Abstract
The aim of this systematic review is to determine the comparative effectiveness and safety of phosphodiesterase 5 inhibitors (PDE5-Is) and α-blockers used alone or combined for the treatment of lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH). An electronic search of PubMed, Cochrane Library and Embase up to January 2014 was performed to identify randomized controlled trials comparing the efficacy and safety of PDE5-Is and α-blockers for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia, which assessed IPSS score, maximum flow rate, postvoided residual urine, quality of life and Erectile Function (IIEF) score as outcomes. Data were analyzed by fixed or random effect models using Cochrane Collaboration review manager software. A total of 12 studies were included. Our novel data demonstrated that there was a trend that α-blockers were more efficacious than PDE5-Is on decreasing IPSS score and increasing maximum flow rate. α-blockers were significantly more effective than PDE5-Is on reduction of postvoided residual urine with a mean difference of 3.67 (95% CI 1.56 to 5.77, P = 0.0006) and PDE5-Is showed greater effect than α-blockers on increasing IIEF score with a mean difference of 9.82 (95% CI 3.80 to 15.85, P = 0.001). In conclusion, our novel data demonstrated that PDE5-Is plus ABs ranked the highest on the improvement of LUTS/BPH. PDE5-Is monotherapy was also effective in this kind of disorder except less reduction of PVR than ABs. In addition, both combined- or mono-therapy were safe.
Keywords: benign prostatic hyperplasia, lower urinary tract symptoms, meta-analysis, phosphodiesterase 5 inhibitors, α-adrenoceptor antagonists
INTRODUCTION
Lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH) are often bothersome and interfere quality of life (QoL) for aging males.1,2 A recent population-based study evaluated in the USA, the UK and Sweden indicated that the prevalence of at least one LUTS at least “sometimes” was 72.3% and 47.9% for at least “often” for males with mean age (range) of 56.6 (40–99) years.3 Pathologic study at autopsy in Asian and Caucasian men showed the overall prevalence of BPH was 74.8% in men with mean age (range) of 64.4 (22–89) years.4 LUTS/BPH led to high personal and societal costs, both in direct medical costs and indirect losses in daily functioning and cause a huge economic burden on the healthcare system and society.5,6
α-adrenoceptor antagonists (α-blockers, ABs) have represented the first-line drug treatment for LUTS/BPH recommended by guidelines, which reduced the sympathetic tone by blocking α-adrenergic receptors, subsequently resulted in the relaxation of smooth muscles in prostate/bladder neck, increase of urinary flow and amelioration of LUTS.7,8 Phosphodiesterase 5 inhibitors (PDE5-Is) have been globally used as the first-line drugs for erectile dysfunction (ED).9 As phosphodiesterase was also expressed and biologically active in the human bladder, urethra and prostatic tissue,10 it was postulated that PDE5-Is will also be efficacious for the treatment of LUTS/BPH. Sairam and his colleagues reported for the first time that PDE5-Is could improve urinary symptom scores in patients with ED in 2002.11 Since then, numerous clinical trials have proved the effectiveness of PDE5-Is for the treatment of LUTS/BPH and tadalafil was recently licensed in USA and in European Union for treating LUTS/BPH with or without ED.12 In addition, the combination of ABs with PDE5-Is was also investigated for the treatment of LUTS/BPH based on the hypothesis that blocking effect of α-adrenergic receptors by ABs could enhance the nitric oxide (NO)-mediated relaxation effect by PDE5-Is on the same smooth muscle targets.13
In 2012, Gacci et al.14 conducted an extensive pair-wise meta-analysis on the use of PDE5-Is alone or in combination with ABs for the treatment of LUTS/BPH, in which they indicated that PDE5-Is could significantly improve LUTS as compared with placebo and similar systematic reviews which compared PDE5-Is with placebo to determine their efficacy for treating LUTS/BPH were available in literature.15,16 But compared with ABs, the first-line drugs for LUTS/BPH, it was controversial whether PDE5-Is showed superior effect. Several clinical trials have assessed the efficacy of PDE5-Is for LUTS/BPH with setting ABs as a positive control, but there was no meta-analysis to summarize the comparative effectiveness of PDE5-Is with ABs. The aim of the present review was to compare the efficacy and safety of PDE5-Is and ABs used alone or combined for treating LUTS/BPH based on existing randomized controlled trials (RCTs), so as to provide a more systematic and comprehensive assessment for the use of PDE5-Is.
MATERIALS AND METHODS
Data sources and searches
We performed database searches of Cochrane Library (Issue 1, January 2014), PubMed (1966–January 2014) and Embase (1984–January 2014) using the following keywords in combination with both medical subject headings terms and text words: lower urinary tract symptom or benign prostatic hyperplasia/enlargement or bladder outlet obstruction plus a-adrenoceptor antagonists or alfuzosin or tamsulosin or doxazosin or terazosin or naftopidil or prazosin plus phosphodiesterase type 5 inhibitor or tadalafil or sildenafil or vardenafil or avanafil or lodenafil or mirodenafil or udenafil plus randomized controlled trials. There was no limitation on publication status or language.
Inclusion criteria
Inclusion criteria used to select studies were based on the principle of participant, intervention, control and outcome (PICO) as follows: (1) patients experienced LUTS/BPH with or without ED; (2) PDE5-Is including sildenafil, vardenafil, tadalafil, avanafil, lodenafil, mirodenafil and udenafil, as study intervention, were orally administered at any regimen and for any duration; (3) ABs including alfuzosin, tamsulosin, doxazosin, terazosin, naftopidil and prazosin or ABs plus PDE5-Is were used as control arms; (4) outcomes were measured by the changes from baseline to endpoint of International Prostate Symptom Score (IPSS), maximum flow rate (Qmax), postvoided residual urine (PVR), quality of life (QoL) and International Index of Erectile Function (IIEF); (5) the studies were RCTs.
Exclusion criteria
Repeat publications, sample size <10 and where studies were only reported superficially, such as in the form of an abstract.
Selection of studies
Three reviewers (MJS, SL and TL) independently screened the title, abstract and keywords of each article retrieved. Full-text papers were screened for further assessment if the information given suggested that the study met the inclusion criteria and did not meet the exclusion criteria.
