Abstract
The effects of diabetes mellitus include long-term damages, dysfunctions, and failures of various organs. An important complication of diabetes is the disturbance in the male reproductive system. Glucose metabolism is an important event in spermatogenesis. Moreover, glucose metabolism is also important for maintaining basic cell activity, as well as specific functions, such as motility and fertilization ability in mature sperm. Diabetic disease and experimentally induced diabetes both demonstrated that either type 1 diabetes or type 2 diabetes could have detrimental effects on male fertility, especially on sperm quality, such as sperm motility, sperm DNA integrity, and ingredients of seminal plasma. Epigenetic modifications are essential during spermatogenesis. The epigenetic regulation represents chromatin modifications including DNA methylation, histone modifications, remodeling of nucleosomes and the higher-order chromatin reorganization and noncoding RNAs. If spermatogenesis is affected during the critical developmental window, embryonic gonadal development, and germline differentiation, environmentally-induced epigenetic modifications may become permanent in the germ line epigenome and have a potential impact on subsequent generations through epigenetic transgenerational inheritance. Diabetes may influence the epigenetic modification during sperm spermatogenesis and that these epigenetic dysregulation may be inherited through the male germ line and passed onto more than one generation, which in turn may increase the risk of diabetes in offspring.
Keywords: diabetes, epigenetic regulation, sperm, spermatogenesis
INTRODUCTION
Globally, it is estimated that 382 million people suffer from diabetes and that the prevalence is 8.3%.1 The term “diabetes mellitus (DM)” describes a metabolic disorder of multiple etiologies characterized by chronic hyperglycemia with the disturbances of carbohydrate, fat, and protein metabolism resulting from defects in insulin secretion, insulin action, or both.2 Type 1 DM is a chronic autoimmune disease with a strong inflammatory component, characterized by loss of the insulin-producing beta cells of the islets of Langerhans in the pancreas, leading to insulin deficiency.3 Type 2 DM is characterized by insulin resistance, which may be combined with relatively reduced insulin secretion.4 Type 2 diabetes is primarily due to lifestyle factors and genetics. A number of lifestyle factors are known to be important to the development of type 2 diabetes, including obesity, lack of physical activity, poor diet, stress, and urbanization.5,6 The DM could induce long-term damages, dysfunctions and failures of various organs, including retinopathy with potential loss of vision, nephropathy leading to renal failure, peripheral neuropathy with risk of foot ulcers, amputations, Charcot joints, autonomic neuropathy causing gastrointestinal, genitourinary, cardiovascular symptoms, and sexual dysfunction.7 An important complication of diabetes is the disturbance in the male reproductive system. Glucose metabolism is an important event in spermatogenesis. Many studies in both human and animals have confirmed the deleterious effect of diabetes on sexual functions, such as semen parameters, nuclear DNA fragment, and chromatin quality.8,9,10
In the following sections, we will review the role of glucose metabolism in sperm as well the clinical and animal studies on type 1 and type 2 diabetes, its effects on male fertility, especially on sperm quality, and the potential molecular mechanism. In addition, the epigenetic regulation of spermatogenesis will be critically reviewed, and the diabetes effects across generation in the germ line will be discussed.
GLUCOSE METABOLISM IN SPERM
Sperm cell is the most differentiated mammalian cell. The main goal of sperm consists of transferring male haploid DNA to female DNA through a series of mechanisms that imply their displacement along the female genital tract and fertilizing ability.11 Energy in sperm cells is mainly used to maintain the motility to complete capacitation and subsequent acrosome reaction.12,13,14
Sperm cells need energy to acquire and maintain motion competence after epididymal maturation because they are actually immotile in testis.14 Much adenosine triphosphate (ATP) in sperms is consumed for maintaining the motility. Except some metabolites, such as lactate and citrate, sperm mainly utilize sugars as an energy fuel including glucose, mannose, and fructose. The two main metabolic pathways involved in energy generation are anaerobic glycolysis and oxidative phosphorylation.15 Inhibitors of either oxidative metabolism or glycolysis in many species show that either pathway alone can maintain mobility independently.16 Sperm metabolism can proceed through glycolysis, mitochondrial oxidative phosphorylation or the pentose phosphate pathway. The predominant pathway used depends on species, oxygen content and/or hexose availability. In fact, sperm have a mitochondrial sheath in the midpiece, where the oxidative processes may take place.15 Therefore, the most important glycolytic enzymes are mainly located in the principal piece of the tail which is connected to the fibrous sheath.15,17,18,19,20,21
