Abstract
P-wave dispersion is defined as the difference between the maximum and the minimum P-wave duration recorded from multiple different-surface ECG leads. It has been known that increased P-wave duration and P-wave dispersion reflect prolongation of intraatrial and interatrial conduction time and the inhomogeneous propagation of sinus impulses, which are well-known electrophysiologic characteristics in patients with atrial arrhythmias and especially paroxysmal atrial fibrillation. Extensive clinical evaluation of P-wave dispersion has been performed in the assessment of the risk for atrial fibrillation in patients without apparent heart disease, in hypertensives, in patients with coronary artery disease, in patients undergoing coronary artery bypass surgery, in patients with congenital heart diseases, as well as in other groups of patients suffering from various cardiac or non-cardiac diseases. In this paper, we aimed to summarize the measurement methods, current use in different clinical situations, strengths and limitations of the of P-wave dispersion.
Keywords: Atrial fibrillation, electrocardiography, P-wave dispersion, P-wave duration
Introduction
Atrial fibrillation (AF) is one of the most common arrhythmias and originated from focal activation and multiple randomly reentrant wavelets that propagate, become extinct or fractionate within the atrial tissue.1,2 P-wave duration, measured from a single-surface electrocardiographic (ECG) lead, has shown significant correlations with the longest duration of the right atrial electrograms, the maximal number of their fragmented deflections and the repetitive atrial firing zone. Although markedly prolonged intra- and interatrial conduction time can be recognized by prolongation of the surface P-wave duration, the question arises as to whether inhomogeneous atrial conduction could be identified by variation in P-wave duration between differently oriented surface ECG leads.1–3 P-wave dispersion (Pd) constitutes a relatively recent contribution to the field of non-invasive electrocardiology and is defined as the difference between the longest and the shortest P-wave duration recorded from multiple different-surface ECG leads.2,4 The Pd has received increasing attention and has been examined in a broad range of clinical settings including cardiovascular and non-cardiovascular diseases.5 In this paper, we aimed to summarize the current use, measurement methods, strengths and limitations of the Pd.
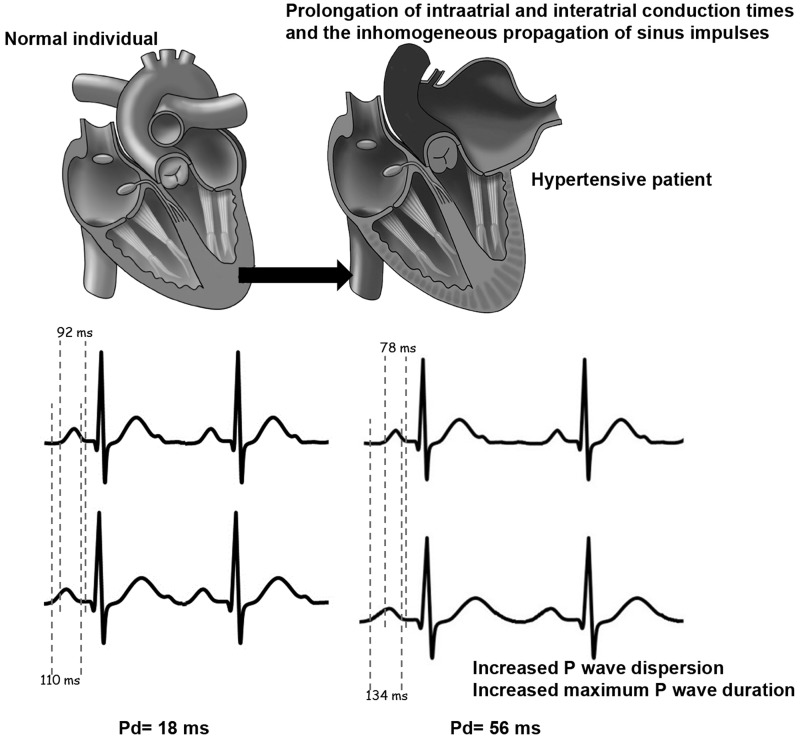
Measurement of Pd
Maximum and minimum P-wave durations are calculated from the standard ECG during sinus rhythm. Pd is derived by subtracting the minimum P-wave duration from the maximum in any of the 12 ECG leads (Figure 1). P-wave onset is determined as the initial deflection from the isoelectric baseline defined by the T-P segment and the P-wave offset is defined as the junction of the end of the P wave and its return to baseline.5 Pd can be calculated by measurements on paper or computerize methods. Manual measurement with hand-held calipers performed by increasing the ECG rate to 50 mm/s and the voltage to 1 mV/cm, accompanied by use of magnification. However, hand-held caliper measurements have less accuracy compared with digital measurements. Comparing manual and digital measurements, for maximum P-wave duration measurements, the intraobserver variability were reduced from 15% to 5% and the interobserver relative errors were diminished from 16% to 8%. Similarly, the intraobserver variability for Pd was reduced from 20% to 8% for intraobserver and from 26% to 10% for interobserver comparing manual and digital measurement acquisition.4,6,7
Figure 1.
Examples for measurement of Pd, minimum and maximum P-wave duration in normal individual and hypertensive patient.
Clinical use of Pd
Extensive clinical evaluation of Pd has been performed in the assessment of the risk for AF in patients without apparent heart disease, coronary artery disease (CAD), hypertension, valvular heart diseases, heart failure (HF), congenital heart diseases, rheumatologic diseases and miscellaneous conditions (Table 1). Clinical applications of Pd are summarized below.
