Abstract
Neuroimaging studies have demonstrated that the medial prefrontal cortex is involved in attributions on enduring and abstract trait characteristics of persons, but not in causal attributions of temporary here-and-now events. Moreover, the neural representation of trait information is thought to be located in the ventral part of the medial prefrontal cortex (vmPFC). In order to verify this latter finding, this study compared the performance of 8 patients with hypoperfusion of the vmPFC, 10 with hypoperfusion excluding the vmPFC and 15 healthy controls on trait and causal attribution questionnaires consisting of several events presented in brief written scenarios. We also investigated whether vmPFC hypoperfusion influenced the experienced intensity of the negative or positive valence of the events. Our results showed that patients with ventral hypoperfusion performed significantly worse on trait attributions in comparison with the non-vmPFC group and healthy controls. All groups performed equally well on causal attributions. These findings support previous research suggesting that the vmPFC is critically involved in enduring trait attribution, but not in temporary causal attribution. Considering the emotional experience of valence, the findings showed more intense valence ratings for negative events and persons. This confirms the role of the vmPFC in the modulation and regulation of negative emotions.
Keywords: trait attribution, causal attribution, valence attribution, hypoperfusion
Introduction
Mentalizing refers to thinking about and inferring the content of other persons’ minds or mental states (including beliefs, goals, desires, intentions, thoughts, etc.). This process of social understanding makes our world more predictable and less threatening. Part of this mentalizing involves attributing observed behaviors to situational causes such as occasional situational constraints or stable features of the situation (e.g., being assaulted vs unpleasant environment), or to temporary mental states or enduring traits of the agent (e.g., angry response vs a horrible person).
Mentalizing is subserved by the medial prefrontal cortex (mPFC) together with the temporo-parietal junction (TPJ), known as core mentalizing brain regions (Frith and Frith, 2001; Mitchell et al., 2005; Amodio and Frith, 2006; Lieberman, 2007; Carrington and Bailey, 2009; Mitchell, 2009; Van Overwalle, 2009; Ma et al., 2011, 2012). The aim of the present study is to investigate the role of the ventral part of the mPFC (vmPFC) in trait, causal and valence attribution, using patients with hypoperfusion of this region.
Neuroimaging research
Neuroimaging studies have demonstrated that the mPFC is preferentially recruited when making attributions to enduring personality traits (Mitchell, 2009; Van Overwalle, 2009; Ma et al., 2011, 2012). It has been assumed that this is so because trait inferences are high-level abstract judgments, encompassing multiple observations of behavior, and hence require the involvement of the medial frontal cortex (Van Overwalle, 2009). Moreover, the neural representation of trait information is thought to be located in the vmPFC, indicating that this region is responsible for encoding, storing and retrieving trait information (Ma et al., 2013, 2014).
In contrast, the TPJ is involved in the detection of goals from observed behavior (Frith and Frith, 2001) and in the representation of beliefs about other persons (Apperly et al., 2004; Saxe and Wexler, 2005). More importantly, the TPJ is strongly recruited when making causal attributions about a here-and-now event (Kestemont et al., 2013, 2014), which requires little abstraction in comparison with trait inferences (Van Overwalle, 2009). Our research group found evidence for TPJ activation in a neuroimaging study comparing situation and person attributions, under intentional vs spontaneous conditions (Kestemont et al., 2013). Intentional attributions refer to social inferences made with an explicit goal, whereas spontaneous attributions refer to social inferences made without explicit intention and even without awareness of making the inference during the observation of others’ behaviors (Uleman, 1999). Other research confirmed the role of the TPJ in intentional causal attributions (Harris et al., 2005; Seidel et al., 2010).
Aside the crucial role of the mPFC in social cognition, the ventral part of this region is generally also important in the processing of emotional stimuli by its interconnectedness with the amygdala (Hornak et al., 2003; Bechara, 2004; Adolphs et al., 2005; Rudebeck et al., 2008). The vmPFC has a role in integrating affective and somatosensorial activation evoked by affective experiences (Damasio et al., 1990; Hornak et al., 2003; Bechara, 2004; Van Overwalle, 2009). The vmPFC–amygdala circuitry integrates and modulates raw initial emotional responses elicited in the amygdala (Kim et al., 2003; Urry et al., 2006). Research has demonstrated that when negative affect is adaptively regulated by the circuitry, vmPFC activity increases and amygdalar activity decreases, suggesting that the activity of the amygdala is inhibited by top-down regulation of the vmPFC (Urry et al., 2006).
Lesion research
Patients with brain lesions in the vmPFC fail to elicit normal autonomic responses and appraisals to emotionally and socially meaningful stimuli (Damasio et al., 1990). The inability to process and experience affective personal meaningful stimuli is associated with the disruption of affective and cognitive mentalizing capacity in patients with vmPFC lesions (Beer et al., 2003; Ferstl et al., 2005; Shamay-Tsoory et al., 2005; Adolphs, 2009; Vandekerckhove et al., 2014). The cognitive component of mentalizing refers to understanding others’ thoughts and beliefs, whereas the affective component reflects the understanding of emotions and feelings of self and others (Leopold et al., 2012). Research demonstrated that the affective component is most strongly impaired in vmPFC-damaged patients (Shamay-Tsoory et al., 2005; Geraci et al., 2010; Leopold et al., 2012). Corradi-Dell’acqua et al. (2014) found that both emotion and belief judgments evoked independent patterns of activity in the vmPFC and dmPFC. Shamay-Tsoory and Aharon-Peretz (2007) also found that affective mentalizing involves the vmPFC, which contributes to the integration of cognitive and affective processes, while cognitive mentalizing requires an intact functioning of the whole mentalizing network (Shamay-Tsoory and Aharon-Peretz, 2007). Kalbe and colleagues (2010) found that inhibiting the right dorsolateral prefrontal cortex by means of transmagnetic stimulation selectively affected cognitive mentalizing.
Present research and hypotheses
Given the limited evidence for deficits in cognitive mentalizing, the first goal of this study is to investigate the capacity of making appropriate trait attributions in patients with vmPFC hypoperfusion (vmPFC group) compared to patients with temporal/parietal hypoperfusion (non-vmPFC group) and a healthy control group (control group). Because the vmPFC is critically involved in the encoding and processing of trait information (Ma et al., 2013, 2014), we predict that the vmPFC group makes less appropriate trait attributions compared to the non-vmPFC and the healthy control group. In contrast, given that the vmPFC is not critically involved in other types of attributions, we expect that there is no detrimental effect on causal attributions to another person or situation in vmPFC-damaged patients compared to the non-vmPFC and the healthy control group.
The second goal of this study is to explore how vmPFC hypoperfusion patients experience and attribute the intensity of emotional valence to persons and events. As the vmPFC is involved in the regulation of negative affect (Kim et al., 2003; Urry et al., 2006) and affective mentalizing (Shamay-Tsoory et al., 2005; Geraci et al., 2010; Leopold et al., 2012), we predict that patients with vmPFC hypoperfusion respond with increased emotional reactivity to both negative and positive events and persons, compared to the non-vmPFC and healthy control participants.
