Abstract
Spontaneous activity levels prior to stimulus presentation can determine how that stimulus will be perceived. It has also been proposed that such spontaneous activity, particularly in the default-mode network (DMN), is involved in self-related processing. We therefore hypothesised that pre-stimulus activity levels in the DMN predict whether a stimulus is judged as self-related or not. Participants were presented in the MRI scanner with a white noise stimulus that they were instructed contained their name or another. They then had to respond with which name they thought they heard. Regions where there was an activity level difference between self and other response trials 2 s prior to the stimulus being presented were identified. Pre-stimulus activity levels were higher in the right temporoparietal junction, the right temporal pole and the left superior temporal gyrus in trials where the participant responded that they heard their own name than trials where they responded that they heard another. Pre-stimulus spontaneous activity levels in particular brain regions, largely overlapping with the DMN, predict the subsequent judgement of stimuli as self-related. This extends our current knowledge of self-related processing and its apparent relationship with intrinsic brain activity in what can be termed a rest-self overlap.
Keywords: self-related processing, default-mode network, own name, pre-stimulus activity, rest–stimulus interaction
Introduction
A number of neuroimaging studies have now shown that the level of brain activity that occurs immediately prior to a stimulus being presented influences how the stimulus will be perceived or responded to (Boly et al., 2007; Hesselmann et al., 2008a, b, 2010; Sadaghiani et al., 2009, 2015; Northoff et al., 2010; Coste et al., 2011). Such pre-stimulus brain activity is taken to be intrinsic in nature (also described as spontaneous) as there is no specific external input in the period prior to the target stimulus being presented. This effect has been shown in simple sensory tasks, such as tone detection, where whether or not the participant will perceive a stimulus is determined by the brain state prior to its presentation (Sadaghiani et al., 2009). It has also been observed in more complex tasks, such as viewing the Rubin’s vase-face picture, where not just whether a stimulus is perceived but how it is perceived is determined by the pre-stimulus activity state (Hesselmann et al., 2008a). Finally, intrinsic activity fluctuations have also been linked to behaviour in a free-decision button press task (Soon et al., 2008). In this, intrinsic activity was found to predict what response the participant was going to make before they were aware of making the decision as to which button to press and pressing it. Taken together, these studies show that intrinsic brain activity can be a key determinant factor in both action and perception.
A set of brain regions known as the default-mode network (DMN) shows a particularly high level of activity when no specific external task or stimuli are present (the so-called resting-state) (Raichle et al., 2001). Such resting-state activity in the DMN is often taken to be analogous to the intrinsic activity studied in the previously described studies and has been linked to internally oriented and self-related processing (Fox et al., 2005; Mason et al., 2007). In addition, many DMN regions overlap with those that respond to self-related stimuli and tasks (Qin et al., 2012; Tacikowski et al., 2012; Nakane et al., 2015), highlighting the possible link between activity in these areas and the self (Qin and Northoff, 2011; Whitfield-Gabrieli et al., 2011; Molnar-Szakacs and Uddin, 2013; Gorgolewski et al., 2014; Lipsman et al., 2014; Nakane et al., 2015; Peer et al., 2015). These previous studies investigating the link between brain activity and self-related processes have focussed upon stimulus-induced activity, however, leaving open the question as to whether or not pre-stimulus intrinsic activity can influence self-related perception. As many of the described intrinsic activity studies find that it is within modality-specific regions that pre-stimulus activity is related to outcomes (e.g. within vision-related regions for visual tasks—Hesselmann et al., 2008a; Sadaghiani et al., 2009), it seems a reasonable assumption that pre-stimulus activity within DMN regions will indeed be related to self-relatedness.
The overall aim of this study, therefore, was to investigate whether pre-stimulus activity, as measured with fMRI, influences whether a stimulus will be perceived as self-related or not. Self-relatedness is a multifaceted phenomenon, covering functions ranging from the processing of self-specific stimuli, such as one’s name, to the embedding of experience in a long-term personal narrative (Qin et al., 2012). To investigate the relationship between pre-stimulus activity and self-relatedness it is necessary to have a discrete stimulus for presentation that can be made ambiguous to allow a binary judgement. The subject’s name is ideal for this role as it is individually specific, is short and self-contained and can be effectively masked to create perceptual ambiguity. As well as this, the presentation of one’s name has also been shown to trigger other self-related processes (Tacikowski et al., 2012).
To make the processing as basic as possible and avoid any confounding effects from the external stimulus we presented contentless auditory stimuli (i.e. white noise) but instructed the participants that either their name or the name of a stranger was audible within it. These stimuli were created by masking recordings of the relevant names with white noise such that the names could not be distinguished, as confirmed behaviourally and from the fMRI responses. Upon hearing the noise, participants had to judge which name they thought they heard. The stimuli where participants reported hearing their own name were classified as self-related and those where they reported hearing another’s name non-self-related.
The task was analysed in two ways. In a first step, auditory stimuli were split into self and non-self (according to the participants judgements) and then the peak of stimulus-induced brain responses compared in order to show (a) that neural responses to self- and non-self-relatedness judgements could be distinguished; and (b) that the regions that showed a difference in response were ones that have previously been implicated in self-related processing. This was further confirmed by applying the regions identified to a previously acquired self-relatedness task dataset and testing for a difference in activity between self- and non-self-related stimuli (Qin et al., 2012). Based on prior results, we hypothesised that stimuli that were perceived as self-related would induce greater activity in cortical midline regions such as the anterior cingulate cortex (Qin and Northoff, 2011). In a second step, we then analysed the activity 2 s prior to the presentation of the stimuli, hypothesising that pre-stimulus levels in sections of the DMN would predict the subsequent assignment of self-relatedness to the stimuli. DMN regions were identified from a separate resting-state scan.