Table 1.
Clinical applications of Pd.
| Atrial fibrillation | Rheumatologic diseases |
| – Prediction3,4,8 | – Rheumatoid arthritis52,53 |
| – Recurrence1,9,10 | – Behcet’s disease54 |
| – After DC shock11 | – Systemic lupus erytematosus55 |
| – After accessory pathway ablation6,12,13 | – Ankylosing spondilitis56 |
| Coronary artery disease14–27 | – Familial Mediterranean Fever57 |
| – After PTCA21 | – Systemic sclerosis58–60 |
| – Acute MI22 | Miscellaneous conditions |
| – Coronary slow flow23,24 | – Obesity61–63 |
| – CABG25–27 | – Metabolic syndrome64 |
| Hypertension28–33 | – Obstructive sleep apnea65 |
| Valvular heart disease | – Hemodialysis66,67 |
| – Mitral stenosis34–37 | – Chronic obstructive pulmonary disease68 |
| – Aortic stenosis38 | – Hypertrophic cardiomyopathy69 |
| – Pulmonary stenosis39 | – Hyperthyroidism70 |
| Heart failure40–42 | – Chemotherapeutics71 |
| – After CRT43,44 | – Acute caffeine ingestion72 |
| Congenital heart diseases | – Alcohol intake73 |
| – Atrial septal defect45–48 | |
| – Atrial septal aneurysm49,50 | |
| – Fontan operation51 |
Atrial Fibrillation
Insults leading to atrial remodeling such as chronically elevated atrial pressure, ischemia and metabolic stress result in slowed conduction with inhomogeneous recovery, defining a substrate for AF.7 Regional delays in atrial depolarization might produce a heterogeneous P-wave duration, because surface P waves in different locations could be affected to a different extent by regional changes in atrial activation times. This local hypothesis explaining the interlead variation in P-waves duration is called Pd.2,3,74 Pd reflects prolonged inhomogeneous and anisotropic distribution of connections between myocardial fibers results in the discontinuous anisotropic propagation of sinus impulses and the inhomogeneous and discontinuous atrial conduction.4
Dilaveris et al.3 found that Pd was significantly higher in 60 patients with paroxysmal lone AF (49 ± 15 ms) than in 40 healthy controls (28 ± 7 ms, p < 0.0001). Pd was proven to be a sensitive and specific ECG marker for the best separation between patients with history of paroxysmal lone AF and healthy subjects.3 A cutoff value of 40 ms proved to have a sensitivity of 83%, a specificity of 85% and a positive predictive accuracy of 89% for the identification of patients with history of paroxysmal lone AF.3 Moreover, during a 12-month follow-up period, the relative risk of an AF recurrence was 2.4 for a Pd value > 40 ms.3 In another publication, Aytemir et al.4 showed that Pd was a sensitive and specific ECG predictor of paroxysmal lone AF. In the same study, Pd showed a significant correlation with maximum P-wave duration (r = 0.702, p < 0.001) and a weak, although significant, association with age (r = 0.270, p < 0.001).4 In another study, Pd was shown to be a significant predictor of frequent symptomatic AF paroxysms but only in the univariate analysis.8 In the same study, Pd was found to be significantly correlated positively with maximum P-wave duration (p < 0.001) and negatively with minimum P-wave duration (p = 0.06). It is obvious that, when beyond Pd both maximum and minimum P-wave duration are incorporated as covariates in multivariate regression models, Pd may not remain an independent predictor of AF in the multivariate analysis.8
Post-electrical cardioversion Pd may help predict recurrences of AF.1,9 In the first minutes after electrical cardioversion of long-lasting AF, short refractory periods present a high risk of re-initiation of AF.1,9 Boriani et al.9 investigated the association of different Pd values and recurrence of AF in the short and longer term in 37 patients and reported significantly higher Pd values in patients with short-term AF recurrence (≤1 month). Furthermore they found that Pd values > 25 ms were associated with a higher short-term relapse rate. But no significant relation was present in the long-term in their study.9 Perzanowski et al.10 reported that a Pd value of 80 ms or greater was both a univariate and independent predictor for AF recurrence after cardioversion.
