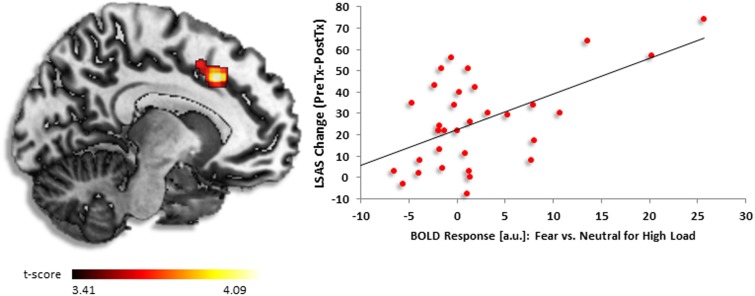
Whole-brain findings from regression analysis revealed greater baseline activation in dorsal anterior cingulate cortex (dACC) [(−8, 22, 38), z = 3.59, k = 357, volume = 2856 mm3] positively corresponded to greater reduction in symptom severity after completing CBT (Figure 2). Follow-up correlations in SPSS illustrated a positive relationship between activation and symptom change (r = 0.56, P < 0.001).
Fig. 2.
(A) Regressing LSAS change (ΔPreTx − PostTx) while initial severity (LSASPreTx) is controlled for as a regressor of no interest, brain map depicts whole-brain analysis of covariance showing dorsal anterior cingulate activity in gSAD as denoted by positive parameter estimates of activation based on fearful vs neutral distractors during high perceptual load displayed on statistical t-map at P < 0.001. (B) Scatter plot of the regression analyses depicting extracted parameter estimates of activation from dorsal anterior cingulate showing greater response to CBT in gSAD was predicted by more dorsal anterior cingulate activity in the presence of fearful vs neutral face distractors during high load.
Additional whole-brain findings showed more symptom improvement was linked to greater pre-CBT activity in anterior insula, frontal inferior triangularis, inferior parietal gyrus and precentral gyrus. The only negative association with symptom change was observed in the paracentral lobule. See Table 2 for all regression results.
Table 2.
Pre-treatment to post-treatment decrease in social anxiety severity, controlling for pre-treatment severity: whole-brain voxel-wise regressiona
| MNI Coordinates |
Volume | Z | ||||
|---|---|---|---|---|---|---|
| Region | x | y | z | (mm3) | ||
| High perceptual load | ||||||
| Fear vs neutral | ||||||
| Positive correlation | Inferior frontal triangularis | 34 | 22 | 12 | 2408 | 4.38 |
| Inferior parietal gyrus | 62 | − 42 | 52 | 640 | 3.83 | |
| Precentral gyrus | − 52 | 8 | 44 | 3432 | 3.74 | |
| Anterior cingulate | − 8 | 22 | 38 | 2856 | 3.59 | |
| Superior frontal gyrus | 16 | −4 | 72 | 248 | 3.51 | |
| Inferior parietal lobule | −66 | −40 | 38 | 104 | 3.47 | |
| Rolandic operculum | 34 | 0 | 18 | 912 | 3.43 | |
| Superior frontal gyrus | 16 | 50 | 38 | 120 | 3.24 | |
| Insula | −32 | 22 | 14 | 144 | 3.23 | |
| Medial frontal gyrus | 26 | 36 | 30 | 88 | 3.18 | |
| Negative correlation | Paracentral lobule | −4 | −34 | 80 | 392 | 3.35 |
| Angry vs neutral | ||||||
| Positive correlation | Insula | −32 | 6 | 16 | 2888 | 4.55 |
| Anterior cingulate | 6 | 38 | 24 | 736 | 3.33 | |
| Paracentral lobule | −6 | −18 | 66 | 1336 | 4.04 | |
| Insula | 28 | −2 | 22 | 808 | 3.88 | |
| Putamen | 28 | −2 | 22 | 872 | 3.75 | |
| Superior frontal medial gyrus | −6 | 42 | 48 | 760 | 3.74 | |
| Rolandic operculum | 56 | −12 | 16 | 600 | 3.65 | |
| Insula | −34 | −32 | 24 | 488 | 3.63 | |
| Supplementary motor area | 8 | −2 | 60 | 392 | 3.34 | |
| Insula | 40 | 0 | −4 | 88 | 3.34 | |
| Supramarginal gyrus | −54 | −22 | 16 | 136 | 3.33 | |
| Postcentral gyrus | 70 | −10 | 18 | 456 | 3.31 | |
| Superior frontal gyrus | −16 | 16 | 14 | 312 | 3.26 | |
| Negative correlation | No suprathreshold clusters | |||||
| Low perceptual load | ||||||
| Fear vs neutral | ||||||
| Positive correlation | No suprathreshold clusters | |||||
| Negative correlation | No suprathreshold clusters | |||||
| Angry vs neutral | ||||||
| Positive correlation | Supramarginal gyrus | 64 | −28 | 46 | 392 | 3.46 |
| Negative correlation | No suprathreshold clusters | |||||
Regions of interest are in bold. MNI, Montreal Neurological Institute; Z, Z-score.
