Abstract
This multi-center, cross-sectional study investigated the association between serum testosterone (T) levels, serum sex hormone-binding globulin (SHBG) levels, and the risk of metabolic syndrome (MS) in 3332 adult Chinese men. The prevalence of MS was 34.7%, and men with MS had lower serum levels of total T (TT) and SHBG than those without MS (P < 0.001). There was no significant difference in serum free T (FT) levels between subjects with and without MS (P = 0.627). In logistic regression analysis, the association between MS and serum SHBG levels persisted after adjusting for age, body mass index (BMI), smoking and drinking status, and serum TT (odds ratio [OR] 0.962, 95% confidence interval [95% CI] 0.954−0.969, P< 0.01). However, the association between serum TT level and the risk of MS was weak after adjusting for age, BMI, SHBG level, and smoking and drinking status (OR 0.981, 95% CI 0.960−1.007). Our study reveals that both serum TT and SHBG levels, but not serum FT, are inversely associated with the prevalence of MS and that serum SHBG is an independent and dominant risk factor for MS.
Keywords: metabolic syndrome, sex hormone-binding globulin, testosterone
INTRODUCTION
Metabolic syndrome (MS) is a cluster of components including visceral obesity, insulin resistance, hypertension, glucose intolerance, and dyslipidemia that substantially increases the risk of type 2 diabetes mellitus and cardiovascular disease.1 It has been reported that the prevalence of MS in the adult population was estimated to be 29.0% in Koreans,2 32.3% in Indians3 and 34.3% in Malaysians,4 according to the definition of the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III).
Serum testosterone (T) consists of three components, which are (1) T bound to sex hormone-binding globulin (SHBG), (2) T bound to albumin, and (3) free T (FT). Traditionally, FT is considered the main component with hormonal activity, and serum SHBG is considered a transport protein for T to target tissues, regulating the circulating concentration of FT. Previous research has shown that low serum T levels are closely associated with MS and its components.5,6,7,8,9,10 There is evidence that T supplement therapy can decrease visceral fat and ameliorate insulin resistance, suggesting that serum T plays a protective role in men with MS.11 However, recent research demonstrated that serum SHBG can directly exert effects on cells by binding to its receptor and acting as a hormone.12 Another study also indicated that serum SHBG concentrations, but not serum T levels, were independently associated with the risk of MS.13 These findings have conflict with the dominant role of serum T on the risk of MS.
Although many studies have investigated the association between serum sex hormones (total T [TT], FT) and the risk of MS,5,7 only a few have focused on the role of serum SHBG in MS especially in the Chinese population. Therefore, we conducted this study in a large sample from the Chinese population to ascertain whether serum T or SHBG is an independent and dominant factor for the risk of MS.
MATERIALS AND METHODS
Study design
This study was a cross-sectional survey of 3600 community-dwelling adult Chinese men aged from 20 to 89 years in three different areas of Hebei, Shanxi, and Jiangsu provinces, selected via cluster and age-stratified sampling. Each survey spot identified a local population register to provide a sampling frame from which participants were randomly selected. A total of 1200 men were recruited from each survey spot between July 2013 and January 2014 after obtaining written informed consent. The study and consent form were approved by the Ethics Committee and Institutional Review Board.
Participants
The participants were invited to attend a local reproductive health service facility to complete interviewer-assisted questionnaires and to undergo a general physical examination, cognitive performance tests, height and weight measurements, and blood tests for biochemical and hormone levels. Because some diseases or treatments may influence sex hormone levels, the exclusion criteria were defined as follows: (1) previously or currently diagnosed malignancies, corticosteroid use, or presence of liver cirrhosis; (2) T replacement, T supplement, or androgen-deprivation therapy use, 5-α reductase inhibitor treatment, or history of orchiectomy; and (3) current hypothalamus-pituitary disease, acute cardiovascular disease, or acute or chronic renal failure. After screening, 3332 participants were included in the data analysis.
Questionnaires
Data regarding sociodemographic and general health status, medical conditions, medications, and lifestyle were obtained from the questionnaires. Alcohol consumption was defined as one or more alcoholic drinks, including beer, wine, and spirits, per week. Smoking status was classified as never or smoking.