Glucides are polar molecules that are rich in -OH groups and can passively cross the lipidic bilayer in a very slow and inefficient manner. Therefore, carriers are required when cells uptake glucides.15 An important role of supplying cells with energy is realized by different membrane proteins that can actively (sodium-dependent glucose transporters [SGLT]) or passively (glucose transporters [GLUT]) transport hexoses through the lipidic bilayer.22,23 The proteins of the SGLT family are active transporters of sugars, particularly glucose.23 GLUTs are 13 proteins of a family that facilitate the transport of sugars and show a peculiar distribution in different tissues, as well as a particular affinity for substrates. Other hexoses (fructose, mannitol), vitamins, and amino sugars as glucosamine can also be transported by GLUTs.24 Glucose passively transport across the blood-testis barrier, which is mediated by GLUTs, is an important event in spermatogenesis. GLUTs also exist in mature sperm cells, which, in fact, require carriers for uptake energetic sources that are important for maintaining basic cell activity, as well as specific functions, such as motility and fertilization ability.15
In human sperm cells, GLUT1 and GLUT2 were in the acrosomal region and the principal and end pieces of the tail, whereas GLUT3 was found in the midpiece.24 GLUT4 did not show any immunologic positivity and GLUT5 was detected in the subequatorial region and the mid and principal pieces.15 GLUT8 was firstly identified in the testes as well as other tissues.25,26 GLUT8 localizes in the midpiece and principal piece as well as in the acrosomal region of the sperm. Immunoelectron microscopic analysis shows that GLUT8 is strongly detectable at the acrosome and neck region of the sperm. In the midpiece, GLUT8 localizes at the outer dense fibers (ODF) as well as at the circumference of the spiral mitochondria. In the principal piece, GLUT8 localizes at the ODF.27,28,29 GLUT8 has an endosomal and lysosomal targeting motif,30 and is only translocated to the plasma membrane in response to insulin in blastocysts.31 Therefore, it might only transport hexoses across endosomes and lysosomes intracellularly. Similar to the lysosome, the acrosome of sperm cells contains lysosomal proteins under a low pH. GLUT8 might play a role in the acrosome reaction upon sperm binding to the oocyte. Indeed, sperm from Glut8−/− mice have showed the decreased ATP and reduced motility compared to wild type. However, these sperms are able to fertilize oocytes. GLUT9 has been recently identified to have a high degree of homology to GLUT5 that transports fructose as well as glucose.32 GLUT9 has two isoforms: a long form containing 12-transmembrane domains (GLUT9a) and a short form without two transmembrane domains (GLUT9b) that exist in sperm cells. Both GLUT9a and GLUT9b are expressed in the midpiece, whereas GLUT9b is also found in the acrosome and principal piece.27 The two isoforms are also high-capacity urate transporters.33 Uric acid inhibits peroxynitrite (ONOO-) generation in sperm and can interact with other reactive oxygen species (ROS) such as hydroxyl radicals.34 Because ROS generated by sperm cells are involved in capacitation, in addition to the role of providing energy substrates, GLUT9 may regulate the redox state and capacitation (Figure 1).
Figure 1.
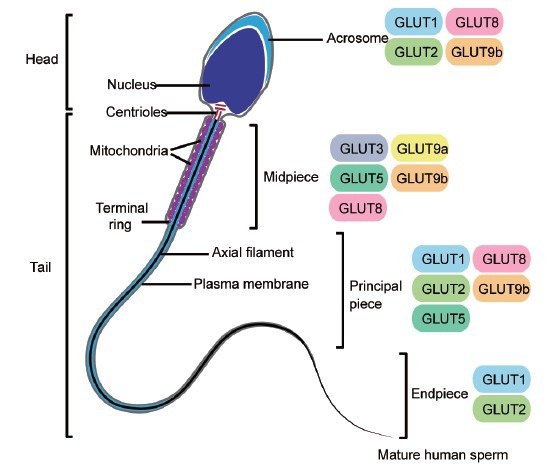
Schematic representation of human sperm cell and localization of several glucose transporter isoforms in the distinct parts of the spermatozoon, namely head (which includes the nucleus and acrosome) and tail (comprehending the midpiece, principal piece and endpiece). GLUT1: glucose transporter 1; GLUT2: glucose transporter 2; GLUT3: glucose transporter 3; GLUT5: glucose transporter 5; GLUT8: glucose transporter 8; GLUT9a: glucose transporter 9a; GLUT9b: glucose transporter 9b.
Various GLUTs in sperms endow them the flexibility to adapt to the changes in the environment, metabolic requirements, etc. However, an abnormal environment like diabetes can cause the dysfunction in nutrient transport, thus leading to the decreased fertility and adverse fetal outcomes.35,36 A previous study examining GLUT expression of GLUT8 and GLUT9 in sperm and testes of two different genetically modified diabetic mice found that mice lacking GLUT9 protein had low sperm motility and decreased fertilization rates. The authors suggested that lack of insulin or hyperglycemia impaired Glut9 transcription and concluded that insulin and glucose were important in sperm maturation and had important roles in the sugar movement in sperm which indirectly controlled motility during capacitation and fertilization. Furthermore, when these same mice were treated with insulin sperm motility and concentration was obviously increased suggesting that insulin signaling improves sperm quality. In addition, it has been found that glucose, not fructose is needed for fertilization and is specifically required during sperm oocyte binding and embryo viability in the mouse.37,38
DIABETIC DISEASE AND EXPERIMENTALLY INDUCED DIABETES – IMPACT ON MALE FERTILITY
Abnormal glucose homeostasis has adverse outcomes for the reproductive function in the male gametes.8 Testicular function and spermatogenesis are affected in both type 1 and type 2 diabetic men.8,39 Traditional light microscopic analysis of the ejaculate suggests that the effect of diabetes on semen quality is negligible and molecular investigation techniques have demonstrated that diabetic men have a dramatically higher percentage of sperm with nuclear and mitochondrial DNA fragmentation and that the damage is oxidative in nature.8,40 Sperm DNA damage is known to be associated with the decreased embryo quality, the lower implantation rates, and, possibly, the early onset of some childhood diseases (Table 1).41
Table 1.
