Abstract
Hormone naïve advanced prostate cancer is subdivided into two disease states: biochemical recurrence and traditional M1 (metastatic) prostate cancer and characterized by no prior hormonal therapy or androgen deprivation therapy (ADT). In biochemical recurrence/prostate-specific antigen (PSA) recurrence, men should be risk-stratified based on their PSA doubling time, the Gleason score and the timing of the recurrence. In general, only men who are at high risk should be considered for early/immediate ADT although this is best done using shared decision with the patient. The type of ADT to be used in biochemical recurrence ranging from oral-only peripheral blockade (peripheral androgen deprivation) to complete hormonal therapy (combined androgen blockade [CAB]) remains in debate owing to lack of randomized controlled trials (RCT). However, there is good RCT support for use of intermittent hormonal therapy (IHT). There is also limited research on biomarker response (PSA and testosterone decline) to predict prognosis. On the other hand, in the setting of M1 hormone naïve prostate cancer, there are many more RCT's to inform our decisions. CAB and gonadotrophin-releasing hormone antagonists perhaps provide a slight efficacy advantage while IHT may be slightly inferior with minimal M1 disease. The PSA nadir at 7 months after starting ADT is a powerful prognostic tool for M1 patients. There is growing recognition that serum testosterone (T) control while on ADT is linked to the development of castrate-resistant prostate cancer. Especially for a M1 patient, maintaining a serum T below 20–30 ng dl−1 prolongs the response to ADT. Novel oral agents (abiraterone and enzalutamide) may soon find use in hormone naïve disease and may alter the treatment landscape. Despite over 75 years of experience with ADT, many questions remain, and the field continues to evolve.
Keywords: androgen deprivation therapy, cancer, gonadotrophin-releasing hormone antagonists, hormonal therapy, luteinizing hormone-releasing hormone agonists, prostate, prostate-specific antigen, testosterone
INTRODUCTION
The use of hormone therapy or androgen deprivation therapy (ADT) in advanced and recurrent prostate cancer is something that all clinicians who manage patients with this disease essentially take for granted.1 Hormonal therapy is so widely embedded in our treatment strategy for the disease that we do not necessarily think about it and use it all the time in our practices.1,2 However, the use of ADT in prostate cancer is a relatively recent phenomenon beginning in 1941 when Huggins and Hodges showed that reduction in testosterone levels by surgical castration or estrogen treatment improved levels of phosphates and relieved symptoms of advanced metastatic prostate cancer.3 Bilateral orchiectomy and estrogen therapy were the standards in therapies from the 1940s to the 1980s.1,2 In the late 70s early 80s estrogen treatment with diethylstilbestrol (DES) was shown to cause significant cardiovascular toxicity.4,5 At about the same time luteinizing hormone-releasing hormone (LH-RH) agonists were launched in the United States and worldwide and soon became widely used in the management of the disease.6 In 2003 the first gonadotrophin-releasing hormone (Gn-RH) antagonist was approved by the Food and Drug Administration (FDA) and a second Gn-RH antagonist was approved in 2008.7,8 For a period of approximately 30 years from the 1980s to the 2000s hormone therapy was used widely and relatively indiscriminately to treat men with advanced prostate cancer as well as biochemical recurrence.1,2,9 However, over the last 3 to 4 years, the side effects and complications of hormonal therapy have come into focus and clinicians in 2015 are taking a more individualized approach to the use of ADT trying to balance the benefit and risk of therapy for the individual patient.10,11,12,13,14,15,16
While virtually every patient with biochemical recurrence and the vast majority of patients with traditionally advanced prostate cancer respond initially to ADT, it is much more difficult to predict the response to therapy and the duration of therapy for the individual patient.17,18,19 A typical patient presenting with hormone naïve prostate cancer will want to know the optimum way to manage his advanced prostate cancer, the types of hormonal therapy available, and, perhaps most difficult to address, an idea of his prognosis and response to therapy.
The typical patient with hormone naïve prostate cancer in 2015 is generally younger, healthier, and better informed.1,2 Particularly as a result of the internet, many patients are armed with much more medical information and have many questions about various treatment strategies. Furthermore, we have seen a marked stage migration during the prostate-specific antigen (PSA) era resulting in less traditional M1 disease at initial presentation.20 However, it is also a broader definition of advanced prostate cancer, and ADT is commonly started for biochemical recurrence.1,9,18 In fact, the most common indication for ADT is biochemical recurrence and this may be one of the most common areas of confusion as physicians try to risk stratify individual patients for proper timing of the use of ADT.9 Certainly, in the setting of biochemical recurrence, there is potential for much longer term use of ADT as survival times will be extended and there is also the controversies surrounding continuous versus intermediate ADT.21,22,23 As noted earlier, there is less blanket acceptance of ADT due to the known recognized side effects profile, particularly the risk of diabetes mellitus and perhaps an increased risk of cardiovascular disease.10,11,12,13,14,15,16 In addition to orchiectomy, LH-RH agonist, and Gn-RH antagonist, urologists and clinicians now have additional oral forms of ADT available over the last several years to manage castrate-resistant prostate cancer (CRPC). These novel oral hormonal therapies, including enzalutamide and abiraterone, while currently approved for CRPC may in the future be used for hormone naïve disease as well.24 Urologists have a growing list of ADT management options and more complex decisions to address with patients. There is also an additional educational burden for our patients.
In any discussion of predicting and maximizing response intervals to ADT, it is important to characterize this discussion based on the type of disease presentation. I will, therefore, discuss biochemical recurrence in a separate discussion from traditional M1 metastatic prostate cancer.
