Abstract
Stress exposure is known to precipitate psychological disorders. However, large differences exist in how individuals respond to stressful situations. A major marker for stress sensitivity is hypothalamus–pituitary–adrenal (HPA)-axis function. Here, we studied how interindividual variance in both basal cortisol levels and stress-induced cortisol responses predicts differences in neural vigilance processing during stress exposure. Implementing a randomized, counterbalanced, crossover design, 120 healthy male participants were exposed to a stress-induction and control procedure, followed by an emotional perception task (viewing fearful and happy faces) during fMRI scanning. Stress sensitivity was assessed using physiological (salivary cortisol levels) and psychological measures (trait questionnaires). High stress-induced cortisol responses were associated with increased stress sensitivity as assessed by psychological questionnaires, a stronger stress-induced increase in medial temporal activity and greater differential amygdala responses to fearful as opposed to happy faces under control conditions. In contrast, high basal cortisol levels were related to relative stress resilience as reflected by higher extraversion scores, a lower stress-induced increase in amygdala activity and enhanced differential processing of fearful compared with happy faces under stress. These findings seem to reflect a critical role for HPA-axis signaling in stress coping; higher basal levels indicate stress resilience, whereas higher cortisol responsivity to stress might facilitate recovery in those individuals prone to react sensitively to stress.
Keywords: cortisol, stress, neural vigilance, interindividual differences, amygdala
Introduction
Stress exposure is known to precipitate mental disorders (Juster et al., 2011). However, whereas stress is part of daily life, only a select population of individuals develops stress-related psychopathology. Elucidating the basis of individual differences in stress vulnerability or resiliency has therefore been at the forefront of clinical and preclinical research.
A well-known physiological factor associated with the vulnerability to develop stress-related mental disorders is hypothalamus–pituitary–adrenal (HPA)-axis functioning. Stress-related psychopathology has been associated with abnormal functioning of the HPA-axis, both under basal conditions and in response to stress. Major depression, for example, generally seems to be characterized by elevated basal cortisol levels and impaired stress recovery, potentially caused by impaired negative feedback on the HPA-axis (Herbert, 2013). On the other hand, post-traumatic stress disorder has been suggested to be related to stronger negative feedback over the HPA-axis, leading to lower basal cortisol levels (Yehuda, 2001) and reduced cortisol responding to stress (Yehuda et al., 1993). However, literature on the exact link between HPA-axis function and psychopathology is rather heterogeneous and these findings may only apply to a subset of individuals (Schatzberg et al., 2014). Importantly, basal levels of cortisol and the stress-induced response involve a differential pattern of corticosteroid receptor activation (de Kloet et al., 1998), and therefore likely have differential neural correlates. Animal studies have indicated that basal cortisol levels mainly involve the activation of nuclear mineralocorticoid receptors (MRs), thought to maintain neuronal homeostasis and limit any disturbances by stress, while the stress-induced rise in cortisol elicits substantial activation of low-affinity MRs and glucocorticoid receptors (GRs) located in the cell membrane, thought to rapidly potentiate the effect of stressors and arousal (Groeneweg et al., 2012). Furthermore, additional nuclear GR activation in response to stress is thought to subsequently contribute to the normalization of stress-induced neuronal activation to help an organism to recover from stress (de Kloet et al., 1998, 2008). These findings suggest that both basal and stress-induced cortisol measures might be important—and to some extent independent—markers of an individual’s stress sensitivity. Initial evidence from healthy participants seems to support this association by showing correlations between HPA-axis activity and psychological traits reflecting stress sensitivity, such as neuroticism and trait anxiety (Everaerd et al., 2015; Laceulle et al., 2015), which are known risk factors for stress-related disorders. However, the neural mechanisms associated with this important link between cortisol signaling and stress sensitivity are currently largely unknown.
A critical brain region involved in stress sensitivity is the amygdala; key regulator of vigilance and emotional processing, and involved in the initiation of the stress response (de Kloet et al., 2005; Phelps and LeDoux, 2005). Stress exposure has been shown to induce a state of neural hypervigilance, in which the amygdala switches to a state of highly sensitive, yet unspecific, processing [e.g. van Marle et al., 2009, but also overall reduced activity has been reported (Pruessner et al., 2008; Khalili-Mahani et al., 2010)]. Patients suffering from stress-related mental disorders display similarly increased amygdala responsivity (Drevets, 2003; Etkin and Wager, 2007), and a compromised ability to suppress its processing of emotional information (Mitterschiffthaler et al., 2008). The exact contribution of cortisol signaling herein is currently however unknown.
Here, we targeted the relationship between individual differences in distinct aspects of HPA-axis function, i.e. tonic basal cortisol levels and the phasic cortisol response to stress, and neural vigilance processing under stress. We chose to study a healthy volunteer cohort to probe general mechanisms unrelated to disease consequences (Lanius et al., 2010). Implementing a randomized, counterbalanced, crossover design, we scanned 120 healthy male participants while they viewed morphing emotional faces (van Marle et al., 2009) preceded by a mild stress-induction or control procedure in two separate sessions. Psychological and physiological measures of the stress response were monitored throughout the experiment, whereas cortisol samples taken at home served as basal measure. Moreover, psychological traits known to confer vulnerability to stress were assessed using questionnaires and tested for associations with HPA-axis function.
Methods
Participants
One hundred twenty young (18–30 years), right-handed, healthy male volunteers gave informed consent to participate in the study. Individuals with any history of or current psychiatric, neurological or endocrine disorders, or receiving any medication that affects central nervous or endocrine systems, were excluded from participation. Because abnormal sleep patterns might heavily influence cortisol secretion at a certain time a day, working night shifts or current jetlags served as additional exclusion criteria. The study was approved by the local ethical reviewing committee (Commissie Mensgebonden Onderzoek region Arnhem-Nijmegen, The Netherlands) and adhering national legislation in accordance to basic international ethical principles (i.e. the Declaration of Helsinki).
Study design
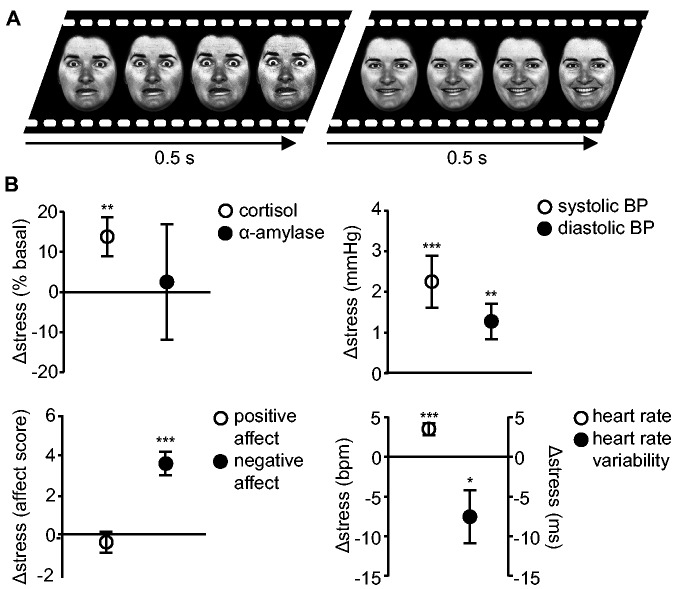
In a randomized, counterbalanced crossover design, all men underwent two afternoon sessions of fMRI scanning, separated by on average 2 weeks (with a minimum of 5 days). Immediately after watching either a stressful or neutral movie clip, participants viewed morphing emotional faces (van Marle et al., 2009) in the magnetic resonance imaging scanner (Figure 1A). Physiological [cortisol and α-amylase level, heart rate, heart rate variability and systolic and diastolic blood pressure (BP)] and psychological (positive and negative affect) indices were measured to confirm successful stress induction (Supplementary Material). To obtain the cleanest measure of basal cortisol levels, not confounded by a potential anticipatory stress response to participation in the experiment, basal cortisol level was measured by two saliva samples taken at home. Participants were instructed to adhere to strict criteria before sampling (Supplementary Material). To facilitate compliance to these instructions and to best possibly match the timing of the samples taken in the laboratory, participants were asked to take one saliva sample just prior to lunch and the other just prior to dinner, on the day before the second session.