Bias assessment
The methodological quality of included studies was appraised with the Cochrane Collaboration bias appraisal tool. In particular, the following factors were evaluated: (1) adequate sequence generation? (2) Allocation concealment? (3) Blinding of participants and personnel? (4) Blinding of outcome assessment? (5) Incomplete outcome data addressed? (6) Free of selective reporting? (7) Free of other bias?
Each question was answered with “low risk”, “high risk” or “unclear” and three reviewers (MJS, SL and TL) assessed each trial. Where differences in opinion existed, they were resolved through open discussion.
Data extraction
Data were extracted independently by three reviewers (MJS, SL and TL) using a standard form. Data of different subgroups were incorporated into one verum arm. Missing information was imputed based on the methods of Cochrane Handbook and was requested from the authors of original studies when necessary.
Pair-wised meta-analysis
The comparative effects of pair-wised meta-analysis were analyzed using Cochrane Collaboration review manager software (RevMan [Computer program] Version 5.0. Copenhagen: the Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Heterogeneity among studies was assessed with the Q test and the I2 index statistic. If P > 0.1 and I2 <<sup> 50%, which meant homogeneity existed among studies, we would use fixed-effect models for the calculation of pooled effect index and if P < 0.1 and I2 >50%, random-effect models would be applied. Summary effect was calculated as mean difference (MD) for continuous variable and odds ratio (OR) for rate variable, together with their 95% confidence intervals (CIs).
Safety assessment
Safety assessment was performed by comparing the adverse events (AEs) among medication arms. AEs of PDE5-Is and ABs reported at least in three studies were included in the meta-analysis.
Network meta-analysis
Comparative effects of PDE5-Is, ABs or combination therapy in the network were calculated using the automated software Aggregate Data Drug Information System (ADDIS).17 We created a consistency model by combining the effect of indirect and direct comparison based on Bayesian approach to get an absolute effect and cumulative probability which was used to rank the three regimens.
RESULTS
Characteristics of included studies
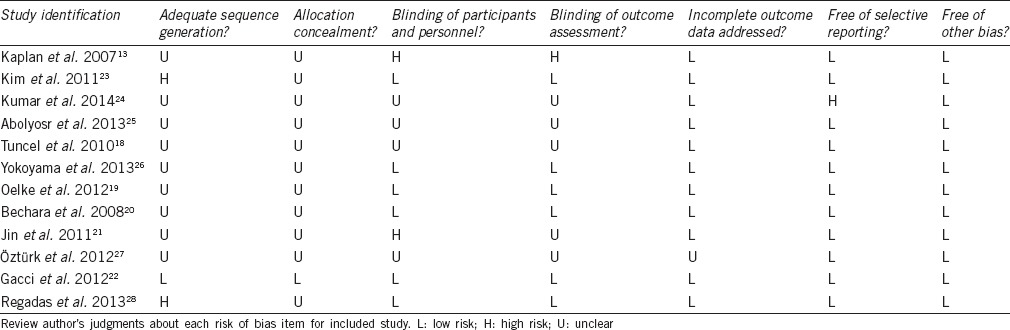
Using the database search strategy, a total of 121 records were retrieved from Cochrane Library, PubMed and Embase, of which 12 RCTs13,18,19,20,21,22,23,24,25,26,27,28 finally met full inclusion criteria for this review. In Jin's study,21 the treatment outcomes were analyzed at 12 and 24 weeks. As the treatment duration of most included studies was 12 weeks, we extracted the results from Jin's study at 12 weeks in order to control the heterogeneity between included trials. Figure 1 depicts the search process. Table 1 provides details of the included trials.
Figure 1.
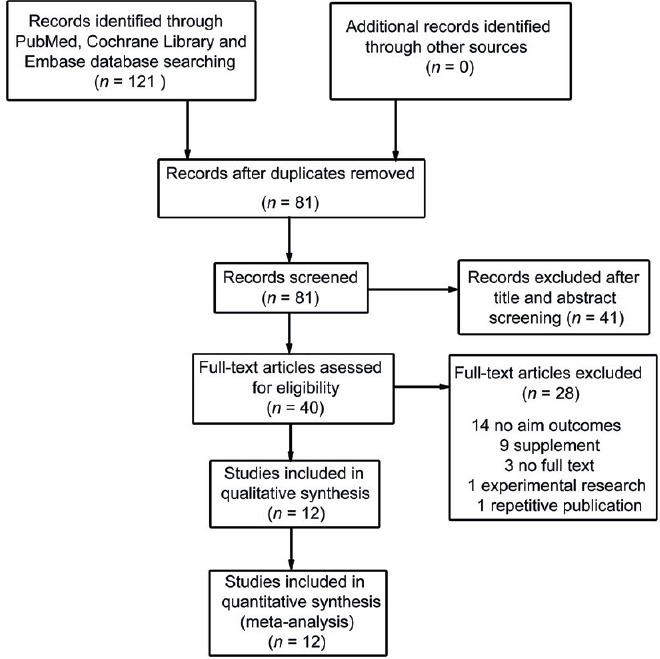
PRISMA flowchart of identification and selection of studies for inclusion in the systematic review.
Table 1.