Methods
Participants
Eighteen healthy participants took part in the experiment (15 females, three males; mean age = 27.1 years, age range = 20–34 years). Participants were screened for any current or previous neurological or psychiatric disorders. All of the participants had first names consisting of two syllables. One participant reported discomfort during scanning and withdrew from the experiment. Written informed consent was obtained from all participants, who were paid for their time. The study was approved by the ethics committee of the Freie Universität Berlin.
Experimental protocol
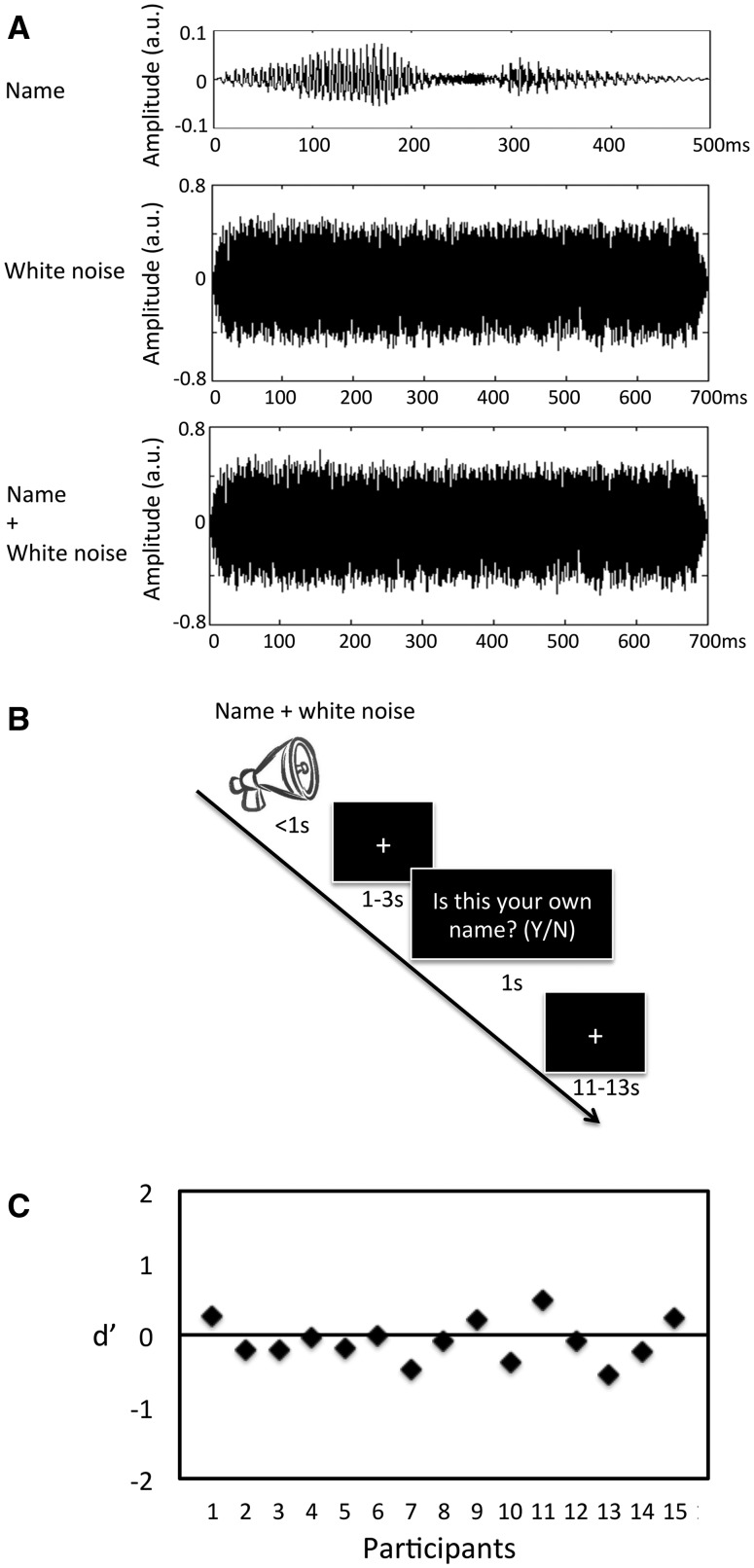
The task that participants undertook in the scanner involved being presented with an auditory stimulus and then being asked to judge whether it was their own name or another that was presented. The stimulus consisted of actual names spoken by the same male researcher, unknown to the participant, overlaid with a burst of white noise. The names used were the participant’s own name and two other names (of a friend and a stranger). The names of others were grouped together in the analysis. As per the inclusion criteria, all names were two syllables long with a mean duration of 529 ± 91 ms (mean ± s.d.). The white noise that was used to obscure the names had a longer duration (700 ms) and a greater intensity than them (see Figure 1A). The stimuli were presented to the participants prior to the experiment and were asked if any names could be heard. All participants reported that they could not hear the names. That the actual names could not be distinguished was confirmed by the behavioural results from the experiment (see ‘Behavioural results’ section). During the experiment, auditory stimuli were delivered via MRI-compatible headphones to both ears.
Fig. 1.
Participants were presented with sounds composed as illustrated in (A). A recording of their name (top) was superimposed with white noise (middle) to give the final stimulus (bottom). The y-axis shows the amplitude in arbitrary units; note that the scale of these units is the same in each plot. As can be seen, the white noise was of a greater amplitude and duration than the name, making the name itself inaudible. The task that participants underwent (B) consisted of these sounds being presented to them, followed by a jittered pause and then a prompt to indicate whether they thought that their own name or another was presented. After this response there was a jittered inter-trial interval during which a fixation cross was displayed. To ensure that the participants could not make out the names within the white noise, individual d’ values were calculated based on the response to each stimulus (C). As can be seen, d’ did not differ from 0, confirming that they responses that the participants were making were not guided by the recorded names.