Clinical and laboratory measurements
Height, weight, body mass index (BMI), hip circumference (HC), and waist circumference (WC) were measured to the nearest 0.1 kg and 0.1 cm. WC was measured at the midpoint between the inferior costal margin and the superior border of the iliac crest on the midaxillary line. BMI was calculated from the weight (kg) divided by height (m). Blood pressures were measured twice after resting for >15 min by well-trained nurses using a mercury sphygmomanometer, and the average values were included in analyses. A single fasting venous blood sample was obtained from each participant in the morning (before 9 a.m.). Serum samples were measured together in batches in the central laboratory of the Beijing Coordinating Center. Fasting plasma glucose levels and serum levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were measured using a Chemix-180 biochemical analyzer (Sysmex, Kobe, Japan). TT, SHBG, and luteinizing hormone (LH) levels were measured using a Beckman UniCel DXI800 automatic chemiluminescence immune analyzer (Beckman Coulter, Fullerton, California, USA). The lower limits of the TT, SHBG, and LH levels were 0.35 nmol l−1, 0.017 nmol l−1, and 0.2 IU l−1, respectively. The intra-assay coefficients of variation (CVs) for TT, SHBG, and LH were 2.7%, 4.8%, and 3.8%, respectively. The mean inter-assay CVs for TT, SHBG, and LH were 5.6%, 5.3%, and 6.4%, respectively. The FT index (FTI) was calculated as T divided by SHBG, and the T secretion index (TSI) was calculated as T divided by LH. Calculated FT (cFT) concentrations were obtained from serum TT, SHBG, and albumin concentrations using the methods proposed by Vermeulen et al.14
Definition of metabolic syndrome
A modified NCEP ATP III definition appropriate for Asian populations was adopted in this study, defined as the presence of three or more of the following MS characteristics: (1) WC ≥90 cm; (2) systolic blood pressure (SBP) ≥130 mmHg, diastolic blood pressure (DBP) ≥85 mmHg, or use of antihypertensive medication; (3) HDL cholesterol <40 mg dl−1 or lipid medication use; (4) fasting plasma glucose ≥110 mg dl−1 or use of antidiabetic medication; and (5) TGs ≥150 mg dl−1 or use of lipid-lowering therapy.15
Statistical analyses
Statistical analyses were performed using SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA). Continuous variables were represented as mean ± standard deviation (s.d.), and categorical data were represented by number (n) and percentage. Comparability between the two groups was tested using the independent two-sample t-test for continuous variables and the Chi-square test for categorical variables. Mean sex hormone levels were compared by one-way analysis of variance by number of MS characteristics in subjects with MS, and post hoc multiple comparisons were assessed using Bonferroni's protected least significant difference test. Spearman's correlations were used to determine the associations between hormones and each MS characteristic. The MS characteristics were dichotomized (1 = the presence or 0 = the absence of a particular criterion, e.g. elevated blood pressure) and entered into the multiple regression equation with hormone level as the predicted outcome. Linear regression models (unadjusted and adjusted) were used to assess the association between MS status (as the independent variable) with various continuous and binary outcome variables. Logistic regression analysis was performed to analyze the odds ratios (ORs) of sex hormone parameters associated with subjects with MS. 95% confidence intervals (95% CIs) were estimated to describe the magnitude of associations. All statistical assessments were two-sided, and P < 0.05 was considered significant.
RESULTS
Subject characteristics
The characteristics of the subjects are shown in Table 1. From a total of 3600 invitees, 3332 Chinese men aged 20-89 years were recruited with a mean age of 54.69 years, including 1, 158 subjects with MS and 2, 174 subjects without MS. The prevalence of MS in this population was 34.7%. Mean weight, height, BMI, WC, HC, waist to hip ratio, TC, and FTI were significantly higher (P < 0.001), whereas mean TT, SHBG, and TSI were notably lower (P < 0.001) in the MS subjects compared to the non-MS subjects. No significant differences in age (P = 0.877) and cFT (P = 0.627), LH (P = 0.087), and LDL (P = 0.076) levels were found between the MS subjects and the non-MS subjects.