The detrimental effects of male diabetes on sperm quality
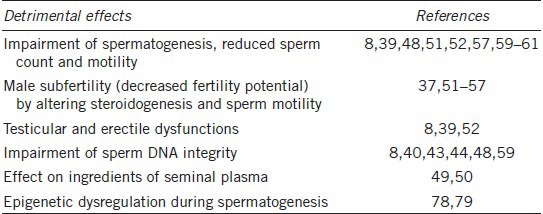
Diabetes and sperm DNA integrity
Sperm cells from men with type 1 diabetes have structural defects with nuclear and mitochondrial DNA fragmentation, reduced motility, and decreased zona pellucida binding.8,39,40 Agbaje et al.42 found that changes in the expression of the genes involved in DNA repair and replication were highly correlated with the increases in sperm DNA fragmentation observed in type 1 DM men. Numerous factors have been shown to increase oxidative stress (OS), ROS production, and sperm DNA damage.43,44 Present at high concentrations in semen, the polyamines (spermine, spermidine, and putrescine) have antioxidant actions, are potent antiglycating agents, and protect against structural/functional advanced glycation end products (AGE) modifications. Therefore, recently, they reported that the changes in the expression of antioxidants could be seen as reactions to, or a cause of, an environment of OS, and, the number of sperm displaying the receptor for advanced glycation end products (RAGE) and that the overall protein amount found in sperm and seminal plasma was prominently higher than that in samples from type 1 diabetic men.43,44 Mallidis et al.43 found that in type 1 diabetic men, mRNA profiles indicated expression perturbations in the genes involved in stress response, DNA metabolism, and replication/repair, particularly owing to their association with OS, glycation, and AGEs/RAGE. RAGE plays a key role in many diabetic complications. The consequences of ligand-RAGE interaction include up-regulation of molecules implicated in inflammatory responses and tissue damage, such as cytokines, adhesion molecules, and matrix metalloproteinases.45 An increment in AGE in seminal plasma of type 1 and type 2 diabetic subjects may suggest a key role of glycation process and increased OS in reproductive system dysfunction.46 These diabetic men showed the obviously higher mean levels of RAGE protein and DNA fragmentation in sperm. The highly positive correlation between RAGE levels and nuclear DNA fragmentation in sperm suggests the central role of RAGE in the disturbances in sexual function of diabetic men. The activation of RAGE increases ROS production, and the high level of ROS can induce DNA fragmentation in turn.47 Furthermore, alterations in mitochondrial DNA in diabetes are responsible for the adverse changes observed in motility of sperm. These are important findings in a clinical perspective for diabetic patients who attend infertility clinics because aggressive diabetic control measures may reverse these changes and improve pregnancy outcomes.48
Diabetes and ingredients of seminal plasma
The researchers found that seminal plasma nitrate/nitrite levels and 8-hydroxydeoxyguanosine (8-OHdG) levels were both markedly increased in the diabetic group (including type 1 and type 2 diabetes).49 Regression analysis results indicated that in diabetic men, nitrate/nitrite levels correlated well with 8-OHdG levels.49 The data suggest that the high levels of nitrate/nitrite in the semen of diabetic men are suggestive of ROS-induced DNA damage that is correlated with 8-OHdG levels but not sperm parameters.49 Because there are no effects to sperm motility, it is likely that these sperm can achieve fertilization and, therefore, this is of clinical significance. The level of malondialdehyde, one of the final products of lipid peroxidation and well-known markers of OS, was notably increased in semen of infertile men with type 2 diabetes and had the negative correlation with sperm density, total sperm count, progressive motility, and normal forms, suggesting that the increase in lipid peroxidation in men with diabetes with poor metabolic control was associated with low sperm quality.50 The glycemic control is an important element for preventing sperm damage in these patients.50 Paasch et al. demonstrated type 1 diabetes, type 2 diabetes, and obesity are all accompanied by multiple changes of the sperm proteome. They found the involvement of semenogelin-1, clusterin, and lactotransferrin, part of the eppin (epididymal proteinase inhibitor) protein complex, which is thought to fulfill fertilization-related functions, such as ejaculate sperm protection, motility regulation, and gain of competence for acrosome reaction in the pathologic alterations of sperm morphology and functions taking place in diabetic and obese individuals.51
Streptozotocin-induced type 1 diabetes model