aAll listed clusters significant at P < 0 .001 (uncorrected) with a cluster extent threshold of at least 10 contiguous voxels.
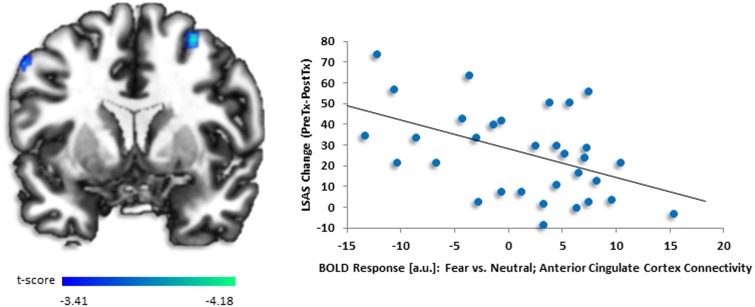
Exploratory PPI analysis with left dACC (−8, 22, 38) as seed region taken from whole-brain regression analysis showed less co-activation with bilateral dorsolateral prefrontal cortex (dlPFC) [right (30, 10, 56), z = 3.65, k = 31, volume = 248 mm3; left (−42, 22, 52), z = 3.43, k = 38, volume = 304 mm3] (Figure 3). Follow-up correlations in SPSS illustrated a negative relationship between baseline dlPFC activity and symptom change (right r = −0.47, P < 0.007; left r = −0.62, P < 0.001). Other regions associated with decreased dACC connectivity were precuneus, precentral gyrus and superior frontal medial gyrus. The only region with increased connectivity with dACC was insula. See Table 3 for all PPI results.
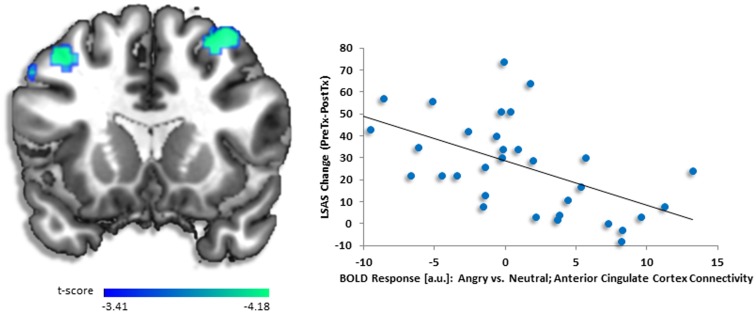
Fig. 3.
(A) Regressing LSAS change (ΔPreTx - PostTx) while initial severity (LSASPreTx) is controlled for as a regressor of no interest, brain map depicts whole-brain analysis of covariance showing less dorsolateral prefrontal cortex functional connectivity with dorsal anterior cingulate cortex denoted by negative parameter estimates of activation based on the fearful vs neutral distractors in high perceptual load displayed on statistical t-map at P < 0.001. (B) Scatter plot of the regression analyses showing extracted parameter estimates of activation from dorsolateral prefrontal cortex region of interest (i.e. 30, 10, 56) demonstrating more symptom change after completing CBT in gSAD corresponded to less activity in dorsolateral prefrontal region to fearful vs neutral face distractors in high load. Scatter plots for other dorsolateral prefrontal regions of interest were similar but are not shown.
Table 3.