Ozdemir et al.11 identified 18 patients in whom an AF attack was induced by urgent or elective cardioversion for a ventricular tachycardia attack and found higher maximum P-wave duration and Pd values compared with a control group of 40 patients without AF. They concluded that the patients with higher Pd values had a greater risk for development of AF after a DC shock.11 Applying these results to the patients with ICD, it may be suggested that the patients with higher Pd and maximum P-wave duration have a greater risk for development of AF after a shock, which may upset the hemodynamic status or result in further inappropriate shocks.11
Although catheter ablation of the accessory pathways significantly decreases the incidence of AF, studies have shown that, in patients with Wolff- Parkinson-White (WPW) syndrome with a history of paroxysmal AF, the high recurrence rate of AF after accessory pathway ablation still persists in up to 26% of patients despite no evidence of accessory pathway conduction.12,75 In a study of Aytemir et al.,6 72 patients with WPW syndrome who had at least one documented AF episode and underwent catheter ablation were enrolled and Pd ≥ 32.5 ms on day 2 after ablation of the accessory pathway was found as an independent predictor of recurrence of paroxysmal AF after catheter ablation in patients with WPW syndrome.6
Amasyali et al.13 investigated the influence of successful slow pathway ablation on P-wave parameters and predictors for the recurrence of paroxysmal AF after catheter ablation for atrioventricular nodal reentrant tachycardia (AVNRT). They found no significant difference in Pd before and after ablation. They also found that maximum P-wave duration and Pd were significantly higher in AVNRT + AF group than only AVNRT group.13
Coronary artery disease
CAD is a significant risk factor for AF because of the anatomic and hemodynamic changes in the left atrium. Among these are left atrial dilatation, increased left atrial pressure and left atrial fibrosis caused by ischemia.14,15 There are several potential relationships between CAD and AF. Increased heterogeneity of refractoriness in CAD is a known substrate for AF. Atrial ischemia causes slow conduction in the ischemic zone and conduction properties become more heterogeneous. The difference in the conduction properties between the ischemic atrial myocardium and the adjacent non-affected tissue may lead to discontinuous propagation of sinus impulses.14,15 Dilaveris et al.16 reported the increase in P-wave duration and Pd in patients with anginal episodes. In a study that analyzed 66 subjects with normal coronary angiogram and 68 patients with significant (>50%) coronary stenosis; Pd was found to be greater in patients with stable CAD than in controls and to be associated with severity of the disease.15 Anatomic and hemodynamic changes in the left atrium as left atrial dilatation, increased left atrial pressure and left atrial fibrosis caused by both ischemia and left ventricular dysfunction may be the underlying causes of increased Pd in patients with CAD. In patients with severe chronic CAD, left atrial overload because of altered left ventricular relaxation caused by ischemic areas and regional fibrosis may be associated with increased Pd.17 Impaired cardiac autonomic function and increased sympathetic activity in patients with CAD may also play a role in the development of AF.18
Diurnal variation of Pd in chronic ischemic heart disease was studied in another study and Yildirim et al.19 demonstrated that patients with CAD had higher morning P-wave duration and Pd values that may be important regarding prediction of timing and treatment of atrial conduction disorders in myocardial ischemia. Increased P-wave duration and Pd values at early morning may be associated with the activation of autonomic nervous system.18,19
AF is one of the most common supraventricular arrhythmias in the setting of acute myocardial infarction (AMI). AF develops for many different reasons, such as left ventricular dysfunction with hemodynamic impairment, atrial ischemia or infarction, right ventricular infarction, excessive release of catecholamines in the course of AMI.20,21 It has been shown that early coronary reperfusion is associated with decreased incidence of AF after AMI.20 Akdemir et al.21 reported that primary percutaneous coronary transluminal angioplasty (PTCA) reduces the incidence of AF, maximum P-wave duration and Pd. They also revealed that primary PTCA has a more favorable effect on reducing P-wave duration and Pd at the end of the first 24 hours in patients with acute anterior MI compared to fibrinolytic therapy. Baykan et al.22 evaluated 147 patients with acute anterior wall myocardial infarction and reported that Pd and maximum P-wave duration are significantly higher in patients with AF by univariate analysis.
In a study reported by Turkmen et al.,23 slow coronary flow was found to be associated with prolonged P-wave duration and increased Pd. These findings may result from microvascular ischemia and/or altered autonomic features in this group of patients.23 Gunes et al.24 found increased P-wave duration in coronary slow flow patients and after nebivolol therapy, they reported significant reduction in P-wave duration and Pd.24
In one study, it was demonstrated that Pd was significantly increased in 47 patients developing AF after coronary artery bypass surgery (49 ± 12 ms) as compared to 60 patients with no postoperative AF (41 ± 12 ms).25 Pd was found to be a significant predictor of postoperative AF but only in the univariate analysis. Other studies have also postulated the predictive ability of Pd in patients undergoing coronary artery bypass surgery.26,27
Hypertension
High blood pressure and subsequent left ventricular hypertrophy and diastolic dysfunction may cause hemodynamic and morphological changes in the left atrium.28,29 It is known that left ventricular hypertrophy and diastolic dysfunction secondary to hypertension can cause an increase in atrial strain, fibrosis and dilation.28–30 Increase in intracavitary pressure and strain triggers disorganization of myocardial fibers and fibrosis. The increase in atrial strain, dilation and fibrosis result in instability and heterogeneity in atrial conduction and these changes can be seen as an increase in maximum P-wave duration and Pd on the electrocardiogram.28–30 Verdecchia et al.31 reported that the risk of AF increased with age and left ventricular mass in hypertensive subjects with sinus rhythm and without other risk factors.
Elevation of catecholamines and angiotensin II in essential hypertension can activate the sympathetic nervous system and affect the autonomic regulation of the heart.28–30 Angiotensin II and catecholamines are important factors for atrial fibrosis.1 Sympathetic nervous system activation and myocardial fibrosis increase atrial conduction time and Pd.30–32 Dagli et al.30 reported that P-wave duration, Pd and left atrial size increased in hypertensive patients than in healthy control subjects. Korkmaz et al.33 showed that nebivolol treatment in hypertensive patients decreased maximum P-wave duration and Pd. Ozben et al.76 found decrease in Pd with blockade of renin-angiotensin-aldosterone system (RAAS) with perindopril in hypertensive population. Korkmaz et al.33 also showed decrease in Pd with blockade of RAAS with quinapril in hypertensives.