In animals, the male reproductive milieu (testis and epididymal sperm) of streptozotocin (STZ)-induced diabetic mice is subjected to significant OS during the early diabetic phase and may apparently contribute to the development of testicular dysfunction, thus leading to altered steroidogenesis and impaired spermatogenesis.52 In insulin-dependent diabetes, leydig cell function and testosterone production decrease due to the absence of the stimulatory effect of insulin on these cells and an insulin-dependent decrease in FSH, which, in turn, reduces LH levels. Sperm output and fertility are reduced because of a decrease in FSH caused by a reduction in insulin.53 Hyperglycemia has an adverse effect on sperm concentration and motility via the changes in energy production and free radical management. Furthermore, rats treated with STZ for 1 month, showed some metabolic adaptations, such as the increase in the efficiency of mitochondrial ATP production, in order to circumvent the deleterious effects promoted by the disease.54 Male diabetes may cause male subfertility by altering steroidogenesis, sperm motility, and GLUT expression. Fertilization rates were distinctly lower in the Akita group, and the STZ-injected male group compared with the normal group. Furthermore, in fertilized zygotes, embryo developmental rates to the blastocyst stage in two diabetic models were lower than that in controls.37 Mallidis et al.55 described the changes in the testicular metabolome after the induction by STZ in an experimental model of type 1 diabetes and identified the perturbations in several important metabolites. Specifically, diabetic mice showed the decreased carnitine, creatine, and choline and the increased lactate, alanine, and myo-inositol.55 Epidermal growth factor deficiency is a potential cause for the pathogenesis of oligozoospermia in diabetic mice.56 STZ-diabetes also notably reduced the epididymal tissue concentrations of testosterone, androgen-binding protein, sialic acid, and glycerylphosphorylcholine, suggesting its adverse effects on the secretory activity and the concentrating capacity of epididymal epithelium. Impaired cauda epididymidal sperm motility and fertility in STZ-diabetic rats implied the defective sperm maturation. Insulin replacement prevented these changes either partially or completely.57
From the above findings, it is evident that STZ-diabetes has an adverse effect on sperm maturation, which may be caused by the decrease in the bioavailability of testosterone and epididymal secretory products. Some scientists indicated that the germ cell abnormalities observed in the hyperglycemic group could be interpreted as the primary effect of STZ, not the hyperglycemia.58 Some researchers demonstrated that STZ-induced DM may influence the male fertility potential via affecting sperm parameters and DNA integrity in mice. However, diabetes has no detrimental effect on histone-protamines replacement during the testicular phase of sperm chromatin packaging.59
High-energy diet-induced prediabetic model
After feeding high-energy diet (HED) for 1 month, rats showed increased glycemic levels, impaired glucose tolerance, and hypoinsulinemia. Moreover, an imbalance of intratesticular and serum testosterone levels was observed. HED also affected the reproductive parameters, and HED rats exhibited a significant increase in abnormal sperm morphology.60 Glycolytic metabolism was favored in testicles of HED rats with an increased expression of GLUT1, GLUT3, and phosphofructokinase 1. Moreover, the lactate production and the expression of metabolism-associated genes and proteins involved in lactate production and transport were also enhanced by HED. Alanine testicular content was decreased, and thus intratesticular lactate/alanine ratio in HED rats was increased, suggesting the increased OS. These results suggest that HED induces a prediabetic state that may impair reproductive function by modulating overall testicular metabolism.60 In prediabetic rats, an alteration in HCO3− homeodynamics in the lumen of the epididymis may affect the establishment of a proper environment for sperm storage and viability, thus influencing male reproductive potential.61
IMPACT OF PARENTAL DIABETES ON MALE GERM CELL EPIGENETIC REGULATION
Epigenetic patterning starts in the germ line and is essential for normal embryo and postnatal development.62 Epigenetic modifications during germ cell development are postulated to play roles in gene expression, meiosis, genomic integrity, and genomic imprinting.63 Epigenetics refers to a collection of mechanisms and phenomena that define the phenotype of a cell without affecting the genotype. Epigenetic states can be modified by environmental factors, which may contribute to the development of abnormal phenotypes. Epidemiological evidence increasingly suggests that environmental exposures early in development have a role in susceptibility to disease in later life. A growing body of data, from animal as well as human studies, has established that the molecular basis of programming involves altered DNA methylation. In molecular terms, it represents chromatin modifications including DNA methylation, histone modifications, remodeling of nucleosomes and the higher-order chromatin reorganization, and noncoding RNAs.64,65,66 Epigenetic modifications are essential during spermatogenesis (Figure 2).
Figure 2.
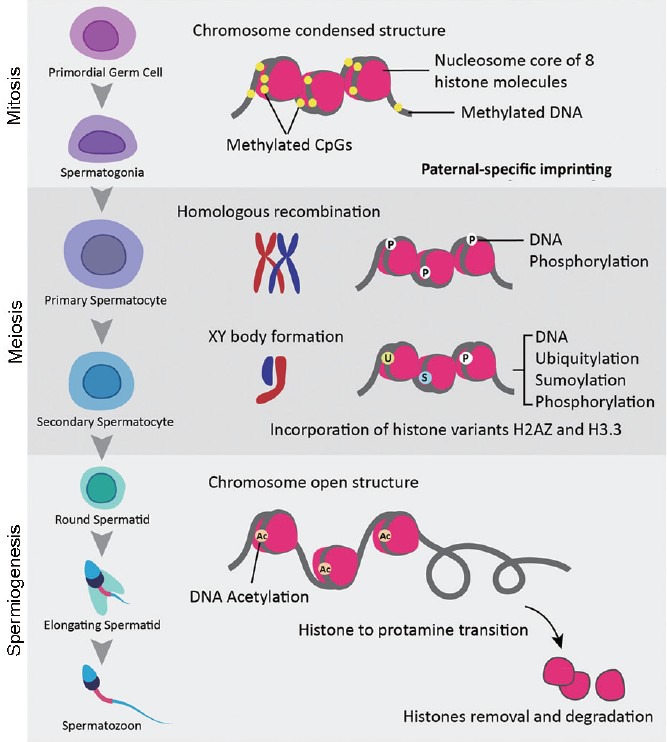
Epigenetic modifications occurring during spermatogenesis. DNA methylation occurs in mitotic germ cells, setting up the paternal specific imprints. Phosphorylation occurs in meiotic cells, assisting in both recombination and XY body formation. Ubiquitylation, sumoylation, and incorporation of the H2AZ and H3.3 variants are all involved in XY body formation. During spermiogenesis, hyperacetylation occurs to assist in the histone-protamine transition.