During the experiment, there was a total of 180 trials spilt over four runs (see Figure 1B for a schemata of the experiment). Participants were asked to make a judgement as to which name was presented in two-thirds of these trials (120 trials) and to passively listen in the other third (60 trials). The passive listening trials were not analysed here. In each trial, the auditory stimulus was first presented (700 ms), followed by a gap of 1–3 s (Boly et al., 2007). A white fixation cross was shown on a black background during both these periods. The question ‘Was this your name?’ (presented in German: ‘War dies Ihr Name?’) was then shown on the screen (white text on black) and the participants required to respond via a button press within 1 s. Trails in which the participant did not respond within 1 s were excluded from the analysis. Trials were separated by an inter-trial interval jittered between 11 and 13 s during which a fixation cross was displayed. Participants were familiarised with the task prior to entering the scanner. They were instructed that their own name would be presented in one-third of the trials.
Behavioural analysis
To ensure that the responses that participants gave were not influenced by the name underlying the white noise, their subjective judgements were analysed according to a signal detection theory approach (Pessoa et al., 2005). For each participant, the probability of reporting ‘own name’ given that the target was not an own name (i.e. false alarm), and the probability of reporting ‘own name’ given that the target was an own name (i.e. hit) was calculated. The sensitivity to their own name, d’, was then calculated from the hit and false alarm rates. A d’ around zero would mean that the participants could not distinguish which name truly underlay the noise (i.e. they could not distinguish signal from noise) and that they were making self/other ascriptions unguided by any external cue. One participant with a d’ >1 was excluded and the d’ for the remaining participants compared with zero using a one-sample t-test (two-tailed).
The absolute frequencies of own name or other judgements were calculated and compared through a paired sample t-test. Each participant had at least 25 instances of each response type. Mean reaction times were compared for the two judgement types (own name or other) using a paired sample t-test. To establish if participants were responding according to some structured pattern we tested whether one choice was more likely to be preceded by a particular other response using Mann–Whitney U-tests for each participant. One participant did show such a pattern (P = 0.012) and so was excluded from further analysis. We also tested to so if a self or other was more likely to be preceded by a particular length of inter-trial interval, there was no such effect in any of the participants.
MRI data acquisition and pre-processing
Magnetic resonance images were acquired on a 3-T Siemens Tim Trio scanner at the Freie Universität Berlin. A 3D anatomical image was first acquired using a fast SPGR sequence (TR = 1.9 ms, TE = 2.25 ms, FOV = 256 × 256 mm2, matrix =256 × 256, slice thickness = 1 mm, 176 slices) for functional image registration and localisation. Data for the self-relatedness task were acquired using an EPI sequence (TR = 2 s, TE = 30 ms, θ = 90°, FOV = 192 × 192 mm2, matrix = 64 × 64, slice thickness = 3 mm, gap = 0 mm). Each volume had 37 axial slices, covering the whole brain. The task was split over four runs, with each run consisting of 362 brain volumes (12.1 min). A resting-state run was also acquired using the same scanner settings (5 min, 150 volumes). For this, participants were instructed to relax with their eyes closed and to not concentrate on anything in particular whilst staying awake.
MRI data were processed using the AFNI software package (Cox, 1996). All functional data underwent 2D and 3D head motion corrections; masking for the removal of the skull; and spatial smoothing using a 6-mm FWHM Gaussian kernel. Time-series were normalised by converting to percent signal change relative to the mean across all time points. Data were then aligned to Talairach standard space and resampled to 3 mm isotropic voxels.
fMRI data analysis—task
Trials were categorised according to the participant’s subjective judgement: own name, where they responded that they thought that they heard their name; and other name, where they responded that they thought that they heard another name. Trial onsets were defined accordingly for a deconvolution analysis using a general linear model approach. The passive listening trials were included in this model but were not used for any analysis, as were trials where the participants missed a response. Also included in the model were the six head motion parameters calculated in the pre-processing steps.
As our focus was on the period preceding stimulus presentation, trials were modelled using tent functions starting 2 s prior to the true onset and extending 14 s past the onset (TENT, 3dDeconvolve). Unlike a conventional modelling approach in which the haemodynamic response is presumed to have a fixed shape, this method does not make any assumptions about the response shape. Instead, the haemodynamic response per condition is estimated through multiple (e.g. 9 in our case) basis functions consisting of a set of equally spaced TENT (piecewise linear) functions or linear splines. In this analysis, each basis function corresponds to an individual time point in the fMRI timeseries surrounding each stimulus onset. The model produces an effect estimate (beta) for each one basis function, representing an estimate of the activity amplitude at the corresponding time relative to stimulus onset. In this way, both the shape and the amplitude of the haemodynamic response can be simultaneously estimated.
For each participant the mean peak stimulus response (6–8 s post-stimulus) was calculated for the subjectively defined self and other trials. These were then contrasted at the group level to identify those regions that showed greater activity following self judgements than other. Similarly, the mean activity levels 2 s prior to stimulus onset were also calculated for each participants and then contrasted for the two conditions at the group level. This identified those brain areas where activity levels were significantly different in trials where the participant would judge the subsequent stimulus as being own name compared with ones where they did not (i.e. own name judgement vs other name judgement). In all these analyses, the number of trials composing each condition and the mean reaction times were included as covariates. A cluster significance level of P < 0.05 following FWE correction was used.
fMRI analysis—resting-state
In order to establish if the clusters identified in the task analysis were located in the DMN we acquired resting-state fMRI data and used this to define this network in these participants. In addition to the pre-processing steps described above (see ‘MRI data acquisition and pre-processing’ section), the mean timeseries from the white matter and the cerebrospinal fluid (CSF) were also regressed out of the functional data, along with the six head motion parameters. To obtain the white matter and CSF timeseries, the anatomical images were first segmented into white matter, grey matter and CSF using the FAST tool from the FSL software package (http://www.fmrib.ox.ac.uk/fsl/). The relevant tissue maps were thresholded at 0.99 (from a range of 0–1) and made into binary masks. These masks were then applied to the functional data and the mean timeseries within each calculated. The functional data were then band-pass filtered between 0.01 and 0.08 Hz (Song et al., 2011). In order to further reduce the effect of head motion on functional connectivity estimates, motion at each time point was estimated as the absolute Euclidean distance moved from the head position in the prior time point, as calculated from the six rigid-body motion parameters. Where there was movement of >0.5 mm between time points, the relevant volume, plus the preceding and subsequent volumes, was removed from the dataset (Huang et al., 2014).