Pre-treatment to post-treatment decrease in social anxiety severity, controlling for pre-treatment severity: whole-brain voxel-wise dorsal anterior cingulate cortex-connectivity regressiona
| Region | MNI Coordinates |
Volume | Z | |||
|---|---|---|---|---|---|---|
| x | y | z | (mm3) | |||
| High perceptual load | ||||||
| Fear vs neutral | ||||||
| Positive correlation | Insula | −36 | −10 | 24 | 112 | 3.64 |
| Negative correlation | Dorsolateral prefrontal cortex | 30 | 10 | 56 | 248 | 3.65 |
| Precuneus | 20 | −60 | 42 | 1064 | 3.62 | |
| Precentral gyrus | −56 | 10 | 44 | 360 | 3.60 | |
| Dorsolateral prefrontal cortex | −42 | 22 | 52 | 304 | 3.43 | |
| Superior frontal medial gyrus | −8 | 50 | 40 | 152 | 3.26 | |
| Angry vs neutral | ||||||
| Positive correlation | No suprathreshold clusters | |||||
| Negative correlation | Dorsolateral prefrontal cortex | 34 | 12 | 56 | 1808 | 4.56 |
| −34 | 32 | 26 | 752 | 3.65 | ||
| Precentral gyrus | −54 | 12 | 40 | 152 | 3.65 | |
| Superior frontal gyrus | −10 | 48 | 42 | 152 | 3.62 | |
| Dorsolateral prefrontal cortex | −40 | 16 | 48 | 784 | 3.60 | |
| Superior frontal gyrus | −16 | 0 | 56 | 192 | 3.58 | |
| 18 | −16 | 64 | 88 | 3.40 | ||
Regions of interest are in bold. MNI, Montreal Neurological Institute; Z, Z-score.
aAll listed clusters significant at P < 0.001 (uncorrected) with a cluster extent threshold of at least 10 contiguous voxels.
Fearful (vs neutral) distractor: low perceptual load
No significant effects were revealed
Angry (vs neutral) distractors: high perceptual load
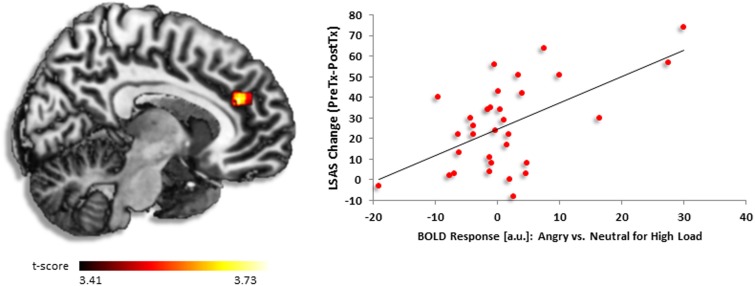
Similar to the fearful (vs neutral) condition, greater reduction in symptom severity after CBT was foretold by more activation in dACC [(6, 38, 24), z = 3.33, k = 92, volume = 736 mm3] (Figure 4). However, here the activation was part of a large insula cluster [(−32, 6, 16), z = 4.55, k = 361, volume = 2888 mm3)]. Follow-up analysis in SPSS verified a positive relationship between dACC activity and symptom change (r = 0.57, P < 0.001).
Fig. 4.
(A) Regressing LSAS change (ΔPreTx − PostTx) while pre-CBT severity (LSASPreTx) is controlled for as a regressor of no interest, brain map demonstrates whole-brain analysis of covariance showing dorsal anterior cingulate activity in gSAD as signified by positive parameter estimates of activation based on the angry vs neutral distractors during high perceptual load displayed on statistical t-map at P < 0.001. B) Scatter plot of the regression analyses depicting extracted parameter estimates of activation from dorsal anterior cingulate indicating greater response to CBT in gSAD was predicted by greater dorsal anterior cingulate activity in the presence of angry vs neutral face distractors during high load.
Additional whole-brain findings, beyond regions of interest, revealed greater symptom change was related to greater baseline activity in a set of regions that included paracentral lobule, putamen, frontal superior medial gyrus and supplementary motor area. There was no evidence CBT outcome was predicted by less baseline activation. See Table 2 for all regression results.
Exploratory PPI analysis conducted with right dACC (6, 38, 24) as seed region taken from whole-brain regression analysis showed anticorrelations with several discrete dlPFC clusters [right (34, 12, 56), z = 4.56, k = 226, volume = 1808 mm3; left (−34, 32, 26), z = 3.65, k = 94, volume = 752 mm3; left (−40, 16, 48), z = 3.60, k = 98, volume = 784 mm3] (Figure 5). Regions that also co-varied less with dACC were precentral gyrus and superior frontal gyrus. No regions were positively coupled with dACC. See Table 3 for all PPI results. In SPSS bilateral dlPFC activation was negatively associated with symptom change (right r = −0.52, P < 0.01; left r = −0.54, P < 0.001; left r = −0.54, P < 0.001), respectively.