Maternal hyperglycemia has significant deleterious effects on the structure and function of both the reproductive endocrine and testicular structures. This detrimental change is likely to occur during fetal life and remains during postnatal life. It shows a similar pattern of change as previously reported in adult male diabetic rats.67 If spermatogenesis is affected during the critical development window, embryonic gonadal development, and germline differentiation, environmentally-induced epigenetic modifications may become permanent in the germ line epigenome and have a potential impact on subsequent generations by way of epigenetic transgenerational inheritance. The resulting diseases may include adult-onset diseases, including infertility, both within individuals and across generations.68 In the prenatal testis, the only germ cells are gonocytes. The gonocytes proliferate for a few days and then become arrested.69 Shortly after birth, the gonocytes move to the basement membrane, become spermatogonia, and resume proliferation.70 Spermatogonia divides in mitosis constitute the pool of stem cells from which meiosis and spermatogenesis proceed. Prior to spermatogenesis, transposable elements (TEs) are silenced in the gonocytes and prospermatogonia.71
The methylation facilitated by DNA methyltransferase (DNMT) 3L is involved in the silencing of TEs in the testis, as evidenced by the loss of methylation at LINE-1 and the intracisternal A-particle transposons caused by the absence of DNMT3L. DNMT3L is expressed in the gonocytes at 14–18 days postcoitum (dpc) when global DNA methylation occurs. Indeed, it has been proposed that the absence of TEs silencing in this model contributes directly to meiotic arrest and infertility in adult mice.72,73,74
Male and female germ cell development is particularly critical for the acquisition of the differential “marking” of imprinted genes to ensure parent-of-origin specific expression.59,75 Paternally imprinted genes are genes that are subjected to monoallelic expression. That is, expression occurs only from the allele inherited from the maternal parent. Paternally imprinted genes are methylated and silenced in male germ cells. Paternal imprinting of genes starts to be established in the gonocytes at about 15.5 dpc and is maintained in the spermatogonia. This methylation and gene silencing are carried out through the remainder of spermatogenesis and maintained in any resultant sperm and offspring. Some paternally imprinted regions have been identified, such as H19-Igf2, Ras-GRF, and Dlk1-Gtl2.76 In male germ cells, the establishment of the paternal imprints involves a factor named BORIS (brother of the regulator of imprinted sites) and the DNMT, DNMT3A, DNMT3B and the closely related DNMT3L.76,77 Deletion of Dnmt3L results in the loss of methylation at paternally imprinted regions. Spermatogonia which were deficient in Dnmt3a and Dnmt3b displayed variations in methylation patterns at paternally imprinted regions.77 In our previous study, altered Igf2 and H19 gene expression was found in sperm of adult F1 offspring of gestational DM, indicating that the changes of epigenetics in germ cells contributed to transgenerational transmission.78 In addition, paternal prediabetes altered the overall methylome patterns in sperms with a large portion of differentially methylated genes overlapping with that of pancreatic islets in offspring, indicating that paternal prediabetes increased the susceptibility to diabetes in offspring through gametic epigenetic alterations.79 The study discovered that paternal prediabetes alters overall methylation patterns in sperm. They isolated sperm from control and prediabetic males and surveyed cytosine methylation patterns across the entire genome by MeDIP-Seq. Notably, global cytosine methylation profiles were altered in prediabetes samples compared with controls, and the methylation of 263 upstream2k, 278 downstream2k, 121 5’UTR, 247 3’UTR, 1299 CDS, and 4354 intron element-associated genes were changed, respectively. They observed that a large proportion of differentially methylated genes identified in sperm overlapped with that of pancreatic islets. Specifically, They observed that certain genes (such as Pik3ca and Pik3r1) can partially resist global demethylation postfertilization and largely inherit cytosine methylation from sperm, further suggesting that there is intergenerational transmission of cytosine methylation at a substantial fraction of the genome.79
New evidence from a variety of model systems demonstrates that noncoding RNAs, such as microRNAs, small RNAs, and long or large RNAs, play a significant role in epigenetic gene regulation and chromosomal dynamics including mechanisms for processes such as dosage compensation, imprinting and gene silencing by RNA interference. Recent reports suggest that gene silencing, mediated by DNA methylation, can be induced by promoter-directed silencing RNAs (siRNAs) in mammalian cells.80,81,82
CONCLUSION AND FUTURE PERSPECTIVES
The studies discussed in this review demonstrate that glucose metabolism is of great important for sperm cells, either type 1 diabetes or type 2 diabetes could have detrimental effects on male fertility, especially on sperm quality, such as sperm motility, sperm DNA integrity, and ingredients of seminal plasma. Diabetes may influence the epigenetic modification during sperm spermatogenesis and that these epigenetic dysregulation may be inherited through the male germ line and passed onto more than one generation, which in turn may increase the risk of diabetes in offspring.
The dramatic increase in diabetes, one of the human metabolic disorders warrants considering the influence of environmental factors on the germ line. Parental nutrition and metabolism are critical determinants of adult offspring health. The effects of maternal or paternal diabetes described in this review have further highlighted the importance of research into intergenerational and even transgenerational effect of diabetes on male fertility and health of offspring. Although much attention has been drawn to the potential implications of transgenerational inheritance for human health, so far there is little support. To address these issues, in future studies, a multigenerational animal model obtained from paternal or maternal hyperglycemia and transgenerational epigenetic inheritance may be worthy of consideration.
AUTHOR CONTRIBUTIONS
GLD, YL, and MEL wrote the main body of the manuscript. JXP participated in the design and helped to draft the manuscript. MXG drew the figures. JZS and HFH contributed to conception and design of the manuscript. All the authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by the National Basic Research Program of China (2012CB944901), the National Natural Science Foundation of China (31171444 and 81200485) and the Research Fund for the Doctoral Program of Higher Education (20120101120051).