Valvular heart disease
Rheumatic mitral stenosis (MS) is frequently seen in developing countries and AF is the most common sustained arrhythmia in patients with rheumatic MS.77 Left atrial dilatation, fibrosis within the wall of the atrium and disorganization of the atrial muscle bundles caused by mitral valve disease and atrial inflammation secondary to rheumatic carditis lead to electrical inhomogeneity and conduction abnormalities within the atrial myocardium.34,35 Guntekin et al.35 found that P-wave duration and Pd were significantly associated with mitral valve area and mean mitral gradient. Although correlation between Pd and left atrial size was not significant at baseline it became significant after increase in left atrial size during their follow-up.35 Turhan et al.34 found progressive shortening of Pd after percutaneous mitral balloon valvuloplasty (PMBV). They explained this finding by the decrease in sympathetic activity and the regression of the pathologic changes in the atrial wall which result in more homogeneous and organized conduction of sinus impulses.34 Erbay et al.36 found a significant correlation between maximum P-wave duration and Pd with mean diastolic gradient of the mitral valve. They also showed that long-term beta-blocker therapy caused a significant decrease in maximum P-wave duration and Pd in patients with rheumatic MS.36 In another study, Ozer et al.37 showed that interatrial electromechanical delay gets longer in MS patients and is correlated with Pd.
AF in a patient with severe aortic stenosis (AS) results in a loss of the atrial contribution to the left ventricular filling and causes a sudden fall in cardiac output and aggravation of symptoms.41 Turhan et al.38 found that patients with AS have longer maximum P-wave duration and higher Pd than healthy control subjects. They also reported that maximum P-wave duration and Pd were higher in patients with paroxysmal AF and AS than those without paroxysmal AF.38 Ozmen et al.39 found that Pd and maximum P-wave duration were increased in pulmonary stenosis. They reported a correlation between the systolic pulmonary valvular pressure gradient and Pd.
Heart failure
Considering left ventricular systolic dysfunction and HF as a risk factor for AF, these patients may be attractive to research for investigating the Pd. In a study consisting of 72 patients with non-ischemic dilated cardiomyopathy (NIDCM) and 72 healthy control subjects, Senen et al.40 found that Pd was significantly higher in patients with NIDCM than in healthy control subjects. They explained that the effects of left ventricular dysfunction on the left atrial pathology and neuroendocrine activity and their effects on intracardiac hemodynamics and atrial electrophysiology might be the underlying mechanisms for these changes in this group of patients.40 Camsari et al.41 reported a significant correlation between left ventricular ejection fraction and Pd. In this study, they also reported that metoprolol treatment was associated with a decreased duration of maximum P-wave duration and Pd in patients with HF.41 Sympathetic activity has been well known to be elevated in patients with HF. P-wave duration and Pd have been reported to be influenced by the autonomic tone as discussed earlier above. Therefore, increased Pd in patients with HF may be also related to increase in sympathetic activity.42 Gunes et al.42 found no significant diurnal variation of Pd in patients having either ischemic or non-ischemic origin of HF treated with optimal drug therapy.
Cardiac resynchronization therapy (CRT) has been proven to be an effective therapy in patients with advanced heart failure and ventricular dyssynchrony. It improves left ventricular function and decreases neurohormonal activation. CRT was also reported to improve atrial function and to reverse atrial remodeling.43,44 In a study of Ding et al.44 CRT decreased maximum P-wave duration and Pd along with an improvement of left ventricular ejection fraction and reduction in left atrial diameter.
Congenital heart diseases
The predictive ability of Pd in patients with congenital heart diseases, before and after surgery or percutaneous interventions was assessed in several studies.45–48 Wong et al.51 demonstrated that patients with atrial tachyarrhythmias late after Fontan operation have longer P-wave duration and Pd and larger right atrial dimension than those without the arrhythmias; these abnormalities are interrelated. Deveci et al.49 found that Pd was greater in patients with atrial septal aneurysms (ASA) with respect to control group. In another study, Okutucu et al.50 recently assessed the Pd and atrial electromechanical delay in patients with ASA. There were statistically significant good correlations of atrial electromechanical delay with Pd. They concluded that Pd and atrial electromechanical delay may provide significant contributions to assess the risk for paroxysmal supraventricular arrhythmia in patients with ASA.50
Rheumatologic diseases
Inflammatory state in the course of rheumatologic disorders may effect atria and therefore change the P-wave parameters. It has been reported that systemic inflammation plays a significant role in AF pathogenesis.1,78 Increased inflammatory activity is associated with poor cardiac prognosis in this group of diseases and ongoing inflammation may affect the myocardium. Elevated inflammatory markers activate the complement system in atrial myocardium. This inflammation leads to tissue injury in the atrial myocardium and myocyte necrosis and fibrosis result in atrial remodelling. This process may alter the membrane potentials of atrial myocytes and might lead to electrical inhomogeneity, conduction abnormalities and inhomogeneous refractory periods throughout the atrial myocardium. These changes might be seen on surface ECG as prolonged P-wave duration and increased Pd.52,53 It was found that Pd gets longer in various rheumatologic disorders such as rheumatoid arthritis,52 Behcet’s disease,54 systemic lupus erythematosus,55 ankylosing spondylitis,56 familial Mediterranean fever57 and systemic sclerosis.58–60
Miscellaneous conditions
It has been shown that obese subjects may be associated with ECG abnormalities even in the absence of clinical symptoms. Wang et al.61 showed that obesity may be an important modifiable risk factor for AF. Kosar et al.62 reported significantly higher maximum P-wave duration and Pd values in obese patients. Seyfeli et al.63 found a significant correlation between body mass index and Pd in women. Metabolic syndrome has been reported to be associated with an increased risk of AF. Yasar et al.64 have shown that patients with metabolic syndrome have higher Pd, indicating increased risk for AF, compared to the control subjects without metabolic syndrome.