Method
Participants
All patients were recruited from the Centre for Epilepsy and Psycho-organic Disorders (Centrum voor Epilepsie en Psycho-Organische Stoornissen or CEPOS, Duffel, Belgium), where they were enrolled in a multidisciplinary rehabilitation program, which consisted of motor and cognitive rehabilitation, occupational therapy, psychotherapy and/or language therapy. A neuropsychological test battery was administered to all patients, to measure attention (Symbol Substitution—a subtest of the WAIS-III-NL, Bourdon and Wiersma et al., 1902; Stroop-task, Stroop, 1935; Wechsler, 2001), memory (Rey Auditory Verbal Learning Test, Rey, 1941; Rey Visual Design Learning Test, Rey, 1964; Complex Figure Test, Meyers and Meyers, 1995; Coetsier Story Recall Test, Coetsier et al., n.d.), visuospatial functioning (Visuospatial Judgment Test, Benton et al., 1978; Complex Figure Test, Meyers and Meyers, 1995; Block Design—a subtest of the WAIS-III-NL, Wechsler, 2001) and executive functioning (Similarities and Matrix reasoning—both subtests of the WAIS-III-NL, Wechsler, 2001; Controlled Oral Word Association Test, Benton and Hamsher, 1976; Semantic Word Enumeration—subtest of the Groningse Intelligentie Test, Luteijn and Van der Ploeg, 1983; Maze Test, Chapuis, 1959). Patients were excluded from our study if they met one of the following criteria: (i) non-native speaker of Dutch, (ii) severe aphasia, (iii) severe visuo-perceptual deficits, (iv) severe memory disturbances, (v) current psychiatric disorder (as measured with the M.I.N.I International Neuropsychiatric Interview, Sheehan et al., 1998; van Vliet et al., 2000; and the Beck Depression Inventory—II, Beck et al., 1996; van der Does, 2002) or additional neurological antecedents, and (vi) a history of alcohol or drug abuse.
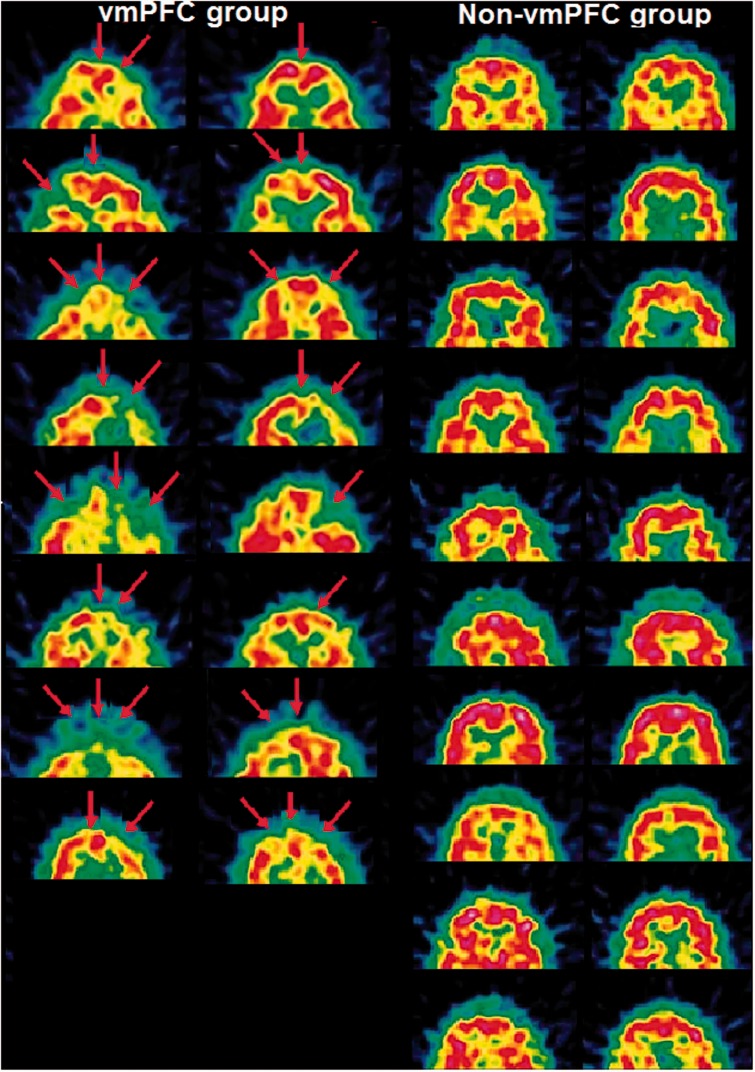
Of the 33 Dutch-speaking participants, 18 were patients. The vmPFC group consisted of eight patients (of which six were men) who showed hypoperfusion of the vmPFC on the basis of a single photon emission computed tomography (SPECT)-scan (Figure 1 and Table 1); their age ranged from 34 to 61 years, with a mean age of 48 years. The non-vmPFC group consisted of 10 patients (of which seven were men) who showed hypoperfusion in temporal and/or parietal regions, with no hypoperfusion in the vmPFC or adjacent regions (Figure 1 and Table 1). Their age ranged from 17 to 70 years, with a mean age of 49 years. All patients had sustained acquired hypoperfusion of different etiology. For the vmPFC group, the etiology was contusion cerebri following traumatic brain injury (n = 6), ischemic cerebrovascular accident (n = 1) and hemorrhage cerebrovascular accident (n = 1). For the non-vmPFC group, the etiology was contusion cerebri following traumatic brain injury (n = 5), ischemic cerebrovascular accident (n = 2), hemorrhage cerebrovascular accident (n = 1) and cerebral anoxia following cardiac arrest (n = 2). The remaining 15 participants (of which 10 were men) composed the healthy control group. Their age ranged from 21 to 67 years, with a mean age of 42 years. The latter group was matched in age, gender, education and professional category to the hypoperfusion groups.
Fig. 1.
SPECT-scans of the patients (two images per patient). Left: vmPFC group consisting of 8 patients with hypoperfusion (indicated by the arrows) of the vmPFC; Right: Non-vmPFC group consisting of 10 patients with hypoperfusion in temporal and/or parietal regions, with no hypoperfusion in the vmPFC and adjacent regions.
Table 1.