REFERENCES
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–26. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 4.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 5.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 6.Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48:44–51. doi: 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, et al. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22:1871–7. doi: 10.1093/humrep/dem077. [DOI] [PubMed] [Google Scholar]
- 9.Kilarkaje N, Al-Hussaini H, Al-Bader MM. Diabetes-induced DNA damage and apoptosis are associated with poly (ADP ribose) polymerase 1 inhibition in the rat testis. Eur J Pharmacol. 2014;737:29–40. doi: 10.1016/j.ejphar.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Cavallini G. Male idiopathic oligoasthenoteratozoospermia. Asian J Androl. 2006;8:143–57. doi: 10.1111/j.1745-7262.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Martinez H. Role of the oviduct in sperm capacitation. Theriogenology. 2007;68(Suppl 1):S138–46. doi: 10.1016/j.theriogenology.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Flesch FM, Gadella BM. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim Biophys Acta. 2000;1469:197–235. doi: 10.1016/s0304-4157(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 13.Harrison RA, Gadella BM. Bicarbonate-induced membrane processing in sperm capacitation. Theriogenology. 2005;63:342–51. doi: 10.1016/j.theriogenology.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Yanagimachi R. Mammalian fertilization. Physiol Reprod. 1994;1:189–317. [Google Scholar]
- 15.Bucci D, Rodriguez-Gil JE, Vallorani C, Spinaci M, Galeati G, et al. GLUTs and mammalian sperm metabolism. J Androl. 2011;32:348–55. doi: 10.2164/jandrol.110.011197. [DOI] [PubMed] [Google Scholar]
- 16.Storey BT. Mammalian sperm metabolism: oxygen and sugar, friend and foe. Int J Dev Biol. 2008;52:427–37. doi: 10.1387/ijdb.072522bs. [DOI] [PubMed] [Google Scholar]
- 17.Eddy EM, Toshimori K, O’Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61:103–15. doi: 10.1002/jemt.10320. [DOI] [PubMed] [Google Scholar]
- 18.Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, et al. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A. 2004;101:16501–6. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford WC. Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum Reprod Update. 2006;12:269–74. doi: 10.1093/humupd/dmi053. [DOI] [PubMed] [Google Scholar]
- 20.Krisfalusi M, Miki K, Magyar PL, O’Brien DA. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol Reprod. 2006;75:270–8. doi: 10.1095/biolreprod.105.049684. [DOI] [PubMed] [Google Scholar]
- 21.Miki K. Energy metabolism and sperm function. Soc Reprod Fertil Suppl. 2007;65:309–25. [PubMed] [Google Scholar]
- 22.Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review) Mol Membr Biol. 2001;18:247–56. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- 23.Scheepers A, Joost HG, Schürmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr. 2004;28:364–71. doi: 10.1177/0148607104028005364. [DOI] [PubMed] [Google Scholar]
- 24.Angulo C, Rauch MC, Droppelmann A, Reyes AM, Slebe JC, et al. Hexose transporter expression and function in mammalian spermatozoa: cellular localization and transport of hexoses and vitamin C. J Cell Biochem. 1998;71:189–203. [PubMed] [Google Scholar]
- 25.Doege H, Schürmann A, Bahrenberg G, Brauers A, Joost HG. GLUT8, a novel member of the sugar transport facilitator family with glucose transport activity. J Biol Chem. 2000;275:16275–80. doi: 10.1074/jbc.275.21.16275. [DOI] [PubMed] [Google Scholar]
- 26.Ibberson M, Uldry M, Thorens B. GLUTX1, a novel mammalian glucose transporter expressed in the central nervous system and insulin-sensitive tissues. J Biol Chem. 2000;275:4607–12. doi: 10.1074/jbc.275.7.4607. [DOI] [PubMed] [Google Scholar]
- 27.Kim ST, Moley KH. The expression of GLUT8, GLUT9a, and GLUT9b in the mouse testis and sperm. Reprod Sci. 2007;14:445–55. doi: 10.1177/1933719107306226. [DOI] [PubMed] [Google Scholar]
- 28.Schürmann A, Axer H, Scheepers A, Doege H, Joost HG. The glucose transport facilitator GLUT8 is predominantly associated with the acrosomal region of mature spermatozoa. Cell Tissue Res. 2002;307:237–42. doi: 10.1007/s00441-001-0499-2. [DOI] [PubMed] [Google Scholar]
- 29.Gómez O, Romero A, Terrado J, Mesonero JE. Differential expression of glucose transporter GLUT8 during mouse spermatogenesis. Reproduction. 2006;131:63–70. doi: 10.1530/rep.1.00750. [DOI] [PubMed] [Google Scholar]
- 30.Augustin R, Riley J, Moley KH. GLUT8 contains a [DE] XXXL[LI] sorting motif and localizes to a late endosomal/lysosomal compartment. Traffic. 2005;6:1196–212. doi: 10.1111/j.1600-0854.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 31.Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, et al. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci U S A. 2000;97:7313–8. doi: 10.1073/pnas.97.13.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manolescu AR, Augustin R, Moley K, Cheeseman C. A highly conserved hydrophobic motif in the exofacial vestibule of fructose transporting SLC2A proteins acts as a critical determinant of their substrate selectivity. Mol Membr Biol. 2007;24:455–63. doi: 10.1080/09687680701298143. [DOI] [PubMed] [Google Scholar]