Can et al.65 found Pd to be greater in patients with obstructive sleep apnea (OSA) and to be associated with severity of the disease. Factors related to OSA, such as repetitive hypoxemia, autonomic nervous system imbalance, systemic inflammation, fluctuations in intrathoracic hemodynamics and diastolic dysfunction may lead to prolongation of intra-atrial and inter-atrial conduction time and provoke inhomogeneous propagation of sinus impulses.65
Significant increases in maximum P-wave duration and Pd have been reported at the end of hemodialysis, which might be responsible for the increased occurrence of AF in these groups of patients.66,67
An insight on the modulatory effects of the autonomic tone changes on Pd were previously given by Tukek and colleagues.79 They showed that the Valsalva maneuver normalized the increased values of P-wave duration and Pd found in patients with history of paroxysmal AF and on the other hand increased P-wave duration and Pd in normal subjects.79 These findings may imply that the Valsalva maneuver decreases the heterogeneity of atrial impulse propagation due to changes in the atrial size and electrophysiology.79
The role of Pd for the prediction of AF episodes was also evaluated in patients with chronic obstructive pulmonary disease,68 in patients with hypertrophic cardiomyopathy,69 in patients with hyperthyroidism,70 in patients under chemotherapy regimen,71 acute caffeine ingestion72 or alcohol intake.73
As a conclusion, Pd has been applied in a wide range of clinical conditions. P-wave duration and Pd reflect prolongation of intraatrial and interatrial conduction time and the inhomogeneous propagation of sinus impulses which are well-known electrophysiologic characteristics in patients with atrial arrhythmias and especially paroxysmal AF. Pd has been used in the assessment of the risk for AF in patients without apparent heart disease, coronary artery disease, hypertension, valvular heart diseases, heart failure, congenital heart diseases, rheumatologic diseases and various clinical conditions. Pd has proven to be a sensitive and specific ECG predictor of AF in the various clinical settings. Prediction of the AF recurrence might guide the physician to determine the antiarrhythmic therapeutic strategies.
The current data on Pd has been limited by studies with modest sample size, limited follow-up and presence of many confounders. Significant challenges remain with regard to our implementation of Pd as a relevant component of screening. Currently there was no prospective, community-based study which developed reference values by identifying a reference population. Reference values from large, community-based studies will help us for standardization of Pd for sex and height.
Measurement techniques of Pd have not been standardized. Investigators continue to use magnification and hand-held calipers and quality-control assessments have been limited. The development of automatic methods able to accurately determine the P-wave duration from standard 12-lead ECGs may contribute to the widespread use of P-wave duration and Pd.
Large-size, population-based cohort studies are necessary to achieve adequate power and adjustment for covariates. Such an effort will determine the clinical utility of Pd to predict future AF, HF and overall mortality, and whether they provide additional data beyond already established clinical, echocardiographic and ECG covariates.
Pd has potential to direct the treatment of AF. Pd may predict success catheter ablation of AF, pharmacological intervention and risk-factor management. Inexpensive and non-invasive, Pd may provide a cost-effective screening mechanism. However, further research is necessary to establish whether Pd will give additional information to predict the development of AF, adverse cardiovascular outcomes and mortality.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
None
Guarantor
SO is the guarantor for all the content presented in this paper.
Contributorship
All authors were involved involved in researching and writing the paper. SO drafted the manuscript, and all authors approved the final version.
References
- 1.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010; 12: 1360–1420. [DOI] [PubMed] [Google Scholar]
- 2.Dilaveris PE, Gialafos JE. P-wave dispersion: a novel predictor of paroxysmal atrial fibrillation. Ann Noninvasive Electrocardiol 2001; 6: 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dilaveris PE, Gialafos EJ, Sideris SK, et al. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J 1998; 135: 733–738. [DOI] [PubMed] [Google Scholar]
- 4.Aytemir K, Ozer N, Atalar E, et al. P wave dispersion on 12-lead electrocardiography in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2000; 23: 1109–1112. [DOI] [PubMed] [Google Scholar]
- 5.Magnani JW, Mazzini MJ, Sullivan LM, et al. P-wave indices, distribution and quality control assessment (from the Framingham Heart Study). Ann Noninvasive Electrocardiol 2010; 15: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aytemir K, Amasyali B, Kose S, et al. Maximum P-wave duration and P-wave dispersion predict recurrence of paroxysmal atrial fibrillation in patients with Wolff-Parkinson-White syndrome after successful radiofrequency catheter ablation. J Interv Card Electrophysiol 2004; 11: 21–27. [DOI] [PubMed] [Google Scholar]
