Abstract
This Letter investigated the efficacy of a decision-support system, designed for respiratory medicine, at predicting asthma exacerbations in a multi-site longitudinal randomised control trial. Adherence to inhaler medication was acquired over 3 months from patients with asthma employing a dose counter and a remote monitoring adherence device which recorded participant's inhaler use: n = 184 (23,656 audio files), 61% women, age (mean ± sd) 49.3 ± 16.4. Data on occurrence of exacerbations was collected at three clinical visits, 1 month apart. The relative risk of an asthma exacerbation for those with good and poor adherence was examined employing a univariate and multivariate modified Poisson regression approach; adjusting for age, gender and body mass index. For all months dose counter adherence was significantly (p < 0.01) higher than remote monitoring adherence. Overall, those with poor adherence had a 1.38 ± 0.34 and 1.42 ± 0.39 (remotely monitored) and 1.25 ± 0.32 and 1.18 ± 0.31 (dose counter) higher relative risk of an exacerbation in model 1 and model 2, respectively. However, this was not found to be statistically significantly different. Remotely monitored adherence holds important clinical information and future research should focus on refining adherence and exacerbation measures. Decision-support systems based on remote monitoring may enhance patient–physician communication, possibly reducing preventable adverse events.
Keywords: diseases, pneumodynamics, decision support systems, regression analysis, Poisson distribution, patient monitoring
Keywords: dose counter adherence, body mass index, gender, age, multivariate modified Poisson regression, univariate modified Poisson regression, remote monitoring adherence device, inhaler medication, multisite longitudinal randomised control trial, respiratory medicine, decision-support system, asthma exacerbations
1. Introduction
Asthma is a common chronic disease globally affecting 300 million people [1]. It is characterised by recurrent attacks of breathlessness and wheezing that may vary in severity and frequency between individuals. Asthma cannot be cured, but appropriate management and adherence to medication can control the disorder, reducing severity and adverse events and increasing quality of life [2].
The biology of an asthma exacerbation is complex, involving many factors. Behaviour, in particular adherence behaviour is a key factor in ensuring the delivery of prescribed treatment. Previous studies have found forming a habit of adherence to be fundamental [3]. Other factors such as personal characteristics and environmental factors also influence clinical outcomes. These personal characteristics can be inherent, some are constant such as gender and height, others stable such as age or have the ability to be changed such as weight or body mass index (BMI).
Determining predictors of asthma exacerbations would be clinically beneficial to prevent or reduce the occurrence of adverse events and to recognise the early signs of an asthma exacerbation. Previous studies have examined predictors of respiratory exacerbations employing conditional probability to examine links with peak expiratory flow rate [4], multivariate logistic regression to examine links with dyspnea scores [5] and exploring links between exacerbations and previous exacerbations [6], among others [7, 8]. Poor or non-adherence to inhaled medication is thought to be a key cause of exacerbations [9]. However, there is limited knowledge on the efficacy of a medication adherence measure at identifying risk of adverse events or as a predictor of asthma exacerbations [10]. More objective measures of adherence are required also, and recent studies have examined the benefit of remote monitoring [11].
This Letter aimed to examine the efficacy of a simple, cost-effective remote monitoring tool at predicting asthma exacerbations. This monitoring tool was designed as a clinical decision-support system. Further validating this tool as a screen for risk of asthma exacerbations could aid preventative clinical strategies by highlighting those patients at risk of adverse events and informing physicians on efficacy of medication dosing.
2. Procedure
This Letter employed longitudinal data from 184 participants with asthma [61% women, age (mean ± sd) 49.3 ± 16.4] who participated in the inhaler compliance assessment study (INCA-1) [12], a randomised, parallel-group, multi-centre trial (NCT01529697) employing a novel remote monitoring device (the INCA™ device) [13–17] to measure and monitor compliance to Seretide Diskus™ dry powder inhaler (DPI).