Fig. 1.
Experimental task and physiological and psychological stress response measures. (A) In each experimental session, participants watched either a stressful or neutral movie clip during fMRI scanning, immediately followed by the dynamic facial expression task; an emotional perception task that consisted of blocks of faces dynamically morphing into overtly fearful or happy expressions. (B) The stress response was defined by the difference measures between the stress and control sessions at t = 95 min, whereas heart rate (HR) responses reflect the difference in HR during the stressful and control movie just prior to the task. Exposure to the stressful movie clip significantly increased salivary cortisol levels, systolic and diastolic blood pressure (BP), and reported negative affect, whereas positive affect and α-amylase levels were not significantly modulated by stress exposure. Furthermore, stressful movie watching significantly increased HR and decreased heart rate variability. ***P < 0.001; **P < 0.01; *P < 0.05.
Procedure
To reduce the impact of diurnal variation in cortisol levels, all testing was performed in the afternoon, between noon and 7:00 P.M. Importantly, overall testing time was not different between sessions [mean ± s.d.; 4 ± 100 min, t(119) < 1]. After arrival, participants were seated in a quiet room and watched a relaxing movie [Life (2009), Martha Holmes] for 30 min to bring them in a relaxed state. At 45 min after arrival their BP was measured, a first saliva sample was taken, and participants were asked to complete the positive and negative affect schedule (PANAS) questionnaire (Watson et al., 1988). At 60 min post-arrival participants were brought to the scanner room and were notified whether they were enrolled in the neutral or stress session.
Movie fragments were used to manipulate stress levels. Scenes shown were either selected from a distressing [Irréversible (2002), Gaspar Noé] or neutral sequence [Comment j'ai tué mon père (2001), Anne Fontaine] and were comparable in amount of speech, human presence, luminance and language (Henckens et al., 2009). The scanning session started with a movie clip of 10 min, followed by the emotional perception task. The task consisted of blocks of faces morphing dynamically from a relatively neutral into either fearful or happy facial expressions (Figure 1A) (van Marle et al., 2009). Participants were instructed to passively view the faces and to make a right index finger response on a button box whenever a fixation cross appeared, as check for their attention. The task lasted ∼4 min. Directly following the task (95 min post-arrival), participants’ BP was assessed again, another saliva sample was taken, and participants completed a second PANAS questionnaire. The difference between both sessions at this time point was used as an index of the stress response. To correct for potential differences in basal activity, cortisol and α-amylase responses were normalized to basal levels. Hence, the cortisol stress response was defined as:
The emotional perception task was first in a series of cognitive tasks participants were asked to perform in the scanner, which were interleaved by shorter-lasting (90 s) ‘reminder’ movie clips. Results on the other tasks will be described elsewhere. At t = 130 min, for the third and final time participants’ BP, a saliva sample was taken, and participants completed a PANAS questionnaire. After completion of all tasks, a structural scan was obtained. The total scan session lasted ∼100 min.
Psychological traits
Participants were asked to complete the Beck depression inventory (Beck et al., 2002), Spielberger’s trait anxiety inventory (van der Ploeg et al., 1980) and the NEO - five factor inventory (Costa and McCrae, 1992), assessing psychological traits linked to stress sensitivity and susceptibility to stress-related psychopathology. Whereas measures of depression, anxiety and neuroticism have been thought to indicate susceptibility to stress-related disease, extraversion scores have been related to stress resilience (Clark et al., 1994; Klein et al., 2011; Everaerd et al., 2015).
Statistical analysis of behavioral and physiological data
Behavioral and physiological data were analyzed in SPSS 19.0 using repeated measures Analysis of variances and paired samples t-tests. For correlational analyses, Pearson correlations were used. To exclude the possibility of potential non-normality or outliers driving correlational significance, all significant correlations were confirmed by additional Spearman correlational (i.e. non-parametric) analyses. Alpha was set at 0.05 throughout.
fMRI acquisition and data analysis
Participants were scanned by a Siemens MAGNETOM Avanto 1.5 Tesla MRI scanner equipped with a 32-channel head coil. Details about the MRI data acquisition protocol and analyses can be found in the Supplementary Data. Following standard preprocessing procedures, we applied a general linear model for each condition, in which regressors represented task blocks separated by emotion type (fear vs happy). Regressors were temporally convolved with the canonical hemodynamic response function of SPM8. Six covariates corresponding to the movement parameters were also included in the model. The single subject parameter estimates from each condition and emotion type were included in subsequent random-effects analyses. For this second-level analysis, factorial ANOVAs were used in SPM, with condition and emotion type as within-subject factors to assess overall effects of stress exposure, emotion type and stress × emotion type interactions (Note: fixation crosses were not modeled as they served as internal baseline). To investigate the neural correlates of ‘individual differences’ in stress sensitivity, identical factorial ANOVAs were used with either the participants’ stress-induced cortisol response or the basal cortisol level as covariate of interest. Due to fMRI data dropout, one participant was excluded from all fMRI analyses. One additional participant was excluded from the basal cortisol level fMRI analyses, since he displayed a highly abnormal value in the correlational plot between basal cortisol level and amygdala responsivity (Figure 4B, >3 s.d. from mean regression).
Fig. 4.