Fig. 5.
(A) Regressing LSAS change (ΔPreTx − PostTx) while baseline severity (LSASPreTx) is controlled for as a regressor of no interest, brain map depicts whole-brain analysis of covariance showing less dorsolateral prefrontal cortex connectivity with dorsal anterior cingulate cortex denoted by negative parameter estimates of activation based on the angry vs neutral distractors during high perceptual load revealed on statistical t-map at P < 0.001. (B) Scatter plot of the regression analyses showing extracted parameter estimates of activation from dorsolateral prefrontal cortex region of interest (i.e. 34, 12, 56) indicating greater response to CBT in gSAD corresponded to less activity in dorsolateral prefrontal cortex region to angry vs neutral face distractors during high load. Scatter plots for other dorsolateral prefrontal regions of interest were comparable but are not shown.
There was no evidence of dACC-related increased functional connectivity.
Angry (vs neutral) distractors: low perceptual load
Regression data revealed symptom improvement was linked to increased activation in supramarginal gyrus. No other effects were observed in the positive direction and no results emerged in the negative direction. See Table 2 for regression result details.
Discussion
Patients with gSAD exhibited a significant reduction in symptom severity after completing CBT and the extent of symptom improvement (i.e. LSAS change (ΔPreTx − PostTx)) was predicted by pre-CBT dorsal anterior cingulate cortex (dACC) activity to threatening (vs neutral) face distractors during high perceptual load when processing resources were restricted. The dACC was a region of interest given its involvement in higher-order functions such as monitoring occurrence of conflict and response selection (Devinsky et al., 1995; Bush et al., 2000; Braver et al., 2001), which plays a role in the recruitment of mechanisms to execute control (Corbetta and Shulman, 2002; Kerns et al., 2004; Fan et al., 2007; Soutschek et al., 2013).
In addition to dACC, increased activation to distractors in a set of cortical regions was shown to correspond with symptom change. For both angry and fearful distractors, CBT response was predicted by regions implicated in conflict detection (e.g. insula, frontal superior cortex) (Braver et al., 2001; Corbetta and Shulman, 2002) and response anticipation/selection (e.g. supplementary motor area, precentral gyrus) (Corbetta and Shulman, 2002; Fan et al., 2007; Soutschek et al., 2013). Also, while responsivity in some structures were more unique to distractor type (e.g. putamen and frontal superior medial gyrus for angry distractors) pre-CBT activation in a frontoparietal network largely predicted CBT success in high perceptual load. Results suggest neural predictors did not generally interact with differences in type of threat distractor.
The following observations indicate gSAD patients with greater deficiency in controlled processes were more likely to respond to CBT. First, there was wide-spread frontopartial responsivity during high perceptual load, as opposed to activation circumscribed to regions integral to emotional conflict resolution (e.g. rostral medial prefrontal cortex; Ochsner et al., 2009; Etkin et al., 2011). In our earlier study of conflict resolution, gSAD was associated with a compensatory response to threat distractors under high perceptual load (Wheaton et al., 2014). Here, more diffuse activation when attentional capacity to process threat was constrained suggests more resources were needed to maintain goal-directed behavior though the insula findings suggests the activation may be due in part to emotional reactivity.
Reactivity in this context could signify the processing of threat distractors or the aversive nature of conflict itself (e.g. experience when confronted with discrepancy between response tendencies; Inzlicht et al., 2015). For example, pre-CBT anterior insula activation, particularly for angry face distractors was robust under high, but not low, perceptual load. Anterior insula and dACC are core structures of the salience network (Seeley et al., 2007; Menon and Uddin, 2010), thus, if activity connoted detection of bottom-up, threat-relevant signals, insula effects in the low load condition would be expected as well. In other words, the cognitive goal during low load was easier to carry out than in the high load condition, as confirmed by behavioral data (i.e. greater accuracy and shorter response time in low relative to high load). Consequently, more resources were ‘left over’ to process threat distractors under low perceptual load. Lack of insula results in the low load condition along with greater task difficulty in high load suggests reactivity to conflict is implicated in insula effects during high perceptual load.