The INCA device is a validated [13, 15] remote monitoring device employed to assess inhaler compliance by audio recording patients using their inhaler. The INCA device which consists of a microphone, a solid-state memory storage, a microprocessor and a battery for recording audio, is attached to an inhaler as shown in Fig. 1 (top). Recording is initiated when the inhaler is opened and ends on closing the inhaler as shown in Fig. 2 (bottom). An electronic real-time clock time stamps the recording and this time stamp is stored as part of the file's metadata. A sampling rate of 8 kHz with an 8-bit sampling resolution is employed to record the audio. During correct inhaler usage, this audio consists of a sequence of distinct features: medication blistering, exhalation away from the inhaler, medication inhalation through the inhaler mouthpiece and subsequent holding of breath as shown in Fig. 2. The audio files are downloaded from the INCA™ device and processed by file transfer protocol software for subsequent analysis of each audio file by a previously validated algorithm [13–16]. Each audio file is classified as (i) used correctly or (ii) used incorrectly which is further classified according to the type of technique error (exhale into mouthpiece, no blister, no inhalation, low inspiratory flow rate, multiple inhalation, multiple blisters). The algorithm outputs this information on inhaler adherence to the clinician along with patient identification number and, date, time and duration of inhaler use. This facilitates inhaler use feedback to the patient resulting in adherence education which may prevent adverse events and improve clinical outcomes.
Fig. 1.
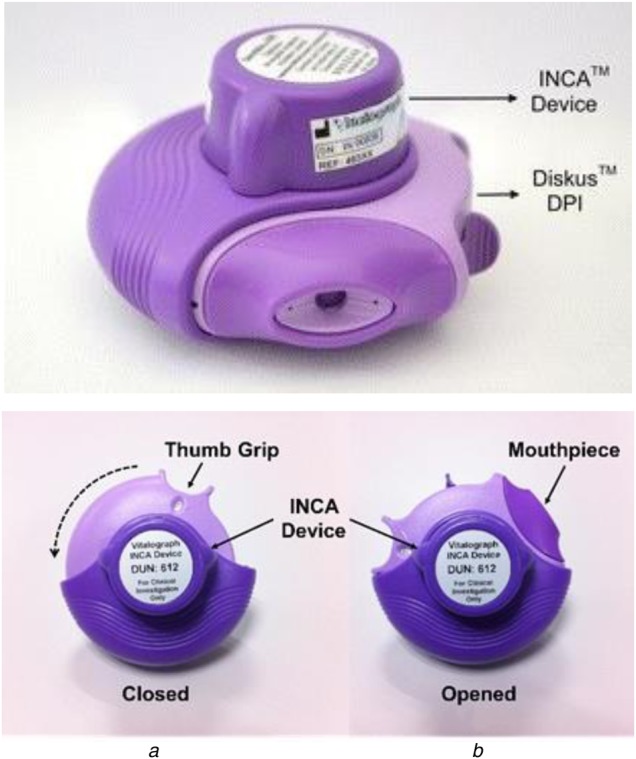
INCA device attached to a DiskusTM DPI (top) [16]. Functional components of the inhaler (bottom) [14]
Fig. 2.
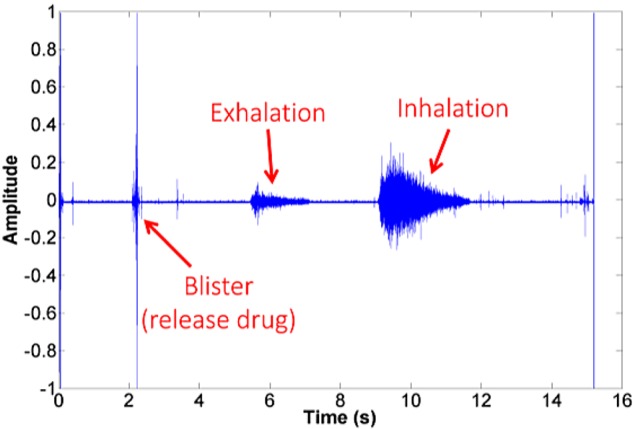
Acoustic signals recorded from the INCA device showing correct inhaler technique [14]
3. Methods
All assessments were performed in a clinical trial centre by either a research nurse or a medical doctor specialising in respiratory medicine. This clinical trial (INCA-1) was a multi-centre clinical trial involving five sites within the Republic of Ireland. Assessments were performed over 3 months which involved four separate visits 1 month apart, beginning with visit 1 and following with visit 2, visit 3 and visit 4. All sites received full education and training on the study protocol [12] ensuring standardisation of all procedures performed. All equipment required was provided to each centre.