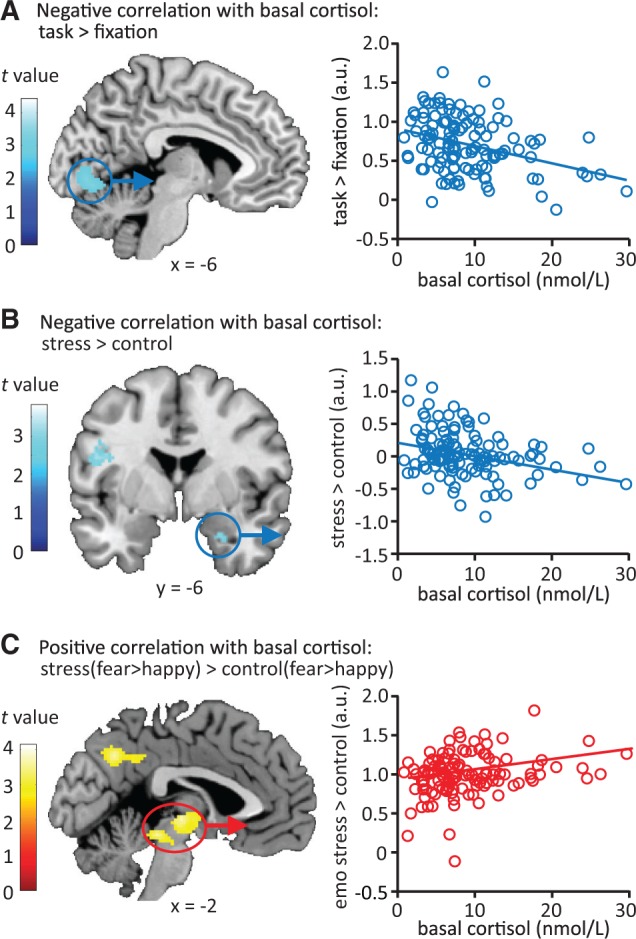
Stress-induced changes in neural emotional processing related to basal cortisol levels. (A) Higher basal cortisol levels were associated with lower overall neural activity in early visual processing areas during the emotional perception task. (B) Participants with higher basal cortisol levels showed reduced stress-induced recruitment of the amygdala. (C) Higher basal cortisol levels were associated with stronger emotion-specific processing (differential processing of negative over positive emotional input) under stress relative to control conditions in the posterior cingulate cortex, precuneus, angular gyrus and thalamus. Correlation plots depict the extracted data (the average beta weights of the whole cluster) from the significant clusters and thus merely serve illustrative purposes. All contrast maps are displayed at P < 0.005 uncorrected. See Table 2 for exact statistics and coordinates.
Statistical tests were family-wise error (FWE) rate corrected (P < 0.05) for multiple comparisons at the cluster level using an initial height threshold at the voxel level of P < 0.005 for the correlational analyses with stress-induced cortisol and basal cortisol levels. Based on our a priori hypothesis about its critical role in emotional processing and stress, the amygdala was treated as a region of interest and was subjected to small-volume correction using its anatomical mask as defined by the WFU PickAtlas Tool (version 2.4). Statistical tests for the amygdala were FWE rate corrected (P < 0.05) for multiple comparisons at the voxel level. We extracted the mean parameter estimates of the anatomically defined [WFU PickAtlas Tool (version 2.4)] bilateral amygdala to test for any correlations between the amygdala response to stress and autonomic stress response measures.
Results
Effects of stress induction on physiological and psychological measures
Physiological measures confirmed successful stress induction (Figure 1B, Supplementary Table S1). Salivary cortisol levels indicated that HPA-axis activity overall was mildly elevated following acute stress induction [stress: 109.12 ± 89.06% of basal, control: 94.08 ± 81.59% of basal, t(118) = 2.72, P = 0.008], but, of interest to this study, this response varied considerably between individuals (ranging from−10.59 to 15.01 nmol/l, Supplementary Figure S1). BP, heart rate and heart rate variability indicated an increase in sympathetic tone following stress induction (Figure 1B). BP was also elevated [mean ± s.d., systolic; stress: 108.88 ± 8.00 mmHg, control: 106.63 ± 7.78 mmHg, t(119) = 3.52, P = 0.001, stress response ranging from −17 to 26 mmHg, diastolic; stress: 69.77 ± 5.60 mmHg, control: 68.49 ± 7.66 mmHg, t(119) = 2.91, P = 0.004, stress response ranging from −12 to 12 mmHg]. Heart rate as recorded during the stressful movie was also increased [stress: 67.53 ± 11.40 bpm, control: 63.80 ± 12.09 bpm, t(113) = −4.63, P < 0.001, stress response ranging from −14.02 to 28.30 bpm], whereas heart rate variability was decreased [stress: 64.05 ± 30.61 ms, control: 72.99 ± 41.94 ms, t(113) = 2.27, P = 0.025, ranging from −89.36 to 88.41 ms]. Salivary α-amylase levels did not lead to measurable stress effects (stress: 112.53 ± 150.43% of basal, control: 111.26 ± 117.22% of basal, stress response ranging from −80.95 to 65.91 U/l). Notably, again there were substantial differences amongst participants in the size of the sympathetic stress response.
Stress also induced an overall increase in subjective stress [negative affect rating, stress: 17.20 ± 7.41, control: 13.60 ± 4.28, t(119) = 6.25, P < 0.001, stress response ranging from −9 to 30, Figure 1B], whereas positive affect was not changed [stress: 27.53 ± 5.80, control: 27.79 ± 6.46, t(119) < 1, stress reponse ranging from −19 to 13]. Interestingly, although they were no primary outcome measure of the task, reaction times during the task were slower in the stress compared with the control session [stress: 711 ± 28 ms, control: 649 ± 20 ms, t(110) = 2.24, P = 0.027, stress response ranging from −841 to 684 ms, Figure 1B]. Such slower responding to non-threatening cues may suggests less focused (task-related) processing, as is indicative of a hypervigilant state, which is characterized by attentional vigilance at the cost of loss of focus (Aston-Jones and Cohen, 2005; Henckens et al., 2009).
Thus, as intended, the stress response measures revealed considerable variability amongst participants, indicating that some participants were more sensitive to the stress-induction procedure than others. The relatively large sample size of this study enabled us to investigate these individual differences in stress responsiveness further. Interestingly, correlational analyses revealed significant correlations between most stress measures related to the sympathetic stress response [i.e. heart rate, heart rate variability, systolic and diastolic BP and α-amylase (Supplementary Table S1)], as well as a significant correlation between these measures and the increase in negative affect in response to stress. Remarkably, none of the sympathetic or psychological measures correlated to the cortisol response to stress (all P > 0.1); suggesting the involvement of two rather independent stress-systems.
Basal cortisol levels as a measure of tonic HPA-axis activity
Basal salivary cortisol levels, reflecting tonic HPA-axis activation, also showed considerable differences between individuals (ranging from 0.86 to 29.66 nmol/l, Supplementary Table S1). Importantly, basal levels did not significantly correlate with the stress-induced (i.e. phasic) cortisol response [r(117) = −0.125, P = 0.174, Supplementary Figure S2], suggesting that tonic and plastic responses were rather independent indices of HPA-axis functioning in our sample, with potentially distinct neural correlates.
To confirm the reliability of our assessment of participants’ basal cortisol levels and their stability over days, we tested for their correlation with other cortisol samples possibly reflecting tonic HPA-axis measures during the experimental session (i.e. those obtained during the neutral control session). Basal cortisol levels correlated significantly with the last cortisol sample obtained in the control session (at t = 130 min, r(118) = 0.244, P = 0.007), confirming the reliability of our assessment and indicating that basal cortisol levels reflect a relatively stable characteristic of a subject’s tonic HPA-axis activity. However, as (anticipation to) MRI scanning has been shown to trigger cortisol responding (Tessner et al., 2006; Muehlhan et al., 2011), no significant correlations were observed between the basal cortisol levels and those observed at the start of the experimental session [at t = 45 min, r(117) = 0.121, P = 0.190], or after entering the MRI scanner [at t = 95 min, r(117) = 0.135, P = 0.142].