The DMN was defined as those regions showing functional connectivity with the posterior cingulate cortex (PCC). A 12-mm diameter sphere was placed as a seed region within the PCC according to previously published coordinates (Greicius et al., 2003). The mean BOLD timeseries in this seed region was then correlated with every other voxel in the brain using Pearson’s linear correlation. The resulting r-values were converted to normally distributed Z-values using Fisher’s Z transform. A group functional connectivity map was then created by comparing voxel-wise Z-values to 0 (one-sample t-tests) and thresholding the resulting statistical map at P < 0.001 (uncorrected) with a cluster extent threshold of 100 voxels. The overlap between the clusters identified from the task analysis and this DMN map was then established.
Independent self-related task dataset analysis
In order to confirm that the brain regions identified as showing greater activity (post-stimulus) following stimuli judged to be self-related were involved in self-processing, these clusters were applied to an independent dataset where own and other names were explicitly presented to participants. In that experiment, Chinese participants listened to their own name, other names (a friend’s and a stranger’s name) and a foreign name (English). All the names were presented such that they were clearly audible and the English names were used as catch trials to ensure that the participants were paying attention (Supplementary Figure 1). Full details of the experiment can be found in Qin et al. (2012). These data were pre-processed and analysed in the same manner as the current experiment. The tent function used to model stimulus response was started at stimulus onset and continued for 12 s. The friend and stranger names were modelled as two separate conditions. Pre-stimulus activity was not modelled as there was only a short gap (4–12 s) between stimulus presentations (in contrast to the longer inter-trial interval used here). Regions of interest were placed at each of the clusters identified in this study as having higher activity following the stimuli judged as the participant’s own name than other name (16 mm spheres at cluster foci). The mean peak values (6–8 s) within these for the independent dataset were then compared for own and other names through paired two-sample t-tests. Bonferroni correction for the four regions [supragenual anterior cingulate cortex (SACC), midcingulate cortex (MCC), left anterior insula (LAI) and right anterior insula (RAI)] and the two other names was done in each of these comparisons (P < 0.05; P < 0.00625 uncorrected).
Results
Behavioural results
The mean d’ for all participants was −0.83 (± 0.29 s.d.), which is not significantly different from zero (t = −1.13, P = 0.28) (Figure 1C). This confirms that the participants were not able to perceive the underlying names and were responding purely to white noise. Overall, the stimulus was judged to be the participant’s own name (n = 43.8 ± 9.9) less often than it was judged to be another name (n = 63.8 ± 12.9; t = 4.31, P < 0.01). Reaction times were longer when the participant responded that it was their own name (own name: 529 ± 111 ms; other name: 476 ± 109 ms; t = 3.85, P < 0.05). The effect whereby the response that is less likely to be made has a longer reaction time has been observed previously (Hesselmann et al., 2008b). For each participant, the number of trials comprising each condition and the mean reaction time was included in the group fMRI analysis as covariates.
Post-stimulus brain activity
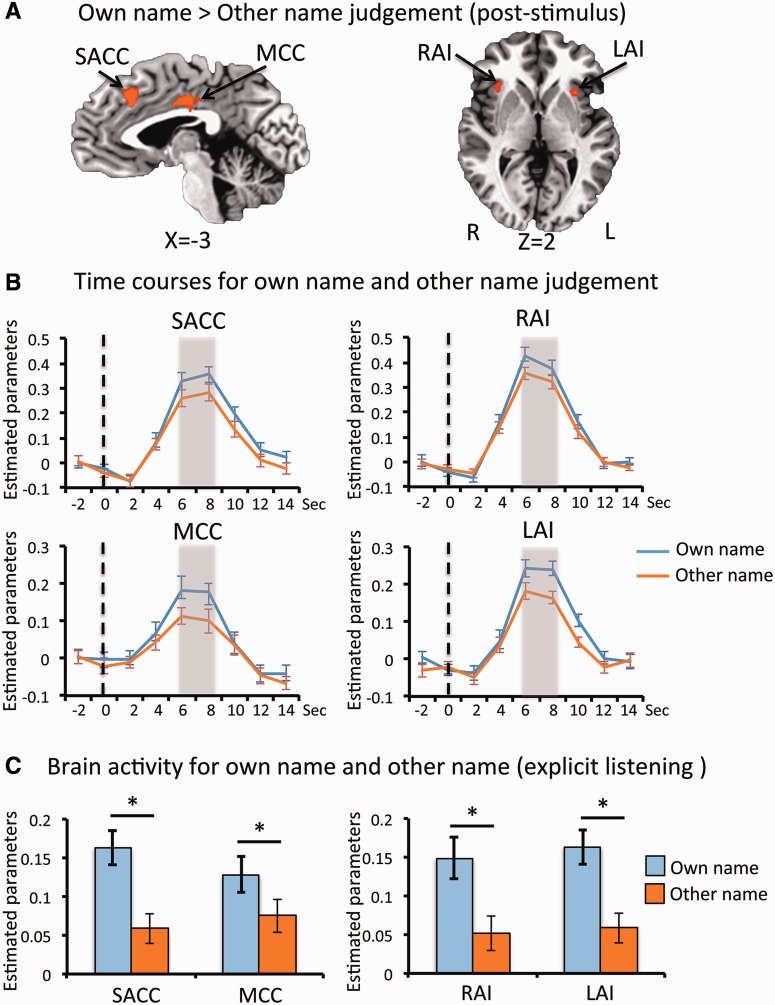
Comparing trials where the participant judged the stimulus to be their own name rather than another, fMRI activity levels were found to be higher in the SACC, MCC, RAI and LAI (Figure 2 and Table 1). The other name judgement did not elicit stronger activity in any regions when compared with the own name judgement.