A case record form was designed to record all data captured. Personal characteristics (age, gender, height and weight) were collected from the INCA-1 monthly assessments. Adverse event data was collected at all follow-up visits (visit 2, visit 3 and visit 4) detailing a description of the event and its duration when participants were asked: ‘Have you experienced an adverse event since your last visit?’. Subsequently, these descriptions were classified into either respiratory or non-respiratory adverse events and further into lower and upper respiratory tract adverse events by clinicians specialising in respiratory medicine. There is no clear definition for an asthma exacerbation. However, it is commonly defined as the need for treatment with systemic corticosteroids, worsening asthma requiring hospital admission or emergency treatment, or a decrease in morning peak flow >25% of baseline on two consecutive days [18, 19]. This Letter has taken a conservative approach and classified an exacerbation as a lower respiratory tract adverse event of 3 days or more duration.
At visit 1, visit 2 and visit 3 all participants were dispensed a Diskus™ inhaler with an INCA™ device attached. At all follow-up visits (at visit 2, visit 3 and visit 4), the research nurse recorded the medication dose counter number displayed on the inhaler and uploaded device audio files for automatic adherence analysis.
Attempted doses were defined as all participant audio recordings regardless of inhaler technique. Actual doses were defined as those recordings which were classified as not having a technique error. Technique errors were: low inspiratory flow rate, multiple inhalations, multiple blisters, blister present no inhalation detected, no blister detected inhalation present, no blister and no inhalation, multiple inhalations and multiple blisters, and exhaling into the mouthpiece.
Remotely monitored adherence and dose counter adherence were calculated as follows:
Where:
Attempted Doses = Attempted Doses per visit per participant.
Actual Doses = Actual Doses per visit per participant
Expected Doses = Prescribed daily dose (2 doses a day) over duration of analysis
Dose Counter Doses = medication dose counter number.
Participants were classified into those who had an exacerbation and those who had not, generating a binary exacerbation measure. Remotely monitored adherence and dose counter adherence were both categorised into those with good adherence (≥80%) and those with poor adherence (<80%). Exacerbation, remotely monitored adherence and dose counter adherence measures were calculated for each participant for the total length of the clinical trial (3 months) and for each month: month 1, month 2 and month 3.
Data was analysed using Stata 14 (StataCorp LP, Texas, USA). A t-test (p < 0.05) was employed to examine differences in dose counter adherence and remotely monitored adherence. A modified Poisson regression (p < 0.05) was employed to estimate the effect of adherence on exacerbation occurrence based on relative risk. Poisson regression can overestimate relative risk, however this can be rectified in a novel modified Poisson regression by employing a robust error variance procedure known as sandwich estimation [20]. This modified Poisson regression has been validated in prospective studies with subject numbers similar to those employed in this Letter [21]. In this Letter, two modified Poisson regression models (model 1 and model 2) were employed to compare the odds ratio and the relative risk of exacerbation occurrence for those with poor adherence against those with good adherence. Model 1 employed a univariate modified Poisson regression. Model 2 also adjusted for age, gender and BMI. Both models were examined over the total duration of the clinical trial (3 months) and for each month (month 1, month 2 and month 3).
4. Results
Personal characteristics of the 184 participants included in the analysis were: 61% female (n = 114), mean ± sd interquartile range (IQR): age 49.3 ± 16.4 (37.1–63.1), BMI 29.3 ± 6.8 (24.9–32.3). Number of respiratory adverse events, lower respiratory tract adverse events and asthma exacerbations can be seen in Table 1. The overall incidence rate of a respiratory adverse event was found to be 31%. There were 23,656 audio files recorded for all participants over 3 months.
Table 1.