Severity of hypoperfusion per patient in each group on a scale from none (empty cell) to severe (3) on the right (R) and left (L) hemisphere and some key behavioral results showing significant differences (see also Table 3)
| Group | Brain region/behavioral results | Patient |
Median | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| vmPFC | ||||||||||||
| Brain region | ||||||||||||
| vmPFC | L3 | R2 | L2 | R3 | L3 | L3 | R1/L2 | L2 | — | — | ||
| Frontal superior | R1/L1 | R1 | — | — | ||||||||
| Temporal | L1 | — | — | |||||||||
| Temporal posterior | R1 | — | — | |||||||||
| Temporal anterior | L3 | L1 | L2 | L1 | — | — | ||||||
| Parietal | — | — | ||||||||||
| Parietal superior | — | — | ||||||||||
| Key behavioral results | ||||||||||||
| Correct trait attributions | 32 | 33 | 33 | 19 | 37 | 25 | 37 | 32 | — | — | 32.50 | |
| Traits negative | 0.7 | 2.7 | 3.6 | 1.1 | 1.1 | 3.3 | 0.4 | 2.6 | — | — | 1.85 | |
| Int. attributions negative | 1.6 | 2.6 | 3.8 | 1.0 | 2.6 | 2.6 | 1.9 | 3.6 | — | — | 2.60 | |
| Int. attributions congruency | 22 | 27 | 25 | 14 | 22 | 16 | 22 | 22 | — | — | 22.00 | |
| non-vmPFC | ||||||||||||
| Brain region | ||||||||||||
| vmPFC | ||||||||||||
| Frontal superior | L1 | R2 | ||||||||||
| Temporal | R2 | R2 | L3 | R3 | ||||||||
| Temporal posterior | L3 | L1 | L1 | R1/L1 | ||||||||
| Temporal anterior | ||||||||||||
| Parietal | L3 | R3 | ||||||||||
| Parietal superior | R1/L1 | L2 | ||||||||||
| Key behavioral results | ||||||||||||
| Correct trait attributions | 34 | 27 | 32 | 33 | 35 | 38 | 40 | 38 | 33 | 38 | 34.50 | |
| Traits negative | 3.4 | 5.3 | 3.3 | 2.2 | 3.0 | 1.4 | 3.4 | 2.2 | 1.4 | — | 3.00 | |
| Int. attributions negative | 4.0 | 4.7 | 4.3 | 3.7 | 5.2 | 4.9 | 4.2 | 2.9 | 2.2 | — | 4.20 | |
| Int. attributions congruency | 21 | 17 | 20 | 18 | 14 | 22 | 30 | 24 | 13 | 27 | 20.50 | |
The informed consent was obtained in a manner approved by the Free University of Brussels.
Anatomical analysis
Cerebral blood flow (CBF) SPECT was performed using Technetium-99m-HMPAO (CeretecR), injecting ∼155MBq of the tracer in a resting supine condition in a quiet room with eyes closed and ears unplugged. The manufacturer’s instructions were followed for the preparation of the tracer. Within 30 min after radiopharmaceutical injection, data acquisition was started. A dual head rotating gamma-camera system (DST-Xli, SMV) was used with low energy high resolution collimators. Patients’ heads were visually positioned along the orbitomeatal line in a dedicated head rest. SPECT data were collected on a 360° rotation, recording 64 views of 35 s each, on a 128×128 matrix. Reconstruction of transaxial, coronal and sagittal slices was made by a filtered back-projection algorithm (Butterworth filter critical frequency 0.5, no attenuation correction) using semi-quantitative analysis with a standard multisegmental template method (segmental analysis, XelerisR, GE Medical Systems) performed on the transaxial slices by an experienced physician. A template with regions of interest (ROI) was adjusted on the cerebral cortex of the first orbito-medial slice and the cerebellum. The cerebral cortex template was divided in 12 segments. The R/L ratios were calculated for all the ROIs as well as the average activity in the ROIs set to the whole cerebral activity used as in internal standard. The segments corresponding with the prefrontal cortex (PFC) were identified on the multisegmental analysis.
Visual analysis of the perfusion in the PFC was performed by an experienced reader of neurological SPECT data using a score system. Score 0 corresponded with no perfusion deficit, score 1 with a light, score 2 with a moderate, and score 3 with a severe perfusion deficit (Table 1). Patients were included in the vmPFC group when the visual perfusion deficit was 2 or more for one or both sides of the PFC which comprises the vmPFC area, which included the orbitofrontal cortex, the rostral anterior cingulate cortex and more anterior parts of the medial PFC. The presence of perfusion abnormalities outside the PFC was also noted with registration of the localization within the rest of the frontal cortex, the temporal, parietal or occipital cortex. When hypoperfusion was exclusively outside of the vmPFC, patients were included in the non-vmPFC group.
Of the eight patients in the vmPFC group, seven showed unilateral and one showed bilateral perfusion abnormalities in the vmPFC. Two patients showed perfusion abnormality limited to the vmPFC (one left and one right); six patients showed additional perfusion abnormalities in the superior frontal cortex and the (anterior) temporal cortex. The 10 patients of the non-vmPFC group showed abnormal perfusion localized within the superior frontal, temporal and parietal cortex, with exclusion of the vmPFC. Table 1 indicates that apart from the vmPFC, other hypoperfusion sites were generally distributed throughout the brain in the vmPFC and non-vmPFC groups, except perhaps for the left anterior temporal lobe where hypoperfusion was present in half the vmPFC group and none of the non-vmPFC group.
Stimulus material and procedure
All participants went through three attribution questionnaires in the same order: the first was the semi-spontaneous attribution questionnaire, the second was the intentional attribution questionnaire, followed by the trait questionnaire. If the participants had questions there was always a researcher nearby to clarify possible ambiguities.
The semi-spontaneous and intentional questionnaires consist of 30 sentences each, describing several everyday events (see Table A1 for all sentences and their implied cause). Half of the sentences in each questionnaire described an event in which the situation was implied as the cause (e.g. Maldron earns a salary—implies that this persons has a job), while the other half implied the person as the cause (e.g. Dilla can work well together—implying that Dilla is social). These implied attributions were identified in pilot studies, where healthy participants categorized each sentence as caused by the situation or person and were used as reference of comparison. Each questionnaire had 15 sentences describing an event with a positive valence and 15 with a negative valence. The sentences were provided in two counterbalanced versions of the questionnaires to avoid order effects. In all sentences, “Star Trek-like” names were used to avoid similarities with familiar others of the participants.
In order to evaluate the quality of participants’ judgments, the correctness or appropriateness of their open-ended responses was scored by two researchers, independently from each other, and blind to the hypoperfusion group to which the participants were assigned to. The inter-rater reliability over these open-ended responses was 92% overall.
Semi-spontaneous questionnaire
In this questionnaire, participants were asked to “search a cause for each event, and describe/give your cause. Hint: To help you find a cause, you can ask yourself a ‘why’-question in every sentence. Always try to give the first answer that comes to mind. There are no correct or incorrect answers.”
The following measures were taken from the participants’ open-ended answers (as reported in Table 3):
Situation and Person Attributions: number of open-ended situation or person attributions.
Correct Situation and Person attributions: number of open-ended responses that were of the same situation or person category as documented in the pilot study (scored by two researchers).
Table 3.