Fig. 2.
(A) Brain regions showing greater activity post-stimulus in trials for own name judgement than other name judgement are shown (P < 0.05, FWE corrected). Time courses extracted from each of these regions are shown in (B). The responses of trials for own name judgement are shown in blue and those for other name judgement in yellow. The vertical bar indicates the point at which stimuli were presented (t = 0 s) and the grey shading the time-period (t = 6–8 s) at which the peak responses were calculated for the contrast shown in (A). To confirm that the regions identified are involved in self-related processing, they were applied to an independent dataset where participants explicitly listened to their own or other names being spoken. Brain responses to own name and one other name from each of the regions are displayed in (C). Asterisk denotes a significant difference between the two conditions (P < 0.05 corrected). LAI = left anterior insula; MCC = middle cingulate cortex; RAI = right anterior insula; SACC = supragenual anterior cingulate cortex. Error bars represent the standard error of the mean.
Table 1.
Coordinates for clusters showing greater activation for own name compared to other name judgement, post- and pre-stimulus
| Brain regions | Coordinates (Talairach) |
||
|---|---|---|---|
| X | Y | Z | |
| Post-stimulus activity (6–8 s post-stimulus onset) | |||
| SACC | 2 | 23 | 36 |
| MCC | −2 | −5 | 33 |
| RAI | 37 | 17 | 6 |
| LAI | −28 | 17 | −4 |
| Pre-stimulus spontaneous activity (2 s prior to the onset of stimuli) | |||
| RTP | 53 | 2 | −27 |
| LSTG | −61 | −19 | 12 |
| RTPJ | 52 | −58 | 24 |
LAI = left anterior insula; LSTG = left super temporal gyrus; MCC = middle cingulate cortex; RAI = right anterior insula; RTP = right temporal pole; RTPJ = right temporoparietal junction; SACC = supragenual anterior cingulate cortex.
These results were based on trial groupings according the participants’ subjective judgement. To confirm that they were responding to pure noise and not the underlying names we also grouped trials according to these objective name classifications and compared fMRI responses with these. No brain regions showed greater activity in response to any of the objectively grouped trials. In addition, the brain responses in the regions found to differ according to the subjective classifications (SACC, MCC and bilateral insula) were not different for the different objective classifications (Supplementary Figure 2).
Finally, to confirm that the brain regions identified by the subjective response contrasts are involved in self-related processing, these ROIs were applied to an independent dataset where self and other names were explicitly presented (Qin et al., 2012). Activity levels within these regions did indeed differ between own and other names when names were explicitly presented (Figure 2 and Supplementary Figure 3).
Pre-stimulus brain activity
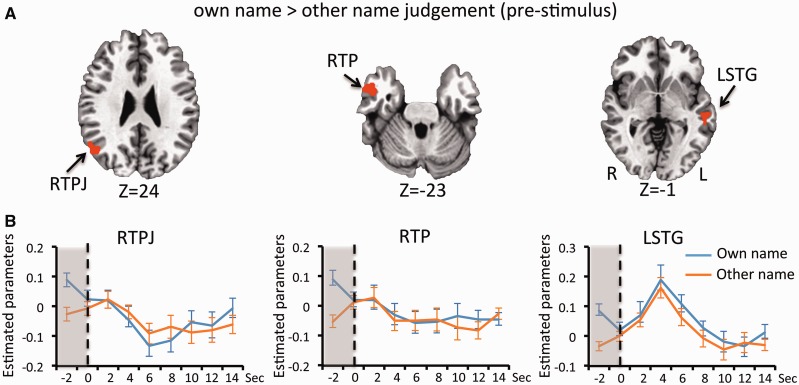
The main aim of this study was to establish that brain activity prior to stimulus presentation could influence whether the stimulus would be judged to be self-related or not. To this end, activity levels 2 s prior to the presentation of the stimulus were contrasted between those trials were the participant would go on to judge the stimuli as their own name and those where they would judge it to be other name. From this contrast it was found that pre-stimulus activity in the right temporoparietal junction (RTPJ), the right temporal pole (RTP) and left superior temporal gyrus (LSTG) was higher in trials where they judged the subsequent stimulus to be their own name (Figure 3 and Table 1). No regions had higher pre-stimulus activity in trials where the stimulus was judged to be other name. When classifying trials according to the objective name presented there were no regions in which there was a difference in pre-stimulus activity levels (Supplementary Figure 4).
Fig. 3.
(A) Brain regions showing greater pre-stimulus activity in trials for own name judgement than for other name judgement are shown (P < 0.05, FWE corrected). Time courses extracted from each of these regions are shown in (B). The responses of trials for own name judgement are shown in blue and those for other name judgement in red. The vertical bar indicates the point at which stimuli were presented (t = 0 s) and the grey shading the time-period (t = −2 s) at which the peak responses were calculated for the contrast shown in (A). Error bars represent the standard error of the mean.
Overlap between pre-stimulus regions and the DMN
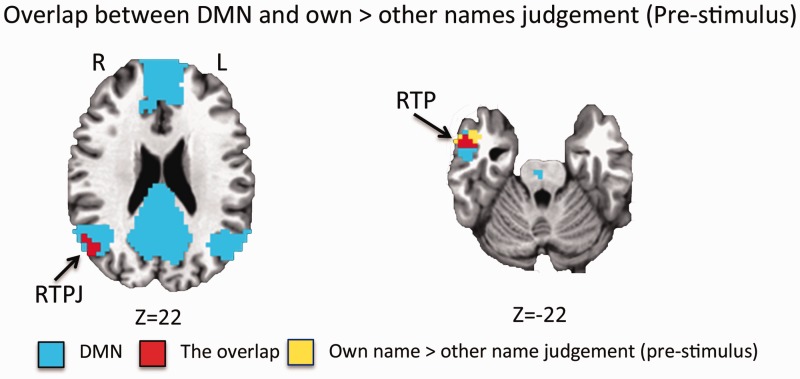
The DMN was outlined in this participant group by calculating resting-state functional connectivity between the PCC and the rest of the brain. This identified a network consisting of the PCC, medial prefrontal cortex (MPFC), bilateral temporoparietal junction and bilateral temporal pole (Figure 4 and Table 2). This network overlaps with the regions found to have higher pre-stimulus activity in own name judgement trails in the RTPL and RTP (Figure 4).