Table 1.
Characteristics of Chinese men with and without MS (n=3332)
The impact of increasing numbers of metabolic syndrome characteristics on serum total testosterone, sex hormone-binding globulin, and calculated free testosterone levels
It is interesting that mean serum TT levels, especially for mean serum SHBG levels, gradually declined with the addition of MS characteristics (P < 0.05), whereas mean serum cFT levels were unchanged (P > 0.05) (Table 2 and Figure 1).
Table 2.
Mean sex hormone concentrations according to the number of MS characteristics
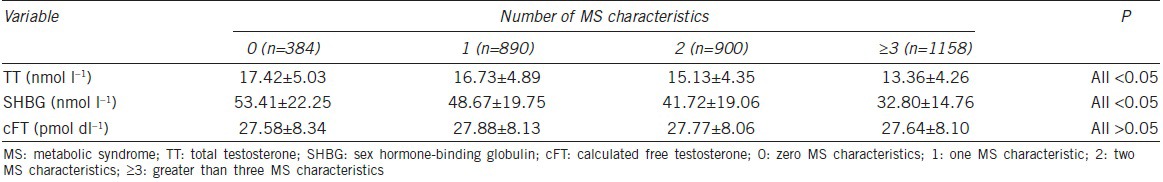
Figure 1.
Means of sex hormone levels by number of components of metabolic syndrome.
Associations between sex hormones and each metabolic syndrome characteristic
The correlations between serum TT, SHBG, and cFT and each MS characteristic are presented in Table 3. There were significant correlations between serum TT and SHBG and each MS characteristic (except for SBP). However, a weak inverse correlation or no correlation was detected between serum cFT and each MS characteristic. Moreover, there was a stronger trend in the correlation coefficient of serum SHBG compared to that of serum TT with regard to WC, HDL, TG, DBP, and BMI.
Table 3.
Correlation between MS characteristics and TT, cFT, and SHBG
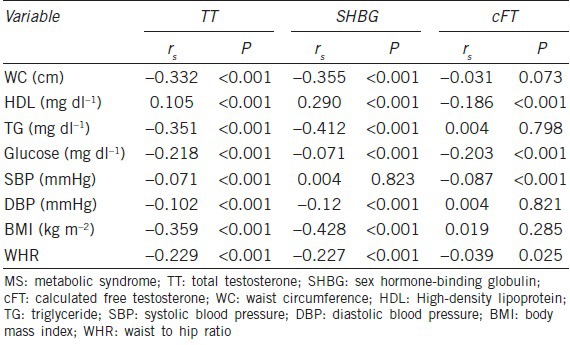
In multiple linear regression analysis, all the characteristics of MS were significantly and inversely associated with serum TT and SHBG levels, and their significance persisted after adjustment for smoking and drinking status and age (Table 4). Nevertheless, elevated BP was still significantly associated with serum SHBG levels (β = −0.062, P < 0.01), but the association with serum TT levels was lost (β = −0.012, P = 0.504), after further adjustment for BMI.
Table 4.