Number of adverse events and exacerbations per month
| Measure | Month 1 | Month 2 | Month 3 |
|---|---|---|---|
| adverse events (AEs) | 36 | 38 | 40 |
| lower respiratory AEs | 28 | 29 | 31 |
| asthma exacerbations | 22 | 26 | 27 |
It was found that dose counter adherence statistically significantly differed from remotely monitored adherence (t-test p < 0.01) with mean ± sd adherence 81.8 ± 52.4, 76.1 ± 42.7 and 74.0 ± 44.0% for dose count adherence compared with 49.3 ± 50.1, 57.0 ± 49.6 and 56.0 ± 50.0% for remotely monitored adherence for month 1, month 2 and month 3, respectively.
Results for statistical model 1 and model 2 can be found in Table 2. In model 1, over the total duration of the clinical trial (3 months) the relative risk of having an asthma exacerbation was 1.38 ± 0.34 (0.82–2.20) (mean ± sd (IQR)) higher for those with poor adherence employing the remotely monitored adherence, and 1.25 ± 0.32 (0.77–2.06) higher employing dose counter adherence, when compared with those with good adherence.
Table 2.
Estimated relative risk of asthma exacerbations when comparing those with poor adherence to those with good adherence for the total 3-month duration of the clinical trial (overall) and for month 1, month 2 and month 3: model 1 and model 2
| All participants | ||||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | Overall | Month 1 | Month 2 | Month 3 | ||||
| Model 1 RR ± SE* (range) (p-value) |
Model 2 RR ± SE* (range) (p-value) |
Model 1 RR ± SE* (range) (p-value) |
Model 2 RR ± SE* (range) (p-value) |
Model 1 RR ± SE* (range) (p-value) |
Model 2 RR ± SE* (range) (p-value) |
Model 1 RR ± SE* (range) (p-value) |
Model 2 RR ± SE* (range) (p-value) |
|
| dose counter adherence | 1.25 ± 0.32 (0.77–2.06) 0.366 |
1.18 ± 0.31 (0.71–1.96) 0.531 |
1.22 ± 0.62 (0.45–3.31) 0.693 |
1.32 ± 0.66 (0.49–3.51) 0.582 |
0.83 ± 0.38 (0.34–2.04) 0.684 |
0.65 ± 0.32 (0.24–1.71) 0.379 |
1.23 ± 0.46 (0.59–2.56) 0.585 |
1.14 ± 0.42 (0.55–2.33) 0.73 |
| remotely monitored adherence | 1.38 ± 0.34 (0.82–2.20) 0.251 |
1.42 ± 0.39 (0.83–2.44) 0.199 |
1.13 ± 0.48 (0.49–2.6) 0.768 |
1.74 ± 0.89 (0.64–4.73) 0.274 |
1.29 ± 0.48 (0.63–2.65) 0.493 |
1.25 ± 0.46 (0.61–2.56) 0.548 |
0.51 ± 0.29 (0.17–1.6) 0.241 |
0.41 ± 0.23 (0.13–1.24) 0.113 |
*Statistical model 1: the estimated relative risk and standard error for the dose counter and remotely monitored adherence, respectively, based on univariate analysis: n = 128, 156, 148 and 137 for overall, month 1, month 2 and month 3, respectively. Statistical model 2: the estimated relative risk for the dose counter and remotely monitored adherence, respectively, adjusting for age, gender and BMI: n = 118, 140, 135 and 126 for overall, month 1, month 2 and month 3, respectively.
In model 1, when examining each month separately and employing remotely monitored adherence, those with poor adherence were found to have a higher relative risk of having an asthma exacerbation when compared with those with good adherence [1.13 ± 0.48 (month 1), 1.29 ± 0.48 (month 2) (mean ± sd)], with the exception of month 3 (0.51 ± 0.29). Similarly, for dose counter adherence those with poor adherence were found to have a higher risk of asthma exacerbations when compared with those with good adherence [1.22 ± 0.62 (month 1), month 3 (1.23 ± 0.46) (mean ± sd)], with the exception of month 2 (0.83 ± 0.38). However, model 1 results were not found to be statistically significantly different.
In model 2, over the total duration of the clinical trial (3 months), the relative risk of having an asthma exacerbation was 1.42 ± 0.39 (0.83–2.44) (mean ± sd (IQR)) higher for those with poor adherence employing the remotely monitored adherence and, 1.18 ± 0.31 (0.71–1.96) higher employing dose counter adherence when compared with those with good adherence.