Second, results from exploratory psychophysiological interactions (PPI) analysis point to a relationship between deficiency in controlled processes and CBT response. Using dACC as the seed region from whole-brain regression analysis, we observed reduced bilateral functional connectivity with dorsolateral prefrontal cortex (dlPFC) to angry and fearful face distractors. Interestingly, in a study of trait anxiety, greater anxiety level was also shown to correlate with less dACC-dlPFC connectivity during emotional conflict (Comte et al., 2015) suggesting reduced cross-talk between these regions may function as a predictor in other anxiety disorders.
The dlPFC is part of a cognitive control network which is recruited, and functionally connected with dACC, when streams of information compete for processing resources (Ochsner et al., 2009; Cieslik et al., 2013). Its connections with prefrontal cortex (Barbas and Pandya, 1989; Miller and Cohen, 2001), medial frontal lobe (Bates and Goldman-Rakic, 1993; Lu et al., 1994; Petrides and Pandya, 1999) and parietal lobes (Petrides and Pandya, 1984; Cavada and Goldman-Rakic, 1989; Andersen et al., 1990) position the dlPFC well as a sensory-behavioral integration area to exert optimal control to override prepotent response tendencies and efficiently shift between different task sets (Miller and Cohen, 2001; Hoshi, 2006; Mansouri et al., 2009). In light of dlPFC involvement in higher-order control and evidence symptom improvement was predicted by reduced dlPFC-dACC coupling, patients with less control capability in the context of high perceptual load were more likely to benefit from CBT.
With regard to inverse relationships between symptom change and pre-CBT neural response during conflict resolution, only paracentral lobule was observed. Specifically, greater reduction in symptom severity was predicted by less paracentral activity to fearful face distractors during high load. It is somewhat surprising results were largely limited to the high perceptual load condition. The only neural predictor to emerge during low perceptual load was supramarginal gyrus with increased activation to angry face distractors foretelling better CBT outcome. Even though these regions are involved in control functions (e.g. target detection, conflict functions) (Corbetta and Shulman, 2002; Fan et al., 2007), overall findings indicate the high load condition was more sensitive at detecting brain-based predictors in CBT response.
Beyond dlPFC, PPI results for dACC under high perceptual load for both fearful (vs neutral) and angry (vs neutral) distractors were reduced functional connectivity with precentral gyrus and superior frontal gyrus, regions associated with conflict resolution (Corbetta and Shulman, 2002; Ochsner et al., 2009). Unique to fearful face distractors was increased dACC-insula coupling indicating cognitive control was modulated by salience of distractor. Additionally, precuneus which is also implicated in cognitive control (Corbetta and Shulman, 2002; Ochsner et al., 2009) co-varied less with dACC in the face of fear distractors. Together, PPI findings point to reduced functional connectivity to dACC in regions associated with controlled processes.
In conclusion, the present study indicates better CBT outcome in gSAD is associated with initial deficiency, or inefficiency, during emotional conflict resolution when demands on control are high. CBT techniques such as cognitive restructuring, aimed at altering maladaptive thoughts (e.g. disputation of negative beliefs, generation of alternative responses), are expected to draw on executive functions. Therefore, patients with greater disruption in regions involved in higher-order processes may be helped more by explicit exercises directed at recruiting prefrontal regions.
Limitations
The study is not without important limitations. First, there was no wait-list control group, therefore, neural and clinical findings cannot be causally attributed to CBT and could be related to a number of factors not related to treatment such as natural course of the illness over the 12-week period and differential regression to the mean in patients. Second, the small sample size may have increased the risk for false negatives and the sample is only partially representative of typical patients with SAD as none were taking a psychotropic medication. Third, the fMRI studies occurred in two different sites, therefore, unknown and un-estimated variance in imaging data not accounted for from the different scanners may have contributed to an increased risk of false negatives. Fourth, lack of independent evaluators for outcome measures, that is, CGI-I across institutions and clinician-administered Liebowitz Social Anxiety Scale at the University of Michigan warrants replication and further investigation as we cannot rule out that findings may have been inadvertently influenced by the therapist. Fifth, findings are based at the group, as opposed to single-subject, level of analysis thus reducing the clinical utility of using baseline fMRI data to predict which patient will likely respond to CBT. Sixth, results are based on a paradigm designed to probe automatic (low perceptual load) and inhibitory (high perceptual load) processes thereby invoking mechanisms beyond cognitive control; therefore, inferences cannot be limited to controlled processes and may not generalize to other indices of control (e.g. Stroop task, explicit emotion regulation). Lastly, neural response predictive of CBT outcome may not be impacted by treatment; future studies should examine brain-based markers of response in the context of post-CBT scans.