- 33.Caulfield MJ, Munroe PB, O’Neill D, Witkowska K, Charchar FJ, et al. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med. 2008;5:e197. doi: 10.1371/journal.pmed.0050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aitken RJ, Ryan AL, Baker MA, McLaughlin EA. Redox activity associated with the maturation and capacitation of mammalian spermatozoa. Free Radic Biol Med. 2004;36:994–1010. doi: 10.1016/j.freeradbiomed.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Cummings EA, O’connell C, Jangaard K. Fetal and neonatal outcomes of diabetic pregnancies. Obstet Gynecol. 2006;108:644–50. doi: 10.1097/01.AOG.0000231688.08263.47. [DOI] [PubMed] [Google Scholar]
- 36.Purcell SH, Moley KH. Glucose transporters in gametes and preimplantation embryos. Trends Endocrinol Metab. 2009;20:483–9. doi: 10.1016/j.tem.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim ST, Moley KH. Paternal effect on embryo quality in diabetic mice is related to poor sperm quality and associated with decreased glucose transporter expression. Reproduction. 2008;136:313–22. doi: 10.1530/REP-08-0167. [DOI] [PubMed] [Google Scholar]
- 38.Sakkas D, Urner F, Menezo Y, Leppens G. Effects of glucose and fructose on fertilization, cleavage, and viability of mouse embryos in vitro. Biol Reprod. 1993;49:1288–92. doi: 10.1095/biolreprod49.6.1288. [DOI] [PubMed] [Google Scholar]
- 39.Baccetti B, La Marca A, Piomboni P, Capitani S, Bruni E, et al. Insulin-dependent diabetes in men is associated with hypothalamo-pituitary derangement and with impairment in semen quality. Hum Reprod. 2002;17:2673–7. doi: 10.1093/humrep/17.10.2673. [DOI] [PubMed] [Google Scholar]
- 40.Roessner C, Paasch U, Kratzsch J, Glander HJ, Grunewald S. Sperm apoptosis signalling in diabetic men. Reprod Biomed Online. 2012;25:292–9. doi: 10.1016/j.rbmo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Aitken RJ, Koopman P, Lewis SE. Seeds of concern. Nature. 2004;432:48–52. doi: 10.1038/432048a. [DOI] [PubMed] [Google Scholar]
- 42.Agbaje IM, McVicar CM, Schock BC, McClure N, Atkinson AB, et al. Increased concentrations of the oxidative DNA adduct 7,8-dihydro-8-oxo-2-deoxyguanosine in the germ-line of men with type 1 diabetes. Reprod Biomed Online. 2008;16:401–9. doi: 10.1016/s1472-6483(10)60602-5. [DOI] [PubMed] [Google Scholar]
- 43.Mallidis C, Agbaje I, O’Neill J, McClure N. The influence of type 1 diabetes mellitus on spermatogenic gene expression. Fertil Steril. 2009;92:2085–7. doi: 10.1016/j.fertnstert.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Mallidis C, Agbaje I, Rogers D, Glenn J, McCullough S, et al. Distribution of the receptor for advanced glycation end products in the human male reproductive tract: prevalence in men with diabetes mellitus. Hum Reprod. 2007;22:2169–77. doi: 10.1093/humrep/dem156. [DOI] [PubMed] [Google Scholar]
- 45.Yan SF, Yan SD, Ramasamy R, Schmidt AM. Tempering the wrath of RAGE: an emerging therapeutic strategy against diabetic complications, neurodegeneration, and inflammation. Ann Med. 2009;41:408–22. doi: 10.1080/07853890902806576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karimi J, Goodarzi MT, Tavilani H, Khodadadi I, Amiri I. Relationship between advanced glycation end products and increased lipid peroxidation in semen of diabetic men. Diabetes Res Clin Pract. 2011;91:61–6. doi: 10.1016/j.diabres.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 47.Karimi J, Goodarzi MT, Tavilani H, Khodadadi I, Amiri I. Increased receptor for advanced glycation end products in spermatozoa of diabetic men and its association with sperm nuclear DNA fragmentation. Andrologia. 2012;44(Suppl 1):280–6. doi: 10.1111/j.1439-0272.2011.01178.x. [DOI] [PubMed] [Google Scholar]
- 48.Rama Raju GA, Jaya Prakash G, Murali Krishna K, Madan K, Siva Narayana T, et al. Noninsulin-dependent diabetes mellitus: effects on sperm morphological and functional characteristics, nuclear DNA integrity and outcome of assisted reproductive technique. Andrologia. 2012;44(Suppl 1):490–8. doi: 10.1111/j.1439-0272.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- 49.Amiri I, Karimi J, Piri H, Goodarzi MT, Tavilani H, et al. Association between nitric oxide and 8-hydroxydeoxyguanosine levels in semen of diabetic men. Syst Biol Reprod Med. 2011;57:292–5. doi: 10.3109/19396368.2011.621508. [DOI] [PubMed] [Google Scholar]
- 50.La Vignera S, Condorelli RA, Vicari E, D’Agata R, Salemi M, et al. High levels of lipid peroxidation in semen of diabetic patients. Andrologia. 2012;44(Suppl 1):565–70. doi: 10.1111/j.1439-0272.2011.01228.x. [DOI] [PubMed] [Google Scholar]
- 51.Paasch U, Heidenreich F, Pursche T, Kuhlisch E, Kettner K, et al. Identification of increased amounts of eppin protein complex components in sperm cells of diabetic and obese individuals by difference gel electrophoresis. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.007187. M110.007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shrilatha B, Muralidhara Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: its progression and genotoxic consequences. Reprod Toxicol. 2007;23:578–87. doi: 10.1016/j.reprotox.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Ballester J, Muñoz MC, Domínguez J, Rigau T, Guinovart JJ, et al. Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J Androl. 2004;25:706–19. doi: 10.1002/j.1939-4640.2004.tb02845.x. [DOI] [PubMed] [Google Scholar]
- 54.Amaral S, Moreno AJ, Santos MS, Seiça R, Ramalho-Santos J. Effects of hyperglycemia on sperm and testicular cells of Goto-Kakizaki and streptozotocin-treated rat models for diabetes. Theriogenology. 2006;66:2056–67. doi: 10.1016/j.theriogenology.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Mallidis C, Green BD, Rogers D, Agbaje IM, Hollis J, et al. Metabolic profile changes in the testes of mice with streptozotocin-induced type 1 diabetes mellitus. Int J Androl. 2009;32:156–65. doi: 10.1111/j.1365-2605.2007.00829.x. [DOI] [PubMed] [Google Scholar]