Fig. 4.
The overlap between the regions showing higher pre-stimulus activity in trials for own name judgement (yellow) and the DMN (blue) are shown in red. RTPJ = temporoparietal junction; RTP = right temporal pole.
Table 2.
Coordinates for the clusters forming the DMN, as defined by functional connectivity with the PCC
| Brain regions | Coordinates (Talairach) |
||
|---|---|---|---|
| X | Y | Z | |
| MPFC | 2 | 60 | 3 |
| PCC | 5 | −47 | 30 |
| LTPJ | −40 | −68 | 33 |
| RTPJ | 43 | −64 | 30 |
| RTP | 58 | −13 | −10 |
| Cerebellum | −40 | −55 | 36 |
| LTP | −58 | −16 | −10 |
| Left Parahippocampal gyrus | −25 | −31 | −10 |
LTP = left temporal pole; LTPJ = left temporoparietal junction; MPFC = medial prefrontal cortex; PCC = posterior cingulate cortex; RTP = right temporal pole; RTPJ = right temporoparietal junction.
Discussion
In this study, we presented white noise stimuli that have no discernible content, as confirmed behaviourally and neurally, and asked participants to judge whether their own name or another name was contained within it. We show that fMRI activity levels 2 s prior to the stimulus being presented predict whether the subject will perceive the noise as containing their own name or another. More specifically, activity in the RTPJ, RTP and LSTG was higher in trials where the participant would go on to judge the stimulus as their own name, as opposed to the other name. The activity clusters identified in the RTPJ and RTP were then shown to overlap with the DMN, as defined from a resting-state scan. Taken together, these findings suggest that spontaneous activity in parts of the DMN may be involved in the ascription of self-relatedness to stimuli. This carries important implications for the understanding of neural mechanism of both spontaneous activity and self-related processing.
Brain regions involved in post-stimulus responses
Previous studies looking at the influence of pre-stimulus activity on subsequent behavioural responses have tended to find that post-stimulus activity levels can be differentiated between the possible responses in regions previously associated with the particular task. For example, in the Rubin vase task, post-stimulus activity levels are different in the fusiform face area in trails where the face is seen, as compared with those where the vase is seen (Hesselmann et al., 2008a). Similarly, in a coherent visual motion task, those trials where coherent motion was perceived induced greater activity in the right motion-sensitive occipito-temporal cortex (hMT+) then trials where random motion was perceived (Hesselmann et al., 2008b).
This was also the case in this study, where trials in which the participant responded that it was their own name that was presented induced stronger brain activity in the MCC, SACC and bilateral anterior insula. These regions have been previously identified as being involved in own-name processing, which we further confirmed by applying the identified regions to an independent dataset in which participants were explicitly presented with either their own name or others. This also lends weight to the conclusion that the differences in brain activity seen are due to the subjectively determined content of the stimulus (i.e. judged to be their own name or other name) and not due to non-specific task effects.
Some prior studies have suggested that there are brain regions which display higher activity levels during other-related processing (Denny et al., 2012). This contrasts with our own results, where post-stimulus activity was not found to be elevated in any regions in trials where the participant responded that they did not hear their own name. This discrepancy may be a result of these prior studies presenting stimuli that are related to specific non-self individuals, as opposed to the ‘other’ stimuli in this study, which was essentially null. This may mean that in the trials in which participants responded that they did not hear their name there was no specific content (such as memories, associations, etc.) to be processed. Without such other-related content to be processed one can hypothesise that there are no brain regions in which activity becomes elevated.
Pre-stimulus activity predicts own-name judgement
Our results show that whether a participant will judge the stimulus as containing their name or other is predicted by the level of activity in the RTPJ, RTP and LSTG 2 s prior to the stimulus being presented. Such an effect of pre-stimulus activity on the perceived content of a subsequent stimulus is in line with prior studies demonstrating such an effect in different sensory and cognitive contexts (Boly et al., 2007; Hesselmann et al., 2008a, b, 2010; Sadaghiani et al., 2009, 2015; Northoff et al., 2010; Coste et al., 2011;). Our results extend these observations by showing an analogous determination of judgements of self-relatedness by pre-stimulus activity.
The temporoparietal junction and temporal pole have consistently been observed to be involved in self-related processing (Churchland, 2002; Tsakiris et al., 2008; Tsakiris, 2010; Justen et al., 2013; Sowden and Catmur, 2013). Our findings suggest that, in addition to this role responding to self-related stimuli, neural activity within these regions may also be involved in determining what incoming stimuli will actually be judged as such. Furthermore, both the RTPJ and RTP regions identified were shown to overlap with the DMN, as delineated from resting-state functional connectivity in the same participants. This is of note given proposals that spontaneous activity within this network may be closely linked to the instantiation of aspects of the self (Qin and Northoff, 2011; Whitfield-Gabrieli et al., 2011; Molnar-Szakacs and Uddin, 2013; Gorgolewski et al., 2014; Lipsman et al., 2014; Peer et al., 2015). Our results lend some support to this idea as they show that the relative level of spontaneous fluctuations within particular DMN regions may interact with incoming stimulus-induced activity (Huang et al., 2015) to influence how self-related that stimulus will be experienced as. Such an effect has also been observed with pre-stimulus low-alpha EEG power related to glutamate + glutamine levels in the MPFC, another component of the DMN (Bai et al., 2015). Taken together, these results point towards the general case that where pre-stimulus spontaneous activity levels are higher, that stimulus will be more likely to be perceived and judged as self-related. Lower pre-stimulus activity in those regions would therefore be associated with other-related judgements. If it is such an interaction between elevated pre-stimulus activity and the incoming stimulus that leads to an ascription of self-relatedness then it would not necessarily require that there be any regions in which elevated pre-stimulus activity is related to other-ascription, which is indeed what we observe. Further work is required to test this hypothesis, however.