In model 2, when examining each month separately and employing remotely monitored adherence, those with poor adherence were found to have a higher relative risk of having an asthma exacerbation when compared with those with good adherence [month 1 (1.74 ± 0.89), month 2 (1.25 ± 0.46) (mean ± sd)], with the exception of month 3 (0.41 ± 0.23). When employing dose counter adherence those with poor adherence were found to have a higher risk of asthma exacerbations when compared with those with good adherence [month 1 (1.32 ± 0.66), month 3 (1.14 ± 0.42) (mean ± sd)], with the exception of month 2 (0.65 ± 0.32). However, results for model 2 were not found to be statistically significantly different.
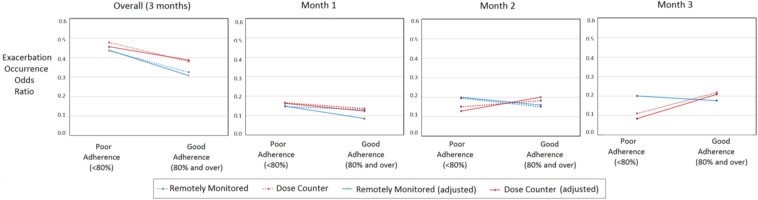
Similar results were found when investigating the odds ratios of a participant having an asthma exacerbation where an overall increase in odds ratio was found for those with poor adherence compared with those with good adherence, however this relationship was less clear when examining the relationship between adherence and exacerbation risk across each month as can be seen in Fig. 3 (for model 1 and model 2).
Fig. 3.
Odds of participants with poor adherence having experienced an exacerbation when compared with those with good adherence, from left to right, over the total 3-month duration of the clinical trial (overall), at month 1, month 2 and month 3, respectively. Dose counter adherence is in blue. Remotely monitored adherence is in red
5. Discussion
The relative risk of asthma exacerbation occurrence was investigated in a multi-site longitudinal randomised control trial. The primary aim was to investigate the efficacy of adherence acquired from a remote monitoring device at predicting risk of asthma exacerbations compared with that from the commonly employed dose counter. It was found that dose counter adherence was significantly (p < 0.01) higher than remote monitoring adherence during month 1, month 2 and month 3. Overall, across the total duration of the clinical trial (3 months) those with poor adherence ( < 80%) were found to have a higher relative risk and odds ratio of asthma exacerbation occurrence (when compared with those with good adherence (≥80%)) if measured by remote monitoring when compared with a dose counter. However, overall both remotely monitored adherence and dose counter adherence were found to predict asthma exacerbations. The INCA device was designed as a decision-support system for physicians and as a tool to improve asthma inhaler adherence by aiding communication between patient and healthcare professional. The results found in this Letter indicate that this adherence data may additionally inform clinicians on exacerbation risk, potentially reducing exacerbations thereby improving quality of life.
Remotely monitored adherence (49.3 ± 50.1, 57.0 ± 49.6 and 56.0 ± 50.0% (mean ± sd)) was significantly (p < 0.01) lower than dose counter adherence (81.8 ± 52.4, 76.1 ± 42.7 and 74.0 ± 44.0%) for month 1, month 2 and month 3, respectively. This shows that the INCA remote monitoring device is acquiring important objective data by excluding incorrect inhaler use. This supports the employment of remote monitoring devices which can acquire relevant objective clinical information, in this instance a more accurate description of the amount of medication delivered to the patient.
For both adherence measures, those participants with poor inhaler adherence over the total duration of the clinical trial (3 months) had a higher relative risk of exacerbation incidence in model 1 and in model 2 after adjusting for confounding factors (age, gender and BMI) when compared with those with good adherence. This relative risk was higher employing remotely monitored adherence (1.38 ± 0.34, 1.42 ± 0.39 (mean ± sd)) compared with dose counter adherence (1.25 ± 0.32, 1.18 ± 0.31) for model 1 and model 2, respectively.