HORMONE NAÏVE DISEASE – BIOCHEMICAL RECURRENCE PATIENTS
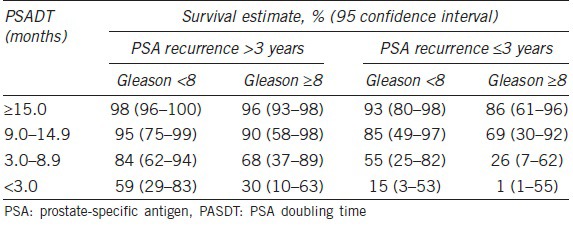
The first major review article on biochemical recurrence or PSA recurrence of prostate cancer was published in 2000.25 Since that time the field has undergone an evolution in the management of patients with biochemical recurrence. Early on, there was generally indiscriminate use of ADT as clinicians did not have the tools available to risk stratify patients with PSA recurrence. In 1999, when Pound et al. from John Hopkins University published the first major paper on risk stratification of biochemical recurrence, the field has continued to evolve in the concept of a risk-stratified approach to PSA recurrence.26 Pound et al. combined Gleason grade, PSA doubling time (PSA-DT), and timing of PSA recurrence after radical prostatectomy to provide estimates on prognosis after biochemical recurrence. This paper along with other seminal studies allowed clinicians to have a better understanding of risk stratification and allowed us to move away from indiscriminate use of hormonal therapy toward a more focused approach for the individual patient.9
There are at least three key questions related to hormone naïve biochemical recurrence patients. The first critical question: at what point in a rising PSA should ADT be introduced? The second question: does the type of ADT matter for maximal response? And the third question: is achieving a very low testosterone level really important in the setting of PSA recurrence?
Regarding the timing of initiation of ADT in biochemical recurrence, we have learned over the last 15 years that the absolute PSA level is less important than PSA kinetics, grade of the disease, and timing of the PSA recurrence.9,17,18 As previously noted, Pound et al. showed that a short PSA-DT in the range of the 3 to 12 months as well as high-grade disease in the primary prostatectomy specimen and a rapid PSA recurrence in the first 1 to 2 years were the key drivers to more serious biochemical recurrence.26 In 2004, Moul et al. used these concepts to study a large cohort of biochemical recurrence patients from the US Military health care system.18 In over 1300 patients studied, PSA-DT < 12 months or high-grade disease (Gleason score 8 or higher) represented a high-risk group of patients with biochemical recurrence. It was only in this high-risk group that the early use of ADT provided a disease-free survival benefit. Specifically, those patients with high risk biochemical recurrence enjoyed a delayed time until clinical metastasis if the ADT was initiated when the PSA was < 10 and before the patient had detectable metastatic disease18 (Figure 1). Conversely, for all the other lower -risk patients with biochemical recurrence, Moul et al. could not demonstrate a clinical metastasis-free survival benefit of the use ADT.18 In 2005, Freedland et al. built further on the John Hopkins database further fine tuning risk stratification in biochemical recurrence.27 Specifically, four levels of PSA-DT were examined: <3 months, 3 to 9 months, 9 to 15 months, and > 15 months as well as Gleason score and timing of PSA recurrence (Table 1). The risk groupings stratified patients by 10-year survival. They found almost universal 10-year survival for patients with a PSA-DT > 15 months, Gleason score < 8 and a PSA recurrence that occurred > 3 years from surgery. Conversely, patients who had a PSA-DT below 3 months as well as Gleason 8 or higher disease and PSA recurrence that occurred during the first 3 years after surgery had a very poor 1% 10-year survival. Freedland et al. published this data in an easy-to-use table nomogram which further helped clinicians consider the timing and usage of ADT. In the setting of biochemical recurrence, early hormonal therapy prior to the development documented metastasis may be beneficial for certain patients but not necessary for other patients. In 2015, clinicians are encouraged to take an individualized approach to the management of biochemical recurrence. Specifically, we should generally not use hormonal therapy for the lower- risk biochemical recurrence patients and should attempt to manage these patients with active surveillance until they develop a higher -risk biochemical recurrence condition.
Figure 1.
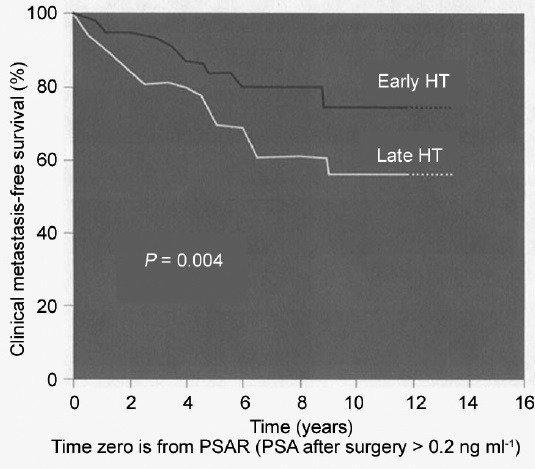
Early hormonal therapy administered at prostate-specific antigen <10 ng ml−1 affects clinical metastasis-free survival. Patients with pathological Gleason Sum > 7 or PSA-DT < 12 months. Used with permission from Moul et al18.
Table 1.