Cortisol indices and psychological traits
Next, we tested whether HPA-axis indices were related to stress sensitivity as indicated by psychological traits. Therefore, we correlated basal cortisol levels and stress-induced cortisol responses to psychological traits indexing stress sensitivity and the risk to develop stress-related psychopathology. Participants with higher cortisol responses to stress reported lower levels of extraversion [r(117) = −0.225, P = 0.014, Figure 2A] and higher depression levels [r(117) = 0.194, P = 0.034] but no differences in neuroticism and trait anxiety scores (P > 0.1). Interestingly, in contrast, participants with relatively high basal cortisol levels were more extravert [r(118) = 0.195, P = 0.032, Figure 2B]. Basal cortisol levels did not show any significant correlations with neuroticism, trait anxiety or depression (all P > 0.1).
Fig. 2.
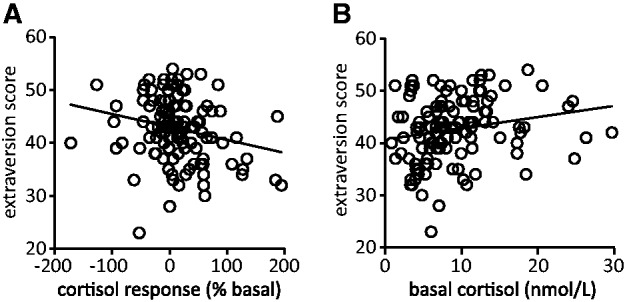
Basal and stress-induced cortisol levels were significantly correlated to extraversion scores. (A) The cortisol response to stress correlated negatively to extraversion scores [r(117) = −0.225, P = 0.014]; the most extravert participants showed the lowest cortisol response to the stressor. Furthermore, the stress-induced cortisol response correlated positively to depression scores [r(117) = 0.194, P = 0.034; not shown in this figure]. (B) Basal cortisol levels were positively correlated to extraversion [r(118) = 0.195, P = 0.032]; the most extravert participants had the highest basal cortisol levels.
Effect of stress on emotional processing
In line with previous studies (van Marle et al., 2009; Henckens et al., 2010; Everaerd et al., 2015), the emotional perception task induced strong activation of bilateral amygdala, hippocampus, prefrontal cortex and a widespread visual processing network (Supplementary Figure S3A and Table S2). Stronger responses toward fearful than happy faces were observed in the inferior occipital and fusiform gyrus but not in the amygdala that processed the emotional stimuli to the same extent (Everaerd et al., 2015). The opposite contrast (happy > fearful) did not yield any significant differences in brain activity (Supplementary Figure S3B and Table S2). Overall, we observed no main effects of stress or stress × emotion interactions, probably because of the large interindividual variability in stress responsivity.
Emotional processing under stress and the cortisol stress response
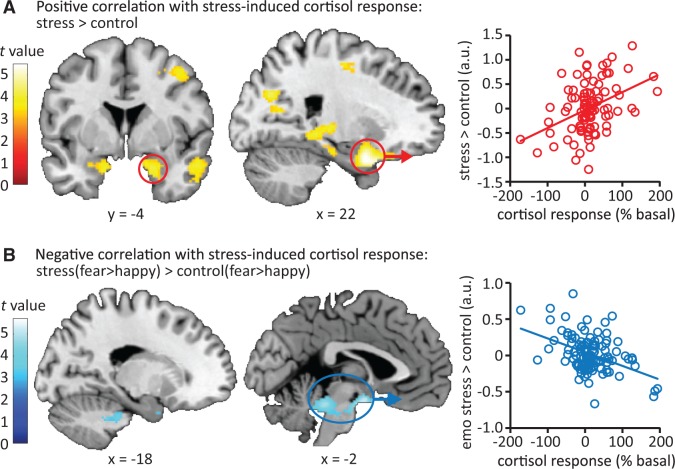
The size of the participants’ cortisol response to the stress-induction procedure was positively correlated to a stress-induced increase in responsivity of the bilateral amygdala, hippocampus, parahippocampal gyrus and inferior temporal gyrus toward emotional faces (Figure 3A, Table 1). Thus, participants displaying a stronger cortisol response showed a stronger stress-related increase in activity in this set of regions. No regions displayed a significant negative correlation between cortisol response and stress-induced differences in activity during emotional processing. Moreover, participants’ cortisol response was associated with a significant reduction in the differential processing of fearful compared with happy faces under stress in a large activation cluster in part of the midbrain, covering the locus coeruleus and extending into the hypothalamus and amygdala (Figure 3B, see Supplementary Figure S4 for illustrative correlational plots on this interaction). We performed post hoc tests to better understand this rather complex correlation. The interaction appeared to be caused by stronger differential processing of fearful vs happy faces in the control condition by individuals with higher cortisol stress responses (Table 1). Further testing of the observed clusters (for the fear > happy contrast in the control session) indicated that the association with stress-induced cortisol levels was primarily caused by reduced activation in response to happy faces in the control session in participants with higher cortisol stress responses (Supplementary Figure S5).
Fig. 3.
Stress-induced changes in neural emotional processing related to the cortisol response to the stressor. (A) The stress-induced increase in cortisol was related to stress-induced emotional processing. A large cluster covering several limbic and temporal regions showed increased activity during stress as a function of salivary cortisol increase. (B) Stress reduced the differential processing of fearful compared with happy faces depending on one’s cortisol response. Correlation plots depict the extracted data (the average beta weights of the whole cluster) from the significant clusters and thus merely serve illustrative purposes. Moreover, for illustrative purposes all contrast maps are displayed at P < 0.001 uncorrected. See Table 1 for exact statistics and coordinates.
Table 1.
Peak voxels and corresponding t values of activation clusters that show significant correlation with the cortisol response to stress
| Region | Cluster size | MNI coordinates |
Peak t value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Positive effect of cortisol response: stress > control | |||||
| Extended cluster of activation, centered around | 21 858*** | 22 | 2 | −22 | 5.50 |
| the amygdala, covering the bilateral | −2 | −66 | −18 | 4.70 | |
| hippocampus, parahippocampal gyrus, inferior | −56 | −24 | 30 | 4.62 | |
| temporal gyrus and supramarginal gyrus | |||||
| Precentral gyrus, R | 1644*** | 28 | −16 | 54 | 4.16 |
| Amygdala, R | 24 | 2 | −20 | 5.37### | |
| Amygdala, L | −18 | −6 | −20 | 3.92## | |
| Negative effect of cortisol response: stress > control | |||||
| / | |||||
| Positive effect of cortisol response: stress(fear > happy) > control(fear > happy) | |||||
| / | |||||
| Negative effect of cortisol response: stress(fear > happy) > control(fear > happy) | |||||
| Midbrain including the locus coeruleus, and hypothalamus | 2670*** | −6 | −8 | −16 | 5.72 |
| Superior frontal gyrus, R | 1016** | 36 | −48 | 36 | 4.65 |
| Amygdala, R | 22 | −4 | −26 | 3.04# | |
| Amygdala, L | −18 | −2 | −24 | 3.24# | |
| Negative effect of cortisol response: stress(fear > happy) / | |||||
| / | |||||
| Positive effect of cortisol response: control(fear > happy) | |||||
| Large cluster covering the hypothalamus, | 4982*** | −6 | −8 | −16 | 5.63 |
| midbrain including the locus coeruleus, | 10 | −40 | −24 | 4.76 | |
| and cerebellum | 0 | −42 | −18 | 4.67 | |
| Insula, R | 638* | 46 | −4 | 4 | 3.85 |
| 630* | 44 | −16 | 10 | 3.70 | |
| Supramarginal gyrus, R | 869** | 64 | −44 | 40 | 4.49 |
| Amygdala, R | 26 | −4 | −26 | 3.27# | |
| Amygdala, L | −18 | −2 | −24 | 3.27# | |
MNI, Montreal Neurological Institute; R, right; L, left. All effects are analyzed using cluster-level statistics, implementing a height threshold at P < 0.005 uncorrected at the voxel level. ***P < 0.001; **P < 0.01 (whole brain corrected); ###P < 0.001 (small-volume corrected for region of interest); ##P < 0.01 (small-volume corrected for region of interest); #P < 0.05 (small-volume corrected for region of interest).