Multiple linear regression analysis of the relationship between serum TT or SHBG and MS characteristics
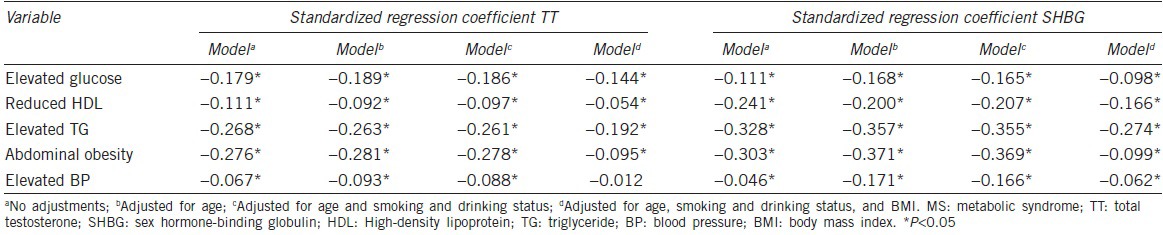
Associations between sex hormones and metabolic syndrome
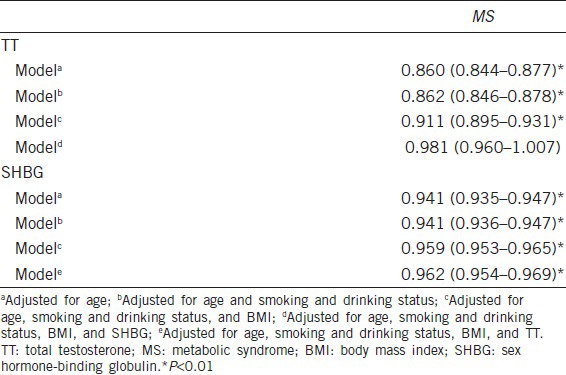
In logistic regression analysis, the significant associations between serum TT and SHBG levels and MS persisted after controlling for potential confounders, such as age, BMI, and smoking and drinking status (P < 0.01) (Table 5). Furthermore, the significant association between MS and serum SHBG persisted after adjusting for TT (OR 0.962, 95% CI 0.954−0.969, P < 0.01) (Table 5). However, the association between serum TT and the risk of MS was not significant after adjusting for age, BMI, SHBG, and smoking and drinking status (OR 0.981, 95% CI 0.960−1.007, P = 0.164) (Table 5).
Table 5.
Multiple logistic regression analysis of the relationship between TT, SHBG and MS
DISCUSSION
This cross-sectional study evaluated the associations between sex hormone levels and MS among Chinese men. The prevalence of MS defined using the NCEP ATP III criteria was found to be 34.7%, which is close to that in previous reports in China.16,17
Our results are consistent with many findings of cross-sectional and longitudinal epidemiological studies in Australia18 and Korea,19 in which serum TT and SHBG levels were lower in men with MS. In accordance with Maggio's findings,20 the results from our study reveal that there is no significant difference in serum cFT levels between subjects with and without MS. Moreover, the mean serum cFT level correlated less with the characteristics of MS than with serum TT and SHBG levels, which is similar to the findings from other studies.19,21,22,23,24 However, some reports were inconsistent with our findings.25,26 Therefore, the association between serum cFT and the risk of MS or MS characteristics is equivocal.25,27 One possible explanation for this discrepancy is the mean age of the study population because a stronger association has been observed in younger men.5 Furthermore, the inverse association between serum cFT level and MS tended to be weaker among studies that adopted the NCEP ATP III criteria.5 Another possible explanation is that serum TT levels decrease in moderately obese men along with a reduction in serum SHBG-binding capacity while serum cFT levels decrease only in severely obese men for whom LH pulse amplitude is reduced.28 Further studies investigating the correlation between serum cFT levels and MS are required to confirm this relationship.
The most interesting finding from our study was that the associations of serum TG, HDL, WC, and DBP with serum SHBG levels were stronger than those with serum TT. The potential mechanisms underlying these associations remain poorly elucidated. Serum SHBG levels positively correlate with serum adiponectin concentrations, and lower levels of serum adiponectin are associated with MS.29,30 In addition, serum SHBG may exert direct or indirect effects on HDL cholesterol metabolism and regulate glucose homeostasis and fatty acid metabolism.31,32 After adjusting for smoking and drinking status, age, and BMI in our multiple regression analysis, all MS characteristics remained significantly associated with serum SHBG concentrations, but the association between BP and serum TT level was eliminated. These findings are consistent with those of epidemiological studies reported previously in Australia.18 Recent research also showed that serum SHBG, but not serum TT, was associated with increasing BP.33 One possible explanation for this contradiction is that there is a stronger association between serum SHBG levels and renin concentrations in men with hypertension, which could be a new pathway for the direct effect of serum SHBG on BP control.34
It is not clear whether serum TT level is associated with the prevalence of MS independently of serum SHBG level and vice versa. In a recent study from southern China reported by Zhang et al.35 both serum SHBG and TT levels were inversely and significantly associated with the prevalence of MS independently of one another. In the logistic regression analysis from the present study, we also found that both serum SHBG and TT levels were inversely and significantly associated with MS after adjusting for age, BMI, and smoking and drinking status. However, after further adjusting for serum TT, serum SHBG remained inversely associated with MS while serum TT was not inversely associated with MS after further adjusting for SHBG. In contrast to Zhang's study,35 in which the mean age of subjects was 37.6 years, our study was performed in a relatively older population of Chinese men, with an average age of 54.69 years. Our findings are consistent with a study reported by Bhasin et al.13 who showed that serum SHBG concentration, but not serum TT concentration, is an independent predictor of incident MS. The effects of serum SHBG concentration on MS may be induced by the following: (1) mediation of steroid hormone signal transduction at the plasma membrane;36 (2) production of a direct effect in endothelial cells through the SHBG receptor;37 and (3) direct effects on the cell by binding to its receptor, acting as a hormone.12 Finally, recent research demonstrated that single nucleotide polymorphisms of the SHBG gene (rs6259, rs3760213) affect not only serum SHBG levels but also the risk of MS in Han Chinese men, suggesting a potential causal role for serum SHBG.38 Thus, serum SHBG concentration may be an independent and dominant risk factor for MS.