The LSTG has also been shown to be directly involved in self-related processing (Uddin et al., 2005; Platek et al., 2006; Devue and Bredart, 2011; Sui et al., 2012). In addition, spontaneous activity within this region has been shown to interact differently with self-related stimulus-induced activity than to non-self-related stimulus-induced activity (Qin et al., 2013). This latter finding highlights the link between spontaneous activity and self-processing that is suggested by our results.
The DMN has been shown to be involved in self-related processing across a wide variety of self-related tasks or stimuli (Sheline et al., 2009; Qin and Northoff, 2011; Denny et al., 2012; Fingelkurts et al., 2012; Murray et al., 2012). These studies have, however, indicated that specific sub-regions within the DMN may be differentially involved depending on the particular task context. For example, midline regions (such as the MPFC or PCC) are more frequently activated during tasks that involve the judgement of trait adjectives or self-evaluative statements (van der Meer et al., 2010). The TPJ, on the other hand, is more involved in switching between self and other representations (Sowden and Catmur, 2013) and may also contribute to the maintenance of a coherent sense of one’s body, a potentially critical component of self-specificity (Tsakiris et al., 2008; Tsakiris, 2010; Sowden and Catmur, 2013). In the current results we also see only the RTPJ and RTP regions, and not other parts of DMN, being identified in the pre-stimulus component of the task. These results point towards a process in which the pre-stimulus activity in the regions identified defines the incoming ambiguous stimulus as self-related, triggering self-related (or self-evaluative) processing in a separate set of brain regions (Decety and Sommerville, 2003; Tsakiris et al., 2008; Tsakiris, 2010; Sowden and Catmur, 2013).
Limitations and future directions
One point to consider about the study design was that actual names were presented overlaid with the white noise and so it could be argued that this may influence our results. This is unlikely, however, as it was shown both behaviourally (i.e. d’ was not different from 0—Figure 1C) and neurally (i.e. that the different name trials did not produce differential BOLD responses—Supplementary Figures 2 and 4) that the participants could not hear the underlying names and that their judgements were not influenced by them. Despite this, repeating the experiment with purely white noise as confirmation would potentially be justified.
A second point to consider is that we use a specific form of self-relatedness here—whether a participant thinks they hear their own name or not. In the future, it would be worth investigating the relationship between spontaneous activity and other forms of self-relatedness. An interesting avenue of investigation in this regard would be to link spontaneous self-related thoughts during a stimulus-free resting-state period with the intrinsic activity in the periods prior to the participant indicating that they have had such thoughts.
Conclusion
To conclude, our results show that the pre-stimulus spontaneous activity level in particular brain regions, largely overlapping with the DMN, predict the subsequent judgement of stimuli as self-related. This extends our current knowledge of self-related processing and its apparent relationship with intrinsic brain activity in what can be termed a rest-self overlap.
Funding
This work was supported by grants to G.N. from the Canadian Institutes of Health Research (201103MOP-244752-BSB-CECA-179644; 201103CCI-248496-CCI-CECA-179644), Michael Smith Foundation (200809EJL-194083-EJL-CECA-179644). N.W.D. acknowledges support from the National Science Foundation of China (No. 31471072). T.L. acknowledges support from the Taiwan’s Ministry of Science and Technology (102-2420-H-038-001-MY3, 103-2811-H-038-002, and 104-2420-H-038-001-MY3). We thank Dr Gang Chen for his support for the data analysis.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared
References
- Bai Y., Nakao T., Xu J., et al. (2015). Resting state glutamate predicts elevated pre-stimulus alpha during self-relatedness: a combined EEG-MRS study on “rest-self overlap”. Social Neuroscience, 21, 1–15. [DOI] [PubMed] [Google Scholar]
- Boly M., Balteau E., Schnakers C., et al. (2007). Baseline brain activity fluctuations predict somatosensory perception in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(29), 12187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland P.S. (2002). Self-representation in nervous systems. Science, 296(5566), 308–10. [DOI] [PubMed] [Google Scholar]
- Coste C. P., Sadaghiani S., Friston K. J., Kleinschmidt A. (2011). Ongoing brain activity fluctuations directly account for intertrial and indirectly for intersubject variability in Stroop task performance. Cerebral Cortex, 21(11), 2612–19. [DOI] [PubMed] [Google Scholar]
- Cox R. W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers And Biomedical Research, 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- Decety J., Sommerville J. A. (2003). Shared representations between self and other: a social cognitive neuroscience view. Trends in Cognitive Sciences, 7(12), 527–33. [DOI] [PubMed] [Google Scholar]
- Denny B. T., Kober H., Wager T. D., Ochsner K. N. (2012). A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive and Neuroscience, 24(8), 1742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devue C., Bredart S. (2011). The neural correlates of visual self-recognition. Consciousness and Cognition, 20(1), 40–51. [DOI] [PubMed] [Google Scholar]
- Fingelkurts A. A., Fingelkurts A. A., Bagnato S., Boccagni C., Galardi G. (2012). DMN operational synchrony relates to self-consciousness: evidence from patients in vegetative and minimally conscious states. Open Neuroimaging Journal, 6, 55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. D., Snyder A. Z., Vincent J. L., Corbetta M., Van Essen D. C., Raichle M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of the America, 102(27), 9673–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K. J., Lurie D., Urchs S., et al. (2014). A correspondence between individual differences in the brain’s intrinsic functional architecture and the content and form of self-generated thoughts. PLoS One, 9(5), e97176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. D., Krasnow B., Reiss A. L., Menon V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann G., Kell C. A., Eger E., Kleinschmidt A. (2008a). Spontaneous local variations in ongoing neural activity bias perceptual decisions. Proceedings of the National Academy of