To test the specificity of the observed association between stress-induced amygdala response and the cortisol response to stress, we also tested for associations between amygdala responding and autonomic stress response measures. Extracted parameter estimated from the amygdala revealed a significant correlation between the amygdala stress response and the systolic BP response to stress [r(118) = 0.199, P = 0.031] but none of the other measures of sympathetic activation (all P > 0.05). However, this association would not survive correction for multiple comparisons. Partial correlation analysis revealed that both physiological stress markers (cortisol and BPsys) were independently associated with the stress-induced increase in amygdala activation [cortisol stress response: ρ(115) = 0.389, P < 0.001, BPsys stress response: ρ(115) = 0.194, P = 0.036]. Thus, this data implies a link between the amygdala stress response and stress-induced cortisol levels, independent of the autonomic stress response.
Emotional processing under stress and basal cortisol levels
Next, we tested for the association of basal cortisol levels with stress-induced alterations in emotional processing. Basal cortisol levels were negatively correlated with overall activity in early visual processing areas (inferior occipital gyrus, lingual gyrus and cerebellum) during emotional processing, regardless of condition (Figure 4A, Table 2). Thus, participants with relatively high basal cortisol levels displayed reduced activation of these regions. No regions displayed a positive correlation in their activity and basal cortisol.
Table 2.
Peak voxels and corresponding t values of activation clusters that show significant correlation with basal cortisol levels
| Region | Cluster size | MNI coordinates |
Peak t value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Positive overall effect basal cortisol | |||||
| / | |||||
| Negative overall effect basal cortisol | |||||
| Inferior occipital gyrus/cerebellum | 473* | 6 | −82 | −14 | 4.42 |
| Positive effect of basal cortisol: stress > control | |||||
| / | |||||
| Negative effect of basal cortisol: stress > control | |||||
| Amygdala, R | 24 | −6 | −20 | 2.99# | |
| Positive effect of basal cortisol: stress(fear > happy) > control(fear > happy) | |||||
| Precuneus/posterior cingulate cortex, R | 848** | 14 | −56 | 40 | 4.08 |
| Angular gyrus, R | 482* | 52 | −54 | 42 | 3.92 |
| Thalamus/midbrain | 497* | 4 | −22 | 0 | 4.13 |
| Positive effect of cortisol response: stress(fear > happy) | |||||
| / | |||||
| Negative effect of cortisol response: control(fear > happy) | |||||
| / | |||||
| Negative effect of basal cortisol: stress(fear > happy) > control(fear > happy) | |||||
| / | |||||
MNI, Montreal Neurological Institute; R, right; L, left. All effects are analyzed using cluster-level statistics, implementing a height threshold at P < 0.005 uncorrected at the voxel level. **P < 0.01; *P < 0.05 (whole brain corrected); #P = 0.05 (small-volume corrected for region of interest).
In contrast to what was observed for stress-induced elevation of cortisol levels, basal cortisol displayed a negative correlation with stress-induced activation of the right amygdala ([t(464) = 2.99, P = 0.053 (small volume correction)], Figure 4B, Table 2). Participants with high basal cortisol levels showed lower stress-induced activation of the amygdala than those with low basal levels. Furthermore, basal cortisol appeared to modulate the effect of stress on emotion-specific neural processing in a direction opposite to the results for the stress-related cortisol response. Higher basal cortisol levels were associated with increased differential responses to fearful compared with happy faces in the posterior cingulate cortex, precuneus, angular gyrus and thalamus under stress relative to control conditions (Figure 4C, Table 2). This correlation was however specific to the stress × emotion interaction effect and could not be pinpointed to a single session.
Discussion
To understand individual differences in stress sensitivity, we tested 120 healthy men under both stressful and neutral conditions and assessed how mild stress exposure affected their vigilance neurocircuitry. Individual differences in neural responding to stress were robustly associated with differences in both stress-induced cortisol release and basal cortisol levels. High cortisol responses to stress exposure were related to increased stress-induced activation of the medial temporal lobe, including the amygdala. Moreover, greater cortisol responses were associated with greater differential processing of fearful compared with happy faces under control conditions. In contrast, relatively high basal cortisol levels were linked to reduced stress-induced amygdala activation with an increased emotional specificity under stress. Thus, individuals with higher basal cortisol levels did not seem to turn in an unspecific hypervigilant state when stressed—a mechanism that may support their relative resilience. Hence, our study demonstrates that basal cortisol levels and stress-related cortisol increases are associated with divergent neural profiles.
Stress does not affect all persons equally (de Kloet et al., 2008). Whereas some individuals thrive under adverse conditions, others break down and are at risk to develop psychopathology. Elucidating the basis of these individual differences in stress vulnerability has been a central question in studying stress-related psychopathology. One important characteristic in stress-related mental disorders is abnormal functioning of the HPA-axis (de Kloet et al., 2006), suggesting that variability in HPA-axis activation might play a role in the development of mental disease. In line with previous studies (Elzinga and Roelofs, 2005; Schwabe et al., 2008; van den Bos et al., 2009), we observed large variability in participants’ HPA-axis and sympathetic responding to our relatively mild stressor. Interestingly, we found a correlation between the stress-induced cortisol response and the stress-related activation of medial temporal lobe during emotional processing. Increased activation of the amygdala and hippocampus following stress has been reported quite consistently in animal studies, on the condition of close spatio-temporal overlap with the actual stressor (Joëls et al., 2006). Two recent human studies also showed increased responsivity (van Marle et al., 2009) and elevated overall activity (Cousijn et al., 2010) of the amygdala during emotional processing following stress exposure. Here, we did not observe overall effects of stress induction on amygdala responding, which might be due to the overall lower cortisol stress responses observed in our study, potentially caused by the repeated testing or the fact that the previous study tested females. Instead, this study demonstrates this potentiation was limited to those individuals that are more sensitive to the stressor in terms of HPA-axis activity. The potentiation of amygdala activity has often been attributed to the increased noradrenergic activation accompanying stress, since pharmacological agents selectively activating or suppressing noradrenergic signaling have been shown to mimic or prevent this effect (van Stegeren et al., 2005; Onur et al., 2009). Here, we show that the individual’s cortisol response is tightly coupled to these alterations in amygdala functioning under stress as well. This association seemed to be rather specific to cortisol, since partialling out the contribution of the sympathetic stress response measures did not change the effect. Interestingly, other studies have reported on a negative association between the cortisol response to stress and activity of the medial temporal lobe during higher-order cognitive functioning [i.e. performing difficult mental arithmetic (Dedovic et al., 2005)] (Pruessner et al., 2008; Khalili-Mahani et al., 2010), which would suggest that the association between cortisol and neural activity is rather task-specific. Also time-dependent associations between cortisol and medial temporal lobe activation have been reported, as resting activity of the amygdala and hippocampus was observed to increase during the first few minutes of corticosteroid infusion, followed by a suppression of activity later on (Lovallo et al., 2010). To further enhance our understanding of the specific nature of these associations, future dedicated studies should assess their exact mechanistic underpinnings by directed manipulation of MRs and GRs.