Assessing 10-year survival: consider PSADT, biochemical recurrence and Gleason score. Used with permission from Freedland et al.27
With regard to the type of ADT used for an individual patient who is deemed to be a candidate for ADT, the choices include LH-RH agonist, Gn-RH antagonist, complete hormonal therapy, intermittent hormonal therapy (IHT), or peripheral androgen blockage.8,22,28,29 While all of these possible treatments are used in individual patients there is limited level 1 evidence to support any ADT in the specific setting of biochemical recurrence.9,25 With regard to LH-RH agonists, FDA and regulatory approval in the 1980s universally involved patients with traditional metastatic prostate cancer. While LH-RH agonists are very commonly used to treat biochemical recurrence prostate cancer, it is important to point out that this is based primarily on tradition but not specific randomized controlled trials (RCTs). With regard to Gn-RH antagonists, the only current FDA approved agent is degarelix, and there are also limited data in PSA recurrence. In the pivotal phase 3 trial by Klotz et al. that compared monthly leuprolide acetate to monthly degarelix, approximately 70% of patients in this trial were nonmetastatic patients.8 There is no question that degarelix provides a more rapid decline in serum testosterone levels in the first month and a superior decline in PSA levels in the first 2 months over leuprolide acetate, however, there is still limited evidence to suggest that Gn-RH antagonists would have a specific benefit in the setting of biochemical recurrence. In the long term follow-up of the Klotz et al. randomize trial, there did appear to be a slight efficacy benefit of degarelix over leuprolide, but this appeared limited to the patients with advanced and metastatic prostate cancer.30 At the last published follow-up of this study, there as yet to be any compelling evidence that antagonists provide any long-term benefit over agonists in the specific setting of biochemical recurrence.31
With regard to combined androgen blockade (CAB), also called maximal androgen blockade, there is also no randomized controlled level 1 evidence to support CAB over hormonal monotherapy in the setting of biochemical recurrence.32,33,34,35,36,37,38,39 The benefit of CAB over monotherapy in traditional metastatic prostate cancer remains hotly debated and any evidence for CAB in the setting of biochemical recurrence is even more controversial and speculative.29 To my knowledge, there are no published data to support CAB in the specific setting of treatment of biochemical recurrence.29,39
With regard to IHT, there have been multiple phase 3 RCTs comparing this therapy to continuous hormonal therapy.21,22,23 While there seems to be some slight evidence for benefit to continuous hormonal therapy over IHT in the setting of traditional metastatic prostate cancer, all the global trials, to my knowledge, seem to support the efficacy of IHT in the setting of biochemical recurrence.40,41 In other words, when a clinician is contemplating starting ADT in the setting of biochemical recurrence IHT would appear to be equally effective as continuous hormonal therapy. Factoring in side effects and morbidity of hormonal therapy, it would appear that IHT would clearly provide an advantage over continuous hormonal therapy when treating patients with biochemical recurrence.
Peripheral androgen blockade (peripheral androgen deprivation [PAD]) is the concept of using oral only ADT to treat patients with advanced prostate cancer.28,42 The largest body of work in this area primarily came from Iversen et al. from Scandinavia with the use of bicalutamide at a dose of 150 mg per day orally.43,44,45 Large randomized trials were conducted that showed that peripheral androgen blockade with bicalutamide was statistically inferior to traditional ADT in the setting of traditional metastatic prostate cancer.43
However, it must be pointed out that the survival benefit of traditional therapy over peripheral blockade was modest with the survival benefit < 2 months.43 Aside from the data on bicalutamide, there were multiple smaller trials using various doses of flutamide with or without finasteride to treat men with biochemically recurrence prostate cancer.28,42 All of these trials showed good safety and tolerability of oral hormonal therapy, however, none of these trials were adequately powered RCTs comparing peripheral blockade to traditional hormonal therapy. As a result, peripheral androgen blockade has never completely gained widespread use in the setting of biochemical recurrence. Nevertheless, peripheral blockade may allow preservation of potency in selected men who remain potent after treatment of their localized prostate cancer.28,42 One of the fundamental concerns regarding peripheral blockade is the response and duration of effectiveness to more traditional ADT when PAD begins to fail. Specifically, if a patient is on peripheral blockade for a period of years then progresses to an LH-RH agonist, for example, what would be the duration of efficacy for the subsequent LH-RH agonist? In other words, will the patient progress to the castrate-resistant disease more quickly after a period of peripheral blockade? It is unclear if the ultimate survival will be any greater with the use peripheral blockade as opposed to managing the patient with surveillance and then prescribing an LH-RH agent later in the course of the disease.
Up until the last several years, the level of testosterone to predict response to hormonal therapy was unused and unappreciated.46,47 Over the last several years, at least in the setting of traditional metastatic prostate cancer, the level of testosterone suppression appears to be important to predict response to ADT. However, in the specific setting of biochemical recurrence, is achieving a very low testosterone level really important? In the specific setting of biochemical recurrence, there is no level 1 evidence to suggest that maximal testosterone suppression will result in better and overall disease-specific survival. We will discuss data in this regard related to traditional advanced prostate cancer below, but in the specific setting of biochemical recurrence the role of measuring testosterone suppression after initiation of ADT for men with biochemical recurrence is unclear.
HORMONE NAÏVE PROSTATE CANCER – TRADITIONAL M1 (METASTATIC) PROSTATE CANCER
In the setting of traditional metastatic prostate cancer, a number of key questions arise with regard to the use of hormone therapy in hormone naïve disease. The first key question: is the PSA response to initial ADT important to predict response and survival? The second question: does the type of ADT matter for maximal response? And the third question: is achieving a very low testosterone level really important in the setting of M1 metastatic prostate cancer?