Notwithstanding limitations, this is the first study we are aware of that examined brain-based markers of CBT response in gSAD to emotional conflict resolution under low and high perceptual load. Findings are inconsistent with previous gSAD studies that predominately focused on neural predictors based on emotion perception, which indicated greater regulatory ability was capitalized on by CBT. Assuming the functional architecture prior to CBT plays a role in CBT effects, mixed results may reflect type of circuitry impinged on by a component of treatment. Core CBT modules include in vivo exposures to fears and strategies directed at reducing maladaptive thoughts (e.g. cognitive restructuring). Consequently, some paradigms may be more suitable at predicting CBT response insofar as type of module is concerned. For example, when attending to social fears, patients with less limbic reactivity and superior regulatory ability may especially benefit from exposures by aptly managing surges in anxiety. Alternatively, those with poor regulatory capability when higher-order functions are targeted may be helped more by exercises that directly challenge negative beliefs. It will be important for future investigations to examine neural predictors of CBT success in the context of threat processing and type of emotional regulation (e.g. implicit vs explicit) to better understand whether certain patients benefit from particular CBT elements or its combination.
Funding
This work was supported by grants from the National Institutes of Health, National Institute of Mental Health (MH076198 to KLP and MH093679 to HK) and the Center for Clinical and Translational Science (CCTS) UL1RR029879.
Conflict of interest. None declared.
References
- American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition: DSM-IV-TR. Washington, DC: American Psychiatric Association. [Google Scholar]
- Amir N., Klumpp H., Elias J., et al. (2005). Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological Psychiatry, 57, 975–81. [DOI] [PubMed] [Google Scholar]
- Andersen R.A., Asanuma C., Essick G., Siegel R.M. (1990). Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. Journal of Comparative Neurology 296, 65–113. [DOI] [PubMed] [Google Scholar]
- Banich M.T., Mackiewicz K.L., Depue B.E., et al. (2009). Cognitive control mechanisms, emotion & memory: a neural perspective with implications for psychopathology. Neuroscience & Biobehavioral Reviews 33, 613–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H., Pandya D.N. (1989). Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology 286, 353–75. [DOI] [PubMed] [Google Scholar]
- Bates J.F., Goldman-Rakic P.S. (1993). Prefrontal connections of medial motor areas in the rhesus monkey. Journal of Comparative Neurology 336, 211–28. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Ball R., Ranieri W.F. (1996). Comparison of Beck Depression Inventories-IA and-II in psychiatric outpatients. J. Pers. Assess 67, 588–97. [DOI] [PubMed] [Google Scholar]
- Bishop S.J. (2007). Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences 11, 307–16. [DOI] [PubMed] [Google Scholar]
- Bishop S.J., Jenkins R., Lawrence A.D. (2007). Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cerebral Cortex 17, 1595–603. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. (2001). Conflict monitoring and cognitive control. Psychological Review 108, 624–52. [DOI] [PubMed] [Google Scholar]
- Braver T.S., Barch D.M., Gray J.R., Molfese D.L., Snyder A. (2001). Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cerebral Cortex 11, 825–36. [DOI] [PubMed] [Google Scholar]
- Brühl A.B., Delsignore A., Komossa K., Weidt S. (2014). Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neuroscience & Biobehavioral Reviews 47, 260–80. [DOI] [PubMed] [Google Scholar]
- Bryant R.A., Felmingham K., Kemp A., et al. (2008). Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychological Medicine 38, 555–61. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences 4, 215–22. [DOI] [PubMed] [Google Scholar]
- Busner J., Targum S.D. (2007). The Clinical Global Impressions Scale. Psychiatry 4, 28–37. [PMC free article] [PubMed] [Google Scholar]
- Butler A.C., Chapman J.E., Forman E.M., Beck A.T. (2006). The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clinical Psychology Review 26, 17–31. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Braver T.S., Barch D.M., et al. (1998). Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280, 747–49. [DOI] [PubMed] [Google Scholar]
- Cavada C., Goldman-Rakic P.S. (1989). Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. Journal of Comparative Neurology 287, 422–45. [DOI] [PubMed] [Google Scholar]
- Cieslik E.C., Zilles K., Caspers S., et al. (2013). Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cerebral Cortex 23, 2677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.M., Wells A. (1995). A cognitive model of social phobia. In: Heimberg R.G., Liebowitz M.R., Hope D.A., Schneier F.R., editors. Social Phobia: Diagnosis, Assessment, and Treatment, pp. 69–93, New York: Guilford Press. [Google Scholar]