- 7.Magnani JW, Williamson MA, Ellinor PT, et al. P wave indices: current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol 2009; 2: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dilaveris PE, Gialafos EJ, Andrikopoulos GK, et al. Clinical and electrocardiographic predictors of recurrent atrial fibrillation. Pacing Clin Electrophysiol 2000; 23: 352–358. [DOI] [PubMed] [Google Scholar]
- 9.Boriani G, Diemberger I, Biffi M, et al. P wave dispersion and short-term vs. late atrial fibrillation recurrences after cardioversion. Int J Cardiol 2005; 101: 355–361. [DOI] [PubMed] [Google Scholar]
- 10.Perzanowski C, Ho AT, Jacobson AK. Increased P-wave dispersion predicts recurrent atrial fibrillation after cardioversion. J Electrocardiol 2005; 38: 43–46. [DOI] [PubMed] [Google Scholar]
- 11.Ozdemir O, Soylu M, Demir AD, et al. Does p-wave dispersion predict the atrial fibrillation occurrence after direct-current shock therapy? Angiology 2006; 57: 93–98. [DOI] [PubMed] [Google Scholar]
- 12.Hamada T, Hiraki T, Ikeda H, et al. Mechanisms for atrial fibrillation in patients with Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol 2002; 13: 223–229. [DOI] [PubMed] [Google Scholar]
- 13.Amasyali B, Kose S, Aytemir K, et al. P wave dispersion predicts recurrence of paroxysmal atrial fibrillation in patients with atrioventricular nodal reentrant tachycardia treated with radiofrequency catheter ablation. Ann Noninvasive Electrocardiol 2006; 11: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lammers WJ, Kirchhof C, Bonke FI, et al. Vulnerability of rabbit atrium to reentry by hypoxia. Role of inhomogeneity in conduction and wavelength. Am J Physiol 1992; 262: H47–H55. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz R, Demirbag R. P-wave dispersion in patients with stable coronary artery disease and its relationship with severity of the disease. J Electrocardiol 2005; 38: 279–284. [DOI] [PubMed] [Google Scholar]
- 16.Dilaveris PE, Andrikopoulos GK, Metaxas G, et al. Effects of ischemia on P wave dispersion and maximum P wave duration during spontaneous anginal episodes. Pacing Clin Electrophysiol 1999; 22: 1640–1647. [DOI] [PubMed] [Google Scholar]
- 17.Elsasser A, Schlepper M, Klovekorn WP, et al. Hibernating myocardium: an incomplete adaptation to ischemia. Circulation 1997; 96: 2920–2931. [DOI] [PubMed] [Google Scholar]
- 18.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J 1994; 15 Suppl A: 9–16. [DOI] [PubMed] [Google Scholar]
- 19.Yildirim N, Topaloglu S, Bozboga S, et al. Diurnal variation of the P-wave dispersion in chronic ischemic heart diseases. Coron Artery Dis 2006; 17: 707–710. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen FE, Sorensen HT, Christensen JH, et al. Reduced occurrence of atrial fibrillation in acute myocardial infarction treated with streptokinase. Eur Heart J 1991; 12: 1081–1083. [DOI] [PubMed] [Google Scholar]
- 21.Akdemir R, Ozhan H, Gunduz H, et al. Effect of reperfusion on P-wave duration and P-wave dispersion in acute myocardial infarction: primary angioplasty versus thrombolytic therapy. Ann Noninvasive Electrocardiol 2005; 10: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baykan M, Celik S, Erdol C, et al. Effects of P-wave dispersion on atrial fibrillation in patients with acute anterior wall myocardial infarction. Ann Noninvasive Electrocardiol 2003; 8: 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turkmen M, Barutcu I, Esen AM, et al. Effect of slow coronary flow on P-wave duration and dispersion. Angiology 2007; 58: 408–412. [DOI] [PubMed] [Google Scholar]
- 24.Gunes Y, Tuncer M, Guntekin U, et al. The effects of nebivolol on P wave duration and dispersion in patients with coronary slow flow. Anadolu Kardiyol Derg 2009; 9: 290–295. [PubMed] [Google Scholar]
- 25.Weber UK, Osswald S, Huber M, et al. Selective versus non-selective antiarrhythmic approach for prevention of atrial fibrillation after coronary surgery: is there a need for pre-operative risk stratification? A prospective placebo-controlled study using low-dose sotalol. Eur Heart J 1998; 19: 794–800. [DOI] [PubMed] [Google Scholar]
- 26.Chandy J, Nakai T, Lee RJ, et al. Increases in P-wave dispersion predict postoperative atrial fibrillation after coronary artery bypass graft surgery. Anesth Analg 2004; 98: 303–310, table of contents. [DOI] [PubMed] [Google Scholar]
- 27.Tsikouris JP, Kluger J, Song J, et al. Changes in P-wave dispersion and P-wave duration after open heart surgery are associated with the peak incidence of atrial fibrillation. Heart Lung 2001; 30: 466–471. [DOI] [PubMed] [Google Scholar]
- 28.Cha YM, Redfield MM, Shen WK, et al. Atrial fibrillation and ventricular dysfunction: a vicious electromechanical cycle. Circulation 2004; 109: 2839–2843. [DOI] [PubMed] [Google Scholar]