Median and range of trait, attribution and valence variables of the vmPFC group, the Non-vmPFC group and the Control group
| vmPFC group |
Non-vmPFC group |
Control group |
||||
|---|---|---|---|---|---|---|
| Behavioral results | Median | R | Median | R | Median | R |
| Trait attribution | ||||||
| Correct trait attributions | 32.50A | 18.00 | 34.50B | 13.00 | 37.00B | 5.00 |
| Valence attributed to event/person | ||||||
| Traits positive | 7.73 | 3.45 | 8.25 | 3.20 | 7.85 | 2.45 |
| Traits negative | 1.85a | 3.25 | 3.00b | 3.95 | 2.40 | 3.15 |
| Intentional attributions positive | 8.13 | 3.80 | 7.43 | 3.74 | 7.53 | 3.07 |
| Intentional attributions negative | 2.60A | 2.80 | 4.20B | 3.00 | 3.40B | 5.40 |
| Semi-spontaneous attribution | ||||||
| Situation attributions | 16.00 | 10.00 | 15.50 | 16.00 | 17.00 | 7.00 |
| Person attributions | 12.50 | 5.00 | 13.50 | 12.00 | 13.00 | 7.00 |
| Correct situation attributions | 14.00 | 6.00 | 13.00 | 8.00 | 13.00 | 4.00 |
| Correct person attributions | 11.00 | 6.00 | 11.00 | 9.00 | 11.00 | 5.00 |
| Intentional attribution | ||||||
| Situation category | 15.00 | 18.00 | 13.00 | 15.00 | 16.00 | 12.00 |
| Person category | 13.50 | 18.00 | 16.00 | 15.00 | 14.00 | 12.00 |
| Congruent response | 22.00A | 13.00 | 20.50A | 17.00 | 27.00B | 6.00 |
| Incongruent response | 8.00 | 13.00 | 8.50 | 17.00 | 4.00 | 6.00 |
| Correct situation category | 11.00 | 12.00 | 8.00 | 10.00 | 12.00 | 7.00 |
| Correct person category | 10.00 | 9.00 | 10.50 | 8.00 | 11.00 | 6.00 |
| Correct situation attributions | 12.50 | 6.00 | 12.50 | 4.00 | 13.00 | 3.00 |
| Correct person attributions | 11.50 | 4.00 | 9.00 | 5.00 | 11.00 | 7.00 |
Note. Medians with different superscript differ within each row according to post hoc Mann–Whitney tests (Bonferroni corrected for semi-spontaneous and intentional attributions). For traits and valence attributions, for which we had a priori predictions, one-sided Mann–Whitney tests were used. Significance level is at P < 0.05 when in capitals, and P < 0.10 (marginal) when in lower case.
Intentional questionnaire
In this questionnaire, participants were informed about whether the cause could be a characteristic of either the situation or the person. In addition, participants had to indicate how pleasant they found the described event. The instruction was: “Search for every event a cause in the person or in the situation. Indicate the category your cause belongs to and describe the cause. Always try to give the first answer that comes to mind. There are no correct or incorrect answers. Hint: To help you find a cause, you can ask yourself a ‘why’-question in every sentence.”
The following measures were taken from the participants’ answers (as reported in Table 3):
Situation and Person Category: number of category responses indicating the situation or the person.
Congruent Response: number of open-ended attribution responses that were congruent with the indicated person or situation category.
Correct Situation and Person Category: number of category responses that were similar to the pilot study.
Correct Situation and Person Attributions: number of open-ended attribution responses that were of the same category as in the pilot study (scored by two researchers).
Finally, for the valence measure, the participants indicated “how pleasant you find the event described in the sentence on a scale ranging from 0 to 10, where 0 = very unpleasant and 10 = very pleasant.”
Trait questionnaire
This questionnaire consisted of 40 pairs of two sentences, each describing a behavior that implied the same trait of the agent performing the behavior. Again half of the sentences had a positive valence, while the other half had a negative valence. All sentences were selected through pilot studies (See Table A2 for all sentences and their implied trait). “Star Trek-like” names were used to avoid similarities with familiar others of the participants. Participants were asked “for every couple of events, search a characteristic in the person. Always try to give the first answer that comes to mind. There are no correct or incorrect answers.”
The following measure was taken from the participants’ open-ended answers (as reported in Table 3):
Correct trait attributions: number of responses that conformed to the trait category as documented in the pilot study (scored by two researchers).
Finally, for the valence measure, the participants indicated “how pleasant you find the person on a scale ranging from 0 to 10, where 0 = very unpleasant and 10 = very pleasant.”
Statistical analyses
All analyses were performed using IBM SPSS Statistics 22 software. As group sample sizes differ, non-parametric Kruskal–Wallis (KW) tests were used to calculate main effects of group, while non-parametric Mann–Whitney (MW) tests were used to compare between the different groups. Hypotheses were tested by computing a priori MW tests (one-sided), while all other (post hoc) MW tests were Bonferroni corrected for the number of tests (two-sided).
Results
To control for group differences in years of education and age, a KW test was performed, with years of education and age as dependent variables and group as grouping variable. No main effect of group was found for years of education or age (respectively, H(2) = 2.84, ns and H(2) = 1.32, ns).
To control for differences on depression (measured with the BDI-II) and weeks since lesion onset between our both patient groups, two MW tests were performed, but they revealed no significant differences (Table 2). Also regarding attention, memory, visuospatial and executive functioning, MW tests could not reveal significant differences between the vmPFC and non-vmPFC groups (all P > .10; Table 2).
Table 2.