- Comte M., Cancel A., Coull J.T., et al. (2015). Effect of trait anxiety on prefrontal control mechanisms during emotional conflict. Human Brain Mapping 36, 2207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neurosciences 3, 201–15. [DOI] [PubMed] [Google Scholar]
- Davidson J.R.T., Foa E.B., Huppert J.D., et al. (2004). Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Archives of General Psychiatry 61, 1005–13. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Morrell M.J., Vogt B.A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain Journal of Neurology 118 (Pt 1), 279–306. [DOI] [PubMed] [Google Scholar]
- Doehrmann O., Ghosh S.S., Polli F.E., et al. (2013). Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA Psychiatry 70, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman P., Friesen W. (1976). Pictures of facial affect. Consulting Psychologists Press. [Google Scholar]
- Egner T., Etkin A., Gale S., Hirsch J. (2008). Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex 18, 1475–84. [DOI] [PubMed] [Google Scholar]
- Etkin A. (2010). Functional neuroanatomy of anxiety: a neural circuit perspective. Current Topics in Behavioral Neurosciences 2, 251–77. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. (2006). Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51, 871–82. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans K.C., Wright C.I., Wedig M.M., et al. (2008). A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depression and Anxiety 25, 496–505. [DOI] [PubMed] [Google Scholar]
- Falconer E., Allen A., Felmingham K.L., Williams L.M., Bryant R.A. (2013). Inhibitory neural activity predicts response to cognitive-behavioral therapy for posttraumatic stress disorder. Journal of Clinical Psychiatry 74, 895–901. [DOI] [PubMed] [Google Scholar]
- Fan J., Kolster R., Ghajar J., et al. (2007). Response anticipation and response conflict: an event-related potential and functional magnetic resonance imaging study. Journal of Neuroscience 27, 2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M., Spitzer R., Gibbon M., Williams J. (1995). Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, NY: Biometrics Research. [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., et al. (1997). Psychophysiological and modulatory interactions in neuroimaging. NeuroImage 6, 218–29. [DOI] [PubMed] [Google Scholar]
- Gentili C., Ricciardi E., Gobbini M.I., et al. (2009). Beyond amygdala: default mode network activity differs between patients with social phobia and healthy controls. Brain Research Bulletin 79, 409–13. [DOI] [PubMed] [Google Scholar]
- Gross J.J. (2002). Emotion regulation: affective, cognitive, and social consequences. Psychophysiology 39, 281–91. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Etkin A., Furman D.J., et al. (2012). Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. American Journal of Psychiatry 169, 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Whalen P.J. (2011). The amygdala: inside and out. F1000 Biology Reports 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg R.G. (2002). Cognitive-behavioral therapy for social anxiety disorder: current status and future directions. Biological Psychiatry 51, 101–8. [DOI] [PubMed] [Google Scholar]
- Heimberg R.G., Liebowitz M.R., Hope D.A., et al. (1998). Cognitive behavioral group therapy vs phenelzine therapy for social phobia: 12-week outcome. Archives of General Psychiatry 55, 1133–41. [DOI] [PubMed] [Google Scholar]
- Heimberg R.G., Horner K.J., Juster H.R., et al. (1999). Psychometric properties of the Liebowitz Social Anxiety Scale. Psychological Medicine 29, 199–212. [DOI] [PubMed] [Google Scholar]
- Hope D.A., Heimberg R.G., Turk C.L. (2006). Managing Social Anxiety: A Cognitive-Behavioral Therapy Approach. New York: Oxford University Press, USA. [Google Scholar]
- Hoshi E. (2006). Functional specialization within the dorsolateral prefrontal cortex: a review of anatomical and physiological studies of non-human primates. Neuroscience Research 54, 73–84. [DOI] [PubMed] [Google Scholar]
- Inzlicht M., Bartholow B.D., Hirsh J.B. (2015). Emotional foundations of cognitive control. Trends in Cognitive Sciences 19, 126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns J.G., Cohen J.D., MacDonald A.W., et al. (2004). Anterior cingulate conflict monitoring and adjustments in control. Science 303, 1023–26. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Chiu W.T., Demler O., Merikangas K.R., Walters E.E. (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry 62, 617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H., Fitzgerald D.A., Phan K.L. (2013). Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry 45, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H., Fitzgerald D.A., Angstadt M., Post D., Phan K.L. (2014). Neural response during attentional control and emotion processing predicts improvement after cognitive behavioral therapy in generalized social anxiety disorder. Psychological Medicine 44, 3109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie N. (1995). Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception and Performance 21, 451–68. [DOI] [PubMed] [Google Scholar]