- 29.Okutucu S, Karakulak UN, Kabakci G. Circadian blood pressure pattern and cardiac autonomic functions: different aspects of same pathophysiology. Anadolu Kardiyol Derg 2011; 11: 168–173. [DOI] [PubMed] [Google Scholar]
- 30.Dagli N, Karaca I, Yavuzkir M, et al. Are maximum P wave duration and P wave dispersion a marker of target organ damage in the hypertensive population? Clin Res Cardiol 2008; 97: 98–104. [DOI] [PubMed] [Google Scholar]
- 31.Verdecchia P, Reboldi G, Gattobigio R, et al. Atrial fibrillation in hypertension: predictors and outcome. Hypertension 2003; 41: 218–223. [DOI] [PubMed] [Google Scholar]
- 32.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 2000; 102: 1388–1393. [DOI] [PubMed] [Google Scholar]
- 33.Korkmaz H, Onalan O, Akbulut M, et al. Nebivolol and quinapril reduce p-wave duration and dispersion in hypertensive patients. Indian Pacing Electrophysiol J 2009; 9: 158–166. [PMC free article] [PubMed] [Google Scholar]
- 34.Turhan H, Yetkin E, Senen K, et al. Effects of percutaneous mitral balloon valvuloplasty on P-wave dispersion in patients with mitral stenosis. Am J Cardiol 2002; 89: 607–609. [DOI] [PubMed] [Google Scholar]
- 35.Guntekin U, Gunes Y, Tuncer M, et al. Long-term follow-up of P-wave duration and dispersion in patients with mitral stenosis. Pacing Clin Electrophysiol 2008; 31: 1620–1624. [DOI] [PubMed] [Google Scholar]
- 36.Erbay AR, Turhan H, Yasar AS, et al. Effects of long-term beta-blocker therapy on P-wave duration and dispersion in patients with rheumatic mitral stenosis. Int J Cardiol 2005; 102: 33–37. [DOI] [PubMed] [Google Scholar]
- 37.Ozer N, Yavuz B, Can I, et al. Doppler tissue evaluation of intra-atrial and interatrial electromechanical delay and comparison with P-wave dispersion in patients with mitral stenosis. J Am Soc Echocardiogr 2005; 18: 945–948. [DOI] [PubMed] [Google Scholar]
- 38.Turhan H, Yetkin E, Atak R, et al. Increased p-wave duration and p-wave dispersion in patients with aortic stenosis. Ann Noninvasive Electrocardiol 2003; 8: 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozmen N, Cebeci BS, Kardesoglu E, et al. P wave dispersion is increased in pulmonary stenosis. Indian Pacing Electrophysiol J 2006; 6: 25–30. [PMC free article] [PubMed] [Google Scholar]
- 40.Senen K, Turhan H, Riza Erbay A, et al. P-wave duration and P-wave dispersion in patients with dilated cardiomyopathy. Eur J Heart Fail 2004; 6: 567–569. [DOI] [PubMed] [Google Scholar]
- 41.Camsari A, Pekdemir H, Akkus MN, et al. Long-term effects of beta blocker therapy on P-wave duration and dispersion in congestive heart failure patients: a new effect? J Electrocardiol 2003; 36: 111–116. [DOI] [PubMed] [Google Scholar]
- 42.Gunes Y, Tuncer M, Guntekin U, et al. Lack of diurnal variation of P-wave and QT dispersions in patients with heart failure. Pacing Clin Electrophysiol 2008; 31: 974–978. [DOI] [PubMed] [Google Scholar]
- 43.Yu CM, Fang F, Zhang Q, et al. Improvement of atrial function and atrial reverse remodeling after cardiac resynchronization therapy for heart failure. J Am Coll Cardiol 2007; 50: 778–785. [DOI] [PubMed] [Google Scholar]
- 44.Ding L, Hua W, Zhang S, et al. Improvement of P wave dispersion after cardiac resynchronization therapy for heart failure. J Electrocardiol 2009; 42: 334–338. [DOI] [PubMed] [Google Scholar]
- 45.Ho TF, Chia EL, Yip WC, et al. Analysis of P wave and P dispersion in children with secundum atrial septal defect. Ann Noninvasive Electrocardiol 2001; 6: 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guray U, Guray Y, Mecit B, et al. Maximum p wave duration and p wave dispersion in adult patients with secundum atrial septal defect: the impact of surgical repair. Ann Noninvasive Electrocardiol 2004; 9: 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janion M, Kurzawski J, Sielski J, et al. Dispersion of P wave duration and P wave vector in patients with atrial septal aneurysm. Europace 2007; 9: 471–474. [DOI] [PubMed] [Google Scholar]
- 48.Baspinar O, Sucu M, Koruk S, et al. P-wave dispersion between transcatheter and surgical closure of secundum-type atrial septal defect in childhood. Cardiol Young 2011; 21: 15–18. [DOI] [PubMed] [Google Scholar]
- 49.Deveci OS, Aytemir K, Okutucu S, et al. Evaluation of the relationship between atrial septal aneurysm and cardiac arrhythmias via P-wave dispersion and signal-averaged P-wave duration. Ann Noninvasive Electrocardiol 2010; 15: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okutucu S, Evranos B, Aytemir K, et al. Relationship between atrial septal aneurysms and atrial electromechanical delay. Int J Cardiovasc Imaging 2010; 27: 505–513. [DOI] [PubMed] [Google Scholar]
- 51.Wong T, Davlouros PA, Li W, et al. Mechano-electrical interaction late after Fontan operation: relation between P-wave duration and dispersion, right atrial size, and atrial arrhythmias. Circulation 2004; 109: 2319–2325. [DOI] [PubMed] [Google Scholar]
- 52.Yavuzkir M, Ozturk A, Dagli N, et al. Effect of ongoing inflammation in rheumatoid arthritis on P-wave dispersion. J Int Med Res 2007; 35: 796–802. [DOI] [PubMed] [Google Scholar]
