To the Editor
Katipamula et al. reported an increase in mastectomy rates for the treatment of early-stage (stages 0, I, and II) breast cancer for the period 2004-2006 at the Mayo Clinic in Rochester, Minnesota 1. This is the first US report of such a trend since the 1990 NIH Consensus Conference recommending breast conserving surgery (BCS) as a viable alternative to mastectomy 2. BCS is generally the preferred treatment for most early-stage breast cancer, given the equivalent survival after BCS and mastectomy and greater morbidity associated with mastectomy. Thus, these and other clinical reports of an increasing trend in contralateral prophylactic mastectomy 3-8 raise the question of a recent “swing of the pendulum” 1 toward use of mastectomy. While mastectomies were more common among Mayo Clinic patients who had received pre-operative magnetic resonance imaging (MRI) (used to detect mammographically occult breast lesions), increased MRI use did not explain the rise in mastectomy rates in this clinic 1.
We and others have shown previously that disadvantaged and minority women with early-stage breast cancer, especially Asians, are more likely than non-Hispanic whites to have mastectomy 9-12. The reasons for this remain unclear, but appear to relate in some part to biological/clinical factors such as smaller breasts and large tumor-to-breast ratios, as well as to sociocultural factors including poor patient-provider communications, language barriers, cultural factors, and possibly transportation difficulties 13-19. However, the paper by Katipamula et al. suggests that we may now be seeing a preference for mastectomy in a distinctly different, predominantly non-Hispanic white, population. If the mastectomy trend observed at the Mayo Clinic is reflective of a similar trend in the general population, then further studies may be warranted to reveal whether this pattern is due to such factors as insufficient provider knowledge or communication about BCS, patient anxiety about radiotherapy or breast cancer recurrence, or the higher financial cost of BCS. Therefore, we examined time trends in mastectomy rates for early-stage breast cancer in population-based data for the state of California, whose registries are supported by both the NCI’s Surveillance, Epidemiology, End Results (SEER) Program and the California Cancer Registry (CCR). We examined the proportions of women receiving mastectomy by time and also by patient race/ethnicity, stage of disease, age, and a neighborhood measure of socioeconomic status (SES) based on block-group level Census 2000 data, as described elsewhere 20. We focused our assessment on cancers for which BCS is a treatment option, namely first primary breast cancers diagnosed at stages 0 (ductal carcinoma in situ (DCIS)), I, or II between the years 1990-2007. Mastectomy was defined based on the first course of surgery treatment including subcutaneous mastectomy, total (simple) mastectomy, modified radical mastectomy, radical mastectomy, extended radical mastectomy, and mastectomy (not otherwise specified), regardless of reconstruction, or removal of the uninvolved contralateral breast.
Across all racial/ethnic groups and stages of disease, the proportion of women receiving mastectomy declined rapidly from 1990-2000 at a rate of 2-4% per year, amounting to a nearly two-fold decrease over this 11-year period. After 2000, the proportion receiving mastectomy either stayed the same or increased about 1-2% per year. Among non-Hispanic whites (Figure 1), we observed an increase in the mastectomy rate that was most pronounced for DCIS, such that the mastectomy proportion for DCIS in 2007 was similar to that for stage I invasive disease. For stages I and II, by contrast, the proportion of patients receiving mastectomy remained constant from 2000 onward, with no evidence of an increase. Figure 2 shows clearly that the recent increase in mastectomy in on-Hispanic whites occurred only among women younger than 50 years at diagnosis. Figure 3 shows that the recent increase also was most prominent in women living in the highest 20% of neighborhoods ranked by SES, with an overall pattern of decreasing variation in mastectomy across SES groups after 2004. In the period 1988-2001, the mastectomy proportion ranged from 25% in the highest-SES quintile to 40% in the lowest-SES quintile, whereas in 2006-2007, this proportion varied by only 5% across the SES groups. Like others, we found racial/ethnic differences in the proportions of patients receiving mastectomy, varying by about 10% among groups, with the highest rates seen for Asians/Pacific Islanders and similar rates for non-Hispanic whites, Blacks, and Hispanics; this pattern was most striking for stage I cancer and remained consistent over time (data not shown).
Figure 1. Time trends in the percentage of non-Hispanic White women diagnosed with breast cancer undergoing mastectomy, by stage, California, 1990-2007.