- 56.Noguchi S, Ohba Y, Oka T. Involvement of epidermal growth factor deficiency in pathogenesis of oligozoospermia in streptozotocin-induced diabetic mice. Endocrinology. 1990;127:2136–40. doi: 10.1210/endo-127-5-2136. [DOI] [PubMed] [Google Scholar]
- 57.Singh S, Malini T, Rengarajan S, Balasubramanian K. Impact of experimental diabetes and insulin replacement on epididymal secretory products and sperm maturation in albino rats. J Cell Biochem. 2009;108:1094–101. doi: 10.1002/jcb.22337. [DOI] [PubMed] [Google Scholar]
- 58.Mangoli E, Talebi AR, Anvari M, Pourentezari M. Effects of experimentally-induced diabetes on sperm parameters and chromatin quality in mice. Iran J Reprod Med. 2013;11:53–60. [PMC free article] [PubMed] [Google Scholar]
- 59.Sasaki H, Matsui Y. Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat Rev Genet. 2008;9:129–40. doi: 10.1038/nrg2295. [DOI] [PubMed] [Google Scholar]
- 60.Rato L, Alves MG, Dias TR, Lopes G, Cavaco JE, et al. High-energy diets may induce a pre-diabetic state altering testicular glycolytic metabolic profile and male reproductive parameters. Andrology. 2013;1:495–504. doi: 10.1111/j.2047-2927.2013.00071.x. [DOI] [PubMed] [Google Scholar]
- 61.Bose R, Adiga SK, D’souza F, Salian SR, Uppangala S, et al. Germ cell abnormalities in streptozotocin induced diabetic mice do not correlate with blood glucose level. J Assist Reprod Genet. 2012;29:1405–13. doi: 10.1007/s10815-012-9873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimmins S, Sassone-Corsi P. Chromatin remodelling and epigenetic features of germ cells. Nature. 2005;434:583–9. doi: 10.1038/nature03368. [DOI] [PubMed] [Google Scholar]
- 63.Turek-Plewa J, Jagodzinski PP. The role of mammalian DNA methyltransferases in the regulation of gene expression. Cell Mol Biol Lett. 2005;10:631–47. [PubMed] [Google Scholar]
- 64.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–63. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 65.Pinney SE, Simmons RA. Metabolic programming, epigenetics, and gestational diabetes mellitus. Curr Diab Rep. 2012;12:67–74. doi: 10.1007/s11892-011-0248-1. [DOI] [PubMed] [Google Scholar]
- 66.Zamudio NM, Chong S, O’Bryan MK. Epigenetic regulation in male germ cells. Reproduction. 2008;136:131–46. doi: 10.1530/REP-07-0576. [DOI] [PubMed] [Google Scholar]
- 67.Jelodar G, Khaksar Z, Pourahmadi M. Endocrine profile and testicular histomorphometry in adult rat offspring of diabetic mothers. J Physiol Sci. 2009;59:377–82. doi: 10.1007/s12576-009-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–9. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huckins C, Clermont Y. Evolution of gonocytes in the rat testis during late embryonic and early post-natal life. Arch Anat Histol Embryol. 1968;51:341–54. [PubMed] [Google Scholar]
- 70.Bourc’his D, Bestor TH. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature. 2004;431:96–9. doi: 10.1038/nature02886. [DOI] [PubMed] [Google Scholar]
- 71.Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Totoki Y, Toyoda A, et al. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev. 2008;22:908–17. doi: 10.1101/gad.1640708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Webster KE, O’Bryan MK, Fletcher S, Crewther PE, Aapola U, et al. Meiotic and epigenetic defects in Dnmt3L-knockout mouse spermatogenesis. Proc Natl Acad Sci U S A. 2005;102:4068–73. doi: 10.1073/pnas.0500702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hata K, Kusumi M, Yokomine T, Li E, Sasaki H. Meiotic and epigenetic aberrations in Dnmt3L-deficient male germ cells. Mol Reprod Dev. 2006;73:116–22. doi: 10.1002/mrd.20387. [DOI] [PubMed] [Google Scholar]
- 74.Davis TL, Trasler JM, Moss SB, Yang GJ, Bartolomei MS. Acquisition of the H19 methylation imprint occurs differentially on the parental alleles during spermatogenesis. Genomics. 1999;58:18–28. doi: 10.1006/geno.1999.5813. [DOI] [PubMed] [Google Scholar]
- 75.Wilkins JF, Haig D. What good is genomic imprinting: the function of parent-specific gene expression. Nat Rev Genet. 2003;4:359–68. doi: 10.1038/nrg1062. [DOI] [PubMed] [Google Scholar]
- 76.Klenova EM, Morse HC, 3rd, Ohlsson R, Lobanenkov VV. The novel BORIS + CTCF gene family is uniquely involved in the epigenetics of normal biology and cancer. Semin Cancer Biol. 2002;12:399–414. doi: 10.1016/s1044-579x(02)00060-3. [DOI] [PubMed] [Google Scholar]
- 77.Kato Y, Kaneda M, Hata K, Kumaki K, Hisano M, et al. Role of the Dnmt3 family in de novo methylation of imprinted and repetitive sequences during male germ cell development in the mouse. Hum Mol Genet. 2007;16:2272–80. doi: 10.1093/hmg/ddm179. [DOI] [PubMed] [Google Scholar]
- 78.Ding GL, Wang FF, Shu J, Tian S, Jiang Y, et al. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes. 2012;61:1133–42. doi: 10.2337/db11-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wei Y, Yang CR, Wei YP, Zhao ZA, Hou Y, et al. Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals. Proc Natl Acad Sci U S A. 2014;111:1873–8. doi: 10.1073/pnas.1321195111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Costa FF. Non-coding RNAs, epigenetics and complexity. Gene. 2008;410:9–17. doi: 10.1016/j.gene.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 81.MacLennan NK, James SJ, Melnyk S, Piroozi A, Jernigan S, et al. Uteroplacental insufficiency alters DNA methylation, one-carbon metabolism, and histone acetylation in IUGR rats. Physiol Genomics. 2004;18:43–50. doi: 10.1152/physiolgenomics.00042.2004. [DOI] [PubMed] [Google Scholar]
- 82.Morris KV, Chan SW, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004;305:1289–92. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]