Sciences of the United States of America, 105(31), 10984–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann G., Kell C. A., Kleinschmidt A. (2008b). Ongoing activity fluctuations in hMT + bias the perception of coherent visual motion. Journal of Neuroscience, 28(53), 14481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann G., Sadaghiani S., Friston K. J., Kleinschmidt A. (2010). Predictive coding or evidence accumulation? False inference and neuronal fluctuations. PLoS One, 5(3), e9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Dai R., Wu X., et al. (2014). The self and its resting state in consciousness: an investigation of the vegetative state. Human Brain Mapping, 35(5), 1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Zhang J., Longtin A., et al. (2015). Is there a nonadditive interaction between spontaneous and evoked activity? Phase-dependence and its relation to the temporal structure of scale-free brain activity. Cerebral Cortex. doi: 10.1093/cercor/bhv288. [DOI] [PubMed] [Google Scholar]
- Justen C., Herbert C., Werner K., Raab M. (2013). Self vs. other: neural correlates underlying agent identification based on unimodal auditory information as revealed by electrotomography (sLORETA). Neuroscience, 259C, 25–34. [DOI] [PubMed] [Google Scholar]
- Lipsman N., Nakao T., Kanayama N., et al. (2014). Neural overlap between resting state and self-relevant activity in human subcallosal cingulate cortex—single unit recording in an intracranial study. Cortex, 60, 139–44. [DOI] [PubMed] [Google Scholar]
- Mason M. F., Norton M. I., Van Horn J. D., Wegner D. M., Grafton S. T., Macrae C. N. (2007). Wandering minds: the default network and stimulus-independent thought. Science, 315(5810), 393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar-Szakacs I., Uddin L. Q. (2013). Self-processing and the default mode network: interactions with the mirror neuron system. Frontiers in Human Neuroscience, 7, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. J., Schaer M., Debbane M. (2012). Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neuroscience and Biobehavioral Reviews, 36(3), 1043–1059. [DOI] [PubMed] [Google Scholar]
- Nakane T., Miyakoshi M., Nakai T., Naganawa S. (2015). How the non-attending brain hears its owner’s name. Cereb Cortex. doi: 10.1093/cercor/bhv184. [DOI] [PubMed] [Google Scholar]
- Northoff G., Qin P., Nakao T. (2010). Rest-stimulus interaction in the brain: a review. Trends in Neuroscience, 33(6), 277–84. [DOI] [PubMed] [Google Scholar]
- Peer M., Salomon R., Goldberg I., Blanke O., Arzy S. (2015). Brain system for mental orientation in space, time, and person. Proceedings of the National Academy of Sciences of United States of America, 112(35), 11072–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., Japee S., Ungerleider L. G. (2005). Visual awareness and the detection of fearful faces. Emotion, 5(2), 243–7. [DOI] [PubMed] [Google Scholar]
- Platek S. M., Loughead J. W., Gur R. C., et al. (2006). Neural substrates for functionally discriminating self-face from personally familiar faces. Human Brain Mapping, 27(2), 91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P., Grimm S., Duncan N. W., et al. (2013). Self-specific stimuli interact differently than non-self-specific stimuli with eyes-open versus eyes-closed spontaneous activity in auditory cortex. Frontiers in Human Neuroscience, 7, 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P., Liu Y., Shi J., et al. (2012). Dissociation between anterior and posterior cortical regions during self-specificity and familiarity: a combined fMRI-meta-analytic study. Human Brain Mapping, 33(1), 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P., Northoff G. (2011). How is our self related to midline regions and the default-mode network?. Neuroimage, 57(3), 1221–33. [DOI] [PubMed] [Google Scholar]
- Raichle M. E., MacLeod A. M., Snyder A. Z., Powers W. J., Gusnard D. A., Shulman G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S., Hesselmann G., Kleinschmidt A. (2009). Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. Journal of Neuroscience, 29(42), 13410–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S., Poline J. B., Kleinschmidt A., D’Esposito M. (2015). Ongoing dynamics in large-scale functional connectivity predict perception. Proceedings of the National Academy of Sciences of the United States of America, 112(27), 8463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y. I., Barch D. M., Price J. L., et al. (2009). The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 1942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X. W., Dong Z. Y., Long X. Y., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One, 6(9), e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon C. S., Brass M., Heinze H. J., Haynes J. D. (2008). Unconscious determinants of free decisions in the human brain. Nature Neuroscience, 11(5), 543–5. [DOI] [PubMed] [Google Scholar]
- Sowden S., Catmur C. (2013). The role of the right temporoparietal junction in the control of imitation. Cerebral Cortex , 25, 1107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Hong Y. Y., Hong Liu C., Humphreys G. W., Han S. (2012). Dynamic cultural modulation of neural responses to one’s own and friend’s faces. Social and Cognitive and Affective Neuroscience , 8, 326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacikowski P., Brechmann A., Nowicka A. (2012). Cross-modal pattern of brain activations associated with the processing of self- and significant other’s name. Human Brain Mapping , 34, 2069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M. (2010). My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia, 48(3), 703–12. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Costantini M., Haggard P. (2008). The role of the right temporo-parietal junction in maintaining a coherent sense of one’s body. Neuropsychologia, 46(12), 3014–18. [DOI] [PubMed] [Google Scholar]
- Uddin L. Q., Kaplan J. T., Molnar-Szakacs I., Zaidel E., Iacoboni M. (2005). Self-face recognition activates a frontoparietal “mirror” network in the right hemisphere: an event-related fMRI study. Neuroimage, 25(3), 926–35. [DOI] [PubMed] [Google Scholar]
- van der Meer L., Costafreda S., Aleman A., David A. S. (2010). Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience and Biobehavioral Reviews, 34(6), 935–46. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Moran J. M., Nieto-Castanon A., Triantafyllou C., Saxe R., Gabrieli J. D. (2011). Associations and dissociations between default and self-reference networks in the human brain. Neuroimage, 55(1), 225–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.