- 53.Guler H, Seyfeli E, Sahin G, et al. P wave dispersion in patients with rheumatoid arthritis: its relation with clinical and echocardiographic parameters. Rheumatol Int 2007; 27: 813–818. [DOI] [PubMed] [Google Scholar]
- 54.Dogan SM, Aydin M, Gursurer M, et al. The increase in P-wave dispersion is associated with the duration of disease in patients with Behcet’s disease. Int J Cardiol 2008; 124: 407–410. [DOI] [PubMed] [Google Scholar]
- 55.Dogdu O, Yarlioglues M, Kaya MG, et al. Assessment of atrial conduction time in patients with systemic lupus erythematosus. J Investig Med 2011; 59: 281–286. [DOI] [PubMed] [Google Scholar]
- 56.Acar G, Sayarlioglu M, Akcay A, et al. Assessment of atrial electromechanical coupling characteristics in patients with ankylosing spondylitis. Echocardiography 2009; 26: 549–557. [DOI] [PubMed] [Google Scholar]
- 57.Acar G, Akcay A, Sayarlioglu M, et al. Assessment of atrial conduction time in patients with familial Mediterranean fever. Pacing Clin Electrophysiol 2009; 32: 308–313. [DOI] [PubMed] [Google Scholar]
- 58.Clements PJ, Furst DE, Cabeen W, et al. The relationship arrhythmias and conduction disturbances to other manifestations of cardiopulmonary disease in progressive systemic sclerosis (PSS). Am J Med 1981; 71: 38–46. [DOI] [PubMed] [Google Scholar]
- 59.Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61: 361–366. [DOI] [PubMed] [Google Scholar]
- 60.Can I, Onat AM, Aytemir K, et al. Assessment of atrial conduction in patients with scleroderma by tissue Doppler echocardiography and P wave dispersion. Cardiology 2007; 108: 317–321. [DOI] [PubMed] [Google Scholar]
- 61.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004; 292: 2471–2477. [DOI] [PubMed] [Google Scholar]
- 62.Kosar F, Aksoy Y, Ari F, et al. P-wave duration and dispersion in obese subjects. Ann Noninvasive Electrocardiol 2008; 13: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seyfeli E, Duru M, Kuvandik G, et al. Effect of obesity on P-wave dispersion and QT dispersion in women. Int J Obes (Lond) 2006; 30: 957–961. [DOI] [PubMed] [Google Scholar]
- 64.Yasar AS, Bilen E, Bilge M, et al. P-wave duration and dispersion in patients with metabolic syndrome. Pacing Clin Electrophysiol 2009; 32: 1168–1172. [DOI] [PubMed] [Google Scholar]
- 65.Can I, Aytemir K, Demir AU, et al. P-wave duration and dispersion in patients with obstructive sleep apnea. Int J Cardiol 2009; 133: e85–e89. [DOI] [PubMed] [Google Scholar]
- 66.Tezcan UK, Amasyali B, Can I, et al. Increased P wave dispersion and maximum P wave duration after hemodialysis. Ann Noninvasive Electrocardiol 2004; 9: 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ozben B, Toprak A, Koc M, et al. P wave dispersion increases during hemodialysis sessions. Nephron Clin Pract 2009; 112: c171–c176. [DOI] [PubMed] [Google Scholar]
- 68.Tukek T, Yildiz P, Akkaya V, et al. Factors associated with the development of atrial fibrillation in COPD patients: the role of P-wave dispersion. Ann Noninvasive Electrocardiol 2002; 7: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kose S, Aytemir K, Sade E, et al. Detection of patients with hypertrophic cardiomyopathy at risk for paroxysmal atrial fibrillation during sinus rhythm by P-wave dispersion. Clin Cardiol 2003; 26: 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aras D, Maden O, Ozdemir O, et al. Simple electrocardiographic markers for the prediction of paroxysmal atrial fibrillation in hyperthyroidism. Int J Cardiol 2005; 99: 59–64. [DOI] [PubMed] [Google Scholar]
- 71.Ceyhan C, Meydan N, Barutca S, et al. Influence of high-dose leucovorin and 5-fluorouracil chemotherapy regimen on P wave duration and dispersion. J Clin Pharm Ther 2004; 29: 267–271. [DOI] [PubMed] [Google Scholar]
- 72.Caron MF, Song J, Ammar R, et al. An evaluation of the change in electrocardiographic P-wave variables after acute caffeine ingestion in normal volunteers. J Clin Pharm Ther 2001; 26: 145–148. [DOI] [PubMed] [Google Scholar]
- 73.Yilmaz R. Effects of alcohol intake on atrial arrhythmias and P-wave dispersion. Anadolu Kardiyol Derg 2005; 5: 294–296. [PubMed] [Google Scholar]
- 74.Papageorgiou P, Monahan K, Boyle NG, et al. Site-dependent intra-atrial conduction delay. Relationship to initiation of atrial fibrillation. Circulation 1996; 94: 384–389. [DOI] [PubMed] [Google Scholar]
- 75.Haissaguerre M, Fischer B, Labbe T, et al. Frequency of recurrent atrial fibrillation after catheter ablation of overt accessory pathways. Am J Cardiol 1992; 69: 493–497. [DOI] [PubMed] [Google Scholar]
- 76.Ozben B, Sumerkan M, Tanrikulu AM, et al. Perindopril decreases P wave dispersion in patients with stage 1 hypertension. J Renin Angiotensin Aldosterone Syst 2009; 10: 85–90. [DOI] [PubMed] [Google Scholar]
- 77.Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48: e1–e148. [DOI] [PubMed] [Google Scholar]
- 78.Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J 2006; 27: 136–149. [DOI] [PubMed] [Google Scholar]
- 79.Tukek T, Akkaya V, Demirel S, et al. Effect of Valsalva maneuver on surface electrocardiographic P-wave dispersion in paroxysmal atrial fibrillation. Am J Cardiol 2000; 85: 896–899, A10. [DOI] [PubMed] [Google Scholar]