- Lavie N. (2000). Selective attention and cognitive control: dissociating attentional functions through different types of load. In Monsell S., Driver J., editors., Control of Cognitive Processes: Attention and Performance XVIII (pp. 175–194). Cambridge, MA: MIT Press. [Google Scholar]
- Lavie N., Lin Z., Zokaei N., Thoma V. (2009). The role of perceptual load in object recognition. Journal of Experimental Psychology: Human Perception and Performance 35, 1346–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience 23, 155–84. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. (2009). Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience 4, 423–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz M.R. (1987). Social phobia. Modern Problems of Pharmacopsychiatry 22, 141–73. [DOI] [PubMed] [Google Scholar]
- Liebowitz M.R., Mangano R.M., Bradwejn J., Asnis G., SAD Study Group (2005). A randomized controlled trial of venlafaxine extended release in generalized social anxiety disorder. Journal of Clinical Psychiatry 66, 238–47. [DOI] [PubMed] [Google Scholar]
- Liu X., Banich M.T., Jacobson B.L., Tanabe J.L. (2006). Functional dissociation of attentional selection within PFC: response and non-response related aspects of attentional selection as ascertained by fMRI. Cerebral Cortex 16, 827–34. [DOI] [PubMed] [Google Scholar]
- Lu M.T., Preston J.B., Strick P.L. (1994). Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. Journal of Comparative Neurology 341, 375–92. [DOI] [PubMed] [Google Scholar]
- MacDonald A.W., Cohen J.D., Stenger V.A., Carter C.S. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288, 1835–38. [DOI] [PubMed] [Google Scholar]
- Mansouri F.A., Tanaka K., Buckley M.J. (2009). Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nature Reviews Neuroscience 10, 141–52. [DOI] [PubMed] [Google Scholar]
- Mennin D.S., Heimberg R.G., Turk C.L., Fresco D.M. (2002). Applying an emotion regulation framework to integrative approaches to generalized anxiety disorder. Clinical Psychological Science and Practice 9, 85–90. [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function 214, 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P. (1998). A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy 36, 809–48. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Hughes B., Robertson E.R., Cooper J.C., Gabrieli J.D.E. (2009). Neural systems supporting the control of affective and cognitive conflicts. Journal of Cognitive Neuroscience 21, 1842–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M., Pandya D.N. (1984). Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. Journal of Comparative Neurology 228, 105–16. [DOI] [PubMed] [Google Scholar]
- Petrides M., Pandya D.N. (1999). Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. European Journal of Neuroscience 11, 1011–36. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Coccaro E.F., Angstadt M., et al. (2013). Corticolimbic brain reactivity to social signals of threat before and after sertraline treatment in generalized social phobia. Biological Psychiatry 73, 329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. (2003). Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry 54, 504–14. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience 27, 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle G.J., Carter C.S., Thase M.E. (2006). Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry 163, 735–38. [DOI] [PubMed] [Google Scholar]
- Soutschek A., Taylor P.C.J., Müller H.J., Schubert T. (2013). Dissociable networks control conflict during perception and response selection: a transcranial magnetic stimulation Study. Journal of Neuroscience 33, 5647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D. (1983). Manual for the State-Trait Anxiety Inventory (form Y). Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Whalen P.J., Shin L.M., McInerney S.C., et al. (2001). A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emot. 1, 70–83. [DOI] [PubMed] [Google Scholar]
- Wheaton M.G., Fitzgerald D.A., Phan K.L., Klumpp H. (2014). Perceptual load modulates anterior cingulate cortex response to threat distractors in generalized social anxiety disorder. Biological Psychology 101, 13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H.U., Stein M.B., Kessler R.C. (1999). Social fears and social phobia in a community sample of adolescents and young adults: prevalence, risk factors and co-morbidity. Psychological Medicine 29, 309–23. [DOI] [PubMed] [Google Scholar]