As early as 1990, it was shown in a small study that PSA declined after the initiation of hormone therapy predicted survival in patients with metastatic prostate cancer.48 Patients who enjoyed a > 80% PSA decrease in the first month of ADT enjoyed a significantly longer progression-free survival compared to those patients who had a < 80% PSA decrease in the first month. In this early study, 42 of the 73 patients had M1 metastatic prostate cancer with the remaining 31 having locally advanced disease. Twenty-five of these patients were treated with bilateral orchiectomy, 25 were treated with DES, 6 received both orchiectomy and DES, and 17 received LH-RH agonist.48
In a subsequent study published by Fowler et al. in 1995, PSA nadir was a very significant predictor of response to hormone therapy.49 In this study, after patient exclusions, 245 patients with localized and 78 patients with newly diagnosed metastatic prostate cancer were treated with ADT in the form of orchiectomy or LH-RH agonist. PSA regression, nadir and doubling times were calculated for the patient cohorts. For the metastatic patients who reached a PSA nadir < 1.0 ng ml−1, they experienced a statistically significant longer time to biochemical recurrence. These authors further stratified PSA nadir on initial ADT from 1 to 1.9, 2 to 3.9, and a PSA nadir of > 4. Patients whose nadir was < 1 enjoyed a much greater disease-free interval compared to patients who experienced a PSA nadir > 4.49
The largest and most noteworthy study to look at PSA levels after initiation of ADT for new M1 prostate cancer was published by Hussain et al. in 2006.50 Specifically in this large Southwest Oncology Group (SWOG) trial, the authors showed that initial PSA nadir 7 months after initiation of LH-RH agonist was a strong predictor of median overall survival (Figure 2). Specifically, patients who enjoyed a PSA nadir ≤ 0.2 at 7 months after the initiation of therapy had a median overall survival of 75 months. In contrast, patients who had a PSA nadir of > 0.2 but < 4.0 had median overall survival of 44 months. Finally, the patients who experienced a PSA nadir at 7 months that were > 4.0 ng ml−1 had a median overall survival of only 13 months. In my practice, I use these publication data to help counsel patients who I initiate on ADT for traditional M1 prostate cancer. I attempt to defer discussion regarding prognosis with these patients until I have been able to examine a 7-month PSA level. Once the patient and I have the 7-month PSA nadir value in hand, we will use the aforementioned survival data to counsel them on prognosis and future treatment strategies. In fact, many of those patients who have suboptimal PSA nadir > 4 at the 7 months interval after starting ADT will have impending CRPC and may benefit from early novel therapeutic agents. Unfortunately, from this SWOG study in 2006 by Hussain et al., we do not have the serum testosterone levels at the 7-month point. It is possible that a combination of PSA response as well as testosterone response at 7 months or even an earlier interval may provide additional prognostic information to help guide future treat strategies for these patients.
Figure 2.
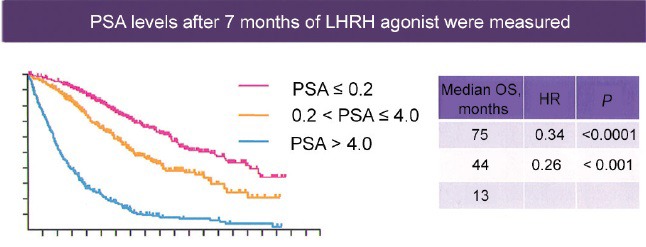
Hormone naïve disease-new M1 prostate-specific antigen level as a biomarker of treatment effect. Used with permission from Hussain et al50.
In the specific setting of traditional M1 metastatic prostate cancer, does the type of ADT matter for maximal response. As previously noted, options for treating these patients are very similar to biochemical recurrence and include orchiectomy, LH-RH agonists, ok-combined hormonal therapy, Gn-RH antagonists, IHT, and peripheral androgen blockade.8,22,28,29 As was the case with biochemical recurrence, our data remain limited on knowing and understanding the optimal treatments for these patients. In regard to CAB, we do have a large body of evidence including several meta-analysis comparing CAB to monotherapy.32,33,34,35,36,37,38,39 Whether CAB provides any survival benefit over monotherapy for the patient with M1 hormone naïve disease has been a controversy for the last 30 years and remains a controversy.29 The bottom line is that there may be a very modest survival benefit to CAB in the setting of M1 metastatic prostate cancer. With regard to the use of an LH-RH agonist versus an antagonist, there is no question of superior testosterone suppression during the first month of therapy with a pure antagonist.8,30 Furthermore, the PSA response is superior for a Gn-RH antagonist during the first 2 months of therapy compared to an LH-RH agonist.8,30 However, beyond this initial effect, the benefit of comparing agonist to antagonist remains much more controversial.30 As previously noted, in the pivotal randomized trial of degarelix versus monthly leuprolide, there appears to be a modest survival benefit of degarelix over leuprolide at the 1-year mark.8 In this trial, patients were permitted to cross over from the leuprolide to degarelix at the 12 months interval. In further follow-up, the data suggested that the patients who switched from the leuprolide to degarelix had an improving survival curve such that by approximately 2 to 3 years of follow-up the survival became equivalent. The authors used these data to suggest a benefit of degarelix versus leuprolide, however, this concept remains controversial.30,31
With regard to IHT versus continuous hormonal therapy in the setting of traditional M1 hormone naïve prostate cancer, concepts up to 2013 suggested no difference in survival rates for continuous versus intermittent ADT.21,22,23 However, in mid-2013 at the ASCO convention, the SWOG reported long-term results of their IHT trial.23 For those patients with M1 limited or minimal metastatic disease, IHT was not proven to be noninferior to continuous hormonal therapy.40,41 In plain English, for men with M1 metastatic hormone naïve prostate cancer and limited metastatic disease, continuous hormone therapy is probably a little bit more effective. In other words, for the patient presenting with newly diagnosed M1 prostate cancer where the metastatic disease burden is limited, continuous hormonal therapy may offer a slight survival advantage over IHT.40,41
As noted earlier, peripheral androgen blockade is the concept of using oral only hormone therapy to treat men with advanced prostate cancer.28,42 In the United States, PAD has been primarily used to treat biochemical recurrence and has been primarily used as a way to limit side effects, particularly loss of libido and impotency. In the setting of traditional metastatic prostate cancer bicalutamide in the dose of 150 mg used alone has been widely studied in Europe compared to traditional ADT.43,44,45 In a summary of these studies, Tyrrell et al. reported that bicalutamide 150 used alone was statically inferior to castration for hormone naïve metastatic prostate cancer.43 However, as previously noted, the absolute survival difference comparing orchiectomy to peripheral androgen blockade was modest with a survival difference only being the matter of several months.43 As a result of this inferiority, peripheral androgen blockade was never FDA approved for the treatment of advanced or biochemical recurrent prostate cancer and is currently very uncommonly used today in the setting of metastatic prostate cancer.