We also observed an association of the stress-induced cortisol response with increased emotion-specific activation of the hypothalamus, insula, amygdala and brain stem under control conditions. Although fMRI as performed here lacks the spatial resolution to pinpoint signal activation to anatomically minute structures such as distinct brainstem nuclei, the activation cluster observed appears to cover the locus coeruleus. This nucleus, as well as the hypothalamus, is known for its role in the initiation and maintenance of the stress response (Abercrombie and Jacobs, 1987; Valentino and van Bockstaele, 2008; Sara, 2009). The strong preferential processing of negative information has been considered an intermediate phenotype for psychopathology (Savitz and Drevets, 2009). Here, we show it might be a predictor of an individual’s cortisol response to stress, and thus, stress sensitivity. Moreover, the reduction of regional emotion-specific processing under stress in strong cortisol responders might indicate a hypervigilant mode caused by stress exposure; typically thought to promote sensitivity of the system at the cost of its specificity (van Marle et al., 2009). Thus, large cortisol responses to stress were associated with a negativity bias under control conditions and a state of hypervigilance during stress. Interestingly, these responses were also linked to lower levels of extraversion and higher levels of depression. Therefore, the strong cortisol response to a stressor might represent a biological risk marker for stress-related psychopathology.
Higher basal cortisol levels were associated with overall lower neural activity in early visual processing areas. Previous studies have shown that stress typically boosts visual processing (Henckens et al., 2009; van Marle et al., 2009), which is thought to reflect a hypervigilant state induced by an increased sympathetic tone (Hermans et al., 2011). This suggests that the reduced recruitment of early visual processing regions might reflect lower vigilance with higher basal cortisol levels. Second, lower basal cortisol was associated with reduced stress-induced recruitment of the amygdala. As mentioned before, stress induction typically increases amygdala activity (van Marle et al., 2009; Cousijn et al., 2010) but apparently less so in participants with higher basal cortisol levels. Our findings are supported by a recent study showing reduced activation of limbic regions during exposure to stressful images in participants secreting more cortisol diurnally (Cunningham-Bussel et al., 2009). Moreover, they are in line with administration studies showing reduced activity in the amygdala following hydrocortisone intake (Henckens et al., 2010; Lovallo et al., 2010).
Basal cortisol levels were also associated with relatively stronger differential processing of fearful over happy faces under stress relative to control conditions in the posterior cingulate cortex, precuneus, angular gyrus and thalamus. Although these findings are not easy to interpret, increased emotion-specific processing under stress is contrary to the typically reduced specificity reported for stress (van Marle et al., 2009). Thus, the pattern of our findings (i.e. overall reduced vigilance processing, reduced stress-induced recruitment of the amygdala and enhanced emotion-specificity under stress) suggests that the presence of higher basal cortisol levels prevents the switch to a hypervigilant state when stressed as done here. This interpretation is supported by a recent study reporting on stronger prefrontal control over the amygdala in participants with high basal cortisol levels (Veer et al., 2012). Together with the observed positive correlation between basal cortisol levels and extraversion scores, these data indicate that high basal cortisol levels reflect relative stress resilience.
Unfortunately, the correlational analyses implemented in this study do not allow inferences on the directionality of the observed relationships between stress-induced cortisol signaling and the neural responding to stress. Electrophysiology studies showing a rapid boost in amygdala processing following stress-level treatment of corticosteroids (Duvarci and Paré, 2007; Karst et al., 2010) might imply the stress-induced cortisol response as the cause of potentiated processing. However, animal and human studies have also reported on anxiolytic effects of cortisol (Andreatini and Leite, 1994; Soravia et al., 2006), and its involvement in stress coping behaviors (Het and Wolf, 2007), emphasizing a role for nuclear GR-activation in stress recovery. Furthermore, a recent meta-analysis by Het et al. (2012) showed that the stress-induced cortisol response was negatively related to the increase in negative affect experienced by an individual, and a recent neuroimaging study (Henckens et al., 2010) showed that the administration of cortisol suppressed amygdala activity. These findings are at odds with the potentiating effects of cortisol seen in rodent brain sections, and support a role of cortisol in stress-coping and recovery. In line with this, recent animal work showed that both higher basal corticosterone levels and a higher stress response predicted resilience to chronic stress (Kim et al., 2013). Moreover, corticosterone administration following trauma was shown to reduce the incidence of PTSD-development (Daskalakis et al., 2014), supposedly rescuing the typically suppressed trauma-induced cortisol response as observed in post-traumatic stress disorder patients (Yehuda et al., 1993). Also recent human studies implicate low basal cortisol levels as a vulnerability factor for stress-related psychopathology (Yehuda and Seckl, 2011).
Overall, our findings support the interpretation that higher basal cortisol levels might protect individuals by reducing the neural stress response, while a hyperexcitable amygdala in stress-sensitive people might require (and thus induce) a larger HPA-axis response to avoid overshoot and aid stress recovery.