Neuropsychological control variables per patient group
| Function | Test/Measure | vmPFC |
Non-vmPFC |
Mann–Whitney |
|||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | U | P | ||
| Depression and Lesion | Beck Depression Inventory-II | 8.14 | 3.18 | 9.44 | 3.00 | 21.5 | 0.284 |
| Weeks since lesion onset | 30.13 | 25.40 | 23.60 | 17.84 | 33.5 | 0.563 | |
| Attention | Bourdon-Wiersma—seconds | 18.32 | 7.97 | 18.92 | 5.89 | 30.0 | 0.564 |
| Symbol Substitution (WAIS-III)—score | 6.83 | 2.48 | 8.40 | 3.27 | 18.5 | 0.210 | |
| Stroop-task Chart 3 minus 2—seconds | 44.86 | 55.13 | 35.38 | 20.06 | 25.5 | 0.771 | |
| Stroop-task Chart 4 minus 2—seconds | 70.29 | 42.89 | 52.50 | 24.65 | 18.0 | 0.668 | |
| Memory | Rey Auditory Verbal Learning Test—short term | 40.38 | 16.17 | 41.00 | 11.87 | 36.5 | 0.755 |
| Rey Auditory Verbal Learning Test—long term | 4.75 | 2.05 | 4.70 | 1.25 | 37.0 | 0.784 | |
| Rey Auditory Verbal Learning Test—recognition | 12.13 | 3.76 | 12.89 | 3.10 | 35.0 | 0.921 | |
| Coetsier Story Recall Test—short term | 61.38 | 20.23 | 56.44 | 23.61 | 33.0 | 0.773 | |
| Coetsier Story Recall Test—long term | 49.63 | 23.78 | 50.44 | 21.32 | 35.5 | 0.962 | |
| Rey Visual Design Learning Test—short term | 44.92 | 17.50 | 35.56 | 11.97 | 19.5 | 0.376 | |
| Rey Visual Design Learning Test—long term | 11.33 | 4.31 | 8.39 | 3.91 | 14.5 | 0.140 | |
| Rey Visual Design Learning Test—recognition | 14.50 | 0.55 | 13.00 | 2.92 | 18.0 | 0.257 | |
| Complex Figure Test—copy (out of 36) | 32.50 | 3.12 | 32.65 | 3.23 | 37.5 | 0.822 | |
| Complex Figure Test—short term | 23.00 | 8.57 | 21.35 | 7.85 | 31.0 | 0.422 | |
| Complex Figure Test—long term | 21.88 | 9.60 | 19.55 | 8.04 | 32.5 | 0.504 | |
| Visuospatial | Visuospatial Judgment Test (out of 30) | 25.63 | 2.50 | 23.60 | 4.12 | 25.0 | 0.178 |
| Block Design (WAIS-III)—score | 9.25 | 3.62 | 7.33 | 2.50 | 15.5 | 0.270 | |
| Executive | Controlled Oral Word Association Test—letter N | 7.29 | 4.86 | 8.57 | 3.64 | 19.0 | 0.479 |
| Controlled Oral Word Association Test—letter A | 7.14 | 4.34 | 9.14 | 3.93 | 17.5 | 0.370 | |
| Controlled Oral Word Association Test—letter K | 10.50 | 5.13 | 12.71 | 4.72 | 16.5 | 0.517 | |
| Semantic Word Enumeration—Professions | 13.13 | 4.05 | 11.50 | 3.78 | 33.0 | 0.529 | |
| Semantic Word Enumeration—Animals | 17.00 | 5.58 | 18.00 | 7.57 | 26.0 | 0.817 | |
| Similarities (WAIS-III) | 8.43 | 3.74 | 9.10 | 3.14 | 32.5 | 0.806 | |
| Matrix reasoning (WAIS-III) | 8.25 | 3.20 | 9.30 | 2.36 | 32.5 | 0.502 | |
| Chapuis Maze Test—seconds to terminate | 333.33 | 176.98 | 347.11 | 202.41 | 13.0 | 0.926 | |
Finally, we found no significant differences between groups with respect to the total number of attributions made using a KW test. Only in three cases were no attributions given: One control participant did not give a trait attribution on one occasion, while one vmPFC patient did not give two intentional causal attributions.
Trait attributions
We predicted that the vmPFC group would make less appropriate trait attributions compared to the non-vmPFC and the healthy control group. A KW test with correct trait attributions as dependent variable and group as grouping variable, revealed a significant main effect of group (H(2) = 9.67, P < 0.005; Table 3). As predicted, a priori (one-sided) MW-tests show that the vmPFC group made significantly less appropriate trait attributions compared to the non-vmPFC group (U = 21.00, z = −1.70, P < 0.05, r = −0.40) and the control group (U = 14.00, z = −2.99, P < 0.001, r = −0.62). No significant differences in appropriate trait attribution between the non-vmPFC and the healthy control group were found (U = 48.00, z = −1.52, ns; two-sided). A parametric analysis of variance (ANOVA) with age, gender and education level as covariates revealed that after controlling for these three variables, the difference between groups remained significant, F(2, 25) = 5.21, P < 0.05. As an exploratory analysis, we computed for the vmPFC group a Pearson correlation between appropriate trait attributions and the severity of the lesion (see Table 1), and found a negative correlation of r = − 0.48, which fell short of significance given the small sample size, P = 0.228. Although, as mentioned earlier, a left anterior temporal hypoperfusion was present in half of the vmPFC group and not in the non-vmPFC group, this does not explain the impairment of the vmPFC group because patients with or without left anterior temporal hypoperfusion did not differ from each other (U = 27.00, z = −0.11, P = 0.95). Thus, taken together, vmPFC patients tended to make less appropriate trait attributions given a more severe lesion.
Causal attributions
We predicted that vmPFC patients would make equally appropriate non-trait attributions compared to the non-vmPFC and the healthy control group. Two KW tests were performed to control for main effects of group, the first with the semi-spontaneous and the second with the intentional attribution variables (as listed in Table 3) as dependent variables and group as grouping variable. To control for the number of comparisons, we applied a Bonferroni correction (i.e., P < 0.05 corrected for 11 comparisons reduces to P < 0.005).
Semi-spontaneous Questionnaire
No main effects of group were found for the semi-spontaneous variables (Table 3).
Intentional Questionnaire
There was a significant main effect of group for congruent responses (H(2) = 13.35, P < 0.05 corrected). As can be seen in Table 3, post hoc (two-sided) MW tests showed that the control group outperformed the vmPFC (U = 11.00, z = −3.19, P < 0.05 corrected, r = −0.67) and non-vmPFC group (U = 23.50, z = −2.87, P < 0.05 corrected, r = −0.57) on congruent responses, while both hypoperfusion groups did not differ from each other (U = 34.00, z = −0.54, ns).
Valence ratings
We predicted that vmPFC patients respond with increased emotional reactivity to both negative and positive events and persons, compared to the non-vmPFC and healthy control participants.
Valence ratings of trait sentences
A KW test was performed with agreeableness of persons in positive and negative trait sentences as dependent variables and group as grouping variable (Table 3). Contrary to our expectations, no main effect of group was found for positive (H(2) = 0.29, ns) or negative trait sentences (H(2) = 2.13, ns). Nevertheless, a priori (one-sided) MW-tests show that the vmPFC group tended to rate persons in negative sentences marginally more negative compared to the non-vmPFC group (U = 21.00, z = −1.44, P < 0.10, r = −0.35). This difference between groups was not significant in an ANOVA with age, gender and education level as covariates. No other differences were (marginally) significant.
Valence ratings of causal attribution sentences
For the intentional questionnaire, a KW test was performed with agreeableness of the event in positive and negative sentences as dependent variables and group as grouping variable (Table 3). A main effect of group was found for negative sentences (H(2) = 7.76, P < 0.05), but not for positive sentences (H(2) = 1.82, ns). A priori (one-sided) MW tests further show that, as predicted, the vmPFC group rated negative sentences significantly more negative than the non-vmPFC group (U = 8.00, z = −2.70, P < 0.01, r = −0.66) and the control group (U = 31.00, z = −1.87, P < 0.05, r = −0.39). An ANOVA with age, gender and education level as covariates revealed that after controlling for these three variables, the difference between groups remained significant, F(2, 25) = 4.59, P < 0.05. Hypoperfusion at the left anterior temporal lobe might also explain this negativity effect, as patients with and without left anterior temporal hypoperfusion differed marginally from each other (U = 10.00, z = −1.81, p < 0.08).
Discussion
This study extends our knowledge on the influence of vmPFC hypoperfusion on deficits in mentalizing and how this damage might influence the reflection on own emotions and feelings. We explored the influence of vmPFC hypoperfusion on social-cognitive mentalizing by means of social attributions, and on emotions and feelings by means of emotional valence ratings towards positive and negative events and persons.