Results were varied when examining the relationship between adherence and exacerbation occurrence each month. In the fully adjusted model those with poor adherence had a 1.74 ± 0.89, 1.25 ± 0.46 and 0.41 ± 0.23 (remotely monitored) (mean ± sd) and 1.13 ± 0.48, 1.29 ± 0.48 and 0.51 ± 0.29 (dose counter) relative risk of an exacerbation in month 1, month 2 and month 3, respectively, when compared with those with good adherence. However, these differences were not found to be statistically significantly different. Similar results were found for model 1 with a minor effect of covariates (age, gender and BMI) as can be seen in Table 2 and Fig. 3. This suggests that the adherence measures employed here are not sufficiently sensitive over this shorter monitoring time (1 month). However, employing a more descriptive adherence measure, in addition to a less conservative definition of exacerbation may yield better results.
The effect of covariates can be seen in the reduced relative risk in Table 2 and odds ratio in Fig. 3 from statistical model 1 to model 2. This shows the importance of adjusting for confounding factors to aid in highlighting variables of most interest. However, other covariates such as smoking status, baseline lung function in addition to know triggers of asthma exacerbations which may hold important clinical information (pollution, season and viruses), may be beneficial to include also. This Letter employed a modified Poisson regression to calculate relative risk of asthma exacerbations. This method is supported by previous literature [22, 23] and has been found to be relatively robust to omitted covariates [21].
The results from this Letter indicate that remotely monitored adherence holds important clinical information. Future research should focus on building a more robust adherence measure which may include duration between administering medication, in addition to cumulative effect of missed doses. We hypothesise that inclusion of such additional elements of adherence would increase the predictive power of remotely monitored adherence.
This Letter examined the efficacy of a design support system at predicting risk of asthma exacerbations. Overall, the remotely monitored adherence measure calculated by this system was found to predict asthma exacerbations over a 3-month clinical trial. In addition, the adherence values calculated were found to be statistically significantly lower than those calculated from a commonly employed dose counter. Over shorter monitoring durations (1 month) the predictive value was unclear. In conclusion, remotely monitored poor adherence (<80%) holds important clinical information as it showed some predictive value for future exacerbations. Further refinement of this system may allow a clinically useful preventative tool be developed which may highlight those patients at risk of adverse events and inform physicians on efficacy of medication dosing. Decision-support systems based on remote monitoring may enhance patient–physician communication to aid clinical outcomes and quality of life, in addition to reducing preventable adverse events.
6. Acknowledgments
The authors express their sincere gratitude for the contributions of the study participants and the INCA 1 team. This work was supported by HRB DI 59/INCASUN.
7. Declaration of Interests
The following authors are named on the INCA device patent application: RB Reilly, RW Costello, I Killane, MS Holmes and C Hughes. The patent, PCT/EP2013/067932, was filed on the 29th of August 2013. This does not alter the authors' adherence to IET policies on sharing data and materials.
8 References
- 1.Masoli M., Fabian D., Holt S., et al. : ‘The global burden of asthma: executive summary of the GINA dissemination committee report’, Allergy, 2004, 59, pp. 469–478 (doi: ) [DOI] [PubMed] [Google Scholar]
- 2.W.H.O. Available at http://www.who.int/topics/asthma/en/
- 3.Ruppar T.M., Russell C.L.: ‘Medication adherence in successful kidney transplant recipients’, Prog. Transplant., 2009, 19, pp. 167–172 (doi: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harm D.L., Kotses H., Creer T.L.: ‘Improving the ability of peak expiratory flow rates to predict asthma’, J. Allergy Clin. Immunol., 1985, 76, pp. 688–694 (doi: ) [DOI] [PubMed] [Google Scholar]