Characteristics of the included studies in meta-analysis
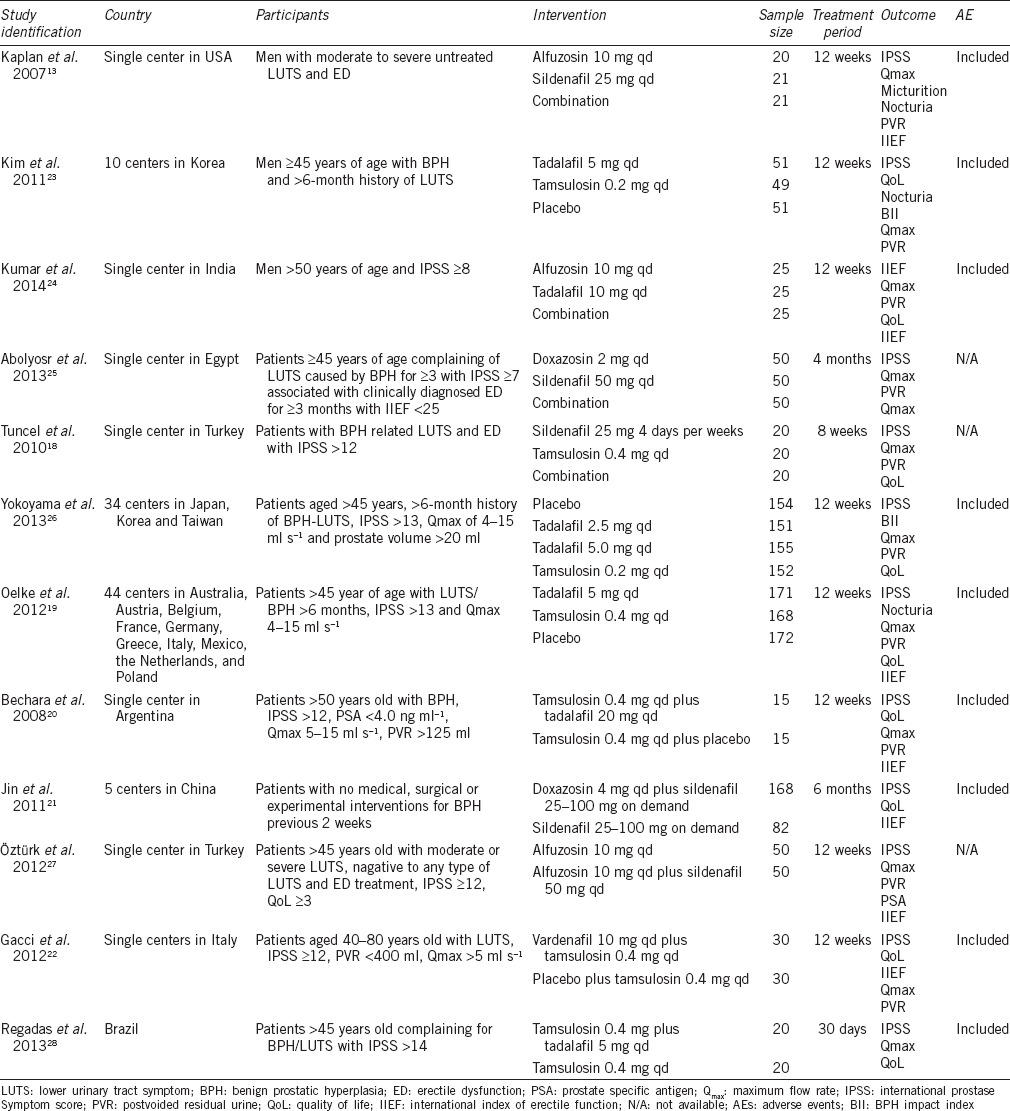
Risk of bias
As described in Table 2, most included studies did not describe their methods of randomization. Thus, it had unclear bias risk for the assessment of adequate sequence generation. Two studies23,28 stratified participants according to their baseline information. Only one study22 showed a method of allocation concealment and the others did not describe their approach. One study21 was designed as open label. One trial13 was performed with studied drug used as on-demand and it was not blind to patients. One study24 did not report all outcomes as their design, and we gave negative judgment for assessment of selective reporting. We gave positive judgment for all the included studies in the assessment of other bias, as we could not detect any risks.
Table 2.
Risk of bias summary
PDE5-Is versus ABs
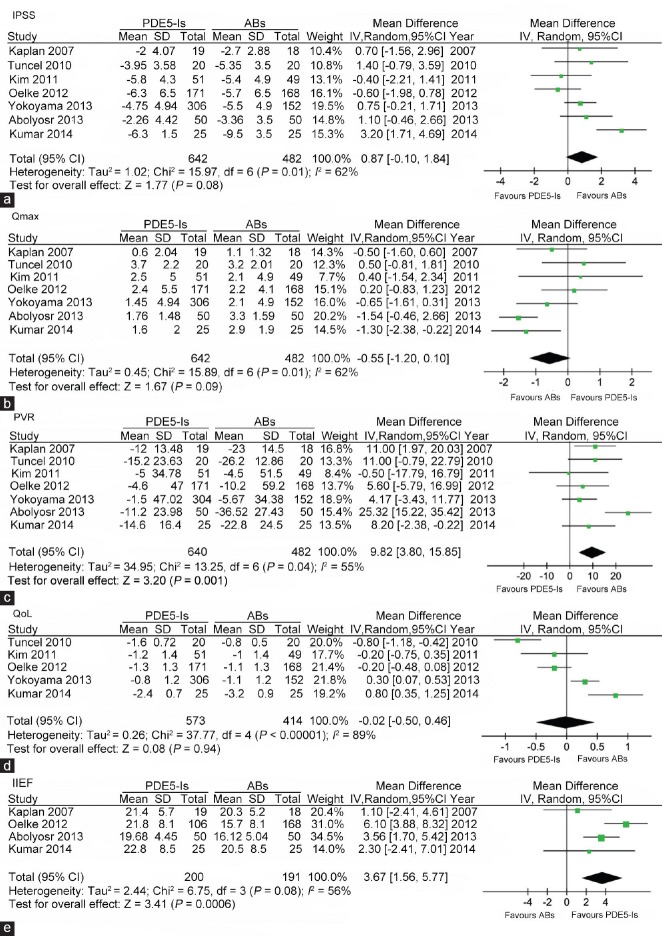
In the comparison of PDE5-Is with ABs, I2 standing for the heterogeneity among the studies was 62%, 62%, 55%, 89% and 56% for the assessment of IPSS, Qmax, PVR, QoL and IIEF, respectively. Thus, random-effect models were applied. As shown in Figure 2a, seven studies included scores of IPSS. The pooled mean difference (MD) for IPSS was 0.87 (95% CI − 0.01 to 1.84, P = 0.08), indicating no significant difference. Figure 2b shows details of seven studies including the assessment of Qmax. The pooled mean difference (MD) for Qmax was −0.55 (95% CI −1.20 to 0.10, P = 0.09) and the difference was not significant, either. Figure 2c-2e displays meta-analysis comparing PDE5-Is with ABs in terms of PVR, QoL and IIEF. The pooled MD was 9.82 (95% CI 3.80 to 15.85, P = 0.001), −0.02 (95% CI −0.50 to 0.46, P = 0.94), 3.67 (95% CI 1.56 to 5.77, P = 0.0006), respectively, which indicated that ABs had significant better effect on reducing PVR and less effect on increasing IIEF score, while both medications had comparable effect on improving QoL.