There are several strengths to our study. First, it included a large sample size (n = 3332), and participants were community-dwelling and comprised a wide age range (aged 20−89 years). Furthermore, potential confounders such as age, drinking, smoking, and BMI were adjusted for our analyses. Finally, three different geographic regions in China were selected, which is more representative of the Chinese population than only one geographical region. However, there are some limitations to this study. First, serum estradiol levels were not measured, so its role in the risk of MS could not be evaluated. Second, the chemiluminescence immunoassay used in the present study is less accurate than LC-MS in determining sex hormone levels, but it is commonly used in epidemiological studies with large sample sizes.39,40
In conclusion, the results from our study reveal that both serum TT and SHBG levels, but not serum cFT levels, are inversely associated with the characteristics and prevalence of MS. Serum SHBG level, but not serum TT level, is an independent and dominant risk factor for MS. The role of serum SHBG concentration in MS should be taken into consideration for further studies.
AUTHOR CONTRIBUTIONS
YHY gathered the data, performed the statistical analysis, interpreted the results, and drafted and revised the manuscript. YQG, CLX, and XJS conceived and designed the study, XJS and YQG coordinated and supervised the project and helped to draft the manuscript. MJZ, CCW, WWL, XWL, and SJZ participated in data collection and performed the laboratory measurements. All authors read and approved the final manuscript.
COMPETING INTEREST
The authors declare no competing interests.
ACKNOWLEDGMENTS
We gratefully acknowledge the help of professors Yuan-Zhong Zhou and Hong-Gang Li for their excellent technical assistance in the statistical data analysis and hormone assays. This work was financed by the National “Twelfth Five-Year” Plan for Science and Technology Support (2012BAI32B03).
REFERENCES
- 1.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 2.Oh JY, Hong YS, Sung YA, Barrett-Connor E. Prevalence and factor analysis of metabolic syndrome in an urban Korean population. Diabetes Care. 2004;27:2027–32. doi: 10.2337/diacare.27.8.2027. [DOI] [PubMed] [Google Scholar]
- 3.Das M, Pal S, Ghosh A. Prevalence of the metabolic syndrome in people of Asian Indian origin: outcomes by definitions. Cardiovasc J Afr. 2011;22:303–5. doi: 10.5830/CVJA-2010-070. [DOI] [PubMed] [Google Scholar]
- 4.Mohamud WN, Ismail AA, Sharifuddin A, Ismail IS, Musa KI, et al. Prevalence of metabolic syndrome and its risk factors in adult Malaysians: results of a nationwide survey. Diabetes Res Clin Pract. 2011;91:239–45. doi: 10.1016/j.diabres.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol. 2011;40:189–207. doi: 10.1093/ije/dyq158. [DOI] [PubMed] [Google Scholar]
- 6.Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, et al. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91:843–50. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez A, Muller DC, Metter EJ, Maggio M, Harman SM, et al. Aging, androgens, and the metabolic syndrome in a longitudinal study of aging. J Clin Endocrinol Metab. 2007;92:3568–72. doi: 10.1210/jc.2006-2764. [DOI] [PubMed] [Google Scholar]
- 8.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762–9. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litman HJ, Bhasin S, Link CL, Araujo AB, McKinlay JB. Serum androgen levels in black, Hispanic, and white men. J Clin Endocrinol Metab. 2006;91:4326–34. doi: 10.1210/jc.2006-0037. [DOI] [PubMed] [Google Scholar]