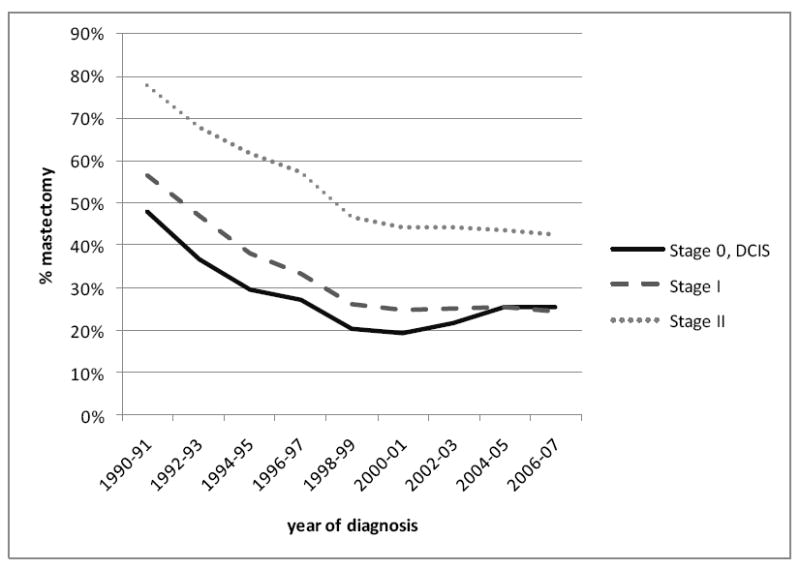
Figure 2. Time trends in the percentage of non-Hispanic White women diagnosed with breast cancer undergoing mastectomy, by age at diagnosis, California, 1990-2007.
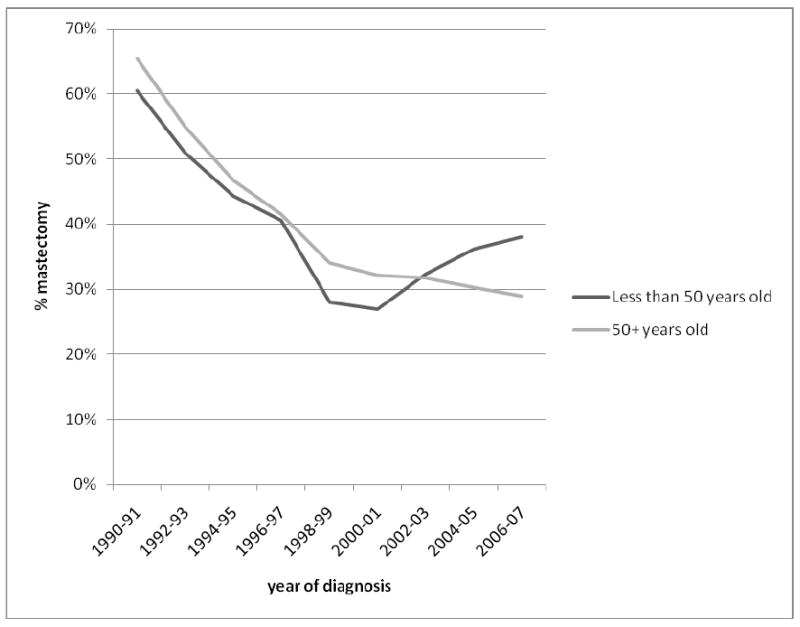
Figure 3. Time trends in the percentage of non-Hispanic White women diagnosed with breast cancer undergoing mastectomy, by neighborhood socioeconomic status (SES), California, 1990-2007.