In the setting of hormone naïve M1 metastatic prostate cancer, is achieving a low testosterone level really important? Up until several years ago, any answers to this question were completely unknown as testosterone levels were rarely measured in the setting of follow-up of advanced prostate cancer patients.46,47 In men that undergo bilateral simple orchiectomy, the mean testosterone level was approximately 20 ng dL−1.51,52 However, the traditional testosterone level to define a castrate state has been 50 ng dL−1.46,47 In fact, all contemporary clinical trials related to prostate cancer have used the level of 50 to define castrate-resistant disease. Furthermore, the efficacy of LH-RH agents has been based on achieving levels at or below 50 ng dL−1. In 2006, Morote et al. showed that over 12% of patients treated with an LH-RH agonist did not achieve a testosterone level < 50.53 Other studies suggested that this rate of insufficient suppression above 50 was in the range of 2%–5%.51,54,55 With regard to not achieving testosterone levels equivalent to orchiectomy or 20 ng dL−1, Morote et al. showed that this rate was between 13% and 37.5%.51,55,56 In other words, up to 1/3 of patients being treated with an LH-RH agonist did not achieve testosterone levels equivalent to orchiectomy. In 2007, Morote et al. further reported patients that failed to achieve testosterone levels below 20 had a more rapid progression toward castrate-resistant disease57 (Figure 3). Examined another way Morote et al. found that patients who had a testosterone level above 32 ng dL−1 had a more rapid course toward castrate-resistant disease. In contrast, patients who achieved a testosterone level < 32 enjoyed an additional 4 years on average until castrate-resistant progression.57 In 2010, Perachino et al. reported similar findings which the testosterone level measured after 6 months of hormone therapy was a strong predictor of progression to castrate-resistant disease.58 More recently, Pickles et al. studied 2196 patients treated with LH-RH agonist during and after radiation therapy.59 Five to 8% of patients experienced breakthrough testosterone levels above 50 ng dL−1 (Figure 4). Importantly, young age < 70 predicted higher risk for testosterone breakthrough above 50. Pickles et al. also demonstrated patients that experienced no testosterone breakthrough levels above 50 enjoyed a better disease free survival after external beam therapy treatment.59 Finally, and most recently, Klotz studied 626 patients on the continuous hormonal therapy arm of PR-7.60 The PR-7 trial was a Canadian study of intermittent versus continuous hormonal therapy in M1 metastatic hormone naïve prostate cancer.61 These authors looked at serum testosterone levels and time to the development of CRPC and studied patients after a median follow-up of 8 years. For those men who had testosterone levels that measured > 50 the hazard ratio for CRPC was 1.91 compared to a control group of men who maintained testosterone levels < 20. For the group who had testosterone levels that measured between 20 and 50 the hazard ration for CRPC was 1.41.60 In summary, these multiple lines of investigation suggest that the level of testosterone suppression during the initiation and early course of ADT for hormone naïve metastatic prostate cancer is a powerful prognostic factor.
Figure 3.
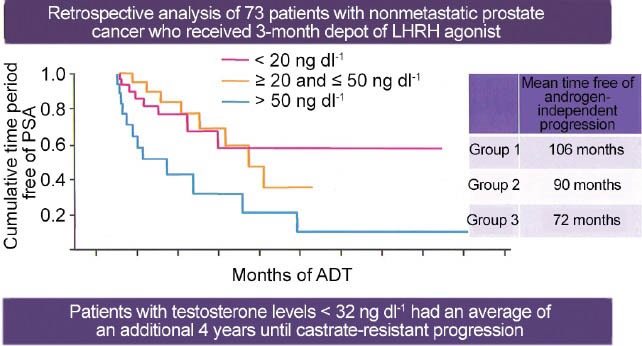
Hormone naïve disease-potential importance of lower T levels. A hypothesis-generating analysis from a retrospective study. Used with persmission from Morote et al57.
Figure 4.
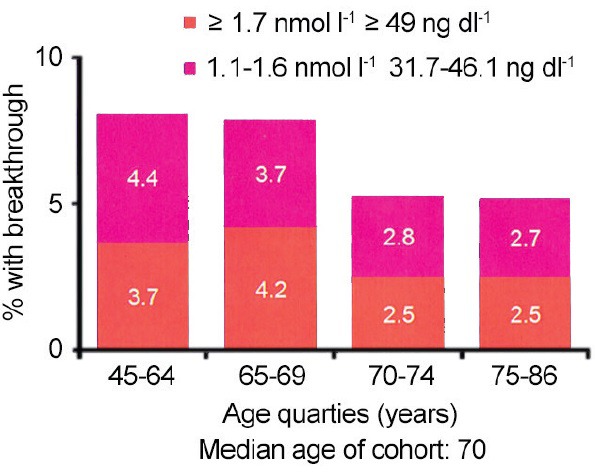
Hormone naïve disease-testosterone suppression with luteinizing hormone-releasing hormone agonists. Used with permission from Pickles59.