Figure 2.
Forest plot for meta-analysis of efficacy of PDE5-Is versus ABs by assessment of IPSS, Qmax, PVR, QoL and IIEF. (a) Pooled MD of score changes from baseline to treatment endpoint of IPSS; (b) Pooled MD of changes from baseline to treatment endpoint of Qmax; (c) Pooled MD of changes from baseline to treatment endpoint of PVR; (d) Pooled MD of changes from baseline to treatment endpoint of QoL; (e) Pooled MD of score of the IIEF at treatment endpoint. ABs: α-blockers; PDE5-Is: phosphodiesterase 5 inhibitors; Qmax: maximum flow rate; IIEF: international index of erectile function; QoL: quality of life; PVR: postvoided residual urine; MD: mean difference; IPSS: international prostate symptom score.
Sensitivity analysis was performed by excluding each of the seven studies. On the assessment of IPSS, when excluding the study of Kim et al. and Oelke et al. the pooled MD was 1.07 (95% CI 0.01 to 2.12, P = 0.05) and 1.16 (95% CI 0.18 to 2.14, P = 0.02), respectively, which meant the pooled effect had significant difference. Regarding Qmax, the pooled MD changed to − 0.72 (95% CI −1.36 to − 0.08, P = 0.03) and − 0.70 (95% CI −1.37 to − 0.04, P = 0.04) after excluding the study of Tuncel et al. and Oelke et al. which indicated statistical significance.
PDE5-Is versus PDE5-Is plus ABs
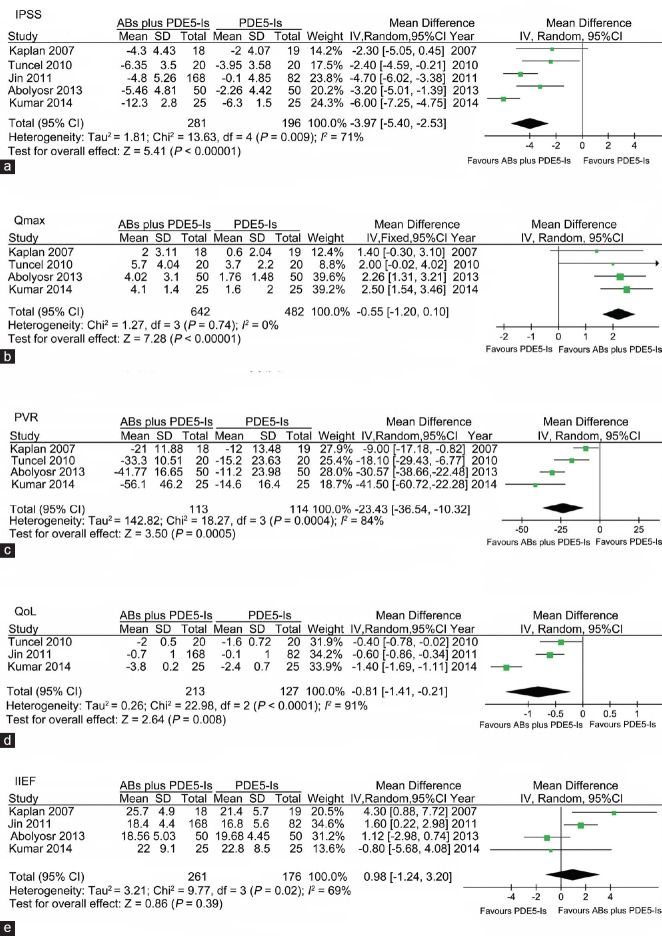
A total of five studies were included in the comparison of PDE5-Is with ABs plus PDE5-Is. I2 standing for the heterogeneity among the studies was 71%, 0%, 84%, 91% and 69% for the assessment of IPSS, Qmax, PVR, QoL and IIEF, respectively. As shown in Figure 3, the pooled MD for assessment of IPSS, Qmax, PVR and QoL was −3.97 (95% CI − 5.40 to −2.53, P < 0.00001), 2.22 (95% CI 1.63 to 2.82, P < 0.00001), −23.43 (95% CI −36.54 to −10.32, P = 0.0005) and −0.81 (95% CI −1.41 to −0.21, P = 0.008), respectively, which favoring combination therapy. In the assessment of IIEF, the pooled MD was 0.98 (95% CI −1.24 to 3.20, P = 0.39), indicating that PDE5-Is monotherapy had comparable effect on increasing IIEF score as compared with the combination therapy. Sensitivity analysis was performed by excluding each of the included studies, and the statistical significance of pooled effect was not changed.
Figure 3.
Forest plot for meta-analysis of efficacy of ABs plus PDE5-Is versus PDE5-Is by assessment of IPSS, Qmax, PVR, QoL and IIEF. (a) Pooled MD of score changes from baseline to treatment endpoint of IPSS; (b) Pooled MD of changes from baseline to treatment endpoint of Qmax; (c) Pooled MD of changes from baseline to treatment endpoint of PVR; (d) Pooled MD of changes from baseline to treatment endpoint of QoL; (e) Pooled MD of score of the IIEF at treatment endpoint. ABs: α-blockers; PDE5-Is: phosphodiesterase 5 inhibitors; Qmax: maximum flow rate; MD: mean difference; IPSS: international prostate symptom score; PVR: postvoided residual urine; QoL: quality of life; IIEF: international index of erectile function.