In regard to social attributions, we found, as predicted, that patients with vmPFC hypoperfusion made less correct trait attributions compared to patients with non-vmPFC hypoperfusion and healthy controls. This is not surprising, as the vmPFC is considered to subserve the neural representation of trait information (Ma et al., 2013, 2014) and is often found to be recruited in neuroimaging studies involving trait attribution (Mitchell, 2009; Van Overwalle, 2009; Ma et al., 2012, 2011). Lesion studies mainly describe the vmPFC to be involved in affective mentalizing, but as Shamay-Tsoory and Aharon-Peretz (2007) argue, it seems that social-cognitive mentalizing requires intact functioning of the whole mentalizing network including the vmPFC. Trait attributions are distinct from causal attributions, because they are abstractions often derived from multiple observations of behaviors, and are therefore considered high-level social judgments that require medial prefrontal cortex involvement (Van Overwalle, 2009). Note that this impairment in trait understanding could not be attributed to a left anterior temporal hypoperfusion, although this damage was present in half of our vmPFC group, and not in the non-vmPFC group.
In contrast to trait attributions, and supporting our research group’s previous results (Kestemont et al., 2013, 2014), we found that the vmPFC is not critically involved in causal attribution. Causal attributions differ from trait attributions in that they are focused on the observation and interpretation of a here-and-now event, without any further abstractions. Therefore, they do not necessarily require the involvement of medial frontal areas (Van Overwalle, 2009). We did find however that both patients with vmPFC and non-vmPFC hypoperfusion have more difficulties in assigning their open-ended answers to the corresponding person or situation category, as they gave less congruent answers compared to the healthy controls. This might reflect a dysfunction in categorizing, in both hypoperfusion groups, which requires some level of abstraction, rather than in causal attribution per se, or another dysfunction that we are currently unaware of.
In regard to emotional valence, we expected the vmPFC-damaged patients to make more intense valence ratings for both negative and positive events and persons as a result of disrupted top-down regulation of the vmPFC on amygdalar activity (Kim et al., 2003; Urry et al., 2006). This hypothesis was mainly confirmed for negative events (see Table 3). The results indicate that events were judged more negative by the vmPFC patients than by the non-vmPFC group and the healthy controls. Taken together, the inhibited top-down regulation of the vmPFC seems to dampen the regulation of negative affect, while the regulation of positive affect remains relatively intact. It seems plausible that negative emotions are more likely to be regulated due to their aversive nature, whereas positive emotions are more agreeable and adaptive for the individuals’ well-being. In line with this interpretation, it has been demonstrated that individuals regulate negative emotions more frequently than positive emotions (Gross, 2007). Moreover, emotion regulation does not always involve a change in intensity of an emotion, but may also involve the maintenance of an emotion, especially in the case of positive emotions (Denham, 1998; Gross, 2001; Tugade and Fredrickson, 2007). Note, however, that this negativity effect might also be explained by hypoperfusion in the left anterior temporal hypoperfusion, a damage which was present among half of the vmPFC patients. As this actually involved only four patients, this finding should be explored in more depth in future research.
A potential limitation of the present study is, as in most lesion studies, the limited number of patients. This shortcoming seems to impact mainly the emotional valence ratings, but seems to be less problematic for the attribution ratings, for which we find support for most of our hypotheses. Nevertheless, there seems to be a trend indicating that causal attributions might also be affected to some limited degree (i.e., reduced correct attribution to person or situation factors). This should be tested in future studies.
Another potential limitation is that there was some degree of overlap between the two types of attribution questionnaires, because the causal attributions questionnaire also involved some trait explanations. However, we want to emphasize that in the trait attribution questionnaire, two behavioral descriptions were presented that implied the same trait, while in the causal attribution questionnaire, a single event was described implying most often causes distinct from traits or person characteristics. Moreover, the intentional causal attributions were preceded by explicit reference to person or situational possibilities, which distinctly focuses on causal rather than trait attributions.
To conclude, the main contribution of this study is that it clearly demonstrates that the vmPFC is required for high-level, abstract trait attribution. This confirms fMRI studies demonstrating that the neural representation of traits resides in the vmPFC (Ma et al., 2013, 2014). For low-level causal attribution, it seems that the vmPFC is not critically involved, confirming and extending earlier findings by Kestemont et al. (2013, 2014). Moreover, with respect to negative emotions towards events, this study confirms the role of the vmPFC in emotion modulation and regulation.
Funding
This research was supported by SRP15 Grant of the Vrije Universiteit Brussel awarded to Frank Van Overwalle and Marie Vandekerckhove, and performed at CEPOS (Centrum voor Epilepsie en Psycho-Organische Stoornissen or CEPOS, Duffel, Belgium).
Appendix
Table A1.
List of experimental sentences that implied either the person or the situation as the cause. (Best possible translation from Dutch)
| Person-implied sentences | Valence | Person cause | Situation-implied sentences | Valence | Situation cause |
|---|---|---|---|---|---|
| Agouk looks at child pornography | − | pedophile | Alnorak swims in the Mediterranean | + | holiday |
| Avosa enjoys the bloodbath | − | psychopath | Angis takes the super glue | − | something broken |
| Dilla can work well together | + | social | Ashram shives from the wind | − | it's cold |
| Jun gives a bouquet at arrival | + | romantic | Birmak pushes the car | − | breakdown |
| Kasj rejoices over another's grief | − | sadist | Caldrik drinks at the wedding | + | party |
| Knarf talks to his colleagues | + | social | Calpo talks loudly during the move | − | much noise |
| Leezon takes care of the homeless | + | helpful | Drelnar avoids her work on public holidays | + | home with family |
| Loma thinks about his girlfriend | + | in love | Fablon puts the plates on the table | + | mealtime |
| Metel kicks the corpse | − | rage | Furaf hides for the storm | − | dangerous |
| Nanik tells the truth | + | honest | Kornap gets a present | + | birthday |
| Neesuw never talks to someone | − | asocial | Mordak buys a bandage | − | accident |
| Smik smiles with pleasure | + | happy | Reshta searches for eggs with the kids | + | Easter |
| Sniel takes advantage of others | − | stingy | Stelvine replaces the tire | − | flat tire |
| Spol works hard to assist others | + | helpful | Tonk quickly retrieves the lifejacket from under the seat | − | danger |
| Telwor sleeps on the sofa | − | tired | Xoyrish undresses in the locker room | + | sports |
| Fafel doesn't lose his courage | + | persistent | Adwan gets off the train | + | reaching destination |
| Jaho loves children | + | motherly | Brennak runs away from the rain | − | wet |
| Kalar joins the conversation | + | social | Ciaro is uninterested in the documentary | − | dull |
| Kale meets with open arms | + | warm | Cyralis pushes the motorbike | − | breakdown |
| Kobar hits a young girl | − | aggressive | Eelram listens to the singing | + | nice singing |
| Kobil calls emergency service for minor injuries | − | anxious | Equis runs over the pedestrian crossing | + | traffic rules |
| Lopo gives about others | + | kind | Listek can go on a holiday | + | has leave |
| Lusam stares at showering children | − | pedophile | Lokas pushes the swing | + | playing |
| Mart carries the luggage of the children | + | helpful | Maldron earns a salary | + | work |
| Olvij pushes the invalid | − | brutal | Nadon spits out the milk | − | sour milk |
| Poliw talks all the time | − | talkative | Nepril trembles in the sea | − | water is cold |
| Radro plays with her feelings | − | playboy | Pheldar pulls the brake | − | danger |
| Ringa leans against the handrail | − | tired | Therus avoids the dunghill | − | stinking |
| Tarin thinks that the future is beautiful | + | optimistic | Weldark throws the dice on a white-black board | + | game |
| Xapo goes to the hospital | − | ill | Whala shows his ticket | + | getting acces |
Table A2.