Some limitations to this study should be mentioned. First, we investigated men only, thus the obtained results cannot be easily generalized to women. Women’s cortisol response to stress is smaller and more variable (Kajantie and Philips, 2006), and depends on their menstrual cycle phase and the use of contraceptives (Kirschbaum et al., 1999), as does their brain response to stress (Ossewaarde et al., 2010; Merz et al., 2013). We here opted to recruit the population with the most robust and stable stress response (i.e. males), and potential sex-differences should be assessed in future studies. Second, the stress-induction method implemented in this study induced levels of mild stress only (explaining the absence of an overall neural stress effect in the participants), and the results can therefore not be readily translated to severe stress, which also affects vigilance processing (van Wingen et al., 2011) and might actually result in the development of psychopathology. Moreover, we only assessed this relationship in healthy individuals, and the link between tonic and phasic cortisol signaling may be different in psychopathologies that have been associated to chronic dysfunction of the HPA-axis (Yehuda et al., 1993; Yehuda, 2001; Herbert, 2013). Future studies should assess whether a similar relationship exists in individuals with (a risk for) psychopathology. Third, we did not assess the full diurnal rhythm of basal cortisol secretion. Cortisol levels typically show a strong response upon awakening, and then decline slowly throughout the day (Weitzman et al., 1971). Dysregulation of the diurnal rhythm of cortisol has been implicated in stress-related psychopathology (Morris et al., 2012) and some studies have even suggested a key role for the cortisol awakening response (Wessa et al., 2006; Vrshek-Schallhorn et al., 2013), although morning and evening measures are often correlated (Morris et al., 2012). We did not assess the awakening response, and future research is needed to determine whether peak levels show a similar relationship to neural vigilance processing under stress. Furthermore, we considered samples taken at home as the cleanest measure of basal HPA-axis activation, since participating in fMRI experiments is known to be (moderately) stressful and to induce both anticipatory and acute increases in state anxiety and cortisol level (Tessner et al., 2006; Muehlhan et al., 2011). However, this saliva collection method (i.e. at home by the participants themselves) has the obvious downside that it occurred out of direct control by the experimenter and the compliance to the instructions could not be checked fully. Nevertheless, we found neural correlates associated with our measures of basal cortisol, endorsing their reliability. Lastly, the associations observed for the basal cortisol levels are rather weak. As higher basal (i.e. tonic) cortisol levels are likely associated with both greater rapid, non-genomic and slow, genomic cortisol effects, it could well be that the contrasting effects of both mechanisms may underlay this rather weak association.
In sum, this study shows that basal cortisol levels and stress-induced cortisol responses have differential neural correlates during stress-induced emotional processing. Our findings seem to support a critical role for cortisol signaling in stress coping; higher basal cortisol levels indicate stress resilience, whereas higher cortisol release following stress might facilitate the return to a homeostatic state in those individuals prone to react sensitively to stress. Thereby, these findings may contribute to the understanding of the role of cortisol in stress-related psychopathology.
Supplementary Material
Acknowledgements
The authors thank N. Driessen, Y. Fang, A. Harteveld and X. Liu for their assistance in data acquisition.
Funding
This work was supported by a grant (918.66.613) from The Dutch Organization for Scientific Research (Nederlandse organisatie voor wetenschappelijk onderzoek).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Abercrombie E.D., Jacobs B.L. (1987). Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli. Journal of Neuroscience, 7(9), 2837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreatini R., Leite J.R. (1994). The effect of corticosterone in rats submitted to the elevated plus-maze and to pentylenetetrazol-induced convulsions. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 18, 1333–47. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Cohen J.D. (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Reviews Neuroscience, 28, 403–50. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R., Brown G.K. (2002). Handleiding. De Nederlandse versie van de Beck Depression Inventory, Beck Depression Inventory-II-NL, 2nd edn. Lisse, The Netherlands: Swets Test Publishers. [Google Scholar]
- Clark L.A., Watson D., Mineka S. (1994). Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology, 103(1), 103–16. [PubMed] [Google Scholar]
- Costa P.T., Jr, McCrae R.R. (1992). Revised NEO Personality Inventory (NEO-PI-R) and the Five Factor Inventory (NEO-FFI): Professional Manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Cousijn H., Rijpkema M., Qin S., et al. (2010). Acute stress modulates genotype effects on amygdala processing in humans. Proceedings of the National Academy of Sciences of the Unites States of America, 107(21), 9867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham-Bussel A.C., Root J.C., Butler T., et al. (2009). Diurnal cortisol amplitude and fronto-limbic activity in response to stressful stimuli. Psychoneuroendocrinology, 34(5), 694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis N.P., Cohen H., Cai G., Buxbaum J.D., Yehuda R. (2014). Expression profiling associates blood and brain glucocorticoid receptor signaling with trauma-related individual differences in both sexes. Proceedings of the National Academy of Sciences of the United States of America, 111(37), 13529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet C.S., Vermetten E., Geuze E., Kavelaars A., Heijnen C.J., Westenberg H.G. (2006). Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. Journal of Psychiatry Research, 40(6), 550–67. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Joëls M., Holsboer F. (2005). Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience, 6(6), 463–75. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Karst H., Joëls M. (2008). Corticosteroid hormones in the central stress response: quick-and-slow. Frontiers in Neuroendocrinology, 29(2), 268–72. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Vreugdenhil E., Oitzl M.S., Joëls M. (1998). Brain corticosteroid receptor balance in health and disease. Endocrinology Reviews, 19(3), 269–301. [DOI] [PubMed] [Google Scholar]
- Dedovic K., Renwick R., Khalili-Mahani N., Engert V., Lupien S.J., Pruessner J.C. (2005). The montreal imaging stress task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry and Neuroscience, 30(5), 319–25. [PMC free article] [PubMed] [Google Scholar]
- Drevets W.C. (2003). Neuroimaging abnormalities in the amygdala in mood disorders. Annals of the New York Academy of Sciences, 985, 420–44. [DOI] [PubMed] [Google Scholar]
- Duvarci S., Paré D. (2007). Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. Journal of Neuroscience, 27(16), 4482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga B.M., Roelofs K. (2005). Cortisol-induced impairments of working memory require acute sympathetic activation. Behavioral Neuroscience, 119(1), 98–103. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerd D., Klumpers F., van Wingen G., Tendolkar I., Fernández G. (2015). Association between neuroticism and amygdala responsivity emerges under stressful conditions. Neuroimage, 112, 218–24. [DOI] [PubMed] [Google Scholar]
- Groeneweg, F.L., Karst, H., de Kloet, E.R., Joëls, M. (2012). Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Molecular and Cellular Endocrinology, 350(2), 299–309. [DOI] [PubMed] [Google Scholar]
- Henckens M.J., Hermans E.J., Pu Z., Joëls M., Fernández G. (2009). Stressed memories: how acute stress affects memory formation in humans. Journal of Neuroscience, 29(32), 10111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens M.J., van Wingen G.A., Joëls M., Fernández G. (2010). Time-dependent effects of corticosteroids on human amygdala processing. Journal of Neuroscience, 30(38), 12725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert J. (2013). Cortisol and depression: three questions for psychiatry. Psychological Medicine, 43(3), 449–69. [DOI] [PubMed] [Google Scholar]