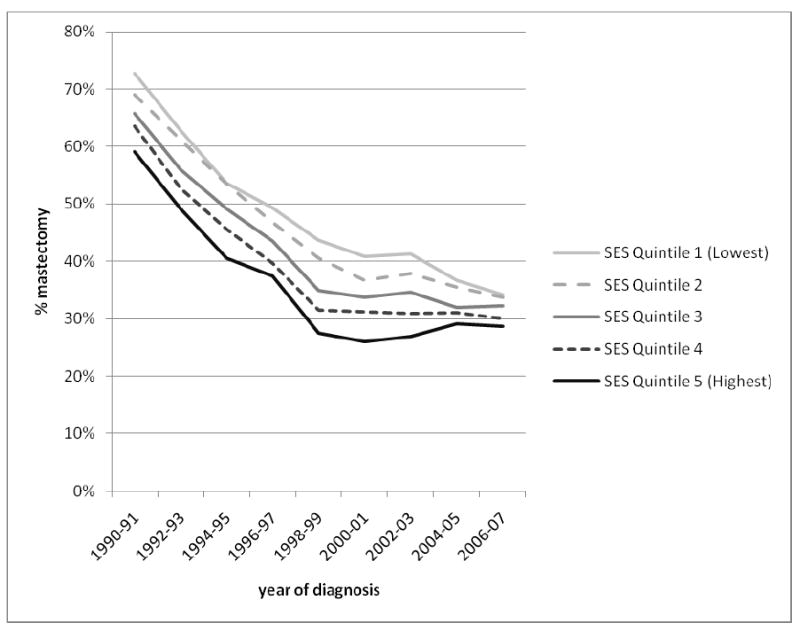
Our analysis of population-based cancer registry data supports either a recent decline in the rate of mastectomy, or, among some groups, a shift from 2000 onward in surgical treatment toward mastectomy for early-stage breast cancer. However, among non-Hispanic white women, this shift was most evident among those who were younger, diagnosed with DCIS, or living in the highest-SES neighborhoods. Across all racial/ethnic, SES, and stage groups, the proportion of women receiving mastectomy has either declined or stayed constant since 2000. To the extent that MRI at breast cancer diagnosis is not covered by many health insurance plans, our finding of increasing mastectomy proportions primarily among women of high SES would support a possible role of MRI.
A number of factors influence the decision to pursue BCS. Factors that may contribute to a recommendation against BCS include increased risk for loco-regional recurrence among younger patients 21,22, recent recommendations for larger margins in the surgical treatment of DCIS 23, greater likelihood of poor cosmetic outcome in small-breasted women, and MRI-detection of more extensive disease. Locoregional management decisions, particularly in younger women, may also be influenced by recent data from the 2005 Early Breast Cancer Trialists’ Collaborative Group demonstrating that overall survival at 15 years is improved when a local recurrence is avoided in the first five years 24. Patient attitudes towards radiotherapy and reconstruction, in addition to individually perceived risks, also factor prominently into the decision about BCS; for patients with DCIS, an additional factor is the recommended use of tamoxifen therapy for five years after BCS, but not mastectomy 25. Non-Hispanic white patients who have greater involvement in the decision-making process have been shown previously to be more likely to choose mastectomy, often in contrast to their surgeon’s recommendations, citing fear of recurrence and effects of radiation in their decision 26. The reasons for mastectomy in this patient sociodemographic group are likely quite different from those responsible for the traditionally high mastectomy rates among Asians and patients of low SES. A better characterization of the specific population groups, the decision-making processes underlying both the traditionally high mastectomy group (i.e., Asian, low SES) and the more recently adopting group (i.e., younger age, DCIS, higher SES), and the long- and short-term symptoms and quality of life associated with each type of treatment would ultimately help ensure that all women are given the opportunity to make fully-informed decisions about their treatment. Decision-making guides should be developed with information on the local recurrence risks, impact of local recurrence on overall survival, and short- and long-term side effects, including quality of life, of each type of treatment.
Acknowledgments
The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author and endorsement by the State of California, Department of Health Services, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Footnotes
The authors have no conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of this manuscript.