PDE5-Is plus ABs versus ABs
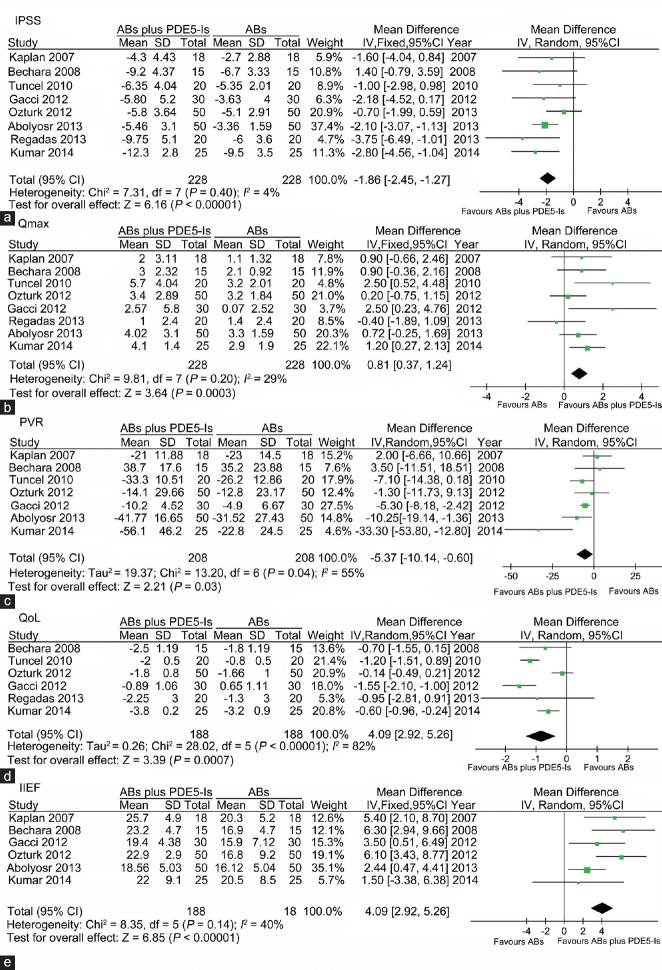
In the comparison of PDE5-Is plus ABs with ABs alone, I2 standing for the heterogeneity among the studies was 4%, 29%, 55%, 82% and 40% for the assessment of IPSS, Qmax, PVR, QoL and IIEF, respectively. As shown in Figure 4, the pooled MD for assessment of IPSS, Qmax, PVR, QoL and IIEF was −1.86 (95% CI -2.45 to − 1.27, P < 0.00001), 0.81 (95% CI 0.37 to 1.24, P = 0.0003), −5.37 (95% CI −10.14 to − 0.60, P = 0.03), −0.84 (95% CI −1.32 to − 0.35, P = 0.0007) and 4.09 (95% CI 2.29 to 5.26, P < 0.00001), respectively, all of which favoring combination therapy. Sensitivity analysis was performed by excluding each of the included studies, and the statistical significance of pooled effect was not changed.
Figure 4.
Forest plot for meta-analysis of efficacy of ABs plus PDE5-Is versus ABs by assessment of IPSS, Qmax, PVR, QoL and IIEF. (a) Pooled MD of score changes from baseline to treatment endpoint of IPSS; (b) Pooled MD of changes from baseline to treatment endpoint of Qmax; (c) Pooled MD of changes from baseline to treatment endpoint of PVR; (d) Pooled MD of changes from baseline to treatment endpoint of QoL; (e) Pooled MD of score of the IIEF at treatment endpoint. ABs: α-blockers; PDE5-Is: phosphodiesterase 5 inhibitors; IPSS: international prostate symptom score; MD: mean difference; PVR: postvoided residual urine; IIEF: international index of erectile function; Qmax: maximum flow rate; QoL: quality of life.
Safety assessment
AEs reported in the included studies are summarized in Table 3. Four studies reported the number of patients with any AEs. Figure 5a shows a total 143 of 549 patients suffering AE in the PDE5-Is arm compared to 94 of 389 in ABs group with an OR of 1.05 (95% CI 0.78 to 1.43), which meant the rate of any AEs between ABs group and PDE5-Is group was not significant. For comparing PDE5-Is with ABs in term of the common AEs, the incidence of dizziness, dyspepsia, headache and nasopharyngitis are analyzed and summarized in Figure 5b-5e, respectively. The corresponding OR was 0.56 (95% CI 0.21 to 1.48), 1.17 (95% CI 0.35 to 3.89), 1.38 (95% CI 0.58 to 3.25) and 0.98 (95% CI 0.39 to 2.44), respectively, indicating no significant difference, either.
Table 3.
Most common reported treatment-related AEs stratified according to trials and treatment arms
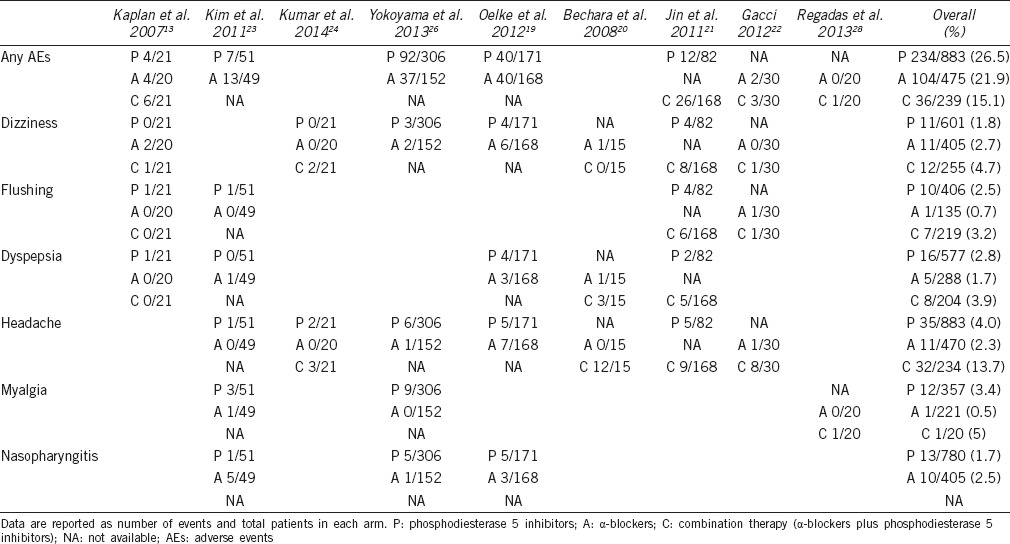
Figure 5.