- 5.Murata G.H., Kapsner C.O., Lium D.J., et al. : ‘A multivariate model for predicting respiratory status in patients with chronic obstructive pulmonary disease’, J. Gen. Intern. Med., 1998, 13, pp. 462–468 (doi: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-ani S., Spigt M., Hofset P., et al. : ‘Predictors of exacerbations of asthma and COPD during one year in primary care’, Fam. Pract., 2013, 30, pp. 621–628 (doi: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topal E., Gücenmez O.A., Harmancı K., et al. : ‘Potential predictors of relapse after treatment of asthma exacerbations in children’, Ann. Allergy Asthma Immunol., 2014, 112, pp. 361–364 (doi: ) [DOI] [PubMed] [Google Scholar]
- 8.Wu A.C., Tantisira K., Li L., et al. : ‘Predictors of symptoms are different from predictors of severe exacerbations from asthma in children’, Chest, 2011, 140, pp. 100–107 (doi: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams L.K., Peterson E.L., Wells K., et al. : ‘Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid non-adherence’, J. Allergy Clin. Immunol., 2011, 128, (6), pp. 1185–1191.e2 (doi: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelkes M., Janssens H.M., de Jongste J.C., et al. : ‘Medication adherence and the risk of severe asthma exacerbations: a systematic review’, Eur. Respir. J., 2015, 45, pp. 396–407 (doi: ) [DOI] [PubMed] [Google Scholar]
- 11.Hacihasanoğlu R., Gözüm S.: ‘The effect of patient education and home monitoring on medication compliance, hypertension management, healthy lifestyle behaviours and BMI in a primary health care setting’, J. Clin. Nurs., 2011, 20, pp. 692–705 (doi: ) [DOI] [PubMed] [Google Scholar]
- 12.Sulaiman I., Mac Hale E., Holmes M., et al. : ‘A protocol for a randomised clinical trial of the effect of providing feedback on inhaler technique and adherence from an electronic device in patients with poorly controlled severe asthma’, BMJ Open, 2016, 6, p. e009350 (doi: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Arcy S., MacHale E., Seheult J., et al. : ‘A method to assess adherence in inhaler use through analysis of acoustic recordings of inhaler events’, PLoS ONE, 2014, 9, p e98701 (doi: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes M.S., D'Arcy S., Costello R.W., et al. : ‘Acoustic analysis of inhaler sounds from community-dwelling asthmatic patients for automatic assessment of adherence’, IEEE J. Transl. Eng. Health Med., 2014, 2, pp. 1–10 (doi: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes M.S., D'Arcy S., Costello R.W., et al. : ‘An acoustic method of automatically evaluating patient inhaler technique’. 35th Annual Int. Conf. of the IEEE Engineering in Medicine and Biology Society (EMBC), 2013, 2013, pp. 1322–1325 [DOI] [PubMed] [Google Scholar]
- 16.Jansen P.O.C., Seheult N., Tee K.C., et al. : ‘The acoustic features of inhalation can be used to quantify aerosol delivery from a Diskus™ dry powder inhaler’, Pharm. Res., 31, (10), pp. 2735–2747 [DOI] [PubMed] [Google Scholar]
- 17.Holmes M.S., Seheult J.N., O'Connell P., et al. : ‘An acoustic-based method to detect and quantify the effect of exhalation into a dry powder inhaler’, J. Aerosol Med. Pulm. Drug Deliv., 2015, 28, pp. 247–253 (doi: ) [DOI] [PubMed] [Google Scholar]
- 18.O'Byrne P.M., Barnes P.J., Rodriguez-Roisin R., et al. : ‘Low dose inhaled budesonide and formoterol in mild persistent asthma’, Am. J. Respir. Crit. Care Med., 2001, 164, pp. 1392–1397 (doi: ) [DOI] [PubMed] [Google Scholar]
- 19.Dougherty R.H., Fahy J.V.: ‘Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype’, Clin. Exp. Allergy, 2009, 39, pp. 193–202 (doi: ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royall R.M.: ‘Model robust confidence intervals using maximum likelihood estimators’, Int. Stat. Rev., 1986, 54, pp. 221–226 (doi: ) [Google Scholar]
- 21.Zou G.: ‘A modified Poisson regression approach to prospective studies with binary data’, Am. J. Epidemiol., 2004, 159, pp. 702–706 (doi: ) [DOI] [PubMed] [Google Scholar]
- 22.Greenland S.: ‘Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case–control studies’, Am. J. Epidemiol., 2004, 160, pp. 301–305 (doi: ) [DOI] [PubMed] [Google Scholar]
- 23.McNutt L.-A., Wu C., Xue X., et al. : ‘Estimating the relative risk in cohort studies and clinical trials of common outcomes’, Am. J. Epidemiol., 2003, 157, pp. 940–943 (doi: ) [DOI] [PubMed] [Google Scholar]