References
- 1.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009;27:4082–8. doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treatment of early-stage breast cancer. JAMA; NIH Consensus Conference; 1991. pp. 391–395. [PubMed] [Google Scholar]
- 3.Arrington AK, Jarosek SL, Virnig BA, et al. Patient and Surgeon Characteristics Associated with Increased Use of Contralateral Prophylactic Mastectomy in Patients with Breast Cancer. Ann Surg Oncol. 2009 doi: 10.1245/s10434-009-0641-z. [DOI] [PubMed] [Google Scholar]
- 4.Tuttle T, Habermann E, Abraham A, et al. Contralateral prophylactic mastectomy for patients with unilateral breast cancer. Expert Rev Anticancer Ther. 2007;7:1117–22. doi: 10.1586/14737140.7.8.1117. [DOI] [PubMed] [Google Scholar]
- 5.Tuttle TM. Counseling breast cancer patients on contralateral prophylactic mastectomy: the physician’s role. Oncology (Williston Park) 2008;22:545–8. [PubMed] [Google Scholar]
- 6.Tuttle TM. Magnetic resonance imaging and contralateral prophylactic mastectomy: the “no mas” effect? Ann Surg Oncol. 2009;16:1461–2. doi: 10.1245/s10434-009-0427-3. [DOI] [PubMed] [Google Scholar]
- 7.Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25:5203–9. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 8.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27:1362–7. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 9.Goel MS, Burns RB, Phillips RS, et al. Trends in breast conserving surgery among Asian Americans and Pacific Islanders, 1992-2000. J Gen Intern Med. 2005;20:604–11. doi: 10.1111/j.1525-1497.2005.0090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez SL, France AM, Lee MM. Socioeconomic status, immigration/acculturation, and ethnic variations in breast conserving surgery, San Francisco Bay area. Ethn Dis. 2004;14:134–40. [PubMed] [Google Scholar]
- 11.Morris CR, Cohen R, Schlag R, et al. Increasing trends in the use of breast-conserving surgery in California. Am J Public Health. 2000;90:281–4. doi: 10.2105/ajph.90.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prehn AW, Topol B, Stewart S, et al. Differences in treatment patterns for localized breast carcinoma among Asian/Pacific islander women. Cancer. 2002;95:2268–75. doi: 10.1002/cncr.10965. [DOI] [PubMed] [Google Scholar]
- 13.Ann Gilligan M, Kneusel RT, Hoffmann RG, et al. Persistent differences in sociodemographic determinants of breast conserving treatment despite overall increased adoption. Med Care. 2002;40:181–9. doi: 10.1097/00005650-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Jubelirer SJ, Harpold R, Miller S, et al. An analysis of factors determining the use of breast conserving surgery for treating early-stage breast cancer. W V Med J. 2001;97:144–7. [PubMed] [Google Scholar]
- 15.Kagawa-Singer M, Wellisch DK, Durvasula R. Impact of breast cancer on Asian American and Anglo American women. Cult Med Psychiatry. 1997;21:449–80. doi: 10.1023/a:1005314602587. [DOI] [PubMed] [Google Scholar]
- 16.Killoran M, Moyer A. Surgical treatment preferences in Chinese-American women with early-stage breast cancer. Psychooncology. 2006;15:969–84. doi: 10.1002/pon.1032. [DOI] [PubMed] [Google Scholar]
- 17.Maskarinec G, Dhakal S, Yamashiro G, et al. The use of breast conserving surgery: linking insurance claims with tumor registry data. BMC Cancer. 2002;2:3. doi: 10.1186/1471-2407-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nattinger AB. Variation in the choice of breast-conserving surgery or mastectomy: patient or physician decision making? J Clin Oncol. 2005;23:5429–31. doi: 10.1200/JCO.2005.04.913. [DOI] [PubMed] [Google Scholar]
- 19.Nattinger AB, Kneusel RT, Hoffmann RG, et al. Relationship of distance from a radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst. 2001;93:1344–6. doi: 10.1093/jnci/93.17.1344. [DOI] [PubMed] [Google Scholar]
- 20.Yost K, Perkins C, Cohen R, et al. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–11. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 21.de Bock GH, van der Hage JA, Putter H, et al. Isolated loco-regional recurrence of breast cancer is more common in young patients and following breast conserving therapy: long-term results of European Organisation for Research and Treatment of Cancer studies. Eur J Cancer. 2006;42:351–6. doi: 10.1016/j.ejca.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Nixon AJ, Neuberg D, Hayes DF, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol. 1994;12:888–94. doi: 10.1200/JCO.1994.12.5.888. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald HR, Silverstein MJ, Mabry H, et al. Local control in ductal carcinoma in situ treated by excision alone: incremental benefit of larger margins. Am J Surg. 2005;190:521–5. doi: 10.1016/j.amjsurg.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 25.NCCN. NCCN Clinical Practice Guidelines in Oncology - Breast Cancer. National Comprehensive Cancer Network V.1.2009. 2009 doi: 10.6004/jnccn.2009.0070. [DOI] [PubMed] [Google Scholar]
- 26.Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol. 2005;23:5526–33. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]