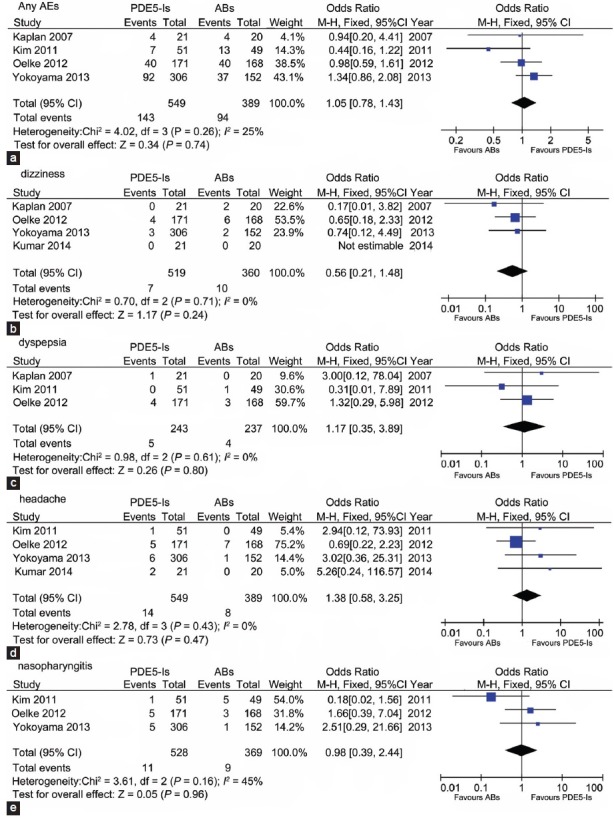
Forest plot for meta-analysis of adverse events of PDE5-Is versus ABs. OR of incidence of any adverse events (a); dizziness (b); dyspepsia (c); headache (d) and nasopharyngitis (e). ABs: α-blockers; PDE5-Is: phosphodiesterase 5 inhibitors; OR: odds ratio.
Network analysis
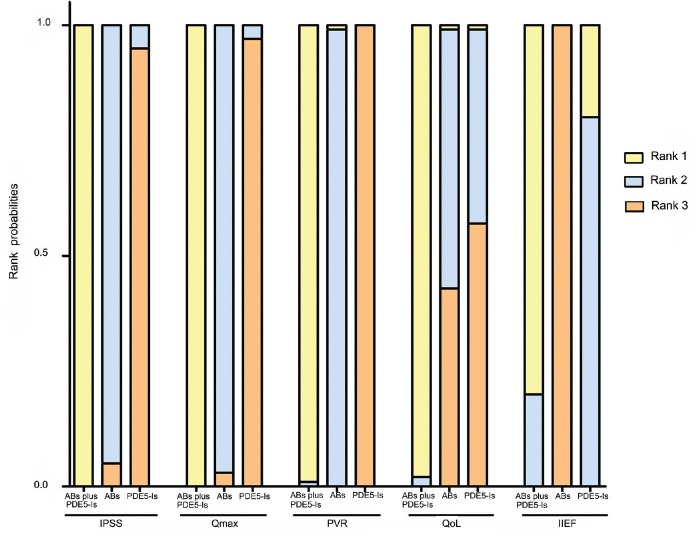
Figure 6 shows the rank probability of the medications, which indicating that the combination of PDE5-Is with ABs had the largest rank probability to gain rank 1 in the improvement of IPSS, Qmax, PVR, QoL and IIEF, while ABs ranked secondly in the assessment of IPSS, Qmax, PVR and QoL and PDE5-Is rank secondly for IIEF.
Figure 6.
Rank probabilities of ABs, PDE5-Is and combination therapy as measured by assessment of IPSS, Qmax, PVR, QoL and IIEF. The Bayesian approach could apply the rank probabilities of each drug therapy. Rank probabilities sum to one, both within a rank over treatments and within a treatment over ranks. Rank 1 represented the best efficacy on the reduction of IPSS total score, PVR, QoL and increase of Qmax and IIEF. ABs: α-blockers; PDE5-Is: phosphodiesterase 5 inhibitors; IPSS: international prostate symptom score; Qmax: maximum flow rate; PVR: postvoided residual urine; QoL: quality of life; IIEF: international index of erectile function.
DISCUSSION
This is the first systematic review and meta-analysis comparing the efficacy and safety of PDE5-Is and ABs used alone or combined for the treatment of LUTS/BPH. Our systematic review and meta-analysis indicated that there was a trend that ABs were more efficacious than PDE5-Is on decreasing IPSS score and increasing Qmax. Additionally, ABs were significantly more effective in reduction of PVR when comparing with PDE5-Is. On the other hand, PDE5-Is showed a better effect than ABs on increasing IIEF score and ranked secondly for improving erectile function. We also found that PDE5-Is plus ABs showed the best effect on the reduction of IPSS, PVR, QoL and the increase of Qmax, while this combination therapy showed comparable effect on increasing IIEF score when compared with PDE5-Is used alone. However, further clinical studies are required for longer duration, larger population size, as well as basic research investigating mechanisms involving PDE5-Is alleviate the symptoms of LUTS/BPH.
Numerous clinical trials have demonstrated the effectiveness of PDE5-Is for the treatment of LUTS/BPH with tadalafil (5 mg once daily) approved in 2011 in the USA and in the European Union in 2012 for the treatment of BPH/LUTS with or without ED. PDE5-Is may alleviate LUTS/BPH through several key mechanisms independently.10,29,30 In general, the plausible mechanism of PDE5-Is in treating LUTS/BPH may be: (1) slight-to-moderate relaxation of muscle tone in prostate and bladder; (2) Significant dilation of local blood vessels which provide adequate blood; (3) Significant augmentation of oxygen perfusion to local organs; (4) Inhibition of afferent nerve activity of bladder; (5) Bluntness of intraprostatic inflammation; (6) Antiproliferation in prostate.10 The effect of PDE5 inhibition leading to increase NO/cGMP concentration in the smooth muscle (SM) of the prostate, urethra, bladder, pelvic neuronal and vascular networks supports lower urinary tract function. Relaxation of the aforementioned SMs results in reduced BPH symptoms including ameliorated detrusor overactivity by increasing blood perfusion and decreasing lower urinary tract tone.31,32 Several reviews and meta-analysis have documented the effectiveness of PDE5-Is for the treatment of LUTS/BPH as compared with placebo.14,15,16 But our systematic review and meta-analysis compared, for the first time, the pooled effect of PDE5-Is versus ABs. We found that PDE5-Is were effective but numerically less effect on the reduction of total IPSS score as compared with ABs. However, the difference was not statistically significant, which could be attributed to the insufficient sample size.