- Hermans E.J., van Marle H.J., Ossewaarde L., et al. (2011). Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science, 334(6059), 1151–3. [DOI] [PubMed] [Google Scholar]
- Het S., Schoofs D., Rohleder N., Wolf O.T. (2012). Stress-induced cortisol level elevations are associated with reduced negative affect after stress: indications for a mood-buffering cortisol effect. Psychosomatic Medicine, 74(1), 23–32. [DOI] [PubMed] [Google Scholar]
- Het S., Wolf O.T. (2007). Mood changes in response to psychosocial stress in healthy young women: effects of pretreatment with cortisol. Behavioral Neuroscience, 121, 11–20. [DOI] [PubMed] [Google Scholar]
- Joëls M., Pu Z., Wiegert O., Oitzl M.S., Krugers H.J. (2006). Learning under stress: how does it work? Trends in Cognitive Sciences, 10, 152–8. [DOI] [PubMed] [Google Scholar]
- Juster R.P., Bizik G., Picard M., et al. (2011). A transdisciplinary perspective of chronic stress in relation to psychopathology throughout lifespan development. Development and Psychopathology, 23(3), 725–76. [DOI] [PubMed] [Google Scholar]
- Kajantie E., Phillips D.I. (2006). The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology, 31, 151–78. [DOI] [PubMed] [Google Scholar]
- Karst H., Berger S., Erdmann G., Schütz G., Joëls M. (2010). Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proceedings of the National Academy of Sciences of the United States of America, 107(32), 14449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili-Mahani N., Dedovic K., Engert V., Pruessner M., Pruessner J.C. (2010). Hippocampal activation during a cognitive task is associated with subsequent neuroendocrine and cognitive responses to psychological stress. Hippocampus, 20, 323–34. [DOI] [PubMed] [Google Scholar]
- Kim J.G., Jung H.S., Kim K.J., Min S.S., Yoon B.J. (2013). Basal blood corticosterone level is correlated with susceptibility to chronic restraint stress in mice. Neuroscience Letters, 555, 137–42. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Kudielka B.M., Gaab J., Schommer N.C., Hellhammer D.H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61, 154–62. [DOI] [PubMed] [Google Scholar]
- Klein D.N., Kotov R., Bufferd S.J. (2011). Personality and depression: explanatory models and review of the evidence. Annual Review of Clinical Psychology, 7, 269–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laceulle O.M., Nederhof E., van Aken M.A., Ormel J. (2015). Adolescent personality: associations with basal, awakening, and stress-induced cortisol responses. Journal of Personality, 83(3), 262–73. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Brewin C.R., Bremner J.D., et al. (2010). Does neuroimaging research examining the pathophysiology of posttraumatic stress disorder require medication-free patients? Journal of Psychiatry & Neuroscience, 35(2), 80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo W.R., Robinson J.L., Glahn D.C., Fox P.T. (2010). Acute effects of hydrocortisone on the human brain: an fMRI study. Psychoneuroendocrinology, 35(1), 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz C.J., Wolf O.T., Schweckendiek J., Klucken T., Vaitl D., Stark R. (2013). Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology, 38(11), 2529–41. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler M.T., Williams S.C., Walsh N.D., et al. (2008). Neural basis of the emotional Stroop interference effect in major depression. Psychological Medicine, 38(2), 247–56. [DOI] [PubMed] [Google Scholar]
- Morris M.C., Compas B.E., Garber J. (2012). Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clinical Psychology Review, 32(4), 301–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlhan M., Lueken U., Wittchen H.U., Kirschbaum C. (2011). The scanner as a stressor: evidence from subjective and neuroendocrine stress parameters in the time course of a functional magnetic resonance imaging session. International Journal of Psychophysiology, 79(2), 118–26. [DOI] [PubMed] [Google Scholar]
- Onur O.A., Walter H., Schlaepfer T.E., et al. (2009). Noradrenergic enhancement of amygdala responses to fear. Social Cognitive and Affective Neuroscience, 4(2), 119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L., Hermans E.J., van Wingen G.A., et al. (2010). Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology, 35(1), 47–55. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron, 48(2), 175–87. [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Dedovic K., Khalili-Mahani N., et al. (2008). Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry, 63, 234–40. [DOI] [PubMed] [Google Scholar]
- Sara S.J. (2009). The locus coeruleus and noradrenergic modulation of cognition. Nature Reviews Neuroscience, 10(3), 211–23. [DOI] [PubMed] [Google Scholar]
- Savitz J.B., Drevets W.C. (2009). Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience, 164, 300–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzberg A.F., Keller J., Tennakoon L., et al. (2014). HPA axis genetic variation, cortisol and psychosis in major depression. Molecular Psychiatry, 19(2), 20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L., Bohringer A., Chatterjee M., Schachinger H. (2008). Effects of pre-learning stress on memory for neutral, positive and negative words: different roles of cortisol and autonomic arousal. Neurobiology of Learning and Memory, 90(1), 44–53. [DOI] [PubMed] [Google Scholar]
- Soravia L.M., Heinrichs M., Aerni A., et al. (2006). Glucocorticoids reduce phobic fear in humans. Proceedings of the National Academy of Sciences of the United States of America, 103, 5585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessner K.D., Walker E.F., Hochman K., Hamann S. (2006). Cortisol responses of healthy volunteers undergoing magnetic resonance imaging. Human Brain Mapping, 27(11), 889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino R.J., van Bockstaele E. (2008). Convergent regulation of locus coeruleus activity as an adaptive response to stress. European Journal of Pharmacology, 583(2–3), 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos R., Harteveld M., Stoop H. (2009). Stress and decision-making in humans: performance is related to cortisol reactivity, albeit differently in men and women. Psychoneuroendocrinology, 34(10), 1449–58. [DOI] [PubMed] [Google Scholar]
- van der Ploeg H.M., Defares P.B., Spielberger C.D. (1980). Handleiding bij de Zelf-Beoordelings Vragenlijst, ZBV: Een Nederlandse vertaling van de Spielberger State-Trait Anxiety Inventory. Lisse, The Netherlands: Swets and Zeitlinger. [Google Scholar]
- van Marle H.J., Hermans E.J., Qin S., Fernández G. (2009). From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biological Psychiatry, 66(7), 649–55. [DOI] [PubMed] [Google Scholar]
- van Stegeren A.H., Goekoop R., Everaerd W., et al. (2005). Noradrenaline mediates amygdala activation in men and women during encoding of emotional material. Neuroimage, 24(3), 898–909. [DOI] [PubMed] [Google Scholar]
- van Wingen G.A., Geuze E., Vermetten E., Fernández G. (2011). Perceived threat predicts the neural sequelae of combat stress. Molecular Psychiatry, 16(6), 664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer I.M., Oei N.Y., Spinhoven P., van Buchem M.A., Elzinga B.M., Rombouts S.A. (2012). Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology, 37(7), 1039–47. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S., Doane L.D., Mineka S., Zinbarg R.E., Craske M.G., Adam E.K. (2013). The cortisol awakening response predicts major depression: predictive stability over a 4-year follow-up and effect of depression history. Psychological Medicine, 43(3), 483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–70. [DOI] [PubMed] [Google Scholar]
- Weitzman E.D., Fukushima D., Nogeire C., Roffwarg H., Gallagher T.F., Hellman L. (1971). Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. Journal of Clinical Endocrinology and Metabolism, 33(1), 14–22. [DOI] [PubMed] [Google Scholar]
- Wessa M., Rohleder N., Kirschbaum C., Flor H. (2006). Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology, 31(2), 209–15. [DOI] [PubMed] [Google Scholar]
- Yehuda R. (2001). Biology of posttraumatic stress disorder. Journal of Clinical Psychiatry, 62(Suppl 17), 41–6. [PubMed] [Google Scholar]
- Yehuda R., Seckl J. (2011). Minireview: stress-related psychiatric disorders with low cortisol levels: a metabolic hypothesis. Endocrinology, 152(12), 4496–503. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Southwick S.M., Krystal J.H., Bremner D., Charney D.S., Mason J.W. (1993). Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. American Journal of Psychiatry, 150(1), 83–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.