- 10.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–35. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 11.Haider A, Gooren LJ, Padungtod P, Saad F. Improvement of the metabolic syndrome and of non-alcoholic liver steatosis upon treatment of hypogonadal elderly men with parenteral testosterone undecanoate. Exp Clin Endocrinol Diabetes. 2010;118:167–71. doi: 10.1055/s-0029-1202774. [DOI] [PubMed] [Google Scholar]
- 12.Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol. 2010;316:79–85. doi: 10.1016/j.mce.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Bhasin S, Jasjua GK, Pencina M, D’Agostino R, Sr, Coviello AD, et al. Sex hormone-binding globulin, but not testosterone, is associated prospectively and independently with incident metabolic syndrome in men: the framingham heart study. Diabetes Care. 2011;34:2464–70. doi: 10.2337/dc11-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 16.Liao CH, Huang CY, Li HY, Yu HJ, Chiang HS, et al. Testosterone and sex hormone-binding globulin have significant association with metabolic syndrome in Taiwanese men. Aging Male. 2012;15:1–6. doi: 10.3109/13685538.2011.597462. [DOI] [PubMed] [Google Scholar]
- 17.He Y, Jiang B, Wang J, Feng K, Chang Q, et al. Prevalence of the metabolic syndrome and its relation to cardiovascular disease in an elderly Chinese population. J Am Coll Cardiol. 2006;47:1588–94. doi: 10.1016/j.jacc.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 18.Chubb SA, Hyde Z, Almeida OP, Flicker L, Norman PE, et al. Lower sex hormone-binding globulin is more strongly associated with metabolic syndrome than lower total testosterone in older men: the Health in Men Study. Eur J Endocrinol. 2008;158:785–92. doi: 10.1530/EJE-07-0893. [DOI] [PubMed] [Google Scholar]
- 19.Hong D, Kim YS, Son ES, Kim KN, Kim BT, et al. Total testosterone and sex hormone-binding globulin are associated with metabolic syndrome independent of age and body mass index in Korean men. Maturitas. 2013;74:148–53. doi: 10.1016/j.maturitas.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Maggio M, Lauretani F, Ceda GP, Bandinelli S, Basaria S, et al. Association between hormones and metabolic syndrome in older Italian men. J Am Geriatr Soc. 2006;54:1832–8. doi: 10.1111/j.1532-5415.2006.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin KY, Ima-Nirwana S, Mohamed IN, Aminuddin A, Ngah WZ. Total testosterone and sex hormone-binding globulin are significantly associated with metabolic syndrome in middle-aged and elderly men. Exp Clin Endocrinol Diabetes. 2013;121:407–12. doi: 10.1055/s-0033-1345164. [DOI] [PubMed] [Google Scholar]
- 22.Liao M, Guo X, Yu X, Pang G, Zhang S, et al. Role of metabolic factors in the association between osteocalcin and testosterone in Chinese men. J Clin Endocrinol Metab. 2013;98:3463–9. doi: 10.1210/jc.2013-1805. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Ford ES, Li B, Giles WH, Liu S. Association of testosterone and sex hormone-binding globulin with metabolic syndrome and insulin resistance in men. Diabetes Care. 2010;33:1618–24. doi: 10.2337/dc09-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajamor S, Després JP, Couillard C, Lemieux S, Tremblay A, et al. Relationship between sex hormone-binding globulin levels and features of the metabolic syndrome. Metabolism. 2003;52:724–30. doi: 10.1016/s0026-0495(03)00066-0. [DOI] [PubMed] [Google Scholar]
- 25.Katabami T, Kato H, Asahina T, Hinohara S, Shin T, et al. Serum free testosterone and metabolic syndrome in Japanese men. Endocr J. 2010;57:533–9. doi: 10.1507/endocrj.k10e-018. [DOI] [PubMed] [Google Scholar]
- 26.Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005;90:2618–23. doi: 10.1210/jc.2004-1158. [DOI] [PubMed] [Google Scholar]