Our meta-analysis also found that PDE5-Is had numerically less effect on the increase of Qmax as compared with ABs and the pooled effect was detected significantly different after excluding some studies. The ineffectiveness of PDE5-Is on urinary flow rate was evidenced by several reviews and meta-analysis when compared with placebo.14,15 Gacci explained that PDE5-Is concomitant relaxation of the detrusor muscle may counteract the relaxation of the prostate and bladder neck. But for detrusor SM, the role of PDE5-Is may not just be limited to relaxation and the mechanism remains to be fully clarified.33
The relaxation effect of PDE5-Is on detrusor could also contribute to the significant less decrease in PVR as compared with ABs, which was found in current study. Although PDE5-Is did not increase PVR in clinical trials when compared with placebo, it should be cautious to prescribe these drugs for patients with a large amount of PVR.
Importantly, our meta-analysis demonstrated that the combination of PDE5-Is with ABs showed more efficacious than both drugs used alone in the reduction of IPSS, QoL, PVR and increase of Qmax and it was consistent with the network analysis. This better effectiveness could be attributed to the synergistic effect of PDE5-NO pathway mediated relaxation and α-adrenoceptor blocking mediated reduction of the sympathetic tone of the same smooth muscles target in the prostate and bladder neck.13 However, there was no significant difference on the increase of IIEF between PDE5-Is plus ABs and PDE5-Is alone, which could be interpreted that ABs did not play a major role in penile erection.
In our present meta-analysis, we did not detect any significant difference in the incidence of any AEs, dizziness, dyspepsia, headache and nasopharyngitis between ABs and PDE5-Is. But it may be improper to compare AEs of different class of drugs, as the mechanism and type of treatment-related AEs were of diversity. As shown in Table 3, the incidence of AEs for the combination therapy was numerically higher than ABs or PDE5-Is used alone. But most cases of AEs were of mild to moderate and none treatment-related serious AEs were reported for the included studies. Therefore, the overall safety of the combination therapy was good.
A major limitation of the present systematic review was the quality of the original studies. As shown in Table 2, many studies had unclear bias risks, and this may limit the quality grade of evidence. In the comparison of PDE5-Is with ABs on assessment of IPSS and Qmax, the significance of the pooled effect changed when excluding some studies, indicating the results were not stable. This may be due to the quality of the original studies and the heterogeneity among included trials. In addition, the duration of the included studies was <12 weeks, studies with longer duration would be worthy.
CONCLUSION
Our novel data demonstrated that PDE5-Is plus ABs ranked the highest on the improvement of LUTS/BPH. PDE5-Is monotherapy was also effective in this kind of disorder except less reduction of PVR than ABs. In addition, both combined- or mono-therapy were safe.
AUTHOR CONTRIBUTIONS
XHW, XW and XHZ conceived and designed the study; conducted the search, selection, risk of bias assessment and data extraction; XW analyzed the data and XHZ. Wrote the manuscript; XHW and XHZ commented on drafts. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing financial interests.
ACKNOWLEDGMENTS
This work is supported by National Natural Science Foundation of China (No. 81172434, to XHW; No. 81270843 and No. 81160086 to XHZ).
REFERENCES
- 1.Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7) Eur Urol. 2003;44:637–49. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Robertson C, Link CL, Onel E, Mazzetta C, Keech M, et al. The impact of lower urinary tract symptoms and comorbidities on quality of life: the BACH and UREPIK studies. BJU Int. 2007;99:347–54. doi: 10.1111/j.1464-410X.2007.06609.x. [DOI] [PubMed] [Google Scholar]
- 3.Coyne KS, Sexton CC, Thompson CL, Milsom I, Irwin D, et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int. 2009;104:352–60. doi: 10.1111/j.1464-410X.2009.08427.x. [DOI] [PubMed] [Google Scholar]
- 4.Zlotta AR, Egawa S, Pushkar D, Govorov A, Kimura T, et al. Prevalence of inflammation and benign prostatic hyperplasia on autopsy in Asian and Caucasian men. Eur Urol. 2014;66:619–22. doi: 10.1016/j.eururo.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Speakman M, Kirby R, Doyle S, Ioannou C. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) – Focus on the UK. BJU Int. 2015;115:508–19. doi: 10.1111/bju.12745. [DOI] [PubMed] [Google Scholar]
- 6.Issa MM, Regan TS. Medical therapy for benign prostatic hyperplasia – Present and future impact. Am J Manag Care. 2007;13(Suppl 1):S4–9. [PubMed] [Google Scholar]
- 7.Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013;64:118–40. doi: 10.1016/j.eururo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 8.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–803. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 9.Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010;57:804–14. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Andersson KE, de Groat WC, McVary KT, Lue TF, Maggi M, et al. Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: pathophysiology and mechanism(s) of action. Neurourol Urodyn. 2011;30:292–301. doi: 10.1002/nau.20999. [DOI] [PubMed] [Google Scholar]
- 11.Sairam K, Kulinskaya E, McNicholas TA, Boustead GB, Hanbury DC. Sildenafil influences lower urinary tract symptoms. BJU Int. 2002;90:836–9. doi: 10.1046/j.1464-410x.2002.03040.x. [DOI] [PubMed] [Google Scholar]