SUMMARY
Hormone naïve advanced prostate cancer includes patients that have biochemical recurrence without documented metastasis as well as those patients with traditional metastatic prostate cancer termed M1 disease. The types of hormonal therapy and efficacy must be examined individually for patients with biochemical recurrence compared to patients with traditional M1 disease. Specifically in the setting of biochemical recurrence, there are very few RCTs to help better our understanding. Specifically, there is no level 1 evidence to know when hormonal therapy should be initiated in biochemical recurrence. However, multiple prognostic factors and a number of well-done retrospective studies suggest that we need to risk stratify biochemical recurrence patients for optimizing use of hormone therapy. Current evidence suggests that only high-risk individuals with biochemical recurrence should be considered for ADT. High-risk individuals include those patients with a PSA-DT < 9 to 12 months and those patients that harbor high-grade disease in their primary tumor. In these high-risk individuals, it is reasonable to consider ADT after a shared decision-making discussion with the patient. There is no level 1 evidence comparing one form of ADT over another in the setting of biochemical recurrence. However, it would appear from multiple RCTs that intermittent deprivation therapy is comparable to continuous hormonal therapy in the setting of biochemical recurrence. Peripheral androgen blockade might be considered in selected patients requiring ADT for biochemical recurrence as a way to avoid side effects and to maintain potency and libido.
In the setting of traditional M1 metastatic prostate cancer, current practice generally supports the use of ADT at the first sign of M1 disease. The type of hormonal therapy to use in M1 hormone naïve metastatic prostate cancer remains hotly contested. There may be a very slight benefit of CAB in M1 disease although this remains very controversial. IHT may be slightly inferior to continuous hormonal therapy in the setting of minimal M1 metastatic prostate cancer. There would appear to be a very limited role for peripheral androgen blockade in the setting of M1 prostate cancer. PSA nadir response to initial ADT for M1 hormone naïve prostate cancer is very important. Specifically measuring the PSA nadir at the 7-month interval after initiation of ADT is a powerful prognostic marker. In addition to PSA nadir, measurement of testosterone during the course of ADT appears to be of added importance. Recent studies show that patients that maintain a serum testosterone level less than the 20–30 ng dL−1 enjoy improved disease control compared to patients who have testosterone breakthrough above these levels. Further study will be necessary to determine the true impact of testosterone suppression in management of advanced prostate cancer with ADT. It is likely that there will be many future changes to consider. Specifically, as novel oral hormonal therapy abiraterone and enzalutamide are used in hormone naïve disease, the concepts we have discussed will require further examination with further data.
EDITORIAL COMMENT—(BY DR JOHN W DAVIS, DEPARTMENT OF UROLOGY, THE UNIVERSITY OF TEXAS, MD ANDERSON CANCER CENTER, HOUSTON, TEXAS, USA)
Androgen deprivation therapy (ADT) for hormone naïve prostate cancer is certainly no longer a novel concept, and is utilized in everyday urologic practice. The novel contribution from Moul is to further refine utilization and timing of ADT, and opportunities to maximize benefit. As we read with excitement new studies on novel agents like abiraterone and enzalutamide, it is equally important to refine our standard pathways. In the category of biochemical recurrence, it is somewhat disappointing that decades of research failed to guide us on definitive decision points for ADT initiation and choice of agents. Nevertheless, Moul cites the best of available research to define low versus high-risk populations that help us to defer ADT as long as possible in the proper patient. As a result, overall utilization of ADT has declined in favor of deferred management and intermittent strategies, although some public health experts have argued that reimbursement adjustments in the mid-2000's (U.S. circumstance) also play a role in decreased utilization.
For patients with traditional (measurable) metastatic disease, the data on PSA nadirs and testosterone levels are highly useful, and should help counsel and troubleshoot patients towards optimum management. I would highlight his “7 month rule” in deferring discussion of prognosis until the initial response to ADT is documented. As we refine our understanding of the androgen receptor and the multiple pathways leading to castrate resistant disease, it is not surprising that certain aggressive biologies of prostate cancer are highly sensitive to the efficacy of ADT, even at the earliest times of exposure.
REFERENCES
- 1.Moul JW, Kibel AS, Roach M, 3rd, Dreicer R. Indications and practice with androgen deprivation therapy. Urology. 2011;78:S478–81. doi: 10.1016/j.urology.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Moul JW, Evans CP, Gomella LG, Roach M, 3rd, Dreicer R. Traditional approaches to androgen deprivation therapy. Urology. 2011;78:S485–93. doi: 10.1016/j.urology.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 3.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate 1941. J Urol. 2002;168:9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 4.Bailar JC, 3rd, Byar DP. Estrogen treatment for cancer of the prostate. Early results with 3 doses of diethylstilbestrol and placebo. Cancer. 1970;26:257–61. doi: 10.1002/1097-0142(197008)26:2<257::aid-cncr2820260203>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Byar DP. Proceedings: the Veterans Administration Cooperative Urological Research Group's studies of cancer of the prostate. Cancer. 1973;32:1126–30. doi: 10.1002/1097-0142(197311)32:5<1126::aid-cncr2820320518>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 6.Dreicer R, Bajorin DF, McLeod DG, Petrylak DP, Moul JW. New data, new paradigms for treating prostate cancer patients – VI: novel hormonal therapy approaches. Urology. 2011;78:S494–8. doi: 10.1016/j.urology.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 7.Debruyne F, Bhat G, Garnick MB. Abarelix for injectable suspension: first-in-class gonadotropin-releasing hormone antagonist for prostate cancer. Future Oncol. 2006;2:677–96. doi: 10.2217/14796694.2.6.677. [DOI] [PubMed] [Google Scholar]