List of experimental sentences that implied a trait (best possible translation from Dutch).
| Actor | Trait-implying sentence 1 | Trait-implying sentence 2 | Valence | Implied trait |
|---|---|---|---|---|
| Bloemak | is crying a lot | is seldom laughing | − | melancholic |
| Blublo | calculated the load very fairly | calculated the income precisely | + | reliable |
| Bollap | talks to people on the train | tells about her thoughts | + | extroverted |
| Brimasy | tells a lot when in a pub | talks about the nice vacation | + | extroverted |
| Burc | showed her admiration to the speaker | smiled at the woman | + | sweet |
| Caltor | made his beloved a bad remark | gave his daughter many beatings | − | aggressiv |
| Choy | gave the beggar 20 euros | gave his secretary a raise | + | generous |
| Deloti | goes to camp with friends | often goes to the pub | + | social |
| Digmo | always creates enormous problems | regulates the orders very poorly | − | irresponsible |
| Elkmo | tells good jokes | grapples his friend | + | funny |
| Fromel | repairs the neighbor's alarm clock | cooks diner for his father | + | helpful |
| Frymi | doesn't go out often | always prepares the food on his own | − | asocial |
| Gimar | calculated the game very correctly | calculated the equipment very appropriately | + | reliable |
| Hanstra | prefers to do many things alone | never goes out of her house | − | asocial |
| Hirma | is in the student association | going to a friend's party | + | social |
| Irmiep | takes his job seriously | takes matters well | + | responsible |
| Ismin | gave millions to 11-11-11 | gave his gold watch to the concierge | + | bounteous |
| Jovop | tells about things quite often very quietly | speaks quite rarely according to them | − | introverted |
| Kavlim | is very often very sad | doesn't know any jokes | − | melancholic |
| Kerbol | did not return his sister's doll | gave his father a mock report | − | unreliable |
| Korbat | gave her secretary her resignation | gave her employees lots of criticism | − | unfriendly |
| Krakla | asked to pay for him | asked nothing and took the money | − | stingy |
| Lemui | asked the travelers' last money | asked his guests a lot of money | − | stingy |
| Melsu | insulted her colleague | made a bad remark to her colleague | − | unfriendly |
| Mortof | made her neighbor a surprise | made her secretary a sandwich | + | friendly |
| Niav | asked his team to cheat | asked his teammate to take dope | − | dishonest |
| Nildre | asked his athlete to lose | asked the man a counterfeit watch | − | dishonest |
| Palsa | prepared food for her colleagues | made her employees very happy | + | friendly |
| Plimeg | contributes the least of everyone | doesn't assist her younger sister | − | unhelpful |
| Quilot | never gives her opinion | rarely formulates her thoughts | − | introverted |
| Surcu | told her beloved her confession | told her friend the truth | + | honest |
| Tamic | fabricates the kitchen of his girlfriend | lends tools to a friend | + | helpful |
| Terp | gave his mother the wrong test | didn't give his grandmother her money | − | unreliable |
| Ulvas | malfunctions in the company | sometimes goes to an appointment | − | irresponsible |
| Ushtom | had confidence in the man | showed her appreciation to the man | + | sweet |
| Vousblo | plays some nice sketches | is often laughing | + | funny |
| Wemblo | follows his schedule perfectly | acts with proper approach | + | responsible |
| Ybar | rarely helps an old man | doesn't repair his mother's broken chair | − | unhelpful |
| Yotono | told everything to her partner | told her beloved the truth | + | honest |
| Zirno | gave his daughter no affection | gave his nephew a beating | − | aggressive |
References
- Adolphs R. (2009). The social brain: neural basis of social knowledge. Annual Review of Psychology , 60, 693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R., Gosselin F., Buchanan T.W., Tranel D., Schyns P., Damasio A.R. (2005). A mechanism for impaired fear recognition after amygdala damage. Nature , 433, 68–72. [DOI] [PubMed] [Google Scholar]
- Amodio D.M., Frith C.D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7(4), 268–77. [DOI] [PubMed] [Google Scholar]
- Apperly I.A., Samson D., Chiavarino C., Humphreys G.W. (2004). Frontal and temporo-parietal lobe contributions to theory of mind: neuropsychological evidence from a false-belief task with reduced language and executive demands. Journal of Cognitive Neuroscience, 16(10), 1773–84. [DOI] [PubMed] [Google Scholar]
- Bechara A. (2004). Disturbances of emotion regulation after focal brain lesions. International Review of Neurobiology , 62, 159–93. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Beer J.S., Heerey E.A., Keltner D., Scabini D., Knight R.T. (2003). The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology , 85, 594–604. [DOI] [PubMed] [Google Scholar]
- Benton A.L., Hamsher K. (1976). Multilingual Aphasia Examination. Iowa City, IA: University of Iowa. [Google Scholar]
- Benton A.L., Varney N.R., Hamsher K.D. (1978). Visuospatial judgment: a clinical test. Archives of Neurology. , 35, 364–7. [DOI] [PubMed] [Google Scholar]
- Bourdon B., Wiersma E.D. (1902). Bourdon-Wiersma Test. Grondingen, NL: Academisch Ziekenhuis, Afdeling Psychologie. [Google Scholar]
- Carrington S.J., Bailey A.J. (2009). Are there theory of mind regions in the brain? A review of the neuroimaging literature. Human Brain Mapping, 30(8), 2313–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis F. (1959). The Maze Test: Two Parallel Procedures. Bern, Switzerland: Huber. [Google Scholar]
- Coetsier L., De Clercq A., De Quindt A., Mortier V., Thienpondt A. (n.d.). Geheugen voor zinvolle samenhang. Deinze, België: Caecilia Boekhandel. [Google Scholar]
- Corradi-Dell' Acqua C., Hofstetter C., Vuilleumier P. (2014). Cognitive and affective theory of mind share the same local patterns of activity in posterior temporal but not medial prefrontal cortex. Social Cognitive and Affective Neuroscience , 9, 1175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A.R., Tranel D., Damasio H. (1990). Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioral Brian Research , 41, 81–94. [DOI] [PubMed] [Google Scholar]
- Denham S.A. (1998). Emotional Development in Young Children. New York, NY: Guilford Press. [Google Scholar]
- Ferstl E.C., Rinck M., von Cramon D.Y. (2005). Emotional and temporal aspects of situation model processing during text comprehension: an event-related fMRI study. Journal of Cognitive Neuroscience , 17, 724–39. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C. (2001). The biological basis of social interaction. Current Directions in Psychological Science, 10(5), 151–5. [Google Scholar]