- 27.Haring R, Völzke H, Spielhagen C, Nauck M, Wallaschofski H. The role of sex hormone-binding globulin and testosterone in the risk of incident metabolic syndrome. Eur J Prev Cardiol. 2013;20:1061–8. doi: 10.1177/2047487312452965. [DOI] [PubMed] [Google Scholar]
- 28.Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab. 1994;79:997–1000. doi: 10.1210/jcem.79.4.7962311. [DOI] [PubMed] [Google Scholar]
- 29.Santaniemi M, Kesäniemi YA, Ukkola O. Low plasma adiponectin concentration is an indicator of the metabolic syndrome. Eur J Endocrinol. 2006;155:745–50. doi: 10.1530/eje.1.02287. [DOI] [PubMed] [Google Scholar]
- 30.Gannagé-Yared MH, Khalife S, Semaan M, Fares F, Jambart S, et al. Serum adiponectin and leptin levels in relation to the metabolic syndrome, androgenic profile and somatotropic axis in healthy non-diabetic elderly men. Eur J Endocrinol. 2006;155:167–76. doi: 10.1530/eje.1.02175. [DOI] [PubMed] [Google Scholar]
- 31.Bataille V, Perret B, Evans A, Amouyel P, Arveiler D, et al. Sex hormone-binding globulin is a major determinant of the lipid profile: the PRIME study. Atherosclerosis. 2005;179:369–73. doi: 10.1016/j.atherosclerosis.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 32.Selva DM, Hammond GL. Peroxisome-proliferator receptor gamma represses hepatic sex hormone-binding globulin expression. Endocrinology. 2009;150:2183–9. doi: 10.1210/en.2008-1289. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Szklo M, Folsom AR, Cook NR, Gapstur SM, et al. Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2012;224:228–34. doi: 10.1016/j.atherosclerosis.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips GB, Jing TY, Laragh JH, Sealey JE. Serum sex hormone levels and renin-sodium profile in men with hypertension. Am J Hypertens. 1995;8:626–9. doi: 10.1016/0895-7061(95)00056-U. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Huang X, Liao M, Gao Y, Tan A, et al. Both total testosterone and sex hormone-binding globulin are independent risk factors for metabolic syndrome: results from Fangchenggang Area Male Health and Examination Survey in China. Diabetes Metab Res Rev. 2013;29:391–7. doi: 10.1002/dmrr.2405. [DOI] [PubMed] [Google Scholar]
- 36.Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA. Sex hormone-binding globulin mediates steroid hormone signal transduction at the plasma membrane. J Steroid Biochem Mol Biol. 1999;69:481–5. doi: 10.1016/s0960-0760(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 37.Daka B, Rosen T, Jansson PA, Larsson CA, Råstam L, et al. Low sex hormone-binding globulin is associated with hypertension: a cross-sectional study in a Swedish population. BMC Cardiovasc Disord. 2013;13:30. doi: 10.1186/1471-2261-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pang XN, Yuan Y, Sun Y, Shen JP, Zha XY, et al. The relationship of sex hormone-binding globulin (SHBG) gene polymorphisms with serum SHBG level and metabolic syndrome in Chinese Han males. Aging Clin Exp Res. 2014;26:583–9. doi: 10.1007/s40520-014-0215-1. [DOI] [PubMed] [Google Scholar]
- 39.Lotti F, Corona G, Vignozzi L, Rossi M, Maseroli E, et al. Metabolic syndrome and prostate abnormalities in male subjects of infertile couples. Asian J Androl. 2014;16:295–304. doi: 10.4103/1008-682X.122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonnet F, Velayoudom Cephise FL, Gautier A, Dubois S, Massart C, et al. Role of sex steroids, intrahepatic fat and liver enzymes in the association between SHBG and metabolic features. Clin Endocrinol (Oxf) 2013;79:517–22. doi: 10.1111/cen.12089. [DOI] [PubMed] [Google Scholar]