- 8.Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–8. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- 9.Freedland SJ, Moul JW. Prostate specific antigen recurrence after definitive therapy. J Urol. 2007;177:1985–91. doi: 10.1016/j.juro.2007.01.137. [DOI] [PubMed] [Google Scholar]
- 10.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–79. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen PL, Alibhai SM, Basaria S, D’Amico AV, Kantoff PW, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2014;67:825–36. doi: 10.1016/j.eururo.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Hakimian P, Blute M, Jr, Kashanian J, Chan S, Silver D, et al. Metabolic and cardiovascular effects of androgen deprivation therapy. BJU Int. 2008;102:1509–14. doi: 10.1111/j.1464-410X.2008.07933.x. [DOI] [PubMed] [Google Scholar]
- 13.Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2009;181:1998–2006. doi: 10.1016/j.juro.2009.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basaria S. Androgen deprivation therapy, insulin resistance, and cardiovascular mortality: an inconvenient truth. J Androl. 2008;29:534–9. doi: 10.2164/jandrol.108.005454. [DOI] [PubMed] [Google Scholar]
- 15.Nobes JP, Langley SE, Laing RW. Metabolic syndrome and prostate cancer: a review. Clin Oncol (R Coll Radiol) 2009;21:183–91. doi: 10.1016/j.clon.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Chung SD, Liu SP, Lin HC, Wang LH. Increased risk of pneumonia in patients receiving gonadotropin-releasing hormone agonists for prostate cancer. PLoS One. 2014;9:e101254. doi: 10.1371/journal.pone.0101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Amico AV, Moul JW, Carroll PR, Cote K, Sun L, et al. Intermediate end point for prostate cancer-specific mortality following salvage hormonal therapy for prostate-specific antigen failure. J Natl Cancer Inst. 2004;96:509–15. doi: 10.1093/jnci/djh086. [DOI] [PubMed] [Google Scholar]
- 18.Moul JW, Wu H, Sun L, McLeod DG, Amling C, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2004;171:1141–7. doi: 10.1097/01.ju.0000113794.34810.d0. [DOI] [PubMed] [Google Scholar]
- 19.Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 20.Ryan CJ, Elkin EP, Small EJ, Duchane J, Carroll P. Reduced incidence of bony metastasis at initial prostate cancer diagnosis: data from CaPSURE. Urol Oncol. 2006;24:396–402. doi: 10.1016/j.urolonc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Calais da Silva FE, Bono AV, Whelan P, Brausi M, Marques Queimadelos A, et al. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the South European Uroncological Group. Eur Urol. 2009;55:1269–77. doi: 10.1016/j.eururo.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Crook JM, O’Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussain M, Tangen CM, Berry DL, Higano CS, Crawford ED, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314–25. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dreicer R, Gleave M, Kibel AS, Thrasher JB, Moul JW. Targeting the androgen receptor – theory and practice. Urology. 2011;78:S482–4. doi: 10.1016/j.urology.2011.05.052. [DOI] [PubMed] [Google Scholar]
- 25.Moul JW. Prostate specific antigen only progression of prostate cancer. J Urol. 2000;163:1632–42. [PubMed] [Google Scholar]
- 26.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 27.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 28.Bañez LL, Blake GW, McLeod DG, Crawford ED, Moul JW. Combined low-dose flutamide plus finasteride vs low-dose flutamide monotherapy for recurrent prostate cancer: a comparative analysis of two phase II trials with a long-term follow-up. BJU Int. 2009;104:310–4. doi: 10.1111/j.1464-410X.2009.08400.x. [DOI] [PubMed] [Google Scholar]
- 29.Moul JW. Twenty years of controversy surrounding combined androgen blockade for advanced prostate cancer. Cancer. 2009;115:3376–8. doi: 10.1002/cncr.24393. [DOI] [PubMed] [Google Scholar]
- 30.Crawford ED, Shore ND, Moul JW, Tombal B, Schröder FH, et al. Long-term tolerability and efficacy of degarelix: 5-year results from a phase III extension trial with a 1-arm crossover from leuprolide to degarelix. Urology. 2014;83:1122–8. doi: 10.1016/j.urology.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Moul JW. Utility of LHRH antagonists for advanced prostate cancer. Can J Urol. 2014;21:22–7. [PubMed] [Google Scholar]
- 32.Labrie F, Dupont A, Belanger A, Cusan L, Lacourciere Y, et al. New hormonal therapy in prostatic carcinoma: combined treatment with an LHRH agonist and an antiandrogen. Clin Invest Med. 1982;5:267–75. [PubMed] [Google Scholar]
- 33.Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–24. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 34.Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–42. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 35.Maximum androgen blockade in advanced prostate cancer: an overview of 22 randomised trials with 3283 deaths in 5710 patients. Prostate Cancer Trialists’ Collaborative Group. Lancet. 1995;346:265–9. [PubMed] [Google Scholar]
- 36.Schmitt B, Bennett C, Seidenfeld J, Samson D, Wilt T. Maximal androgen blockade for advanced prostate cancer. Cochrane Database Syst Rev. 2000;2:CD001526. doi: 10.1002/14651858.CD001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klotz L, Schellhammer P. Combined androgen blockade: the case for bicalutamide. Clin Prostate Cancer. 2005;3:215–9. doi: 10.3816/cgc.2005.n.002. [DOI] [PubMed] [Google Scholar]