- Geraci A., Surian L., Ferraro M., Cantagallo A. (2010). Theory of Mind in patients with ventromedial or dorsolateral prefrontal lesions following traumatic brain injury. Brain Injury: [BI], 24(7–8), 978–87. [DOI] [PubMed] [Google Scholar]
- Gross J.J. (2001). Emotion regulation in adulthood: timing is everything. Current Directions in Psychological Sciences , 10, 214–19. [Google Scholar]
- Gross J.J. (2007). The Handbook of Emotin Regulation. New York, NY: Guilford. [Google Scholar]
- Harris L.T., Todorov A., Fiske S.T. (2005). Attributions on the brain: neuro-imaging dispositional inferences, beyond theory of mind. NeuroImage, 28(4), 763–9. [DOI] [PubMed] [Google Scholar]
- Hornak J., Bramham J., Rolls E.T., et al. (2003). Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain , 126, 1691–712. [DOI] [PubMed] [Google Scholar]
- Kalbe E., Schlegel M., Sack A.T., et al. (2010). Dissociating cognitive from affective theory of mind: a TMS study. Cortex , 46, 769–80. [DOI] [PubMed] [Google Scholar]
- Kestemont J., Ma N., Baetens K., Clément N., Van Overwalle F., Vandekerckhove M. (2014). Neural correlates of attributing causes to the self, another person and the situation. Social Cognitive and Affective Neuroscience, 10(1), 114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestemont J., Vandekerckhove M., Ma N., Van Hoeck N., Van Overwalle F. (2013). Situation and person attributions under spontaneous and intentional instructions: an fMRI study. Social Cognitive and Affective Neuroscience, 8(5), 481–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Somerville L.H., Johnstone T., Alexander A.L., Whalen P.J. (2003). Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport, 14(18), 2317–22. [DOI] [PubMed] [Google Scholar]
- Leopold A., Krueger F., Dal Monte O., et al. (2012). Damage to the left ventromedial prefrontal cortex impacts affective theory of mind. Social Cognitive and Affective Neuroscience. 7(8), 871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D. (2007). Social cognitive neuroscience: a review of core processes. Annual Review of Psychology, 58, 259–89. [DOI] [PubMed] [Google Scholar]
- Luteijn F., Van der Ploeg F.A.E. (1983). Handleiding GIT. Lisse, NL: Swets & Zeitlinger. [Google Scholar]
- Ma N., Baetens K., Vandekerckhove M., Kestemont J., Fias W., Van Overwalle F. (2013). Traits are represented in the medial prefrontal cortex: an fMRI adaptation study. Social Cognitive and Affective Neuroscience, 9(8), 1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Baetens K., Vandekerckhove M., Van der Cruyssen L., Van Overwalle F. (2014). Dissociation of a trait and a valence representation in the mPFC. Social Cognitive and Affective Neuroscience, 9(10), 1506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Vandekerckhove M., Baetens K., Van Overwalle F., Seurinck R., Fias W. (2012). Inconsistencies in spontaneous and intentional trait inferences. Social Cognitive and Affective Neuroscience, 7(8), 937–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Vandekerckhove M., Van Overwalle F., Seurinck R., Fias W. (2011). Spontaneous and intentional trait inferences recruit a common mentalizing network to a different degree: spontaneous inferences activate only its core areas. Social Neuroscience, 6(2), 123–38. [DOI] [PubMed] [Google Scholar]
- Meyers J.E., Meyers K.R. (1995). Complexe FIguurtest van Rey Met Recognitietrial. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Mitchell J.P. (2009). Social psychology as a natural kind. Trends in Cognitive Sciences, 13(6), 246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.P., Banaji M.R., Macrae C.N. (2005). General and specific contributions of the medial prefrontal cortex to knowledge about mental states. NeuroImage, 28(4), 757–62. [DOI] [PubMed] [Google Scholar]
- Rey A. (1941). L'examen psychologique dans les cas d'encephalopathie traumatique. Archives de Psychologie , 28, 21. [Google Scholar]
- Rey A. (1964). L'examen clinique en psychologie. Paris, France: Presses universitair de France. [Google Scholar]
- Rudebeck P.H., Bannerman D.M., Rushworth M.F.S. (2008). The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cognitive, Affective, and Behavioral Neuroscience , 8, 485–97. [DOI] [PubMed] [Google Scholar]
- Saxe R.R., Wexler A. (2005). Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia, 43(10), 1391–9. [DOI] [PubMed] [Google Scholar]
- Seidel E.-M., Eickhoff S.B., Kellermann T., et al. (2010). Who is to blame? Neural correlates of causal attribution in social situations. Social Neuroscience, 5(4), 335–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Aharon-Peretz J. (2007). Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia, 45(13), 3054–67. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Tomer R., Berger B.D., Goldsher D., Aharon-Peretz J. (2005). Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology, 18(1), 55–67. Available: http://www.ncbi.nlm.nih.gov/pubmed/15761277 [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Harnett-Sheehan K., et al. (1998). The Mini International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry , 59, 22–33. [PubMed] [Google Scholar]
- Stroop J. (1935). Studies of interferences in serial verbal reaction. Journal of Experimental Psychology , 18, 643–62. [Google Scholar]
- Tugade M.M., Fredrickson B.L. (2007). Regulation of positive emotions: emotion regulation strategies that promote resilience. Journal of Happiness Studies , 8, 311–33. [Google Scholar]
- Uleman J.S. (1999). Spontaneous versus intentional inferences in impression formation. In: Chaiken Y., Trope S., editors. Dual-process Theories in Social Psychology. New York, NY: The Guilford Press, pp. 141–60. [Google Scholar]
- Urry H.L., van Reekum C.M., Johnstone T., et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience, 26(16), 4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove M., Plessers M., Van Mieghem A., et al. (2014). Impaired facial emotion recognition in patients with ventromedial prefrontal hypoperfusion. Neuropsychology , 28, 605–12. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. (2009). Social cognition and the brain: a meta-analysis. Human Brain Mapping, 30(3), 829–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet I.M., Leroy H., van Megen H.J.G.M. (2000). M.I.N.I. plus: M.I.N.I. Internationaal Neuropsychiatrisch Interview: Nederlandse versie 5.0.0. [Google Scholar]
- Wechsler D. (2001). WAIS-III Nederlandstalige bewerking. Afname en scoringshandleiding. Lisse, NL: Swets & Zeitlinger. [Google Scholar]