- 12.Hatzimouratidis K. A review of the use of tadalafil in the treatment of benign prostatic hyperplasia in men with and without erectile dysfunction. Ther Adv Urol. 2014;6:135–47. doi: 10.1177/1756287214531639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan SA, Gonzalez RR, Te AE. Combination of alfuzosin and sildenafil is superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. Eur Urol. 2007;51:1717–23. doi: 10.1016/j.eururo.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Gacci M, Corona G, Salvi M, Vignozzi L, McVary KT, et al. A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with alpha-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol. 2012;61:994–1003. doi: 10.1016/j.eururo.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 15.Laydner HK, Oliveira P, Oliveira CR, Makarawo TP, Andrade WS, et al. Phosphodiesterase 5 inhibitors for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a systematic review. BJU Int. 2011;107:1104–9. doi: 10.1111/j.1464-410X.2010.09698.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Zheng S, Han P, Wei Q. Phosphodiesterase-5 inhibitors for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a systematic review and meta-analysis. Urology. 2011;77:123–9. doi: 10.1016/j.urology.2010.07.508. [DOI] [PubMed] [Google Scholar]
- 17.van Valkenhoef G, Tervonen T, Zwinkels T, de Brock B, Hillege H. ADDIS: a decision support system for evidence-based medicine. Decis Support Syst. 2013;55:459–75. [Google Scholar]
- 18.Tuncel A, Nalcacioglu V, Ener K, Aslan Y, Aydin O, et al. Sildenafil citrate and tamsulosin combination is not superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. World J Urol. 2010;28:17–22. doi: 10.1007/s00345-009-0484-z. [DOI] [PubMed] [Google Scholar]
- 19.Oelke M, Giuliano F, Mirone V, Xu L, Cox D, et al. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol. 2012;61:917–25. doi: 10.1016/j.eururo.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Bechara A, Romano S, Casabé A, Haime S, Dedola P, et al. Comparative efficacy assessment of tamsulosin vs. tamsulosin plus tadalafil in the treatment of LUTS/BPH. Pilot study. J Sex Med. 2008;5:2170–8. doi: 10.1111/j.1743-6109.2008.00940.x. [DOI] [PubMed] [Google Scholar]
- 21.Jin Z, Zhang ZC, Liu JH, Lu J, Tang YX, et al. An open, comparative, multicentre clinical study of combined oral therapy with sildenafil and doxazosin GITS for treating Chinese patients with erectile dysfunction and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Asian J Androl. 2011;13:630–5. doi: 10.1038/aja.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gacci M, Vittori G, Tosi N, Siena G, Rossetti MA, et al. A randomized, placebo-controlled study to assess safety and efficacy of vardenafil 10 mg and tamsulosin 0.4 mg vs. tamsulosin 0.4 mg alone in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Sex Med. 2012;9:1624–33. doi: 10.1111/j.1743-6109.2012.02718.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim SC, Park JK, Kim SW, Lee SW, Ahn TY, et al. Tadalafil administered once daily for treatment of lower urinary tract symptoms in Korean men with benign prostatic hyperplasia: results from a placebo-controlled pilot study using tamsulosin as an active control. LUTS Low Urin Tract Symptoms. 2011;3:86–93. doi: 10.1111/j.1757-5672.2011.00088.x. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Kondareddy C, Ganesamoni R, Nanjappa B, Singh SK. Randomized controlled trial to assess the efficacy of the combination therapy of alfuzosin and tadalafil in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. LUTS Low Urin Tract Symptoms. 2014;6:35–40. doi: 10.1111/luts.12016. [DOI] [PubMed] [Google Scholar]
- 25.Abolyosr A, Elsagheer GA, Abdel-Kader MS, Hassan AM, Abou-Zeid AM. Evaluation of the effect of sildenafil and/or doxazosin on benign prostatic hyperplasia-related lower urinary tract symptoms and erectile dysfunction. Urol Ann. 2013;5:237–40. doi: 10.4103/0974-7796.120293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoyama O, Yoshida M, Kim SC, Wang CJ, Imaoka T, et al. Tadalafil once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a randomized placebo- and tamsulosin-controlled 12-week study in Asian men. Int J Urol. 2013;20:193–201. doi: 10.1111/j.1442-2042.2012.03130.x. [DOI] [PubMed] [Google Scholar]
- 27.Öztürk MI, Kalkan S, Koca O, Günes M, Akyüz M, et al. Efficacy of alfuzosin and sildenafil combination in male patients with lower urinary tract symptoms. Andrologia. 2012;44(Suppl 1):791–5. doi: 10.1111/j.1439-0272.2011.01268.x. [DOI] [PubMed] [Google Scholar]
- 28.Regadas RP, Reges R, Cerqueira JB, Sucupira DG, Josino IR, et al. Urodynamic effects of the combination of tamsulosin and daily tadalafil in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia: a randomized, placebo-controlled clinical trial. Int Urol Nephrol. 2013;45:39–43. doi: 10.1007/s11255-012-0317-7. [DOI] [PubMed] [Google Scholar]
- 29.Giuliano F, Ückert S, Maggi M, Birder L, Kissel J, et al. The mechanism of action of phosphodiesterase type 5 inhibitors in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Eur Urol. 2013;63:506–16. doi: 10.1016/j.eururo.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Brock GB, McVary KT, Roehrborn CG, Watts S, Ni X, et al. Direct effects of tadalafil on lower urinary tract symptoms versus indirect effects mediated through erectile dysfunction symptom improvement: integrated data analyses from 4 placebo controlled clinical studies. J Urol. 2014;191:405–11. doi: 10.1016/j.juro.2013.09.057. [DOI] [PubMed] [Google Scholar]
- 31.Morelli A, Sarchielli E, Comeglio P, Filippi S, Mancina R, et al. Phosphodiesterase type 5 expression in human and rat lower urinary tract tissues and the effect of tadalafil on prostate gland oxygenation in spontaneously hypertensive rats. J Sex Med. 2011;8:2746–60. doi: 10.1111/j.1743-6109.2011.02416.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Zang N, Wei Y, Yin J, Teng R, et al. Testosterone regulates smooth muscle contractile pathways in the rat prostate: emphasis on PDE5 signaling. Am J Physiol Endocrinol Metab. 2012;302:E243–53. doi: 10.1152/ajpendo.00458.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kajioka S, Nakayama S, Seki N, Naito S, Brading AF. Oscillatory membrane currents paradoxically induced via NO-activated pathways in detrusor cells. Cell Calcium. 2008;44:202–9. doi: 10.1016/j.ceca.2007.11.008. [DOI] [PubMed] [Google Scholar]