- 38.Akaza H, Hinotsu S, Usami M, Arai Y, Kanetake H, et al. Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer. 2009;115:3437–45. doi: 10.1002/cncr.24395. [DOI] [PubMed] [Google Scholar]
- 39.Akaza H. Combined androgen blockade for prostate cancer: review of efficacy, safety and cost-effectiveness. Cancer Sci. 2011;102:51–6. doi: 10.1111/j.1349-7006.2010.01774.x. [DOI] [PubMed] [Google Scholar]
- 40.Alva A, Hussain M. Intermittent androgen deprivation therapy in advanced prostate cancer. Curr Treat Options Oncol. 2014;15:127–36. doi: 10.1007/s11864-013-0272-2. [DOI] [PubMed] [Google Scholar]
- 41.Wolff JM, Abrahamsson PA, Irani J, da Silva FC. Is intermittent androgen-deprivation therapy beneficial for patients with advanced prostate cancer? BJU Int. 2014;114:476–83. doi: 10.1111/bju.12626. [DOI] [PubMed] [Google Scholar]
- 42.Monk JP, Halabi S, Picus J, Hussain A, Philips G, et al. Efficacy of peripheral androgen blockade in prostate cancer patients with biochemical failure after definitive local therapy: results of Cancer and Leukemia Group B (CALGB) 9782. Cancer. 2012;118:4139–47. doi: 10.1002/cncr.26732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyrrell CJ, Kaisary AV, Iversen P, Anderson JB, Baert L, et al. A randomised comparison of ‘Casodex’ (bicalutamide) 150 mg monotherapy versus castration in the treatment of metastatic and locally advanced prostate cancer. Eur Urol. 1998;33:447–56. doi: 10.1159/000019634. [DOI] [PubMed] [Google Scholar]
- 44.Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB, Van Poppel H, et al. Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup. J Urol. 2000;164:1579–82. [PubMed] [Google Scholar]
- 45.Iversen P, McLeod DG, See WA, Morris T, Armstrong J, et al. Antiandrogen monotherapy in patients with localized or locally advanced prostate cancer: final results from the bicalutamide Early Prostate Cancer programme at a median follow-up of 9.7 years. BJU Int. 2010;105:1074–81. doi: 10.1111/j.1464-410X.2010.09319.x. [DOI] [PubMed] [Google Scholar]
- 46.Djavan B, Eastham J, Gomella L, Tombal B, Taneja S, et al. Testosterone in prostate cancer: the Bethesda consensus. BJU Int. 2012;110:344–52. doi: 10.1111/j.1464-410X.2011.10719.x. [DOI] [PubMed] [Google Scholar]
- 47.Moul JW, Dreicer R. Focusing on testosterone. Urology. 2011;78:S476–7. doi: 10.1016/j.urology.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Arai Y, Yoshiki T, Yoshida O. Prognostic significance of prostate specific antigen in endocrine treatment for prostatic cancer. J Urol. 1990;144:1415–9. doi: 10.1016/s0022-5347(17)39757-4. [DOI] [PubMed] [Google Scholar]
- 49.Fowler JE, Jr, Pandey P, Seaver LE, Feliz TP, Braswell NT. Prostate specific antigen regression and progression after androgen deprivation for localized and metastatic prostate cancer. J Urol. 1995;153:1860–5. [PubMed] [Google Scholar]
- 50.Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24:3984–90. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 51.Oefelein MG, Cornum R. Failure to achieve castrate levels of testosterone during luteinizing hormone releasing hormone agonist therapy: the case for monitoring serum testosterone and a treatment decision algorithm. J Urol. 2000;164:726–9. doi: 10.1097/00005392-200009010-00025. [DOI] [PubMed] [Google Scholar]
- 52.Oefelein MG, Resnick MI. Effective testosterone suppression for patients with prostate cancer: is there a best castration? Urology. 2003;62:207–13. doi: 10.1016/s0090-4295(03)00331-5. [DOI] [PubMed] [Google Scholar]
- 53.Morote J, Esquena S, Abascal JM, Trilla E, Cecchini L, et al. Failure to maintain a suppressed level of serum testosterone during long-acting depot luteinizing hormone-releasing hormone agonist therapy in patients with advanced prostate cancer. Urol Int. 2006;77:135–8. doi: 10.1159/000093907. [DOI] [PubMed] [Google Scholar]
- 54.Wechsel HW, Zerbib M, Pagano F, Coptcoat MJ. Randomized open labelled comparative study of the efficacy, safety and tolerability of leuprorelin acetate 1M and 3M depot in patients with advanced prostatic cancer. Eur Urol. 1996;30(Suppl 1):7–14. doi: 10.1159/000474238. [DOI] [PubMed] [Google Scholar]
- 55.McLeod D, Zinner N, Tomera K, Gleason D, Fotheringham N, et al. A phase 3, multicenter, open-label, randomized study of abarelix versus leuprolide acetate in men with prostate cancer. Urology. 2001;58:756–61. doi: 10.1016/s0090-4295(01)01342-5. [DOI] [PubMed] [Google Scholar]
- 56.Morote J, Esquena S, Abascal JM, Trilla E, Cecchini L, et al. Failure to maintain a suppressed level of serum testosterone during long-acting depot luteinizing hormone-releasing hormone agonist therapy in patients with advanced prostate cancer. Urol Int. 2006;77:135–8. doi: 10.1159/000093907. [DOI] [PubMed] [Google Scholar]
- 57.Morote J, Orsola A, Planas J, Trilla E, Raventós CX, et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol. 2007;178:1290–5. doi: 10.1016/j.juro.2007.05.129. [DOI] [PubMed] [Google Scholar]
- 58.Perachino M, Cavalli V, Bravi F. Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance? BJU Int. 2010;105:648–51. doi: 10.1111/j.1464-410X.2009.08814.x. [DOI] [PubMed] [Google Scholar]
- 59.Pickles T, Hamm J, Morris WJ, Schreiber WE, Tyldesley S. Incomplete testosterone suppression with luteinizing hormone-releasing hormone agonists: does it happen and does it matter? BJU Int. 2012;110:E500–7. doi: 10.1111/j.1464-410X.2012.11190.x. [DOI] [PubMed] [Google Scholar]
- 60.Klotz LR. Amelia Island, FL: American Association of Genitourinary Surgeons Annual Meeting; 2014. Apr 26, Nadir T on ADT predicts for time to castrate resistant progression: a secondary analysis of the PR-7 Intermittent versus continuous trial. [Google Scholar]
- 61.Klotz L. Intermittent versus continuous androgen deprivation therapy in advanced prostate cancer. Curr Urol Rep. 2013;14:159–67. doi: 10.1007/s11934-013-0325-x. [DOI] [PubMed] [Google Scholar]


